A Label-Free Photoelectrochemical Biosensor Based on ZnO/Cs3MnBr5 Heterogeneous Films for Alpha-Fetoprotein Detection
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Apparatus
2.3. Preparation of ZnO IO
2.4. Preparation and Modification of Cs3MnBr5 NCs
2.4.1. Synthesis of Cs-Oleates
2.4.2. Synthesis of Cs3MnBr5 NCs
2.5. Preparation of FTO/ZnO/Cs3MnBr5 Electrode
2.6. PEC Measurements
3. Results and Discussion
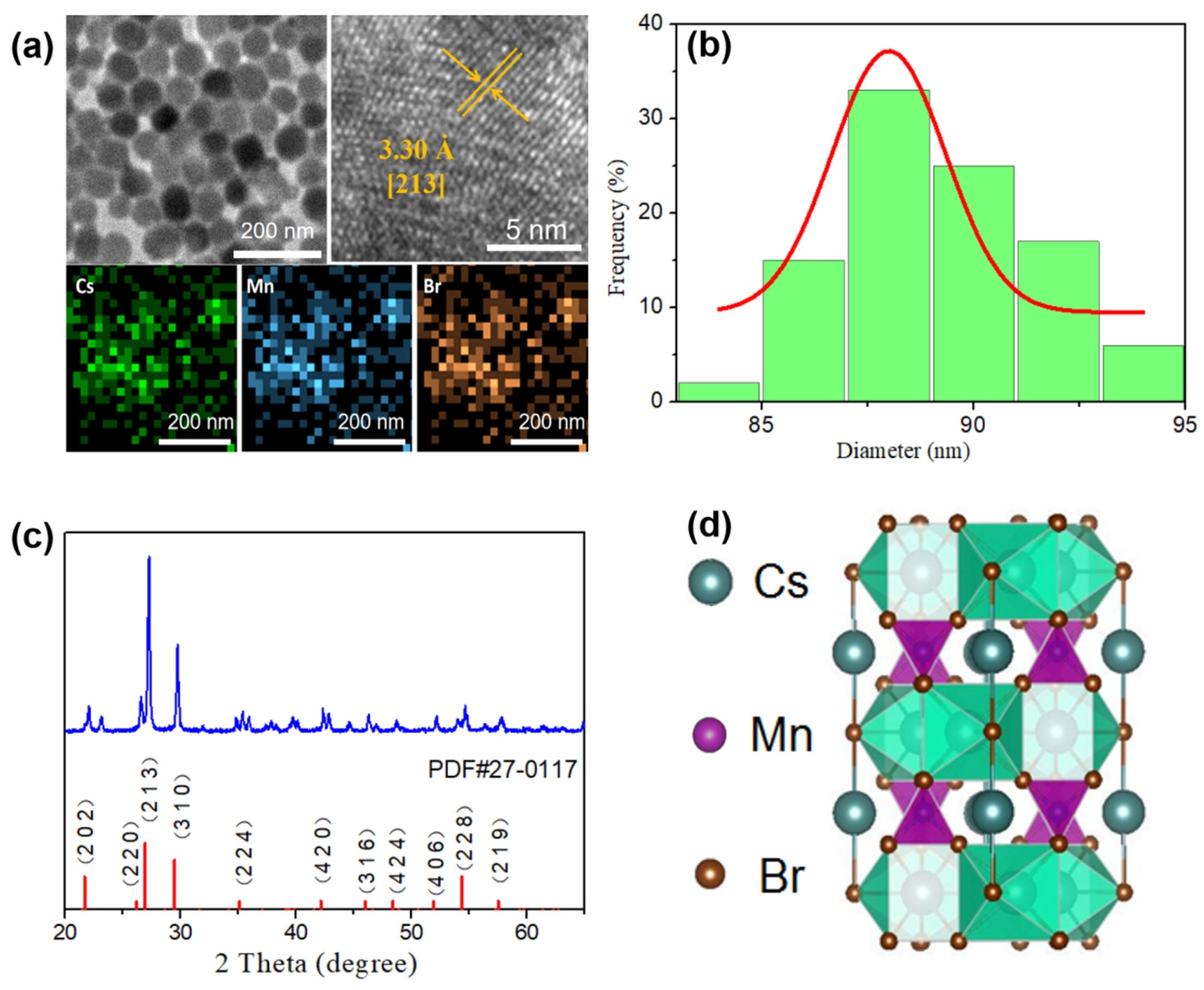
3.1. Characterization
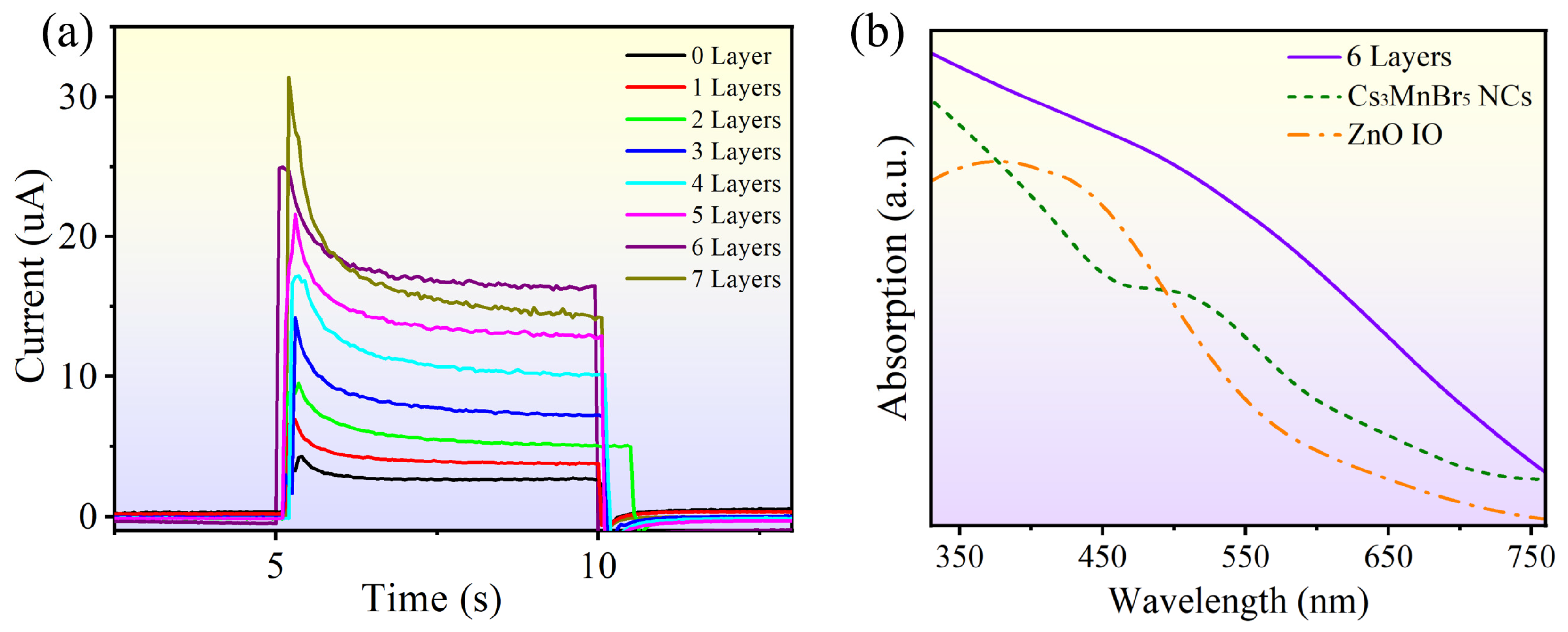
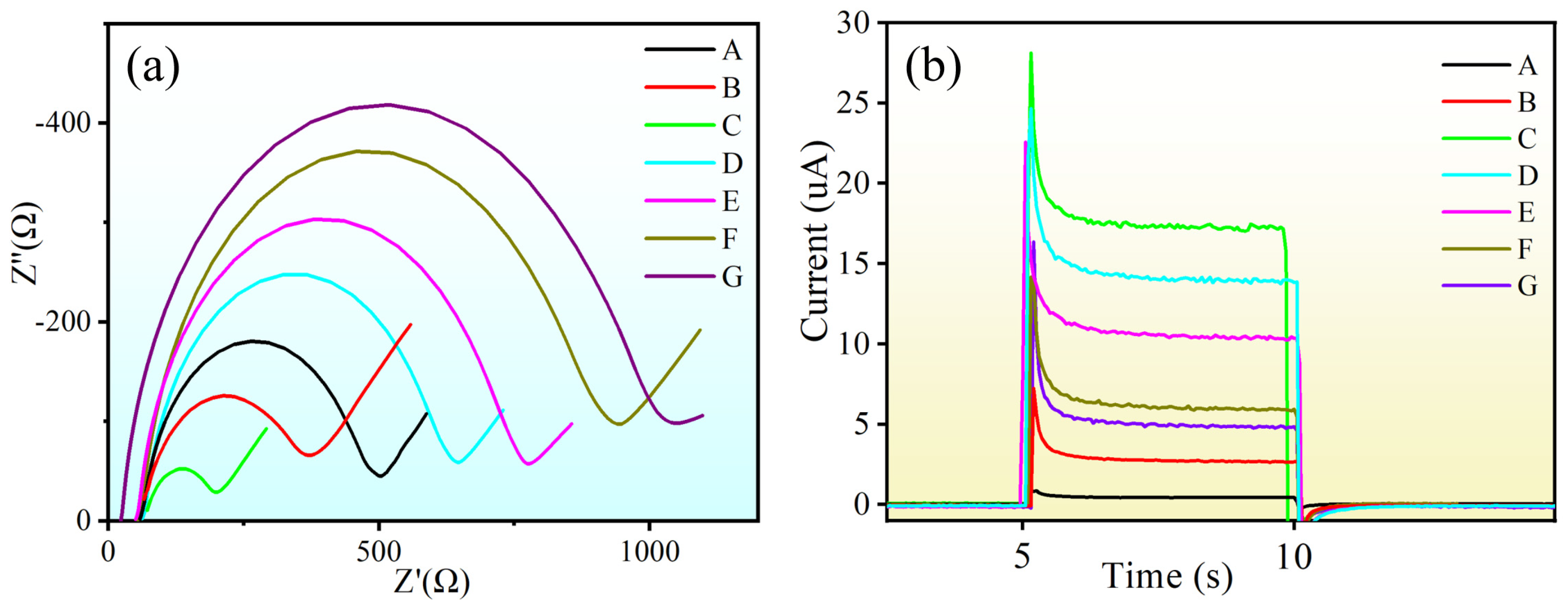
3.2. Characterization of Photoelectrochemical Biosensor Construction Process
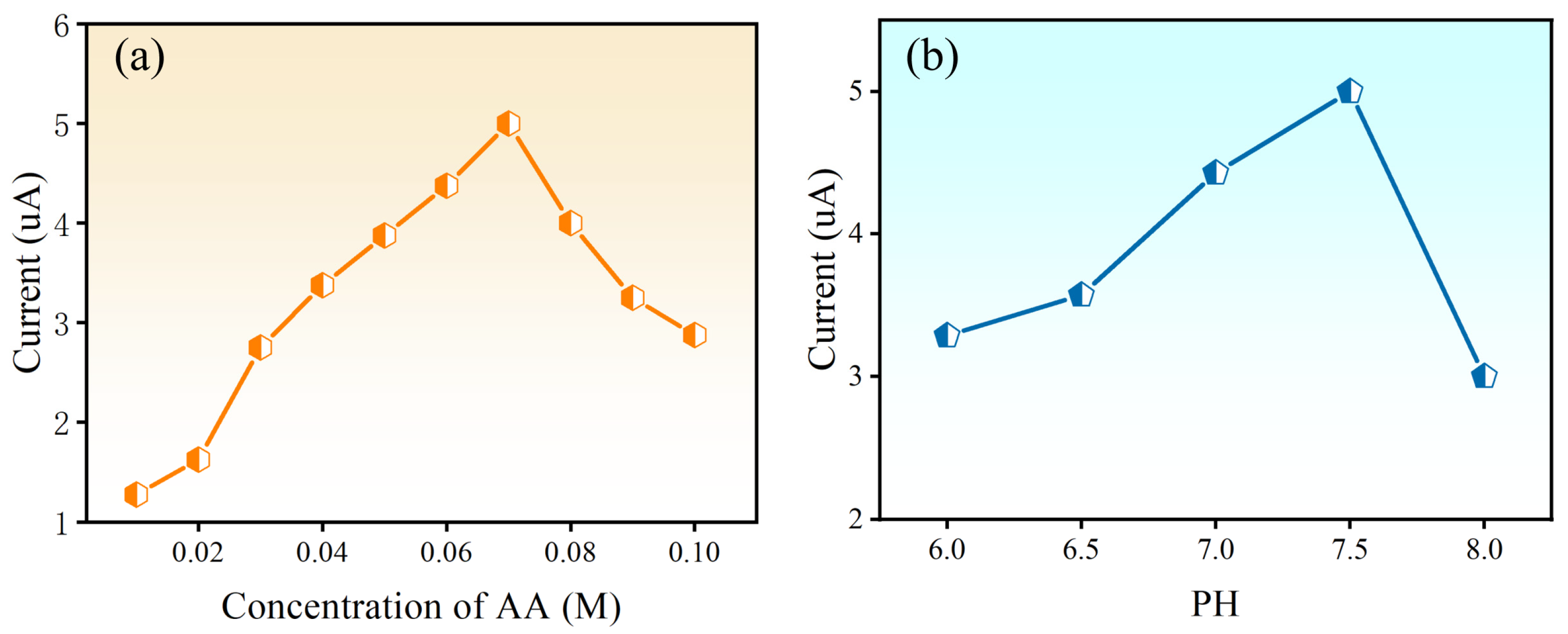
3.3. Optimization of the Detection Conditions
3.4. Photoelectrochemical Detection of AFP
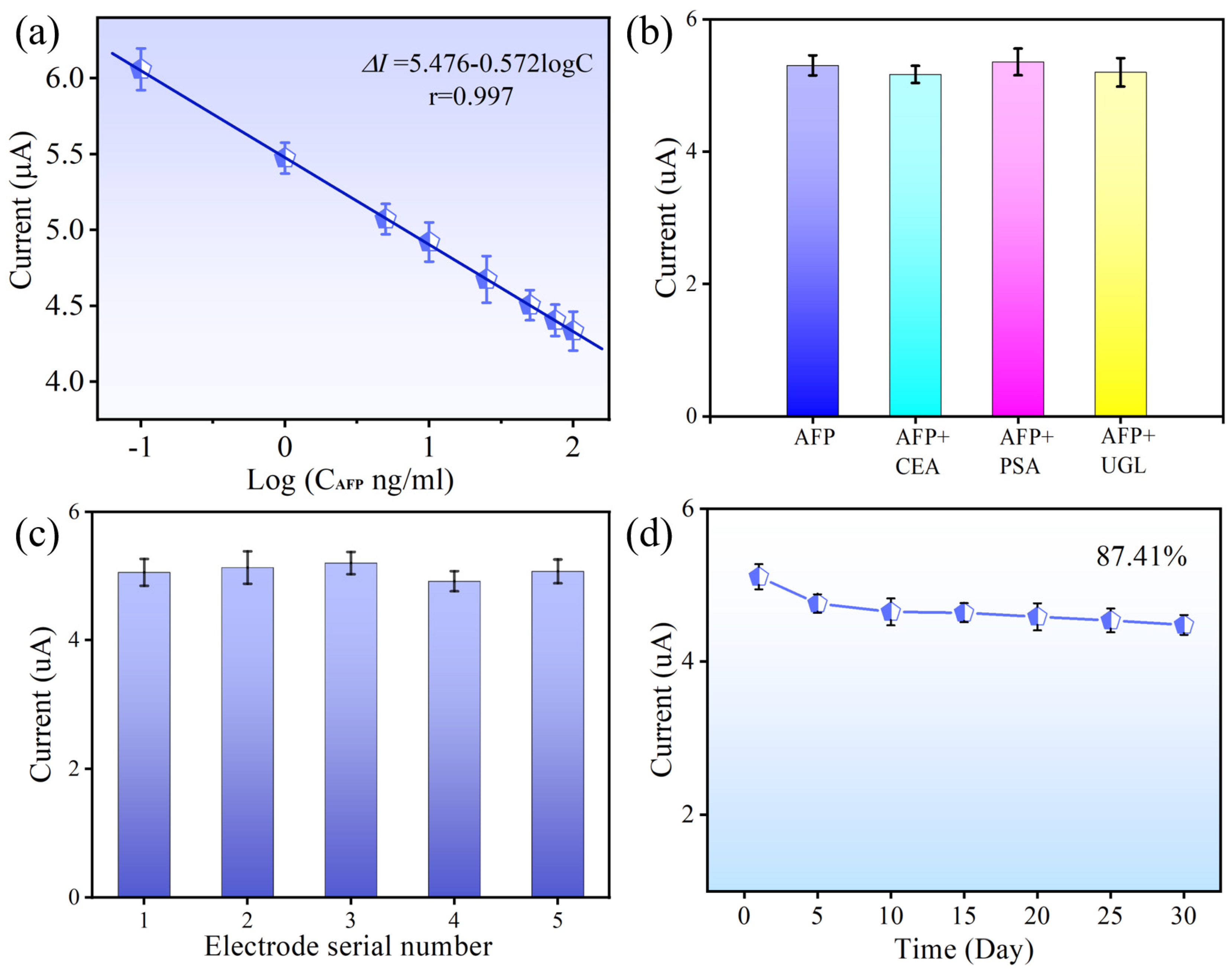
3.5. Specificity, Reproducibility, and Stability of the PEC Biosensor
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Ganesan, P.; Kulik, L.M. Hepatocellular Carcinoma New Developments. Clin. Liver Dis. 2023, 27, 85–102. [Google Scholar] [CrossRef] [PubMed]
- Greten, T.F.; Lai, C.W.; Li, G.; Staveley-O’Carroll, K.F. Targeted and Immune-Based Therapies for Hepatocellular Carcinoma. Gastroenterology 2019, 156, 510–524. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.; Sun, A.; Zhao, Y.; Ying, W.; Sun, H.; Yang, X.; Xing, B.; Sun, W.; Ren, L.; Hu, B.; et al. Proteomics identifies new therapeutic targets of early-stage hepatocellular carcinoma. Nature 2019, 567, 257–261. [Google Scholar] [CrossRef] [PubMed]
- Tan, D.J.H.; Ng, C.H.; Lin, S.Y.; Pan, X.H.; Tay, P.; Lim, W.H.; Teng, M.; Syn, N.; Lim, G.; Yong, J.N.; et al. Clinical characteristics, surveillance, treatment allocation, and outcomes of non-alcoholic fatty liver disease-related hepatocellular carcinoma: A systematic review and meta-analysis. Lancet Oncol. 2022, 23, 521–530. [Google Scholar] [CrossRef] [PubMed]
