Rocket Dynamics of Capped Nanotubes: A Molecular Dynamics Study
Abstract
1. Introduction
2. Methods
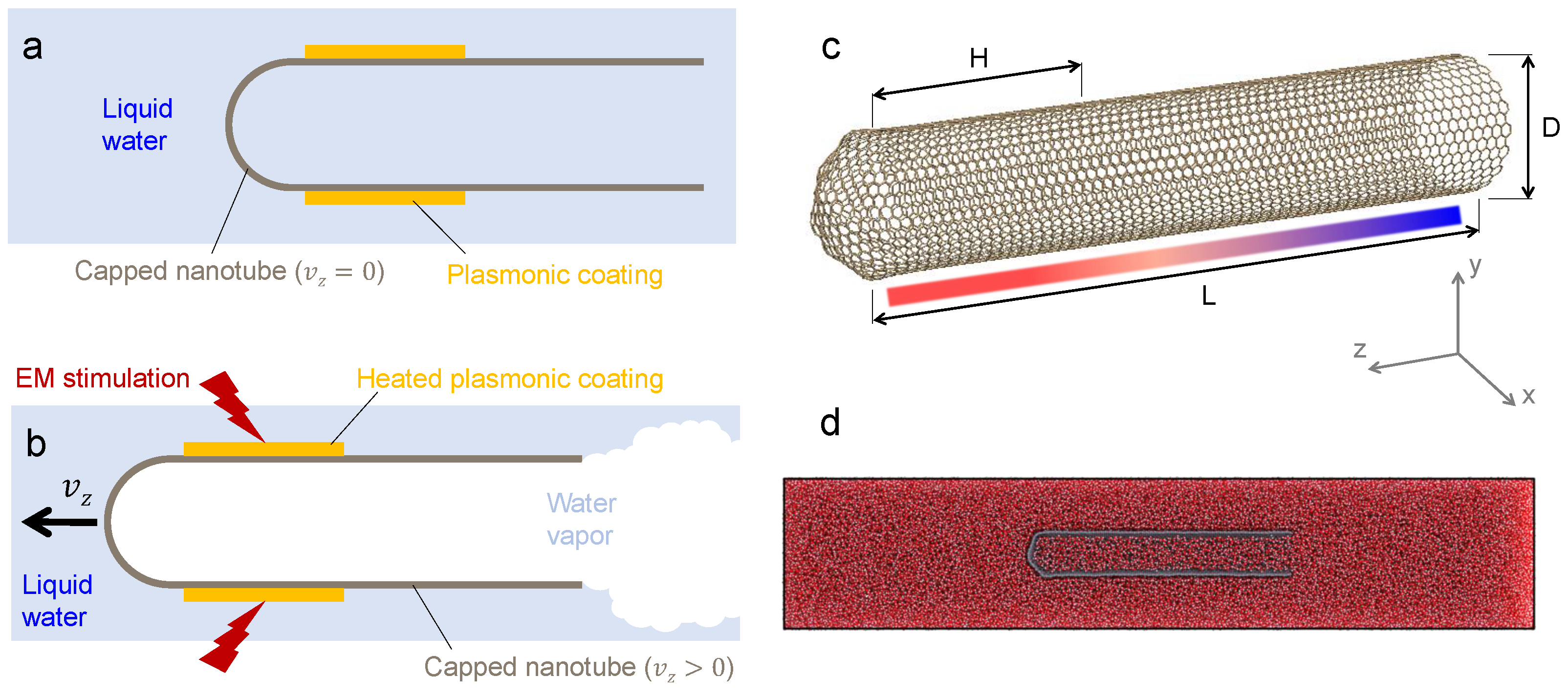
2.1. Nanorocket Concept
2.2. Simulation Details
3. Results and Discussions
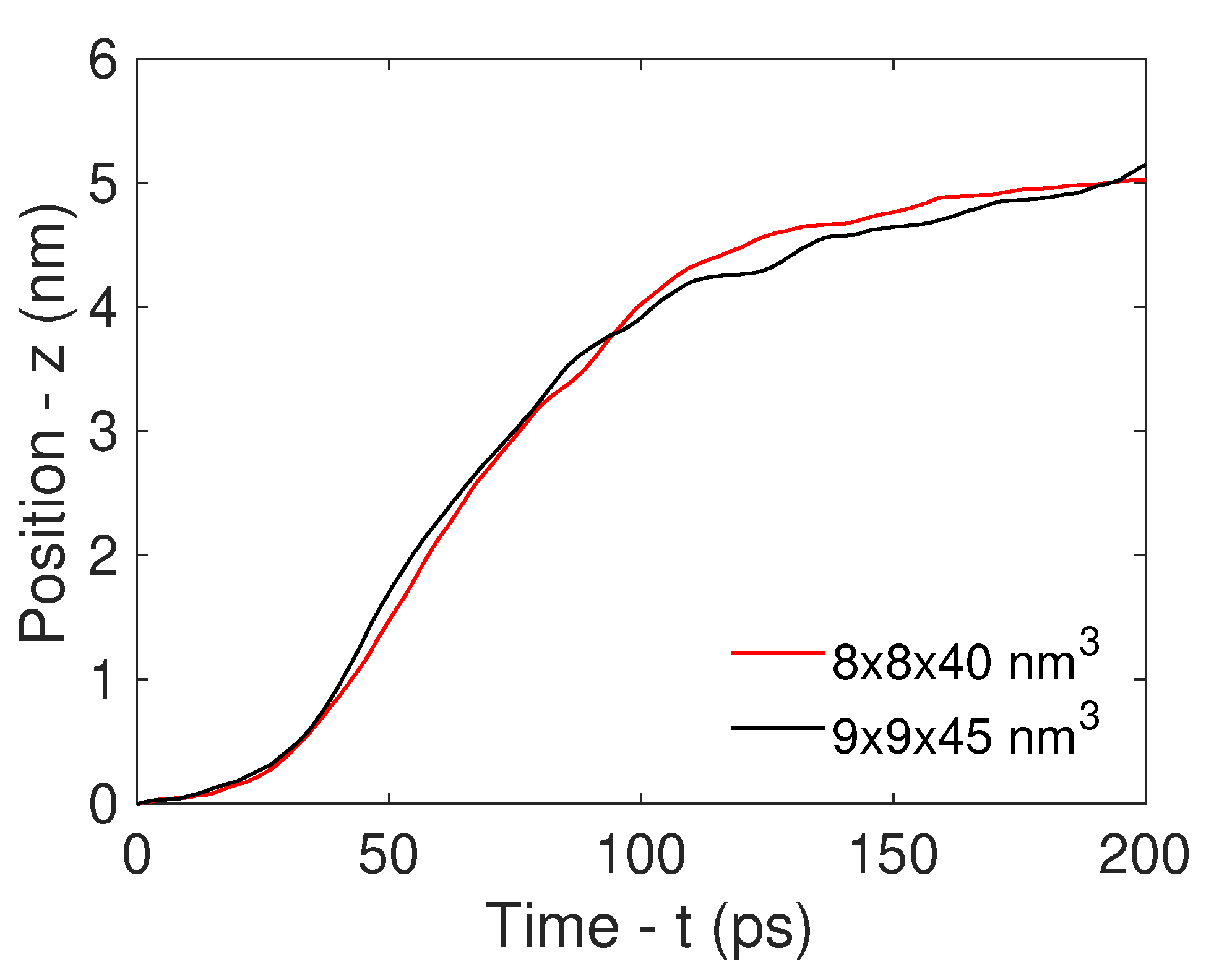
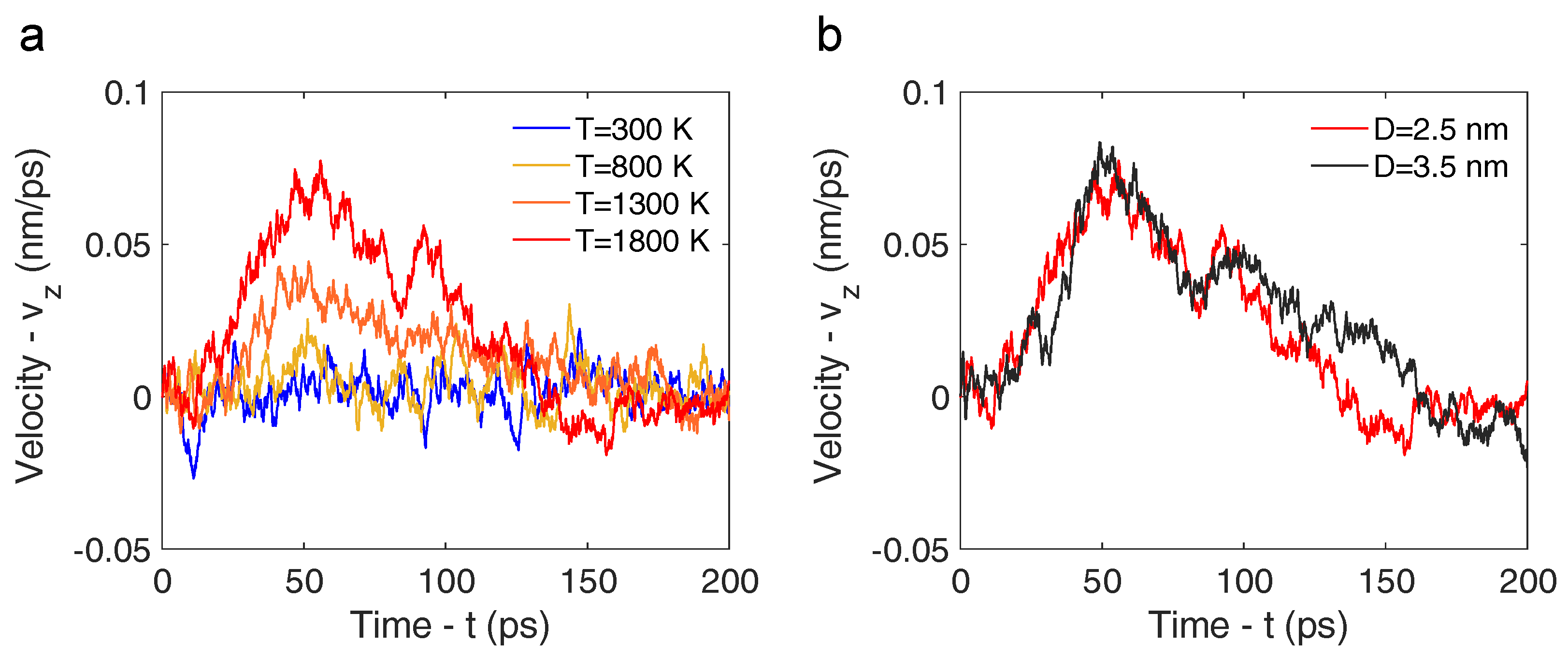
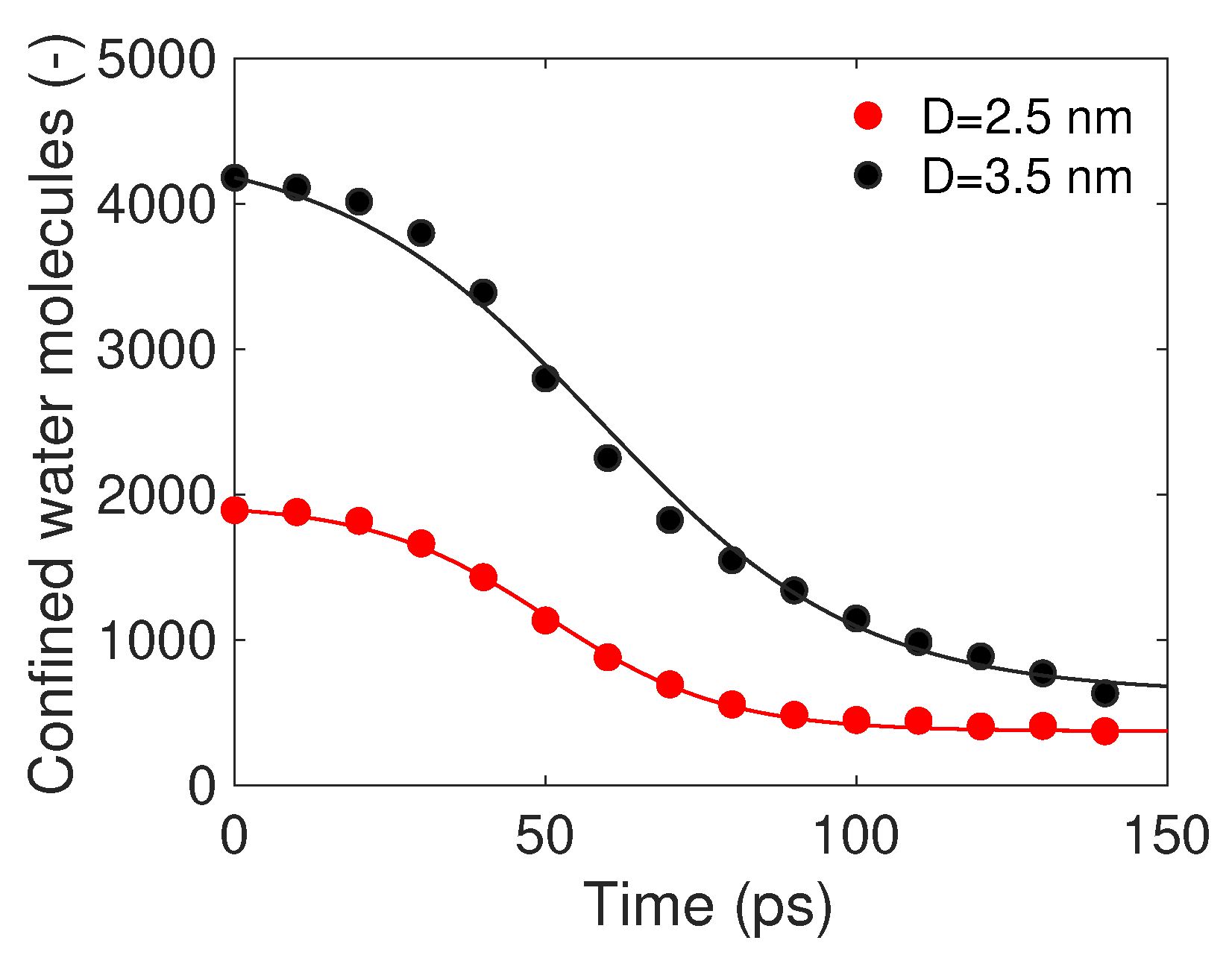
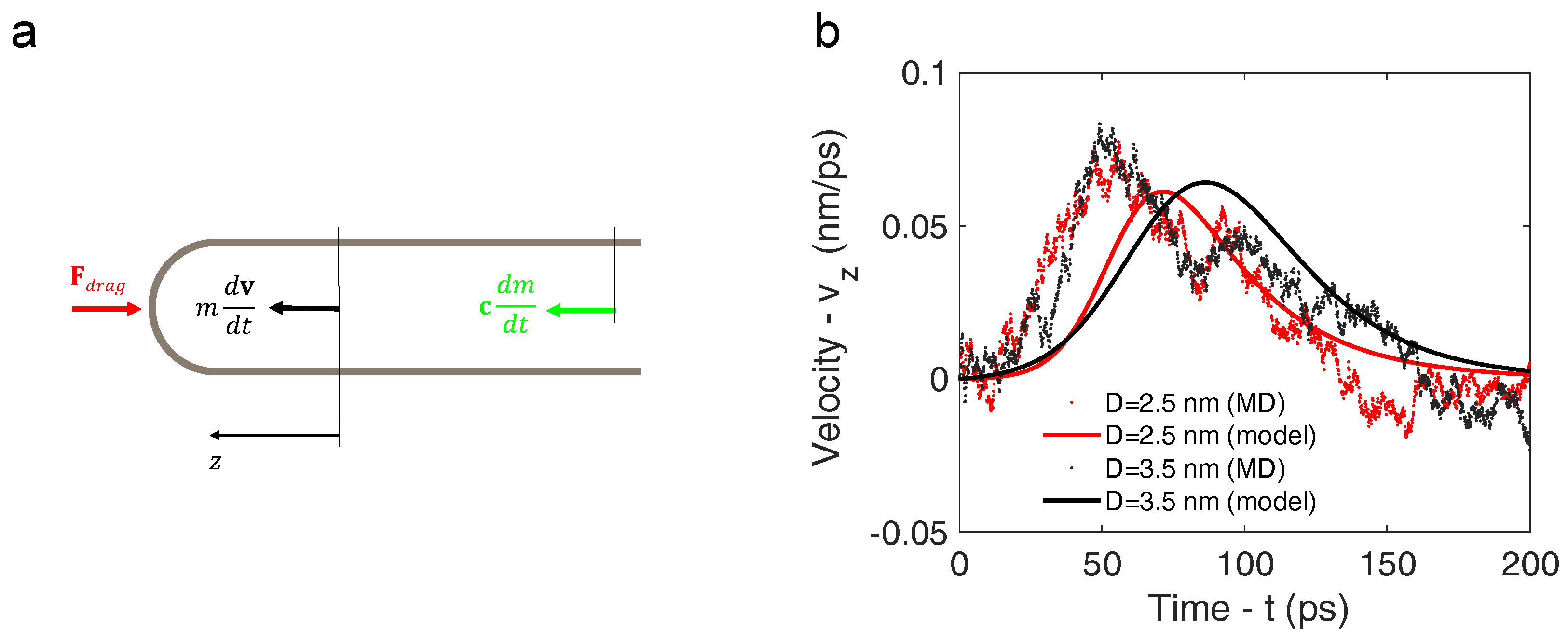
3.1. Molecular Dynamics Simulation
3.2. Analytical Motion Formulation: Rocket Model
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ozin, G.A.; Manners, I.; Fournier-Bidoz, S.; Arsenault, A. Dream nanomachines. Adv. Mater. 2005, 17, 3011–3018. [Google Scholar] [CrossRef]
- Jalili, N.A.; Muscarello, M.; Gaharwar, A.K. Nanoengineered thermoresponsive magnetic hydrogels for biomedical applications. Bioeng. Transl. Med. 2016, 1, 297–305. [Google Scholar] [CrossRef] [PubMed]
- Abdelmohsen, L.K.; Peng, F.; Tu, Y.; Wilson, D.A. Micro-and nano-motors for biomedical applications. J. Mater. Chem. B 2014, 2, 2395–2408. [Google Scholar] [CrossRef] [PubMed]
- Prabhu, V.; Uzzaman, S.; Grace, V.M.B.; Guruvayoorappan, C. Nanoparticles in drug delivery and cancer therapy: The giant rats tail. J. Cancer Ther. 2011, 2, 325–334. [Google Scholar] [CrossRef]
- Cavalcanti, A.; Shirinzadeh, B.; Freitas, R.A., Jr.; Hogg, T. Nanorobot architecture for medical target identification. Nanotechnology 2007, 19, 015103. [Google Scholar] [CrossRef]
- Chen, Y.; Lavacchi, A.; Miller, H.; Bevilacqua, M.; Filippi, J.; Innocenti, M.; Marchionni, A.; Oberhauser, W.; Wang, L.; Vizza, F. Nanotechnology makes biomass electrolysis more energy efficient than water electrolysis. Nat. Commun. 2014, 5, 4036. [Google Scholar] [CrossRef] [PubMed]
- Kang, J.W.; Hwang, H.J. Nanoscale carbon nanotube motor schematics and simulations for micro-electro-mechanical machines. Nanotechnology 2004, 15, 1633. [Google Scholar] [CrossRef]
