TiO2 Nanoparticles with Adjustable Phase Composition Prepared by an Inverse Microemulsion Method: Physicochemical Characterization and Photocatalytic Properties
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
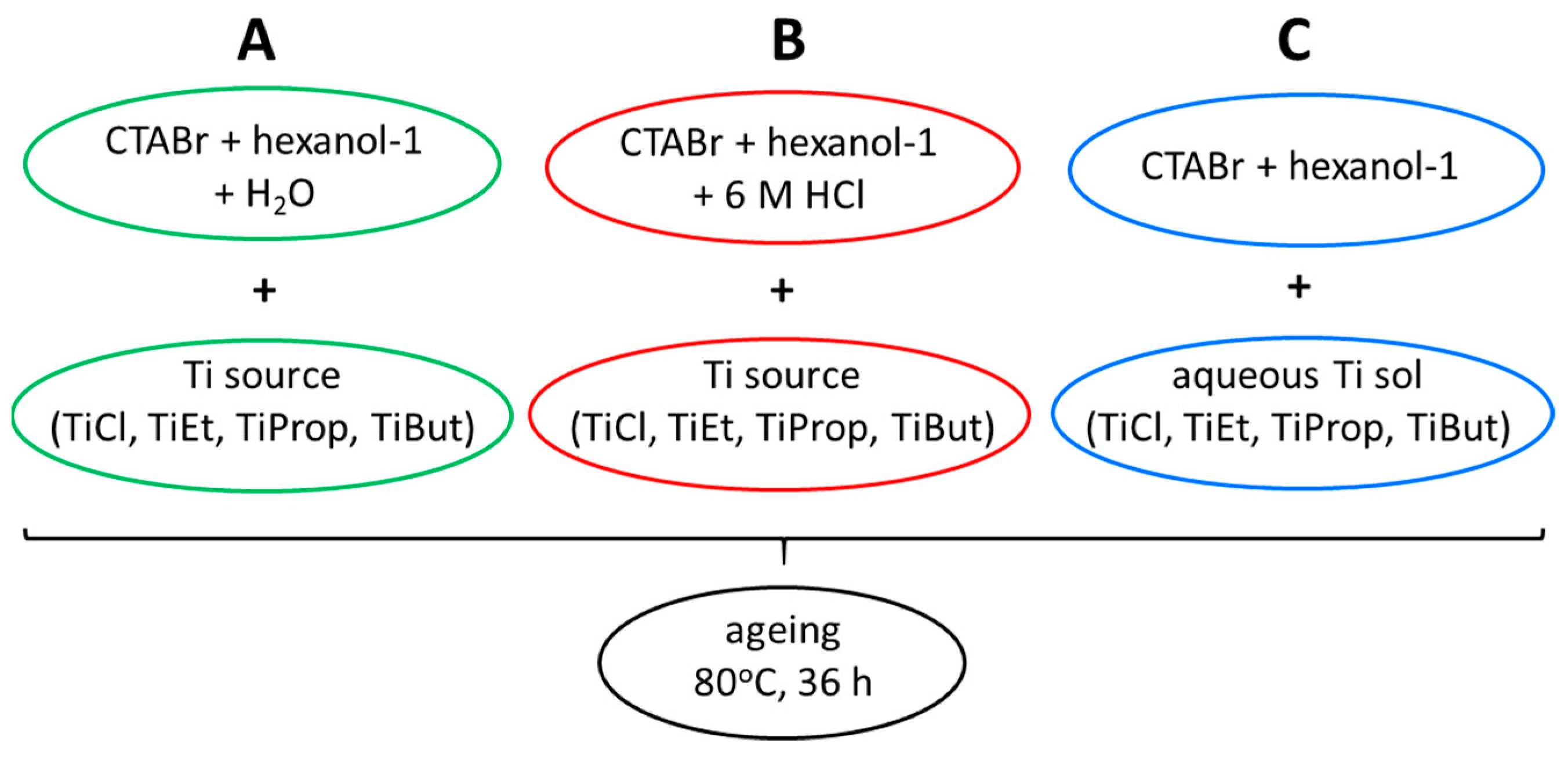
- The first reverse microemulsion was prepared using distilled water as the aqueous phase. In a typical experiment, water, CTABr, and 1-hexanol were mixed together in a glass beaker in the weight ratio 17:28:55 and stirred magnetically until the mixture turned transparent, indicating the formation of an inverse microemulsion. Subsequently, the required Ti compound was added dropwise to the stirred microemulsion in the amount yielding 3.7 wt.% Ti in the aqueous phase. The liquid remained clear;
- The second reverse microemulsion was prepared using 6 M HCl solution as the aqueous phase. Otherwise, the synthetic procedure was identical to that of variant A. Also, here the obtained transparent inverse microemulsion remained clear after the addition of any of the Ti sources;
- The third reverse microemulsion was prepared using one of the prepared Ti sols as the aqueous component. In a typical experiment, aqueous Ti sol, CTABr, and 1-hexanol were mixed together in the weight ratio 17:28:55 and stirred magnetically until the mixture turned transparent.
