Ultra-Sensitive Simultaneous Detection of Dopamine and Acetaminophen over Hollow Porous AuAg Alloy Nanospheres
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials and Characterization
2.2. Synthesis of AuAg HPNSs
2.3. Characterization Tests
2.4. Fabrication of the AuAg HPNS-Modified GCE
2.5. Simultaneous Detection of DA and AC in Human Serum
3. Results and Discussion
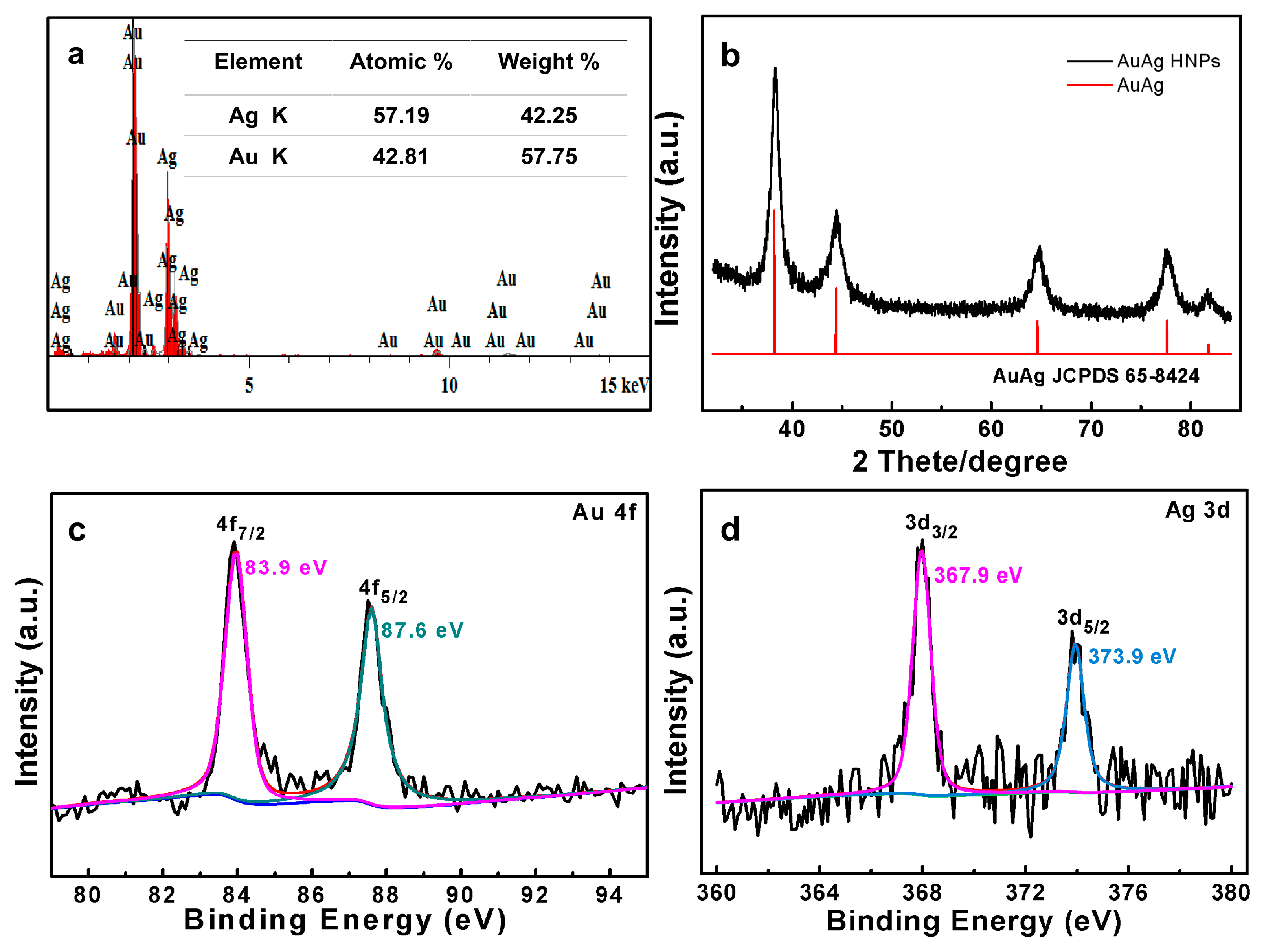
3.1. Structure and Morphology of AuAg HPNSs
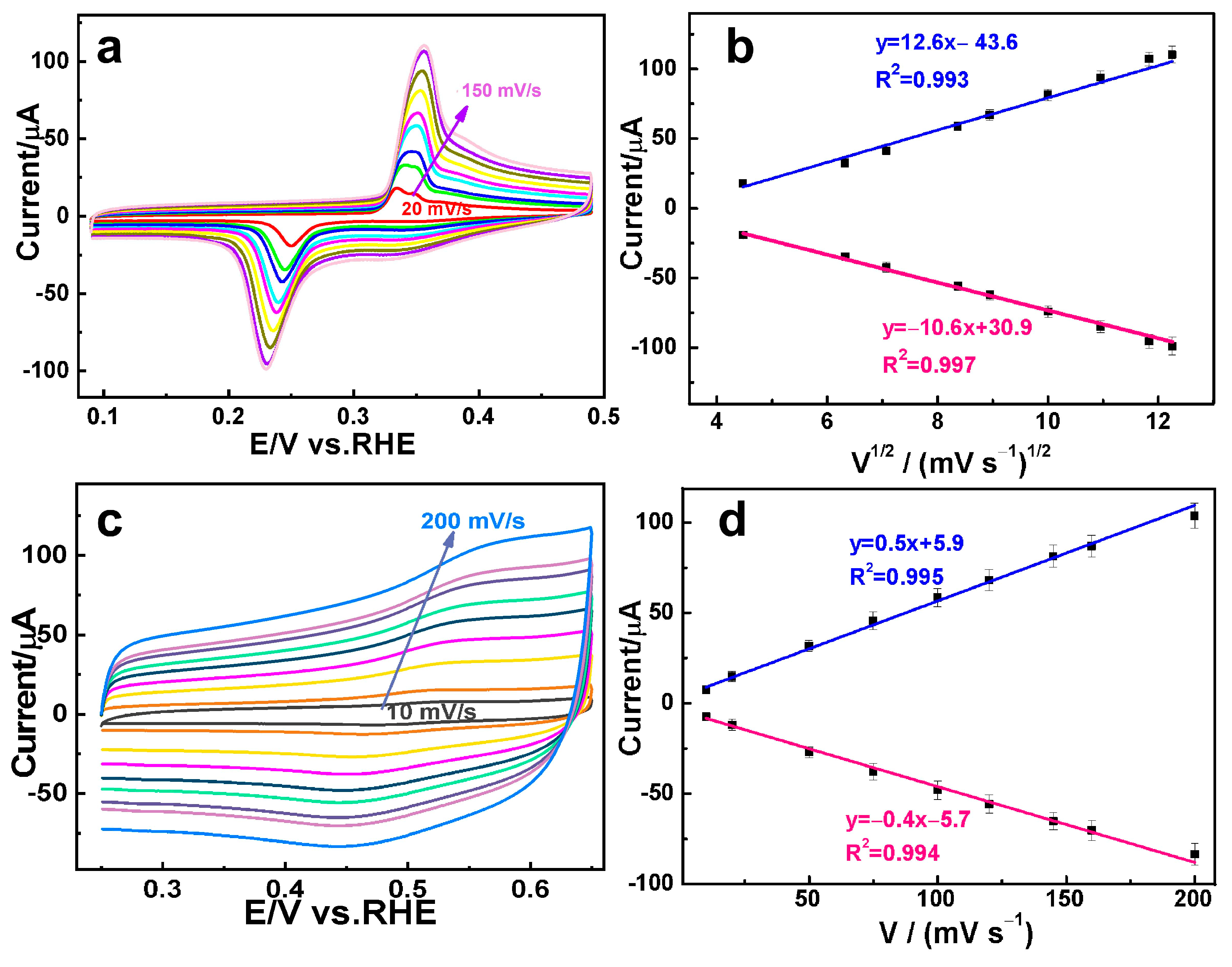
3.2. Electrochemical Analysis of DA and AC on the AuAg HPNSs
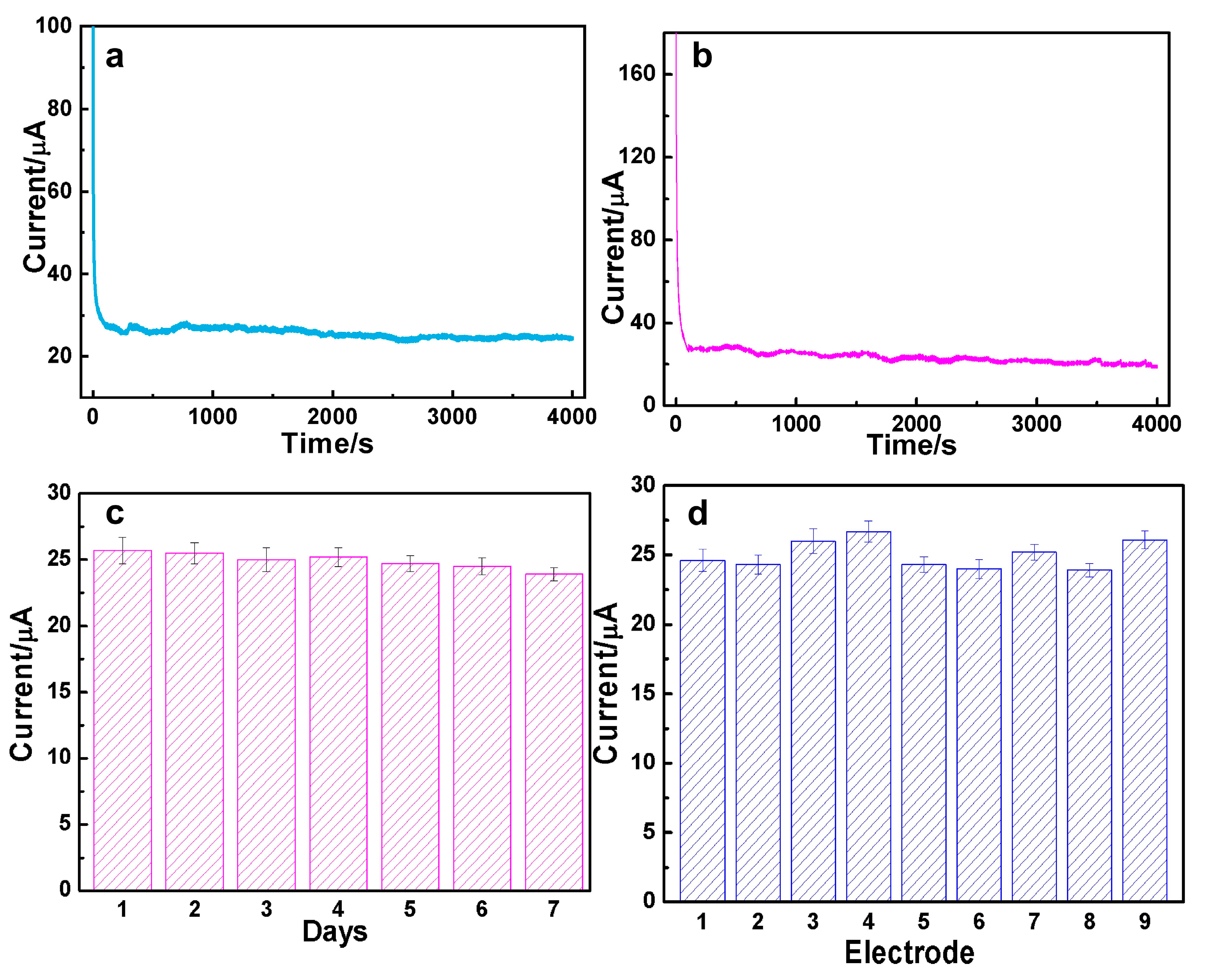
3.3. The Durability and Reproducibility for DA and AC Determination over AuAg HPNSs
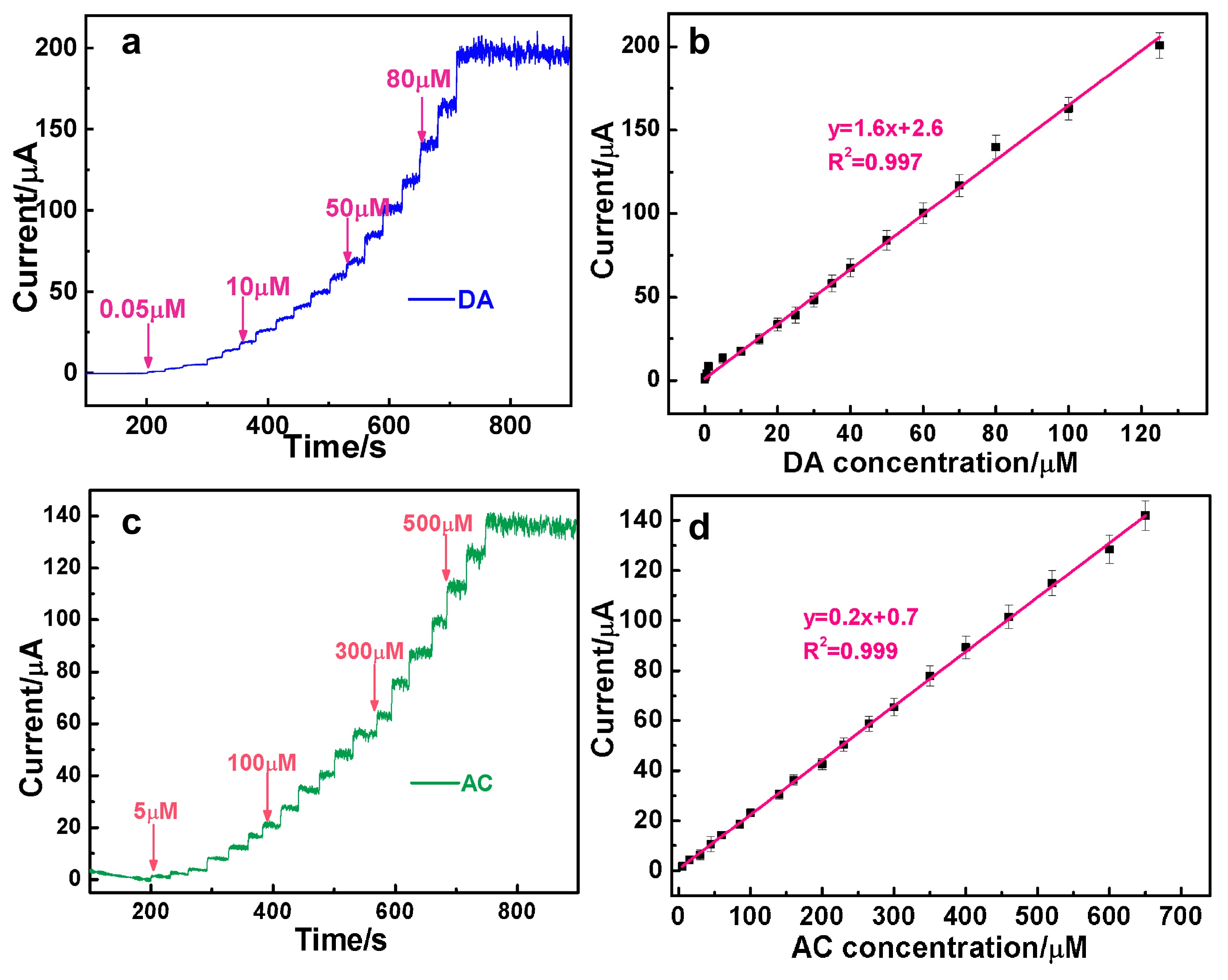
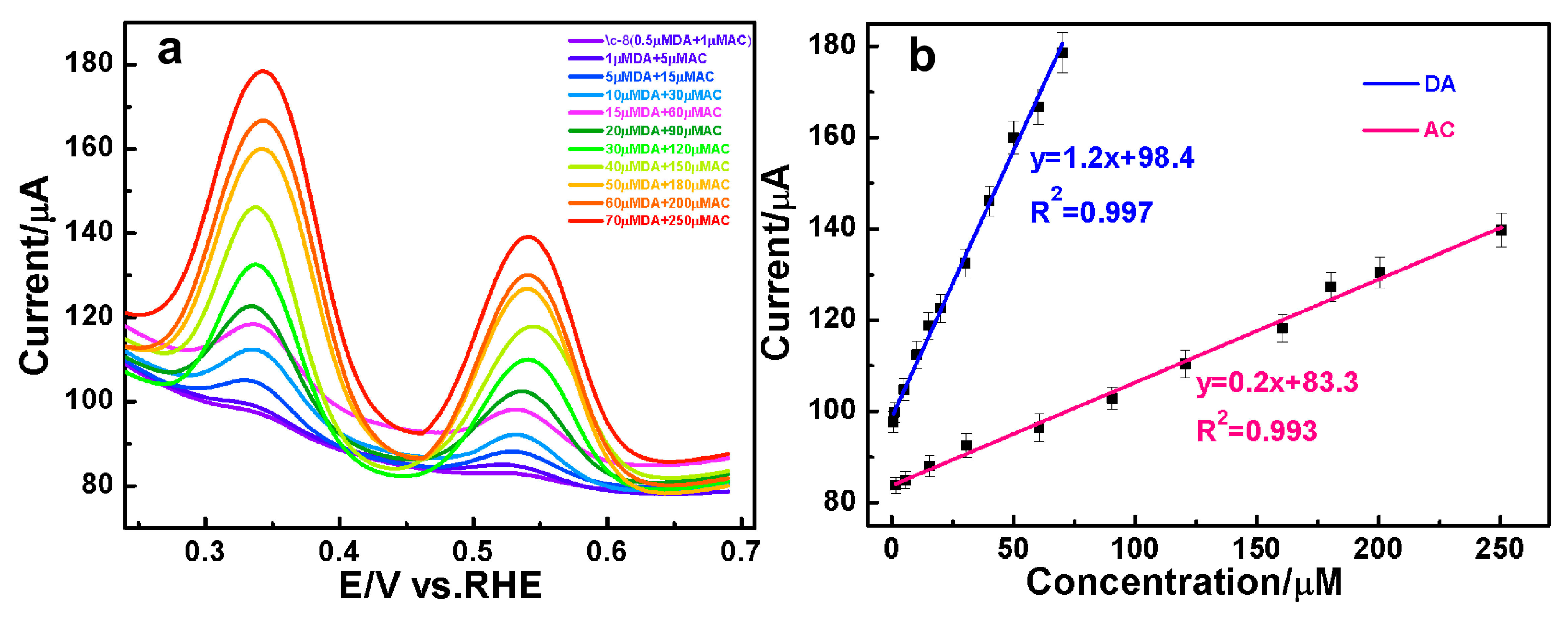
3.4. The Simultaneous Quantification of DA and AC over AuAg HPNSs
3.5. The Simultaneous Analysis of DA and AC in Human Serum
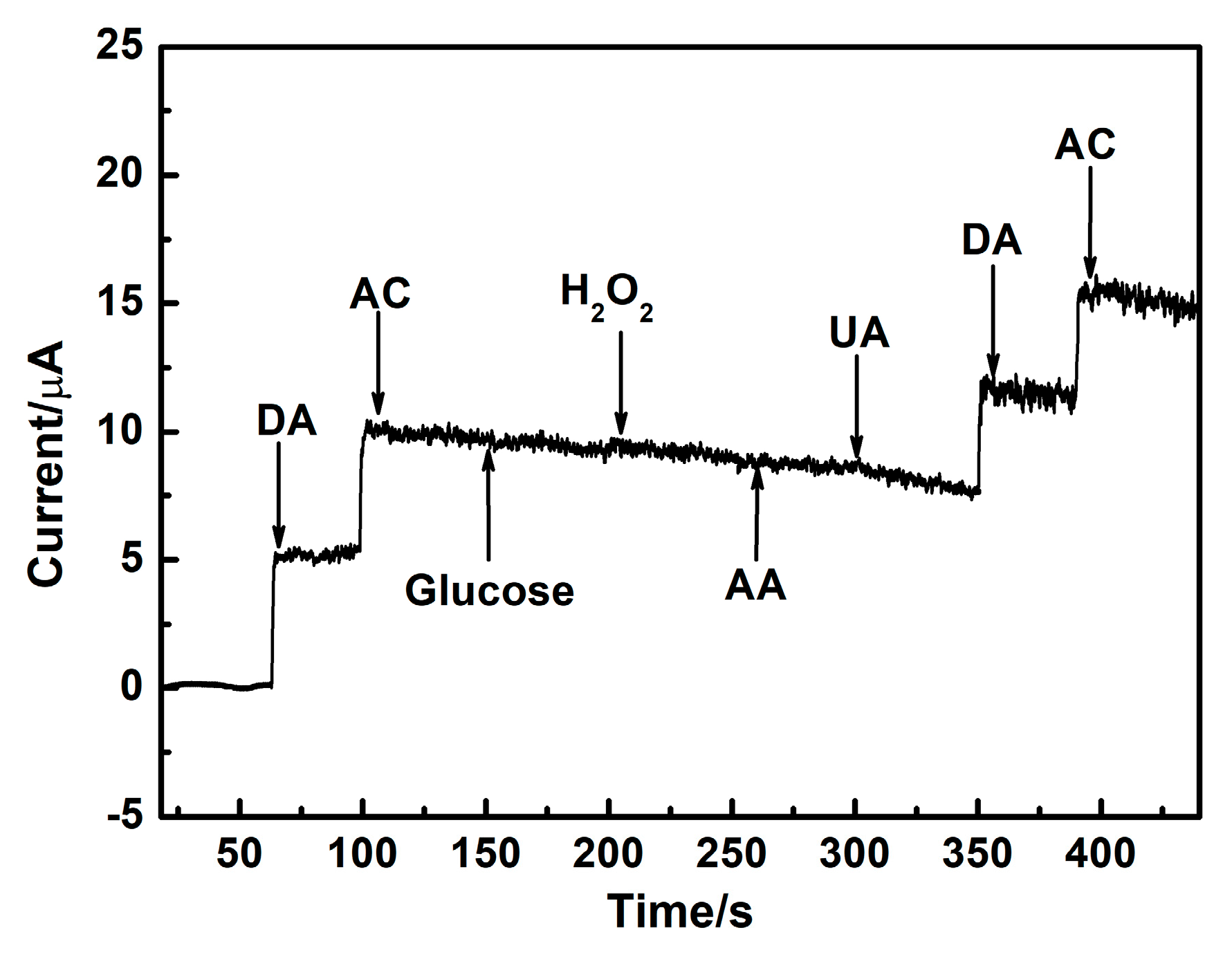
3.6. The Selectivity for DA and AC Detection over AuAg HPNSs
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Wan, X.; Yang, S.; Cai, Z.; He, Q.; Ye, Y.; Xia, Y.; Li, G.; Liu, J. Facile synthesis of MnO2 nanoflowers/N-doped reduced graphene oxide composite and its application for simultaneous determination of dopamine and uric acid. Nanomaterials 2019, 9, 847. [Google Scholar] [CrossRef] [PubMed]
- Cai, Z.; Ye, Y.; Wan, X.; Liu, J.; Yang, S.; Xia, Y.; Li, G.; He, Q. Morphology–dependent electrochemical sensing properties of iron oxide-graphene oxide nanohybrids for dopamine and uric acid. Nanomaterials 2019, 9, 835. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Wang, K.; Zhang, L.; Zhang, Y.; Shen, L. Phosphorus-doped graphene-based electrochemical sensor for sensitive detection of acetaminophen. Anal. Chim. Acta 2018, 1036, 26–32. [Google Scholar] [CrossRef] [PubMed]