- Liu, M.; Zentgraf, T.; Liu, Y.; Bartal, G.; Zhang, X. Light-driven nanoscale plasmonic motors. Nat. Nanotechnol. 2010, 5, 570. [Google Scholar] [CrossRef] [PubMed]
- Cha, T.G.; Pan, J.; Chen, H.; Salgado, J.; Li, X.; Mao, C.; Choi, J.H. A synthetic DNA motor that transports nanoparticles along carbon nanotubes. Nat. Nanotechnol. 2014, 9, 39. [Google Scholar] [CrossRef]
- Wu, Z.; Lin, X.; Wu, Y.; Si, T.; Sun, J.; He, Q. Near-infrared light-triggered “on/off” motion of polymer multilayer rockets. ACS Nano 2014, 8, 6097–6105. [Google Scholar] [CrossRef]
- Gong, X.; Li, J.; Lu, H.; Wan, R.; Li, J.; Hu, J.; Fang, H. A charge-driven molecular water pump. Nat. Nanotechnol. 2007, 2, 709. [Google Scholar] [CrossRef] [PubMed]
- Morciano, M.; Fasano, M.; Nold, A.; Braga, C.; Yatsyshin, P.; Sibley, D.N.; Goddard, B.D.; Chiavazzo, E.; Asinari, P.; Kalliadasis, S. Nonequilibrium molecular dynamics simulations of nanoconfined fluids at solid-liquid interfaces. J. Chem. Phys. 2017, 146, 244507. [Google Scholar] [CrossRef] [PubMed]
- Bergamasco, L.; Alberghini, M.; Fasano, M. Nano-metering of Solvated Biomolecules Or Nanoparticles from Water Self-Diffusivity in Bio-inspired Nanopores. Nanoscale Res. Lett. 2019, 14, 336. [Google Scholar] [CrossRef] [PubMed]
- Fasano, M.; Falciani, G.; Brancato, V.; Palomba, V.; Asinari, P.; Chiavazzo, E.; Frazzica, A. Atomistic modelling of water transport and adsorption mechanisms in silicoaluminophosphate for thermal energy storage. Appl. Therm. Eng. 2019, 160, 114075. [Google Scholar] [CrossRef]
- Ghosh, A.; Fischer, P. Controlled propulsion of artificial magnetic nanostructured propellers. Nano Lett. 2009, 9, 2243–2245. [Google Scholar] [CrossRef] [PubMed]