- Torimura, T.; Iwamoto, H. Treatment and the prognosis of hepatocellular carcinoma in Asia. Liver Int. 2022, 42, 2042–2054. [Google Scholar] [CrossRef] [PubMed]
- Vogel, A.; Meyer, T.; Sapisochin, G.; Salem, R.; Saborowski, A. Hepatocellular carcinoma. Lancet 2022, 400, 1345–1362. [Google Scholar] [CrossRef]
- Beaufrere, A.; Calderaro, J.; Paradis, V. Combined hepatocellular-cholangiocarcinoma: An update. J. Hepatol. 2021, 74, 1212–1224. [Google Scholar] [CrossRef]
- El-Mahdy, H.A.; Sallam, A.-A.M.; Ismail, A.; Elkhawaga, S.Y.; Elrebehy, M.A.; Doghish, A.S. miRNAs inspirations in hepatocellular carcinoma: Detrimental and favorable aspects of key performers. Pathol. Res. Pract. 2022, 233, 153886. [Google Scholar] [CrossRef]
- Hanif, H.; Ali, M.J.; Susheela, A.T.; Khan, I.W.; Luna-Cuadros, M.A.; Khan, M.M.; Lau, D.T.-Y. Update on the applications and limitations of alpha-fetoprotein for hepatocellular carcinoma. World J. Gastroenterol. 2022, 28, 216–229. [Google Scholar] [CrossRef]
- Llovet, J.M.; Pinyol, R.; Kelley, R.K.; El-Khoueiry, A.; Reeves, H.L.; Wang, X.W.; Gores, G.J.; Villanueva, A. Molecular pathogenesis and systemic therapies for hepatocellular carcinoma. Nat. Cancer 2022, 3, 386–401. [Google Scholar] [CrossRef]
- Hu, X.; Chen, R.; Wei, Q.; Xu, X. The Landscape of Alpha Fetoprotein in Hepatocellular Carcinoma: Where Are We? Int. J. Biol. Sci. 2022, 18, 536–551. [Google Scholar] [CrossRef] [PubMed]
- Johnson, P.; Zhou, Q.; Dao, D.Y.; Lo, Y.M.D. Circulating biomarkers in the diagnosis and management of hepatocellular carcinoma. Nat. Rev. Gastroenterol. Hepatol. 2022, 19, 670–681. [Google Scholar] [CrossRef] [PubMed]