- Anuar, N.; Basirun, W.J.; Ladan, M.; Shalauddin, M.; Mehmood, M.S. Fabrication of platinum nitrogen-doped graphene nanocomposite modified electrode for the electrochemical detection of acetaminophen. Sens. Actuators B 2018, 266, 375–383. [Google Scholar] [CrossRef]
- Gu, J.; An, Q.; Chen, J.; He, Y.; Huang, W. Preparation and responsive performance study of AuNPs/RGO-MoO2/GCE composite modified electrodes based on its high sensitivity to acetaminophen and dopamine. Inorg. Chem. Commun. 2023, 147, 110282. [Google Scholar] [CrossRef]
- Daneshinejad, H.; Chamjangali, M.A.; Goudarzi, N.; Roudbari, A. Application of a thin film of poly(solochrome black T) as a redox mediator for the electro-catalytic simultaneous determination of dopamine and acetaminophen in the pharmaceutical and biological samples. Mater. Sci. Eng. C 2016, 58, 532–540. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Zhang, Y.; Cui, M.; Lu, Y.; Peng, D. Molecular-scale cage-confinement pyrolysis route to size-controlled molybdenum carbide nanoparticles for electrochemical sensor. Biosens. Bioelectron. 2020, 165, 112373. [Google Scholar] [CrossRef] [PubMed]
- Glavanović, S.; Glavanović, M.; Tomišić, V. Simultaneous quantitative determination of paracetamol and tramadol in tablet formulation using UV spectrophotometry and chemometric methods. Spectrochim. Acta Part A 2016, 157, 258–264. [Google Scholar] [CrossRef] [PubMed]
- Qi, D.; Zhang, Q.; Zhou, W.H.; Zhao, J.; Zhang, B.; Sha, Y.; Pang, Z. Quantification of dopamine in brain microdialysates with high-performance liquid chromatography-tandem mass spectrometry. Anal. Sci. 2016, 32, 419–424. [Google Scholar] [CrossRef]
- Burgot, G.; Auffret, F.; Burgot, J.-L. Determination of acetaminophen by thermometric titrimetry. Anal. Chim. Acta 1997, 343, 125–128. [Google Scholar] [CrossRef]