- Regan, B.; Aloni, S.; Jensen, K.; Ritchie, R.; Zettl, A. Nanocrystal-powered nanomotor. Nano Lett. 2005, 5, 1730–1733. [Google Scholar] [CrossRef] [PubMed]
- Somada, H.; Hirahara, K.; Akita, S.; Nakayama, Y. A molecular linear motor consisting of carbon nanotubes. Nano Lett. 2008, 9, 62–65. [Google Scholar] [CrossRef] [PubMed]
- Alaghemandi, M.; Spohr, E. A new class of nanoengines based on thermoresponsive polymers: Conceptual design and behavior study. Chem. Phys. Lett. 2013, 581, 80–84. [Google Scholar] [CrossRef]
- Fasano, M.; Crisafulli, A.; Cardellini, A.; Bergamasco, L.; Chiavazzo, E.; Asinari, P. Thermally triggered nanorocket from double-walled carbon nanotube in water. Mol. Simul. 2019, 45, 417–424. [Google Scholar] [CrossRef]
- Delogu, F. Molecular Dynamics of a Nanomotor Based on Carbon Nanotubes. J. Phys. Chem. C 2009, 113, 15909–15913. [Google Scholar] [CrossRef]
- Kang, J.W.; Hwang, H.J.; Lee, J.H.; Lee, H.J. Fluidic gas-driven carbon-nanotube motor: Molecular dynamics simulations. J. Korean Phys. Soc. 2004, 45, 573–576. [Google Scholar]
- Barreiro, A.; Rurali, R.; Hernández, E.R.; Moser, J.; Pichler, T.; Forro, L.; Bachtold, A. Subnanometer motion of cargoes driven by thermal gradients along carbon nanotubes. Science 2008, 320, 775–778. [Google Scholar] [CrossRef] [PubMed]
- Dai, Y.; Tang, C.; Guo, W. Simulation studies of a “nanogun” based on carbon nanotubes. Nano Res. 2008, 1, 176–183. [Google Scholar] [CrossRef]
- Coluci, V.; Timoteo, V.; Galvao, D. Thermophoretically driven carbon nanotube oscillators. Appl. Phys. Lett. 2009, 95, 253103. [Google Scholar] [CrossRef]
- Jiang, H.R.; Yoshinaga, N.; Sano, M. Active motion of a Janus particle by self-thermophoresis in a defocused laser beam. Phys. Rev. Lett. 2010, 105, 268302. [Google Scholar] [CrossRef] [PubMed]