- Pilvenyte, G.; Ratautaite, V.; Boguzaite, R.; Ramanavicius, A.; Viter, R.; Ramanavicius, S. Molecularly Imprinted Polymers for the Determination of Cancer Biomarkers. Int. J. Mol. Sci. 2023, 24, 4105. [Google Scholar] [CrossRef] [PubMed]
- Cimbalo, A.; Alonso-Garrido, M.; Font, G.; Manyes, L. Toxicity of mycotoxins in vivo on vertebrate organisms: A review. Food Chem. Toxicol. 2020, 137, 111161. [Google Scholar] [CrossRef] [PubMed]
- Tan, G.; Wang, S.; Yu, J.; Chen, J.; Liao, D.; Liu, M.; Nezamzadeh-Ejhieh, A.; Pan, Y.; Liu, J. Detection mechanism and the outlook of metal-organic frameworks for the detection of hazardous substances in milk. Food Chem. 2024, 430, 136934. [Google Scholar] [CrossRef] [PubMed]
- Haupt, K.; Rangel, P.X.M.; Bui, B.T.S. Molecularly Imprinted Polymers: Antibody Mimics for Bioimaging and Therapy. Chem. Rev. 2020, 120, 9554–9582. [Google Scholar] [CrossRef]
- Hlavacek, A.; Farka, Z.; Mickert, M.J.; Kostiv, U.; Brandmeier, J.C.; Horak, D.; Skladal, P.; Foret, F.; Gorris, H.H. Bioconjugates of photon-upconversion nanoparticles for cancer biomarker detection and imaging. Nat. Protoc. 2022, 17, 1028–1072. [Google Scholar] [CrossRef]
- Khursheed, R.; Dua, K.; Vishwas, S.; Gulati, M.; Jha, N.K.; Aldhafeeri, G.M.; Alanazi, F.G.; Goh, B.H.; Gupta, G.; Paudel, K.R.; et al. Biomedical applications of metallic nanoparticles in cancer: Current status and future perspectives. Biomed. Pharmacother. 2022, 150, 112951. [Google Scholar] [CrossRef] [PubMed]
- Nejabat, M.; Samie, A.; Ramezani, M.; Alibolandi, M.; Abnous, K.; Taghdisi, S.M. An Overview on Gold Nanorods as Versatile Nanoparticles in Cancer Therapy. J. Control. Release 2023, 354, 221–242. [Google Scholar] [CrossRef]