2.2. Methods
3. Results and discussion
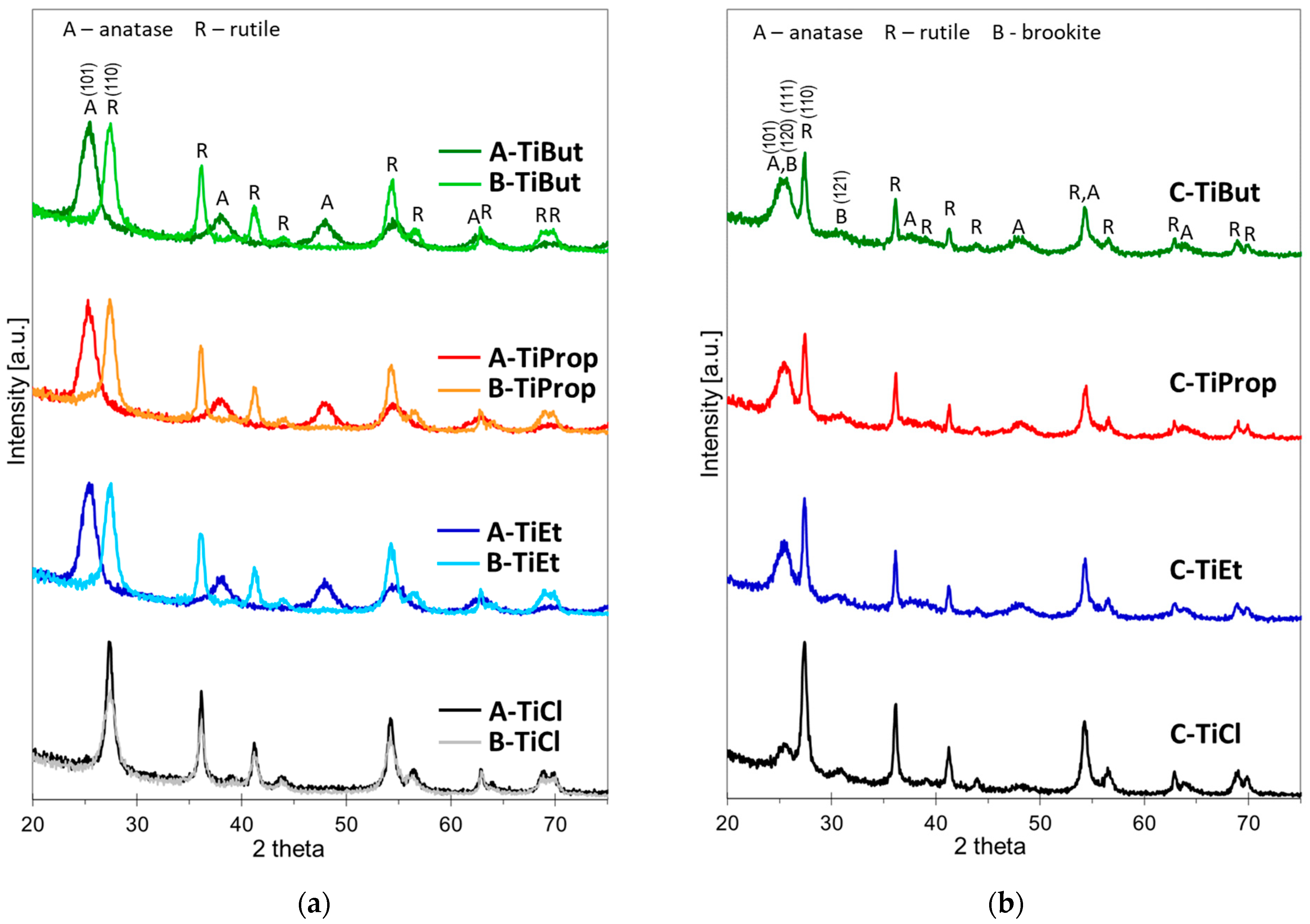
3.1. X-ray Diffraction Analysis
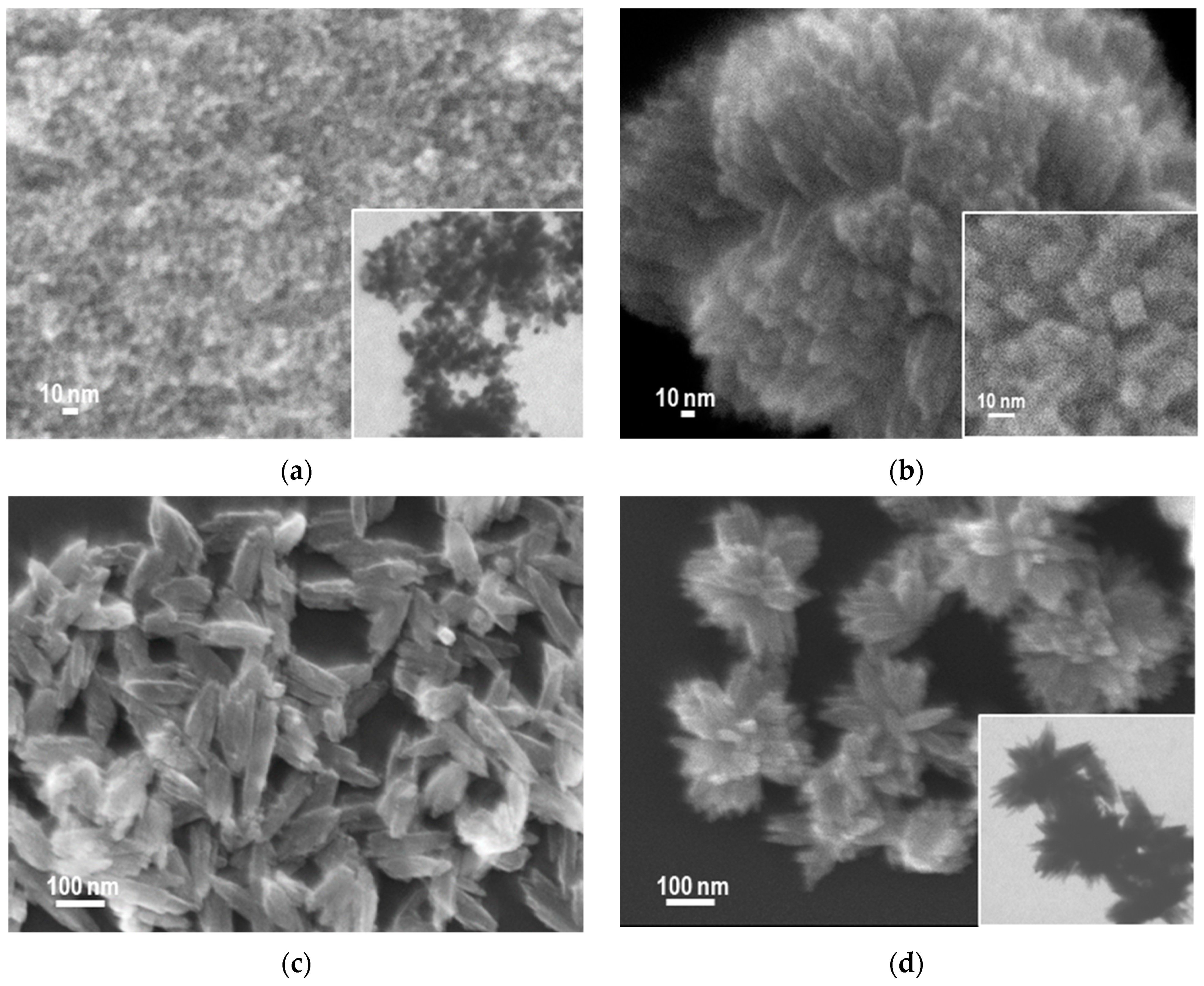
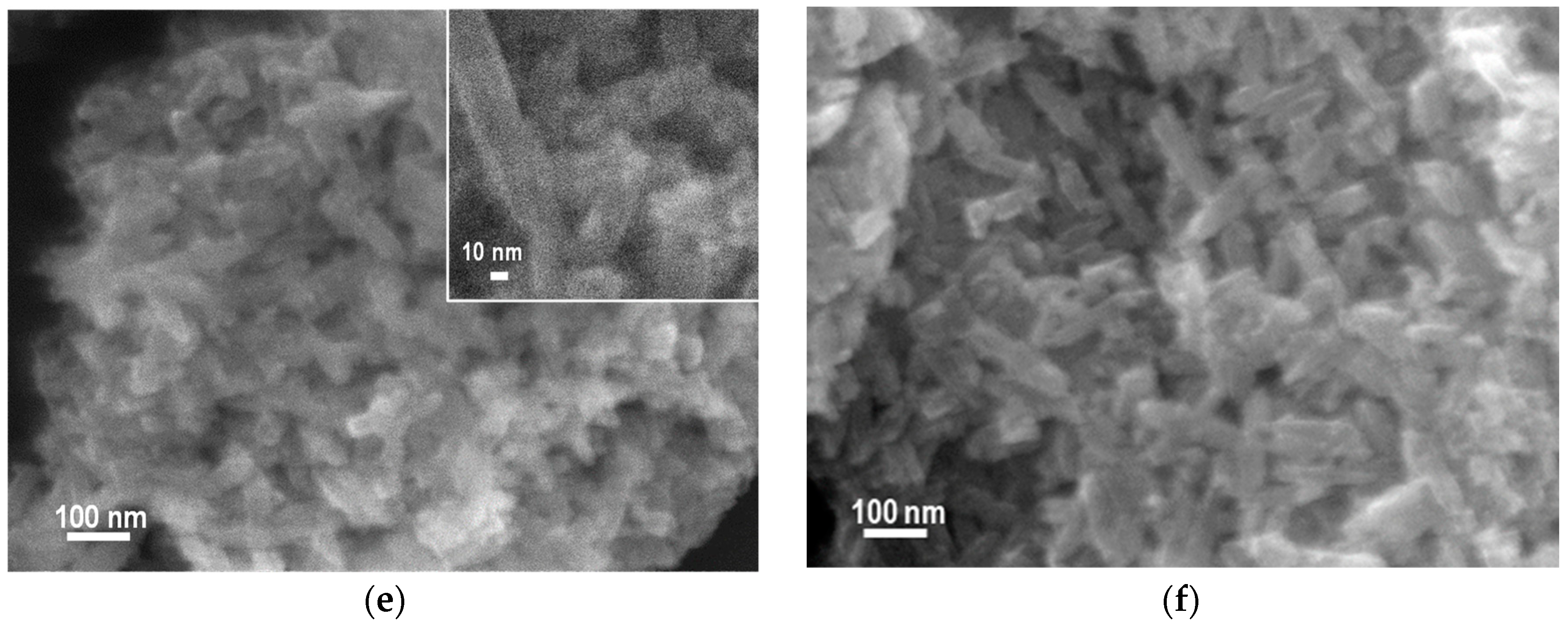
3.2. SEM/TEM Analysis
3.3. Textural Properties
3.4. Vibrational Spectroscopy
3.4.1. FTIR Spectroscopy
3.4.2. Raman Spectroscopy
3.5. UV–vis Diffuse Reflectance Spectroscopy
3.6. Photocatalytic Experiments
4. Conclusions
- Anatase NPs were formed when the alkoxides were added to a microemulsion containing pure H2O as the aqueous phase (method A);
- Rutile NPs crystallized when the alkoxides were added to a microemulsion containing 6 M HCl as the aqueous phase (method B), and when TiCl4 was added to a microemulsion containing water (method A) or 6 M HCl (method B) as the aqueous phase;
- Mixed-phase anatase/rutile/brookite NPs were obtained when the aqueous phase used to form the microemulsions contained Ti precursor (method C).
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Verma, R.; Gangwar, J.; Srivastava, A.K. Multiphase TiO2 nanostructures: A review of efficient synthesis, growth mechanism, probing capabilities, and applications in bio-safety and health. RSC Adv. 2017, 7, 44199–44224. [Google Scholar]
- Haider, A.J.; Jameel, Z.N.; Al-Hussaini, I.H.M. Review on: Titanium dioxide applications. Energy Procedia 2019, 157, 17–29. [Google Scholar] [CrossRef]
- Mironyuk, I.F.; Soltys, L.M.; Tatarchuk, T.R.; Tsinurchyn, V.I. Ways to improve the efficiency of TiO2-based photocatalysts (Review). Phys. Chem. Solid State 2020, 21, 300–311. [Google Scholar]
- Serwicka, E.M. Titania-clay mineral composites for environmental catalysis and photocatalysis. Catalysts 2021, 11, 1087. [Google Scholar] [CrossRef]
- Tian, X.; Cui, X.; Lai, T.; Ren, J.; Yang, Z.; Xiao, M.; Wang, B.; Xiao, X.; Wang, Y. Gas sensors based on TiO2 nanostructured materials for the detection of hazardous gases: A review. Nano Mater. Sci. 2021, 3, 390–403. [Google Scholar]
- Benčina, M.; Iglič, A.; Mozetič, M.; Junkar, I. Crystallized TiO2 Nanosurfaces in Biomedical Applications. Nanomaterials 2020, 10, 1121. [Google Scholar] [CrossRef] [PubMed]
- Liang, S.; Wang, X.; Cheng, Y.J.; Xia, Y.; Müller-Buschbaum, P. Anatase titanium dioxide as rechargeable ion battery electrode—A chronological review. Energy Storage Mater. 2022, 45, 201–264. [Google Scholar] [CrossRef]
- Daghrir, R.; Drogui, P.; Robert, D. Modified TiO2 for environmental photocatalytic applications: A review. Ind. Eng. Chem. Res. 2013, 52, 3581–3599. [Google Scholar]
- Wang, J.; Wang, Z.; Wang, W.; Wang, Y.; Hu, X.; Liu, J.; Gong, X.; Miao, W.; Ding, L.; Li, X.; et al. Synthesis, modification and application of titanium dioxide nanoparticles: A review. Nanoscale 2022, 14, 6709. [Google Scholar] [CrossRef]