- Pagano, R.; Syrgiannis, Z.; Bettini, S.; Ingrosso, C.; Valli, L.; Giancane, G.; Prato, M. Localized and surface plasmons coupling for ultrasensitive dopamine detection by means of SPR-based perylene bisimide/Au nanostructures thin film. Adv. Mater. Interfaces 2021, 8, 2101023. [Google Scholar] [CrossRef]
- Borahan, T.; Unutkan, T.; Şahin, A.; Bakırdere, S. A rapid and sensitive reversed phase-HPLC method for simultaneous determination of ibuprofen and paracetamol in drug samples and their behaviors in simulated gastric conditions. J. Sep. Sci. 2019, 42, 678–683. [Google Scholar] [CrossRef]
- Lee, C.S.; Yu, S.H.; Kim, T.H. One-step electrochemical fabrication of reduced graphene oxide/gold nanoparticles nanocomposite-modified electrode for simultaneous detection of dopamine, ascorbic acid, and uric acid. Nanomaterials 2018, 8, 17. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Lei, W.; Xu, Y.; Xia, X.; Hao, Q. Simultaneous detection of dopamine and uric acid using a Poly(l-lysine)/Graphene oxide modified electrode. Nanomaterials 2016, 6, 178. [Google Scholar] [CrossRef]
- Gholivand, M.B.; Amiri, M. Simultaneous detection of dopamine and acetaminophen by modified gold electrode with polypyrrole/aszophloxine film. J. Electroanal. Chem. 2012, 676, 53–59. [Google Scholar] [CrossRef]
- Shen, L.; Dong, J.; Wen, B.; Wen, X.; Li, J. Facile synthesis of hollow Fe3O4-rGO hanocomposites for the electrochemical detection of acetaminophen. Nanomaterials 2023, 13, 707. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Hou, J.; Xu, C.; Chen, Z.; Qin, R.; Liu, H. TiO2 particles wrapped onto macroporous germanium skeleton as high performance anode for lithium-ion batteries. Chem. Eng. J. 2020, 381, 122649. [Google Scholar] [CrossRef]