- Pan, C.; Mao, Z.; Yuan, X.; Zhang, H.; Mei, L.; Ji, X. Heterojunction Nanomedicine. Adv. Sci. 2022, 9, 2105747. [Google Scholar] [CrossRef]
- Singh, S.K.; Mazumder, S.; Vincy, A.; Hiremath, N.; Kumar, R.; Banerjee, I.; Vankayala, R. Review of Photoresponsive Plasmonic Nanoparticles That Produce Reactive Chemical Species for Photodynamic Therapy of Cancer and Bacterial Infections. ACS Appl. Nano Mater. 2023, 6, 1508–1521. [Google Scholar] [CrossRef]
- Chaudhary, V.; Khanna, V.; Awan, H.T.A.; Singh, K.; Khalid, M.; Mishra, Y.K.; Bhansali, S.; Li, C.-Z.; Kaushik, A. Towards hospital-on-chip supported by 2D MXenes-based 5th generation intelligent biosensors. Biosens. Bioelectron. 2023, 220. [Google Scholar] [CrossRef] [PubMed]
- Iravani, S.; Varma, R.S. Green synthesis, biomedical and biotechnological applications of carbon and graphene quantum dots. A review. Environ. Chem. Lett. 2020, 18, 703–727. [Google Scholar] [CrossRef] [PubMed]
- Jafari, S.; Mahyad, B.; Hashemzadeh, H.; Janfaza, S.; Gholikhani, T.; Tayebi, L. Biomedical Applications of TiO2 Nanostructures: Recent Advances. Int. J. Nanomed. 2020, 15, 3447–3470. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Q.; Tang, D. Recent advances in photoelectrochemical biosensors for analysis of mycotoxins in food. Trac-Trends Anal. Chem. 2020, 124, 115814. [Google Scholar] [CrossRef]
- Li, J.; Liu, D.; Zhou, D.; Shao, L.; Chen, X.; Song, H. Label-free photoelectrochemical biosensor for alpha-fetoprotein detection based on Au/CsxWO3 heterogeneous films. Talanta 2021, 225, 122074. [Google Scholar] [CrossRef]