- Zambrano, H.A.; Walther, J.H.; Koumoutsakos, P.; Sbalzarini, I.F. Thermophoretic motion of water nanodroplets confined inside carbon nanotubes. Nano Lett. 2008, 9, 66–71. [Google Scholar] [CrossRef] [PubMed]
- Schoen, P.A.; Walther, J.H.; Poulikakos, D.; Koumoutsakos, P. Phonon assisted thermophoretic motion of gold nanoparticles inside carbon nanotubes. Appl. Phys. Lett. 2007, 90, 253116. [Google Scholar] [CrossRef]
- Zhang, X.; Hu, M.; Poulikakos, D. A low-frequency wave motion mechanism enables efficient energy transport in carbon nanotubes at high heat fluxes. Nano Lett. 2012, 12, 3410–3416. [Google Scholar] [CrossRef] [PubMed]
- Tascini, A.S.; Armstrong, J.; Chiavazzo, E.; Fasano, M.; Asinari, P.; Bresme, F. Thermal transport across nanoparticle–fluid interfaces: The interplay of interfacial curvature and nanoparticle–fluid interactions. Phys. Chem. Chem. Phys. 2017, 19, 3244–3253. [Google Scholar] [CrossRef]
- Khodayari, A.; Fasano, M.; Bigdeli, M.B.; Mohammadnejad, S.; Chiavazzo, E.; Asinari, P. Effect of interfacial thermal resistance and nanolayer on estimates of effective thermal conductivity of nanofluids. Case Stud. Therm. Eng. 2018, 12, 454–461. [Google Scholar] [CrossRef]
- Crisafulli, A.; Khodayari, A.; Mohammadnejad, S.; Fasano, M. Sliding dynamics of parallel graphene sheets: Effect of geometry and van der Waals interactions on nano-spring behavior. Crystals 2018, 8, 149. [Google Scholar] [CrossRef]
- Chaban, V.V.; Prezhdo, V.V.; Prezhdo, O.V. Confinement by carbon nanotubes drastically alters the boiling and critical behavior of water droplets. ACS Nano 2012, 6, 2766–2773. [Google Scholar] [CrossRef]
- Chaban, V.V.; Prezhdo, O.V. Water boiling inside carbon nanotubes: Toward efficient drug release. ACS Nano 2011, 5, 5647–5655. [Google Scholar] [CrossRef] [PubMed]