- He, Q.; Liu, J.; Liu, X.; Li, G.; Chen, D.; Deng, P.; Liang, J. Fabrication of amine-modified magnetite-electrochemically reduced graphene oxide nanocomposite modified glassy carbon electrode for sensitive dopamine determination. Nanomaterials 2018, 8, 194. [Google Scholar] [CrossRef]
- Wu, P.; Liu, H.; Cao, Y.; Xi, S.; Li, Z.; He, Z.; Song, L.; Xu, J.; Bai, P.; Zhao, L.; et al. Mesostructured cellular foam silica supported Au-Pt nanoalloy: Enrichment of D-state electrons for promoting the catalytic synergy. Microporous Mesoporous Mater. 2021, 316, 110982. [Google Scholar] [CrossRef]
- Ma, K.; Yang, L.; Liu, J.; Liu, J. Electrochemical sensor nanoarchitectonics for sensitive detection of uric acid in human whole blood based on screen-printed carbon electrode equipped with vertically-ordered mesoporous silica-nanochannel film. Nanomaterials 2022, 12, 1157. [Google Scholar] [CrossRef]
- Liu, M.; Chen, Q.; Lai, C.; Zhang, Y.; Deng, J.; Li, H.; Yao, S. A double signal amplification platform for ultrasensitive and simultaneous detection of ascorbic acid, dopamine, uric acid and acetaminophen based on a nanocomposite of ferrocene thiolate stabilized Fe3O4@Au nanoparticle with graphene sheet. Biosens. Bioelectron. 2013, 48, 75–81. [Google Scholar] [CrossRef]
- Soltani, N.; Tavakkoli, N.; Shahdost-fard, F.; Salavati, H.; Abdoli, F. A carbon paste electrode modified with Al2O3-supported palladium nanoparticle for simultaneous voltammetric determination of melatonin, dopamine, and acetaminophen. Microchim. Acta 2019, 186, 540. [Google Scholar] [CrossRef]
- Liu, B.; Ouyang, X.; Ding, Y.; Luo, L.; Xu, D.; Ning, Y. Electrochemical preparation of nickel and copper oxides-decorated graphene composite for simultaneous determination of dopamine, acetaminophen and tryptophan. Talanta 2016, 146, 114–121. [Google Scholar] [CrossRef]