- Li, N.; Wang, C.; Chen, L.; Ye, C.; Peng, Y. Ultrathin Covalent Organic Framework Nanosheets/Ti3C2Tx-Based Photoelectrochemical Biosensor for Efficient Detection of Prostate-Specific Antigen. Molecules 2022, 27, 6732. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.; Zhang, X.; Zhong, S.; Gao, Y.; Cui, X. Aggregation-induced emission property of pectin from orange peel and its multiple applications. Colloids Surf. A-Physicochem. Eng. Asp. 2022, 647, 129087. [Google Scholar] [CrossRef]
- Xing, Y.; Chen, X.; Jin, B.; Chen, P.; Huang, C.; Jin, Z. Photoelectrochemical Aptasensors Constructed with Photosensitive PbS Quantum Dots/TiO2 Nanoparticles for Detection of Kanamycin. Langmuir 2021, 37, 3612–3619. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Li, X.; Xiang, M.-H.; Gao, F.; Qu, F.; Li, M.; Lu, L. Enzyme-free nucleic acid dual-amplification strategy combined with mimic enzyme catalytic precipitation reaction for the photoelectrochemical detection of microRNA-21. Microchim. Acta 2022, 189, 249. [Google Scholar] [CrossRef]
- Qiu, Z.; Lei, Y.; Lin, X.; Zhu, J.; Zeng, R.; Sa, R.; Tang, D.; Chen, Q.; Chen, Y. A laser-induced zinc oxide/graphene photoelectrode for a photocurrent-polarity-switching photoelectrochemical biosensor with bipedal DNA walker amplification. J. Mater. Chem. B 2024, 12, 984–990. [Google Scholar] [CrossRef]
- Tseng, K.-W.; Hsiao, Y.-P.; Jen, C.-P.; Chang, T.-S.; Wang, H.-C. Cu2O/PEDOT:PSS/ZnO Nanocomposite Material Biosensor for Esophageal Cancer Detection. Sensors 2020, 20, 2455. [Google Scholar] [CrossRef]
- Ge, L.; Hou, R.; Cao, Y.; Tu, J.; Wu, Q. Photoelectrochemical enzymatic sensor for glucose based on Au@C/TiO2 nanorod arrays. Rsc Adv. 2020, 10, 44225–44231. [Google Scholar] [CrossRef]