- Arun, J.; Nachiappan, S.; Rangarajan, G.; Alagappan, R.P.; Gopinath, K.P.; Lichtfouse, E. Synthesis and application of titanium dioxide photocatalysis for energy, decontamination and viral disinfection: A review. Environ. Chem. Lett. 2023, 21, 339–362. [Google Scholar] [CrossRef]
- Zhang, H.; Banfield, J.F. Understanding Polymorphic Phase Transformation Behavior during Growth of Nanocrystalline Aggregates: Insights from TiO2. J. Phys. Chem. B 2000, 104, 3481–3487. [Google Scholar] [CrossRef]
- Wang, Y.; He, Y.; Lai, Q.; Fan, M. Review of the progress in preparing nano TiO2: An important environmental engineering material. J. Environ. Sci. 2014, 26, 2139–2177. [Google Scholar]
- Irshad, M.A.; Nawaz, R.; Rehman, M.Z.; Adrees, M.; Rizwan, M.; Ali, S.; Ahmad, S.; Tasleem, S. Synthesis, characterization and advanced sustainable applications of titanium dioxide nanoparticles: A review. Ecotoxicol. Environ. Saf. 2021, 212, 111978. [Google Scholar] [CrossRef] [PubMed]
- Nyamukamba, P.; Okoh, O.; Mungondori, H.; Taziwa, R.; Zinya, S. Synthetic Methods for Titanium Dioxide Nanoparticles: A Review. In Titanium Dioxide—Material for a Sustainable Environment; IntechOpen: London, UK, 2018. [Google Scholar]
- Qamar, O.A.; Jamil, F.; Hussain, M.; Bae, S.; Inayat, A.; Shah, N.S.; Waris, A.; Akhterf, P.; Kwon, E.E.; Park, Y.K. Advances in synthesis of TiO2 nanoparticles and their application to biodiesel production: A review. Chem. Eng. J. 2023, 460, 141734. [Google Scholar] [CrossRef]
- Ganguli, A.K.; Ganguly, A.; Vaidya, S. Microemulsion-based synthesis of nanocrystalline materials. Chem. Soc. Rev. 2010, 39, 474–485. [Google Scholar] [CrossRef]
- Khadzhiev, S.N.; Kadiev, K.M.; Yampolskaya, G.P.; Kadieva, M.K. Trends in the synthesis of metal oxide nanoparticles through reverse microemulsions in hydrocarbon media. Adv. Colloid Interface Sci. 2013, 197-198, 132–145. [Google Scholar] [CrossRef]
- Sharma, S.; Yadav, N.; Chowdhury, P.K.; Ganguli, A.K. Controlling the Microstructure of Reverse Micelles and Their Templating Effect on Shaping Nanostructures. J. Phys. Chem. B 2015, 119, 11295–11306. [Google Scholar] [CrossRef] [PubMed]
- Hsu, C.-Y.; Mahmoud, Z.H.; Abdullaev, S.; Ali, F.K.; Naeem, Y.A.; Mizher, R.M.; Karim, M.M.; Abdulwahid, A.S.; Ahmadi, Z.; Habibzadeh, S.; et al. Nano titanium oxide (nano-TiO2): A review of synthesis methods, properties, and applications. Case Stud. Chem. Environ. Eng. 2024, 9, 100626. [Google Scholar] [CrossRef]
- Wu, M.; Long, J.; Huang, A.; Luo, Y.; Feng, S.; Xu, R. Microemulsion-Mediated Hydrothermal Synthesis and Characterization of Nanosize Rutile and Anatase Particles. Langmuir 1999, 15, 8822–8825. [Google Scholar] [CrossRef]