- Fasano, M.; Borri, D.; Chiavazzo, E.; Asinari, P. Protocols for atomistic modeling of water uptake into zeolite crystals for thermal storage and other applications. Appl. Therm. Eng. 2016, 101, 762–769. [Google Scholar] [CrossRef]
- Kotaidis, V.; Dahmen, C.; Von Plessen, G.; Springer, F.; Plech, A. Excitation of nanoscale vapor bubbles at the surface of gold nanoparticles in water. J. Chem. Phys. 2006, 124, 184702. [Google Scholar] [CrossRef] [PubMed]
- Lapotko, D. Pulsed photothermal heating of the media during bubble generation around gold nanoparticles. Int. J. Heat Mass Transf. 2009, 52, 1540–1543. [Google Scholar] [CrossRef]
- Beqa, L.; Singh, A.K.; Fan, Z.; Senapati, D.; Ray, P.C. Chemically attached gold nanoparticle–carbon nanotube hybrids for highly sensitive SERS substrate. Chem. Phys. Lett. 2011, 512, 237–242. [Google Scholar] [CrossRef]
- Ismaili, H.; Lagugne-Labarthet, F.; Workentin, M.S. Covalently assembled gold nanoparticle-carbon nanotube hybrids via a photoinitiated carbene addition reaction. Chem. Mater. 2011, 23, 1519–1525. [Google Scholar] [CrossRef]
- Alias, S.H.; Sondig, S.S.S.; Jani, M.; Mutalib, A.; Yazid, H. Fabrication of Gold Nanoparticles on Multiwalled Carbon Nanotubes Nanohybrids. In Advanced Materials Research; Trans Tech Publications, Ltd.: Wollerau, Switzerland, 2014; Volume 1024, pp. 79–82. [Google Scholar]
- Abraham, M.J.; Murtola, T.; Schulz, R.; Páll, S.; Smith, J.C.; Hess, B.; Lindahl, E. GROMACS: High performance molecular simulations through multi-level parallelism from laptops to supercomputers. SoftwareX 2015, 1, 19–25. [Google Scholar] [CrossRef]
- Berendsen, H.; Grigera, J.; Straatsma, T. The missing term in effective pair potentials. J. Phys. Chem. 1987, 91, 6269–6271. [Google Scholar] [CrossRef]
- Walther, J.H.; Jaffe, R.; Halicioglu, T.; Koumoutsakos, P. Carbon nanotubes in water: Structural characteristics and energetics. J. Phys. Chem. B 2001, 105, 9980–9987. [Google Scholar] [CrossRef]