- Wang, H.; Lin, G.; Li, X.; Lu, W.; Peng, Z. Self-standing hollow porous AuPt nanospheres and their enhanced electrocatalytic performance. J. Colloid Interface Sci. 2019, 544, 396–403. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Sui, G.; Guo, D.; Li, J.; Zhang, L.; Li, S.; Xin, J.; Chai, D.; Guo, W. Structure-designed synthesis of hollow/porous cobalt sulfide/phosphide based materials for optimizing supercapacitor storage properties and hydrogen evolution reaction. J. Colloid Interface Sci. 2021, 599, 577–585. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Zhang, H.; Zhang, Z.; Ma, Q.; Kong, C.; Min, S. Carbonized wood membrane decorated with AuPd alloy nanoparticles as an efficient self-supported electrode for electrocatalytic CO2 reduction. J. Colloid Interface Sci. 2022, 607, 312–322. [Google Scholar] [CrossRef]
- Thanha, T.D.; Balamurugana, J.; Hiena, H.V.; Kima, N.H.; Lee, J.H. A novel sensitive sensor for serotonin based on high-quality of AuAg nanoalloy encapsulated graphene electrocatalyst. Biosens. Bioelectron. 2017, 96, 186–193. [Google Scholar] [CrossRef]
- Li, J.; Zhang, X. Fabrication of poly(aspartic acid)-nanogold modified electrode and its application for simultaneous determination of dopamine, ascorbic acid, and uric acid. Am. J. Anal. Chem. 2012, 3, 195–203. [Google Scholar] [CrossRef]
- Yang, H.; Hou, J.; Wang, Z.; Zhang, T.; Xu, C. An ultrasensitive biosensor for superoxide anion based on hollow porous PtAg nanospheres. Biosens. Bioelectron. 2018, 117, 429–435. [Google Scholar] [CrossRef]
- Jiao, A.; Dong, X.; Zhang, H.; Xu, L.; Tian, Y.; Liu, X.; Chen, M. Construction of pure worm-like AuAg nanochains for ultrasensitive SERS detection of pesticide residues on apple surfaces. Spectrochim. Acta Part A 2019, 209, 241–247. [Google Scholar] [CrossRef]