- Mori, Y.; Okastu, Y.; Tsujimoto, Y. Titanium dioxide nanoparticles produced in water-in-oil emulsion. J. Nanoparticle Res. 2001, 3, 219–225. [Google Scholar] [CrossRef]
- Andersson, M.; Österlund, L.; Ljungström, S.; Palmqvist, A. Preparation of Nanosize Anatase and Rutile TiO2 by Hydrothermal Treatment of Microemulsions and Their Activity for Photocatalytic Wet Oxidation of Phenol. J. Phys. Chem. B 2002, 106, 10674–10679. [Google Scholar] [CrossRef]
- Zhang, D.; Qi, L.; Ma, J.; Cheng, H. Formation of crystalline nanosized titania in reverse micelles at room temperature. J. Mater. Chem. 2002, 12, 3677–3680. [Google Scholar] [CrossRef]
- Yan, M.; Chen, F.; Zhang, J.; Anpo, M. Preparation of Controllable Crystalline Titania and Study on the Photocatalytic Properties. J. Phys. Chem. B 2005, 109, 8673–8678. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.S.; Park, S.S.; Lee, G.D.; Ju, C.S.; Hong, S.S. Synthesis of TiO2 particles by reverse microemulsion method using nonionic surfactants with different hydrophilic and hydrophobic group and their photocatalytic activity. Catal. Today 2005, 101, 283–290. [Google Scholar] [CrossRef]
- Andersson, M.; Kiselev, A.; Österlund, L.; Palmqvist, A.E.C. Microemulsion-Mediated Room-Temperature Synthesis of High-Surface-Area Rutile and Its Photocatalytic Performance. J. Phys. Chem. C 2007, 111, 6789–6797. [Google Scholar] [CrossRef]
- Wang, Y.; Zhou, A.; Yang, Z. Preparation of hollow TiO2 microspheres by the reverse microemulsions. Mater. Lett. 2008, 62, 1930–1932. [Google Scholar]
- Zubieta, C.E.; Soltero-Martínez, J.F.A.; Luengo, C.V.; Schulz, P.C. Preparation, characterization and photoactivity of TiO2 obtained by a reverse microemulsion route. Powder Technol. 2011, 212, 410–417. [Google Scholar]
- Nilsson, E.; Furusho, H.; Terasaki, O.; Palmqvist, A.E.C. Synthesis of nanoparticulate anatase and rutile crystallites at low temperatures in the Pluronic F127 microemulsion system. J. Mater. Res. 2011, 26, 288–295. [Google Scholar] [CrossRef]
- Hosseini Zori, M. Synthesis of TiO2 Nanoparticles by Microemulsion/Heat Treated Method and Photodegradation of Methylene Blue. J. Inorg. Organomet. Polym. Mater. 2010, 21, 81–90. [Google Scholar] [CrossRef]
- Li, X.; Zheng, W.; He, G.; Zhao, R.; Liu, D. Morphology Control of TiO2 Nanoparticle in Microemulsion and Its Photocatalytic Property. ACS Sustain. Chem. Eng. 2013, 2, 288–295. [Google Scholar] [CrossRef]
- Ye, X.; Zheng, C.; Ma, L.; Huang, X. Microemulsion-assisted hydrothermal preparation and infrared radiation property of TiO2 nanomaterials with tunable morphologies and crystal form. Mater. Sci. Semicond. Process. 2015, 31, 295–301. [Google Scholar] [CrossRef]