- Chiavazzo, E.; Asinari, P. Enhancing surface heat transfer by carbon nanofins: Towards an alternative to nanofluids? Nanoscale Res. Lett. 2011, 6, 249. [Google Scholar] [CrossRef] [PubMed]
- Chiavazzo, E.; Fasano, M.; Asinari, P.; Decuzzi, P. Scaling behaviour for the water transport in nanoconfined geometries. Nat. Commun. 2014, 5, 3565. [Google Scholar] [CrossRef] [PubMed]
- Fasano, M.; Chiavazzo, E.; Asinari, P. Water transport control in carbon nanotube arrays. Nanoscale Res. Lett. 2014, 9, 1–8. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Miyamoto, S.; Kollman, P.A. Settle: An analytical version of the SHAKE and RATTLE algorithm for rigid water models. J. Comput. Chem. 1992, 13, 952–962. [Google Scholar] [CrossRef]
- Mark, P.; Nilsson, L. Structure and dynamics of the TIP3P, SPC, and SPC/E water models at 298 K. J. Phys. Chem. A 2001, 105, 9954–9960. [Google Scholar] [CrossRef]
- Robinson, M.; Suarez-Martinez, I.; Marks, N. Generalized method for constructing the atomic coordinates of nanotube caps. Phys. Rev. B 2013, 87, 155430. [Google Scholar] [CrossRef]
- Robinson, M.; Marks, N. NanoCap: A framework for generating capped carbon nanotubes and fullerenes. Comput. Phys. Commun. 2014, 185, 2519–2526. [Google Scholar] [CrossRef][Green Version]
- Bussi, G.; Donadio, D.; Parrinello, M. Canonical sampling through velocity rescaling. J. Chem. Phys. 2007, 126, 014101. [Google Scholar] [CrossRef]
- Allen, M.P.; Schmid, F. A thermostat for molecular dynamics of complex fluids. Mol. Simul. 2007, 33, 21–26. [Google Scholar] [CrossRef]
- Nosé, S.; Klein, M. Constant pressure molecular dynamics for molecular systems. Mol. Phys. 1983, 50, 1055–1076. [Google Scholar] [CrossRef]
- Parrinello, M.; Rahman, A. Polymorphic transitions in single crystals: A new molecular dynamics method. J. Appl. Phys. 1981, 52, 7182–7190. [Google Scholar] [CrossRef]