- Yang, J.; Fu, S.; Luo, F.; Guo, L.; Qiu, B.; Lin, Z. Homogeneous photoelectrochemical biosensor for microRNA based on target-responsive hydrogel coupled with exonuclease III and nicking endonuclease Nb.BbvCI assistant cascaded amplification strategy. Microchim. Acta 2021, 188, 267. [Google Scholar] [CrossRef]
- Yu, Q.; Fu, Y.; Xiao, K.; Zhang, X.; Du, C.; Chen, J. A label-free photoelectrochemical biosensor with ultra-low-background noise for lead ion assay based on the Cu2O-CuO-TiO2 heterojunction. Anal. Chim. Acta 2022, 1195, 339456. [Google Scholar] [CrossRef]
- Zhao, C.; Jing, T.; Tian, J.; Guo, J.; Wu, M.; Shi, D.; Zhao, Z.; Guo, Z. Visible light-driven photoelectrochemical enzyme biosensor based on reduced graphene oxide/titania for detection of glucose. J. Nanostruct. Chem. 2022, 12, 193–205. [Google Scholar] [CrossRef]
- Zhao, K.; Yan, X.; Gu, Y.; Kang, Z.; Bai, Z.; Cao, S.; Liu, Y.; Zhang, X.; Zhang, Y. Self-Powered Photoelectrochemical Biosensor Based on CdS/RGO/ZnO Nanowire Array Heterostructure. Small 2016, 12, 245–251. [Google Scholar] [CrossRef]
- Xiao, M.; Zhu, M.; Yuan, R.; Yuan, Y. Dual-sensitized heterojunction PDA/ZnO@MoS2 QDs combined with multilocus domino-like DNA cascade reaction for ultrasensitive photoelectrochemical biosensor. Biosens. Bioelectron. 2023, 227, 115151. [Google Scholar] [CrossRef]
- Weng, Q.; Zheng, X.; Zhang, S.; Zhu, L.; Huang, Q.; Liu, P.; Li, X.; Kang, J.; Han, Z. A photoelectrochemical immunosensor based on natural pigment sensitized ZnO for alpha-fetoprotein detection. J. Photochem. Photobiol. A-Chem. 2020, 388, 112200. [Google Scholar] [CrossRef]
- Huang, J.; Cui, K.; Li, L.; Li, X.; Wang, F.; Wang, Y.; Zhang, Y.; Ge, S.; Yu, J. Paper-Supported Photoelectrochemical Biosensor for Dual-Mode miRNA-106a Assay: Integration of Luminescence-Confined Upconversion-Actuated Fluorescent Resonance Energy Transfer and CRISPR/Cas13a-Powered Cascade DNA Circuits. Langmuir 2023, 39, 16048–16059. [Google Scholar] [CrossRef]