- Cui, X.; Wang, J.; Zhang, X.; Wang, Q.; Song, M.; Chai, J. Preparation of Nano-TiO2 by Surfactant-Free Microemulsion-Hydrothermal Method and Its Photocatalytic Activity. Langmuir 2019, 35, 9255–9263. [Google Scholar] [CrossRef] [PubMed]
- Keswani, R.K.; Ghodke, H.; Sarkar, D.; Khilar, K.C.; Srinivasa, R.S. Room temperature synthesis of titanium dioxide nanoparticles of different phases in water in oil microemulsion. Colloids Surf. A Physicochem. Eng. Asp. 2010, 369, 75–81. [Google Scholar] [CrossRef]
- Liu, Z.; Jian, Z.; Fang, J.; Xu, X.; Zhu, X.; Wu, S. Low-Temperature Reverse Microemulsion Synthesis, Characterization, and Photocatalytic Performance of Nanocrystalline Titanium Dioxide. Int. J. Photoenergy 2012, 702503. [Google Scholar] [CrossRef]
- Zielińska-Jurek, A.; Kowalska, E.; Sobczak, J.W.; Lisowski, W.; Ohtani, B.; Zaleska, A. Preparation and characterization of monometallic (Au) and bimetallic (Ag/Au) modified-titania photocatalysts activated by visible light. Appl. Catal. B Environ. 2011, 101, 504–514. [Google Scholar] [CrossRef]
- Tatarchuk, T.; Danyliuk, N.; Shyichuk, A.; Macyk, W.; Naushad, M. Photocatalytic degradation of dyes using rutile TiO2 synthesized by reverse micelle and low temperature methods: Real-time monitoring of the degradation kinetics. J. Mol. Liq. 2021, 342, 117407. [Google Scholar] [CrossRef]
- Bakhtiari, F.; Foruhar, N.; Zelati, A.; Amouamouha, M. Morphology, size control, and photocatalytic activities of titanium dioxide nanoparticles synthetized by microemulsion method. AIP Adv. 2023, 13, 085124. [Google Scholar] [CrossRef]
- Ahmad, S.I.; Friberg, S. Catalysis in micellar and liquid-crystalline phases. I. System water-hexadecyltrimethylammonium bromide-hexanol. J. Am. Chem. Soc. 1972, 94, 5196–5199. [Google Scholar] [CrossRef]
- Dong, S.; Feng, J.; Fan, M.; Pi, Y.; Hu, L.; Han, X.; Liu, M.; Sun, J.; Sun, J. Recent developments in heterogeneous photocatalytic water treatment using visible light responsive photocatalysts: A review. RSC Adv. 2015, 5, 14610–14630. [Google Scholar] [CrossRef]
- Jiang, X.; Manawan, M.; Feng, T.; Qian, R.; Zhao, T.; Zhou, G.; Kong, F.; Wang, Q.; Dai, S.; Pan, J.H. Anatase and rutile in evonik aeroxide P25: Heterojunctioned or individual nanoparticles? Catal. Today 2018, 300, 12–17. [Google Scholar] [CrossRef]
- Sterte, J. Synthesis and properties of titanium oxide cross-linked montmorillonite. Clay Clay Miner. 1986, 34, 658–664. [Google Scholar] [CrossRef]
- Wojdyr, M. Fityk: A general-purpose peak fitting program. J. Appl. Cryst. 2010, 43, 1126–1127. [Google Scholar] [CrossRef]