- Hockney, R.; Goel, S.; Eastwood, J. Quiet high-resolution computer models of a plasma. J. Comput. Phys. 1974, 14, 148–158. [Google Scholar] [CrossRef]
- Yang, F.; Wang, M.; Zhang, D.; Yang, J.; Zheng, M.; Li, Y. Chirality pure carbon nanotubes: Growth, sorting, and characterization. Chem. Rev. 2020, 120, 2693–2758. [Google Scholar] [CrossRef]
- Shiomi, J.; Maruyama, S. Water transport inside a single-walled carbon nanotube driven by a temperature gradient. Nanotechnology 2009, 20, 055708. [Google Scholar] [CrossRef]
- Wei, X.; Wang, M.S.; Bando, Y.; Golberg, D. Thermal stability of carbon nanotubes probed by anchored tungsten nanoparticles. Sci. Technol. Adv. Mater. 2011, 12, 044605. [Google Scholar] [CrossRef]
- Markesteijn, A.; Hartkamp, R.; Luding, S.; Westerweel, J. A comparison of the value of viscosity for several water models using Poiseuille flow in a nano-channel. J. Chem. Phys. 2012, 136, 134104. [Google Scholar] [CrossRef] [PubMed]
- Wei, N.; Wang, H.Q.; Zheng, J.C. Nanoparticle manipulation by thermal gradient. Nanoscale Res. Lett. 2012, 7, 154. [Google Scholar] [CrossRef]
- Bellussi, F.M.; Roscioni, O.M.; Ricci, M.; Fasano, M. Anisotropic electrostatic interactions in coarse-grained water models to enhance the accuracy and speed-up factor of mesoscopic simulations. J. Phys. Chem. B 2021, 125, 12020–12027. [Google Scholar] [CrossRef]
- Casto, A.; Bellussi, F.M.; Diego, M.; Del Fatti, N.; Banfi, F.; Maioli, P.; Fasano, M. Water filling in carbon nanotubes with different wettability and implications on nanotube/water heat transfer via atomistic simulations. Int. J. Heat Mass Transf. 2023, 205, 123868. [Google Scholar] [CrossRef]
- Stuart, S.J.; Tutein, A.B.; Harrison, J.A. A reactive potential for hydrocarbons with intermolecular interactions. J. Chem. Phys. 2000, 112, 6472–6486. [Google Scholar] [CrossRef]