- Zheng, D.; Yang, J.; Zheng, Z.; Peng, M.; Ng, K.-M.; Chen, Y.; Huang, L.; Gao, W. Sensitive photoelectrochemical detection of colitoxin DNA based on NCDs@CuO/ZnO heterostructured nanocomposites with efficient separation capacity of photo-induced carriers. Microchim. Acta 2022, 189, 166. [Google Scholar] [CrossRef]
- Zhao, Y.; Gong, J.; Zhang, X.; Kong, R.; Qu, F. Enhanced biosensing platform constructed using urchin-like ZnO-Au@CdS microspheres based on the combination of photoelectrochemical and bioetching strategies. Sens. Actuators B-Chem. 2018, 255, 1753–1761. [Google Scholar] [CrossRef]
- Shao, L.; Zhou, D.; Ding, N.; Sun, R.; Xu, W.; Wang, N.; Xu, S.; Liu, D.; Song, H. Broadband Ultraviolet Photodetectors Based on Cerium Doped Lead-Free Cs3MnBr5 Metal Halide Nanocrystals. Acs Sustain. Chem. Eng. 2021, 9, 4980–4987. [Google Scholar] [CrossRef]
- You, X.; Pikul, J.H.; King, W.P.; Pak, J.J. Zinc oxide inverse opal enzymatic biosensor. Appl. Phys. Lett. 2013, 102, 253103. [Google Scholar] [CrossRef]
- Jiang, Y.; Liu, D.; Yang, Y.; Xu, R.; Zhang, T.; Sheng, K.; Song, H. Photoelectrochemical detection of alpha-fetoprotein based on ZnO inverse opals structure electrodes modified by Ag2S nanoparticles. Sci. Rep. 2016, 6, 38400. [Google Scholar] [CrossRef]
- Xu, R.; Jiang, Y.; Xia, L.; Zhang, T.; Xu, L.; Zhang, S.; Liu, D.; Song, H. A sensitive photoelectrochemical biosensor for AFP detection based on ZnO inverse opal electrodes with signal amplification of CdS-QDs. Biosens. Bioelectron. 2015, 74, 411–417. [Google Scholar] [CrossRef]