- Chen, X.; Xiang, Y.; Xu, L.; Liu, G. Recovery and reduction of Au(III) from mixed metal solution by thiourearesorcinol formaldehyde microspheres. J. Hazard. Mater. 2020, 397, 122812. [Google Scholar] [CrossRef] [PubMed]
- Zhou, K.; Han, C.; Li, C.; Jiu, J.; Yang, Y.; Li, L.; Wang, H.; Liu, J.; Liu, Z.; Yan, H.; et al. Highly stable transparent conductive electrodes based on silver-platinum alloy-walled hollow nanowires for optoelectronic devices. ACS Appl. Mater. Interfaces 2018, 10, 36128–36135. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Liu, B.; Li, Y.; Xu, C.; Hou, J. Highly sensitive detection of dopamine based on hierarchical nanoporous NiCoO2/Ni composite. J. Electroanal. Chem. 2020, 873, 114380. [Google Scholar] [CrossRef]
- Vatrá, J.; Boča, R.; Linert, W. Oxidation properties of dopamine at and near physiological conditions. Monatsh Chem. 2015, 146, 1799–1805. [Google Scholar] [CrossRef]
- Kim, D.; Oh, Y.B.; Shin, H.J.; Blaha, C.D.; Bennet, K.E.; Lee, K.H.; Kim, I.Y.; Jang, D.P. Investigation of the reduction process of dopamine using paired pulse voltammetry. J. Electroanal. Chem. 2014, 717–718, 157–164. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Liu, M.; Xiang, C.; Xi, Q.; Yao, S. Electrochemical quartz crystal microbalance study on growth and property of the polymer deposit at gold electrodes during oxidation of dopamine in aqueous solutions. Thin Solid Films 2006, 497, 270–278. [Google Scholar] [CrossRef]
- Nematollahi, D.; Shayani-Jam, H.; Alimoradi, M.; Niroomand, S. Electrochemical oxidation of acetaminophen in aqueous solutions: Kinetic evaluation of hydrolysis, hydroxylation and dimerization processes. Electrochimi Acta 2009, 54, 7407–7415. [Google Scholar] [CrossRef]