- Kim, J.H.; Pham, T.V.; Hwang, J.H.; Kim, C.S.; Kim, M.J. Boron nitride nanotubes: Synthesis and applications. Nano Converg. 2018, 5, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Sekino, T. Synthesis and applications of titanium oxide nanotubes. In Inorganic and Metallic Nanotubular Materials: Recent Technologies and Applications; Springer: Berlin/Heidelberg, Germany, 2010; pp. 17–32. [Google Scholar]
- Perepichka, D.F.; Rosei, F. Silicon nanotubes. Small 2006, 2, 22–25. [Google Scholar] [CrossRef] [PubMed]
| Setup | D (nm) | Chirality | Box Size [nm × nm × nm] | T (K) | Water Molecules [-] |
|---|---|---|---|---|---|
| 1 | 2.5 | 19 × 19 | 8 × 8 × 40 | 300 | 83,287 |
| 2 | 2.5 | 19 × 19 | 8 × 8 × 40 | 800 | 83,287 |
| 3 | 2.5 | 19 × 19 | 8 × 8 × 40 | 1300 | 83,287 |
| 4 | 2.5 | 19 × 19 | 8 × 8 × 40 | 1800 | 83,287 |
| 5 | 3.5 | 27 × 27 | 12 × 12 × 60 | 1800 | 285,769 |
| 6 | 2.5 | 19 × 19 | 9 × 9 × 45 | 1800 | 119,414 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hamad, M.S.; Morciano, M.; Fasano, M. Rocket Dynamics of Capped Nanotubes: A Molecular Dynamics Study. Nanomaterials 2024, 14, 1134. https://doi.org/10.3390/nano14131134
Hamad MS, Morciano M, Fasano M. Rocket Dynamics of Capped Nanotubes: A Molecular Dynamics Study. Nanomaterials. 2024; 14(13):1134. https://doi.org/10.3390/nano14131134
Chicago/Turabian StyleHamad, Mustafa S., Matteo Morciano, and Matteo Fasano. 2024. "Rocket Dynamics of Capped Nanotubes: A Molecular Dynamics Study" Nanomaterials 14, no. 13: 1134. https://doi.org/10.3390/nano14131134
APA StyleHamad, M. S., Morciano, M., & Fasano, M. (2024). Rocket Dynamics of Capped Nanotubes: A Molecular Dynamics Study. Nanomaterials, 14(13), 1134. https://doi.org/10.3390/nano14131134