- Xia, L.; Song, J.; Xu, R.; Liu, D.; Dong, B.; Xu, L.; Song, H. Zinc oxide inverse opal electrodes modified by glucose oxidase for electrochemical and photoelectrochemical biosensor. Biosens. Bioelectron. 2014, 59, 350–357. [Google Scholar] [CrossRef]
- Rahmanian, R.; Mozaffari, S.A. Electrochemical fabrication of ZnO-polyvinyl alcohol nanostructured hybrid film for application to urea biosensor. Sens. Actuators B-Chem. 2015, 207, 772–781. [Google Scholar] [CrossRef]
- Chen, X.; Xu, W.; Jiang, Y.; Pan, G.; Zhou, D.; Zhu, J.; Wang, H.; Chen, C.; Li, D.; Song, H. A novel upconversion luminescence derived photoelectrochemical immunoassay: Ultrasensitive detection to alpha-fetoprotein. Nanoscale 2017, 9, 16357–16364. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shao, L.; Zhang, B.; Wu, W.; Cui, G.; Liu, M. A Label-Free Photoelectrochemical Biosensor Based on ZnO/Cs3MnBr5 Heterogeneous Films for Alpha-Fetoprotein Detection. Nanomaterials 2024, 14, 1127. https://doi.org/10.3390/nano14131127
Shao L, Zhang B, Wu W, Cui G, Liu M. A Label-Free Photoelectrochemical Biosensor Based on ZnO/Cs3MnBr5 Heterogeneous Films for Alpha-Fetoprotein Detection. Nanomaterials. 2024; 14(13):1127. https://doi.org/10.3390/nano14131127
Chicago/Turabian StyleShao, Long, Biyu Zhang, Wei Wu, Gengyan Cui, and Mao Liu. 2024. "A Label-Free Photoelectrochemical Biosensor Based on ZnO/Cs3MnBr5 Heterogeneous Films for Alpha-Fetoprotein Detection" Nanomaterials 14, no. 13: 1127. https://doi.org/10.3390/nano14131127
APA StyleShao, L., Zhang, B., Wu, W., Cui, G., & Liu, M. (2024). A Label-Free Photoelectrochemical Biosensor Based on ZnO/Cs3MnBr5 Heterogeneous Films for Alpha-Fetoprotein Detection. Nanomaterials, 14(13), 1127. https://doi.org/10.3390/nano14131127