- Yang, L.; Huang, N.; Lu, Q.; Liu, M.; Li, H.; Zhang, Y.; Yao, S. A quadruplet electrochemical platform for ultrasensitive and simultaneous detection of ascorbic acid, dopamine, uric acid and acetaminophen based on a ferrocene derivative functional Au NPs/carbon dots nanocomposite and graphene. Anal. Chim. Acta 2015, 903, 69–80. [Google Scholar] [CrossRef] [PubMed]
- Yao, W.; Guo, H.; Liu, H.; Li, Q.; Xue, R.; Wu, N.; Li, L.; Wang, M.; Yang, W. Simultaneous electrochemical determination of acetaminophen and dopamine based on metal-organic framework/multiwalled carbon nanotubes-Au@Ag nanocomposites. J. Electrochem. Soc. 2019, 166, B1258. [Google Scholar] [CrossRef]
- Claude, J.; Mbouguen, K.; Ngameni, E. Simultaneous quantification of dopamine, acetaminophen and tyrosine at carbon paste electrode modified with porphyrin and clay. Anal. Methods 2017, 9, 4157–4166. [Google Scholar]
- Alothman, Z.; Bukhari, N.; Wabaidur, S.; Haider, S. Simultaneous electrochemical determination of dopamine and acetaminophen using multiwall carbon nanotubes modified glassy carbon electrode. Sens. Actuators B 2010, 146, 314–320. [Google Scholar] [CrossRef]
- Soltani, N.; Tavakkoli, N.; Ahmadi, N. Simultaneous determination of acetaminophen, dopamine and ascorbic acid using a PbS nanoparticle Schiff base-modified carbon paste electrode. Comptes Rendus. Chim. 2015, 18, 438–448. [Google Scholar] [CrossRef]
- Babaei, A.; Yousefi, A.; Afrasiabi, M.; Shabanian, M. A sensitive simultaneous determination of dopamine, acetaminophen and indomethacin on a glassy carbon electrode coated with a new composite of MCM-41 molecular sieve/nickel hydroxide nanoparticle/multiwalled carbon nanotubes. J. Electroanal. Chem. 2015, 740, 28–36. [Google Scholar] [CrossRef]