- Welter, E.S.; Garg, S.; Glaser, R.; Goepel, M. Methodological Investigation of the Band Gap Determination of Solid Semiconductors via UV/Vis Spectroscopy. ChemPhotoChem 2023, 7, e202300001. [Google Scholar] [CrossRef]
- Elgh, B.; Palmqvist, A.E.C. A facile low-temperature synthesis of TiO2 nanoparticles with excellent polymorph control. J. Sol-Gel Sci. Techn. 2015, 76, 395–401. [Google Scholar] [CrossRef]
- Thommes, M.; Kaneko, K.; Neimark, A.V.; Olivier, J.P.; Rodriguez-Reinoso, F.; Rouquerol, J.; Sing, K.S.W. Physisorption of gases, with special reference to the evaluation of surface area and pore size distribution (IUPAC Technical Report). Pure Appl. Chem. 2015, 87, 1052–1069. [Google Scholar] [CrossRef]
- Busca, G.; Ramis, G.; Amores, J.M.G.; Escribano, V.S.; Piaggio, P. FT Raman and FTIR studies of titanias and metatitanate powders. J. Chem. Soc. Faraday Trans. 1994, 90, 3181–3190. [Google Scholar] [CrossRef]
- Sun, J.; Gao, L.; Zhang, Q. Synthesizing and Comparing the Photocatalytic Properties of High Surface Area Rutile and Anatase Titania Nanoparticles. J. Am. Ceram. Soc. 2003, 86, 1677–1682. [Google Scholar] [CrossRef]
- Wang, J.; Li, R.; Zhang, Z.; Sun, W.; Wang, X.; Xu, R.; Xing, Z.; Zhang, X. Degradation of Hazardous Dyes in Wastewater using Nanometer Mixed Crystal TiO2 Powders under Visible Light Irradiation. Water Air Soil Pollut. 2008, 189, 225–237. [Google Scholar] [CrossRef]
- Sugapriya, S.; Sriram, R.; Lakshmi, S. Effect of annealing on TiO2 nanoparticles. Optik 2013, 124, 4971–4975. [Google Scholar] [CrossRef]
- Praveen, P.; Viruthagiri, G.; Mugundan, S.; S hanmugam, N. Structural, optical and morphological analyses of pristine titanium di-oxide nanoparticles – Synthesized via sol–gel route. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2014, 117, 622–629. [Google Scholar] [CrossRef] [PubMed]
- García-Contreras, L.A.; Flores-Flores, J.O.; Arenas-Alatorre, J.Á.; Chávez-Carvayar, J.Á. Synthesis, characterization and study of the structural change of nanobelts of TiO2 (H2Ti3O7) to nanobelts with anatase, brookite and rutile phases. J. Alloys Compd. 2022, 923, 166236. [Google Scholar] [CrossRef]
- Wachs, I.E. Raman and IR studies of surface metal oxide species on oxide supports: Supported metal oxide catalysts. Catal. Today 1996, 27, 437–455. [Google Scholar] [CrossRef]
- Balachandran, U.; Eror, N.G. Raman spectra of titanium dioxide. J. Sol. State Chem. 1982, 42, 276–282. [Google Scholar] [CrossRef]
- Gotić, M.; Ivanda, M.; Popović, S.; Musić, S.; Sekulić, A.; Turković, A.; Furić, K. Raman investigation of nanosized TiO2. J. Raman Spectrosc. 1997, 28, 555–558. [Google Scholar]
- Xue, X.; Ji, W.; Mao, Z.; Mao, H.; Wang, Y.; Wang, X.; Ruan, W.; Zhao, B.; Lombardi, J.R. Raman Investigation of Nanosized TiO2: Effect of Crystallite Size and Quantum Confinement. J. Phys. Chem. C 2012, 116, 8792–8797. [Google Scholar]
- Lubas, M.; Jasinski, J.J.; Sitarz, M.; Kurpaska, L.; Podsiad, P.; Jasinski, J. Raman spectroscopy of TiO2 thin films formed by hybrid treatment for biomedical applications. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2014, 133, 867–871. [Google Scholar] [PubMed]
- Ocaña, M.; Fornés, V.; Ramos, J.V.G.; Serna, C.J. Factors Affecting the Infrared and Raman Spectra of Rutile Powders. J. Solid State Chem. 1988, 75, 364–372. [Google Scholar] [CrossRef]
- Swamy, V. Size-Dependent Modifications of the First-Order Raman Spectra of Nanostructured Rutile TiO2. Phys. Rev. B-Condens. Matter Mater. Phys. 2008, 77, 15–18. [Google Scholar] [CrossRef]
- Tompsett, G.A.; Bowmaker, G.A.; Cooney, R.P.; Metson, J.B.; Rodgers, K.A.; Seakins, J.M. The Raman spectrum of brookite, TiO2 (Pbca, Z = 8). J. Raman Spectrosc. 1995, 26, 57–62. [Google Scholar] [CrossRef]
- Zhang, J.; Yan, S.; Fu, L.; Wang, F.; Yuan, M.; Luo, G.; Xu, Q.; Wang, X.; Li, C. Photocatalytic Degradation of Rhodamine B on Anatase, Rutile, and Brookite TiO2. Chin. J. Catal. 2011, 32, 983–991. [Google Scholar] [CrossRef]
- Žerjav, G.; Žižek, K.; Zavašnik, J.; Pintar, A. Brookite vs. rutile vs. anatase: What`s behind their various photocatalytic activities? J. Environ. Chem. Eng. 2022, 10, 107722. [Google Scholar] [CrossRef]
- Paul, S.; Choudhury, A. Investigation of the optical property and photocatalytic activity of mixed-phase nanocrystalline titania. Appl. Nanosci. 2013, 4, 839–847. [Google Scholar] [CrossRef]
- Zhang, J.; Zhou, P.; Liu, J.; Yu, J. New understanding of the difference of photocatalytic activity among anatase, rutile and brookite TiO2. Phys. Chem. Chem. Phys. 2014, 16, 20382–20386. [Google Scholar] [CrossRef] [PubMed]
- Djokić, V.R.; Marinković, A.D.; Petrović, R.; Ersen, O.; Zafeiratos, S.; Mitrić, M.; Ophus, C.; Radmilović, V.R.; Janacković, D. Highly active rutile TiO2 nanocrystalline photocatalysts. ACS Appl. Mater. Interfaces 2020, 12, 33058–33068. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, T.T.; Edalati, K. Brookite TiO2 as an active photocatalyst for photoconversion of plastic wastes to acetic acid and simultaneous hydrogen production: Comparison with anatase and rutile. Chemosphere 2024, 355, 141785. [Google Scholar] [CrossRef] [PubMed]
- Sarngan, P.P.; Lakshmanan, A.; Sarkar, D. Influence of Anatase-Rutile Ratio on Band Edge Position and Defect States of TiO2 Homojunction Catalyst. Chemosphere 2022, 286, 131692. [Google Scholar] [CrossRef]