- Nasirizadeh, N.; Shekari, Z.; Zare, H.R.; Ardakani, S.A.Y.; Braz, H.A.J. Developing a sensor for the simultaneous determination of dopamine, acetaminophen and tryptophan in pharmaceutical samples using a multi-walled carbon nanotube and oxadiazole modified glassy carbon electrode. J. Braz. Chem. Soc. 2013, 24, 1846–1856. [Google Scholar] [CrossRef]
- Liu, W.; Shi, Q.; Zheng, G.; Zhou, J.; Chen, M. Electrocatalytic oxidation toward dopamine and acetaminophen based on AuNPs@TCnA/GN modified glassy carbon electrode. Anal. Chim. Acta 2019, 1075, 81–90. [Google Scholar] [CrossRef]

| No. of Serum Sample | Added (μM) | Found (μM) | RSD (%) (n = 3) | Recovery (%) | ||||
|---|---|---|---|---|---|---|---|---|
| DA | AC | DA | AC | DA | AC | DA | AC | |
| 1 | 15 | 60 | 15.6 | 59.6 | 3.14 | 3.06 | 104.0 | 99.3 |
| 2 | 40 | 150 | 40.9 | 148.6 | 3.08 | 3.17 | 102.2 | 99.1 |
| 3 | 60 | 200 | 62.2 | 203.5 | 2.53 | 2.95 | 103.7 | 101.8 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, M.; Liu, X.; Sun, C.; Cao, X.; Zhang, Y.; Hou, L.; Yang, H.; Xu, C. Ultra-Sensitive Simultaneous Detection of Dopamine and Acetaminophen over Hollow Porous AuAg Alloy Nanospheres. Nanomaterials 2024, 14, 1131. https://doi.org/10.3390/nano14131131
Li M, Liu X, Sun C, Cao X, Zhang Y, Hou L, Yang H, Xu C. Ultra-Sensitive Simultaneous Detection of Dopamine and Acetaminophen over Hollow Porous AuAg Alloy Nanospheres. Nanomaterials. 2024; 14(13):1131. https://doi.org/10.3390/nano14131131
Chicago/Turabian StyleLi, Menghua, Xinzheng Liu, Changhui Sun, Xiaorong Cao, Yuanyuan Zhang, Linrui Hou, Hongxiao Yang, and Caixia Xu. 2024. "Ultra-Sensitive Simultaneous Detection of Dopamine and Acetaminophen over Hollow Porous AuAg Alloy Nanospheres" Nanomaterials 14, no. 13: 1131. https://doi.org/10.3390/nano14131131