- Nimmy, A.V.; Anandakumar, V.M.; Biju, V. Band alignment and interfacial charge transfer in sol–gel derived anatase/rutile heterophase TiO2: Explaining the synergistic photocatalytic activity. Phys. Chem. Chem. Phys. 2024, 26, 13937–13948. [Google Scholar]
- Li, W.; Du, D.; Yan, T.; Kong, D.; You, J.; Li, D. Relationship between surface hydroxyl groups and liquid-phase photocatalytic activity of titanium dioxide. J. Colloid Interface Sci. 2015, 444, 42–48. [Google Scholar] [CrossRef]


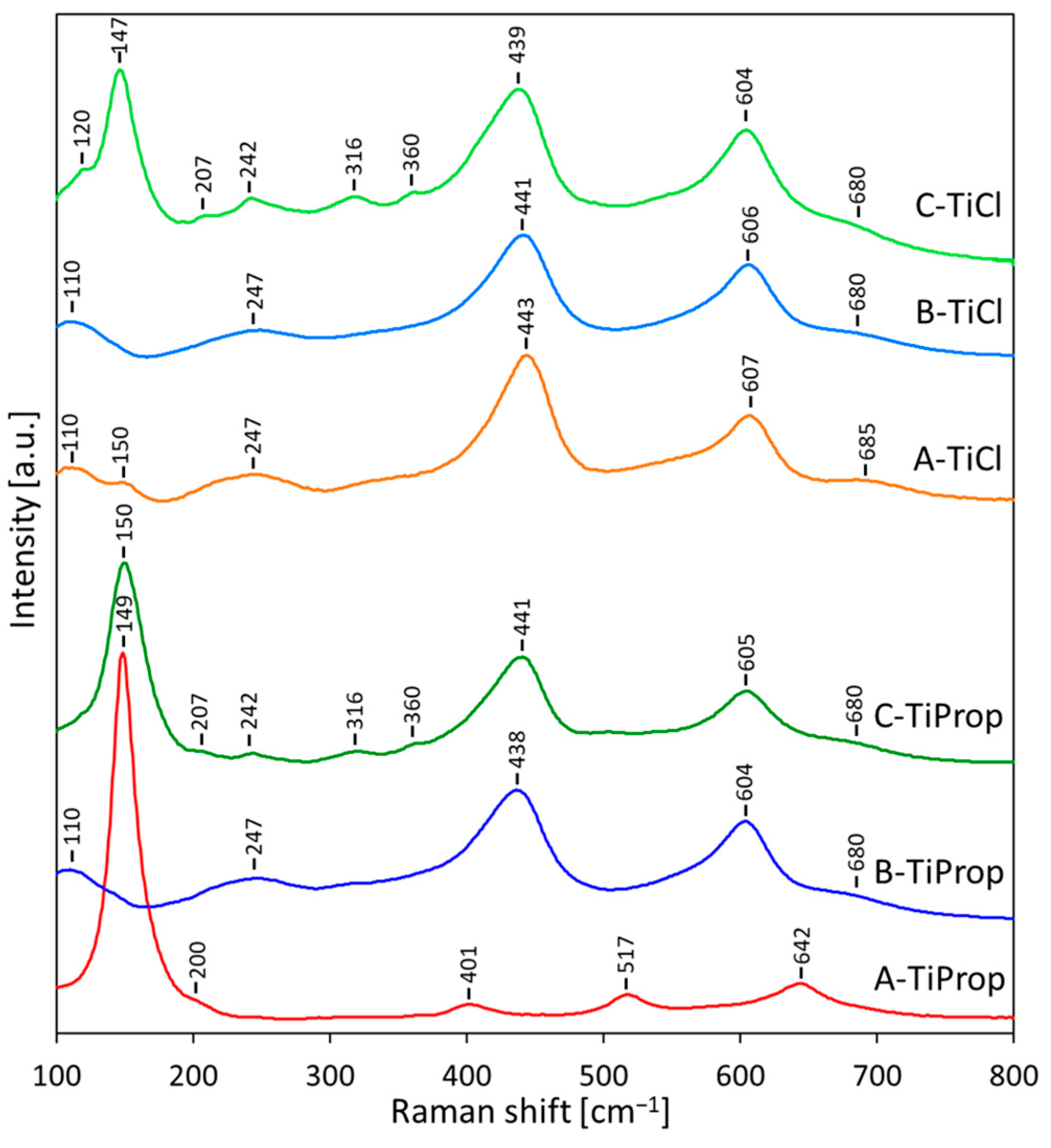
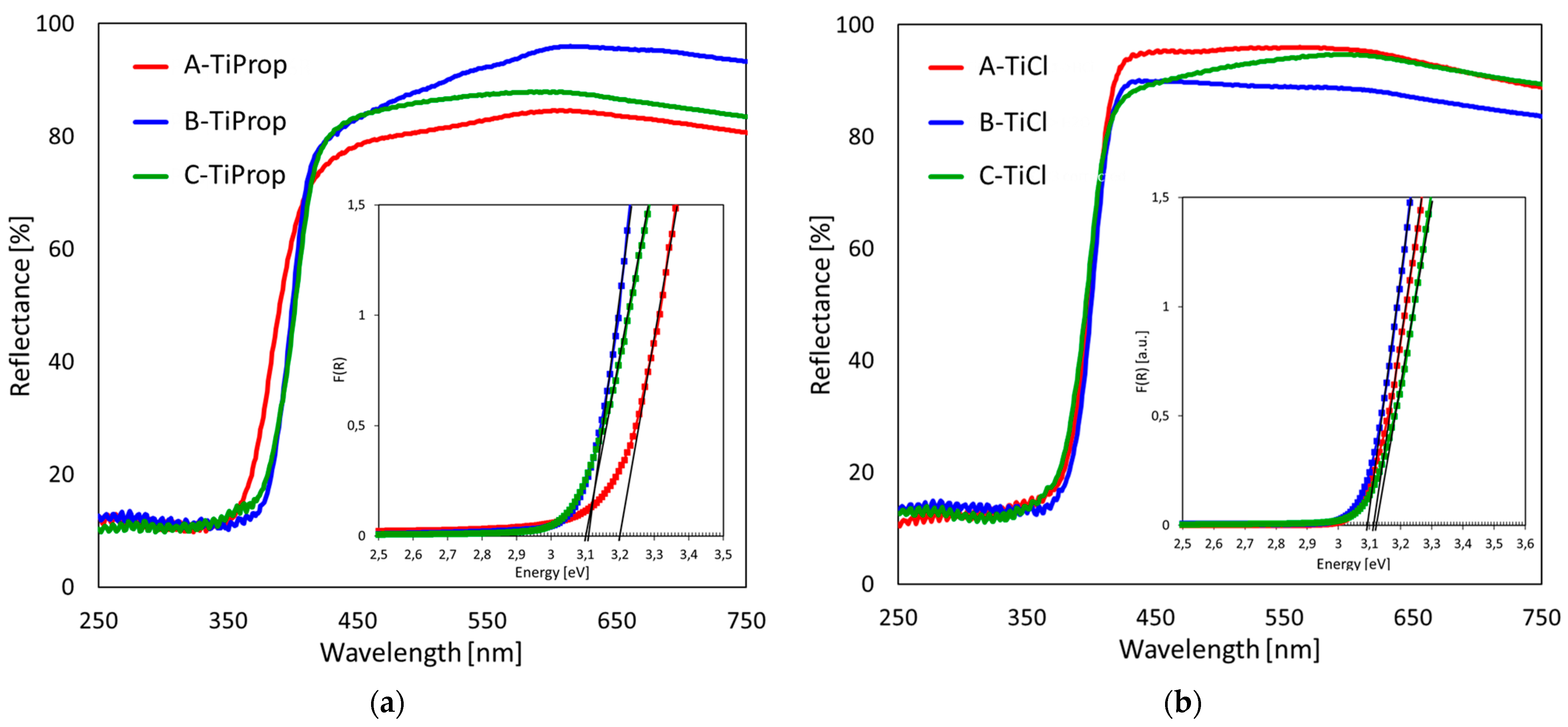
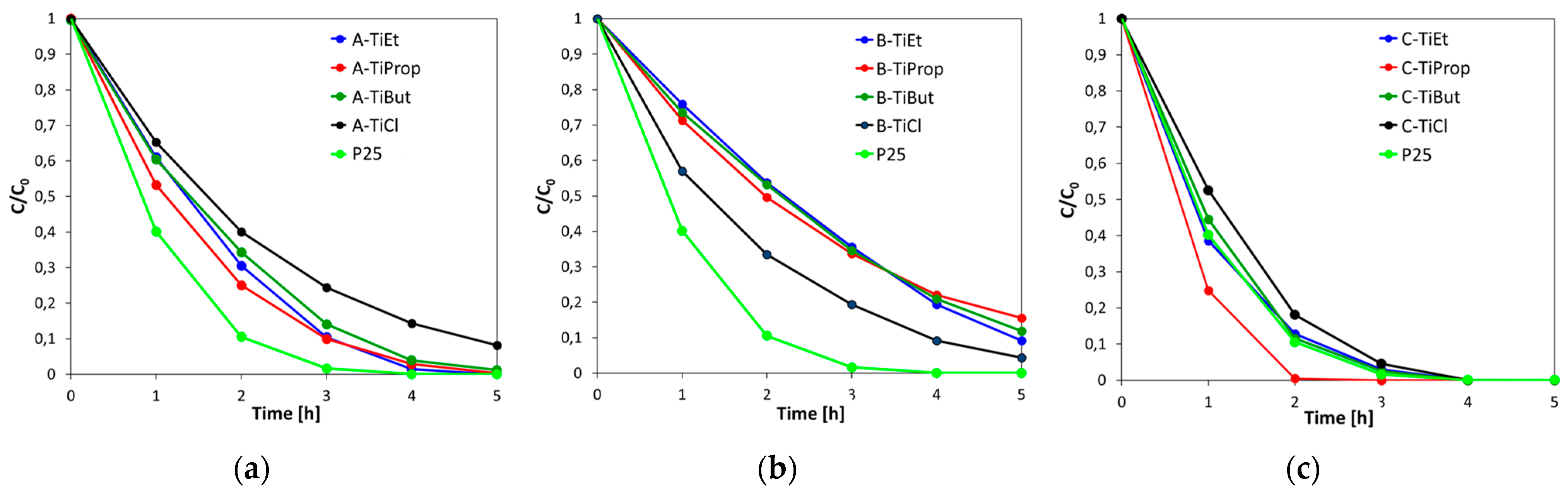
| Sample | SBET [m2g−1] | Vtot [cm3g−1] | Dav [nm] | Ddom [nm] | Anatase Content/ Crystal Size [wt.%]/[nm] | Rutile Content/ Crystal Size [wt.%]/[nm] | Brookite Content/ Crystal Size [wt.%]/[nm] | Eg [eV] |
|---|---|---|---|---|---|---|---|---|
| A-TiCl | 56 | 0.279 | 19.8 | 6.1 | - | 100/11.3 | - | 3.11 |
| A-TiEt | 206 | 0.318 | 6.2 | 6.1 | 100/6.5 | - | - | 3.18 |
| A-TiProp | 216 | 0.326 | 6.0 | 6.1 | 100/6.2 | - | - | 3.20 |
| A-TiBut | 235 | 0.324 | 5.5 | 6.1 | 100/6.3 | - | - | 3.18 |
| B-TiCl | 114 | 0.095 | 3.4 | 2.9 | - | 100/7.4 | - | 3.09 |
| B-TiEt | 41 | 0.125 | 12.2 | 5.9 | - | 100/8.7 | - | 3.09 |
| B-TiProp | 47 | 0.153 | 12.9 | 6.6 | - | 100/8.1 | - | 3.11 |
| B-TiBut | 42 | 0.128 | 12.2 | 6.1 | - | 100/8.2 | - | 3.12 |
| C-TiCl | 83 | 0.270 | 13.0 | 4.9 | 13/5.1 | 63/18.2 | 24/9.3 | 3.12 |
| C-TiEt | 100 | 0.313 | 12.5 | 4.9 | 43/5.9 | 35/23.0 | 22/8.4 | 3.10 |
| C-TiProp | 112 | 0.291 | 10.4 | 4.9 | 39/5.9 | 30/24.8 | 31/7.6 | 3.10 |
| C-TiBut | 99 | 0.347 | 14.0 | 6.1 | 47/5.6 | 29/23.0 | 23/7.6 | 3.09 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Napruszewska, B.D.; Walczyk, A.; Duraczyńska, D.; Kryściak-Czerwenka, J.; Karcz, R.; Gaweł, A.; Nowak, P.; Serwicka, E.M. TiO2 Nanoparticles with Adjustable Phase Composition Prepared by an Inverse Microemulsion Method: Physicochemical Characterization and Photocatalytic Properties. Nanomaterials 2024, 14, 1130. https://doi.org/10.3390/nano14131130
Napruszewska BD, Walczyk A, Duraczyńska D, Kryściak-Czerwenka J, Karcz R, Gaweł A, Nowak P, Serwicka EM. TiO2 Nanoparticles with Adjustable Phase Composition Prepared by an Inverse Microemulsion Method: Physicochemical Characterization and Photocatalytic Properties. Nanomaterials. 2024; 14(13):1130. https://doi.org/10.3390/nano14131130
Chicago/Turabian StyleNapruszewska, Bogna D., Anna Walczyk, Dorota Duraczyńska, Joanna Kryściak-Czerwenka, Robert Karcz, Adam Gaweł, Paweł Nowak, and Ewa M. Serwicka. 2024. "TiO2 Nanoparticles with Adjustable Phase Composition Prepared by an Inverse Microemulsion Method: Physicochemical Characterization and Photocatalytic Properties" Nanomaterials 14, no. 13: 1130. https://doi.org/10.3390/nano14131130





