Flexible CNT-Interpenetrating Hierarchically Porous Sulfurized Polyacrylonitrile (CIHP-SPAN) Electrodes for High-Rate Lithium-Sulfur (Li-S) Batteries
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals and Materials
2.2. Preparation of SPAN and CNT Interpenetrating SPAN Electrode
2.3. Synthesis of V2O5/Carbon Fiber (V-CF) Interlayers
2.4. In Situ UV–Vis Absorption Spectra
2.5. Characterizations
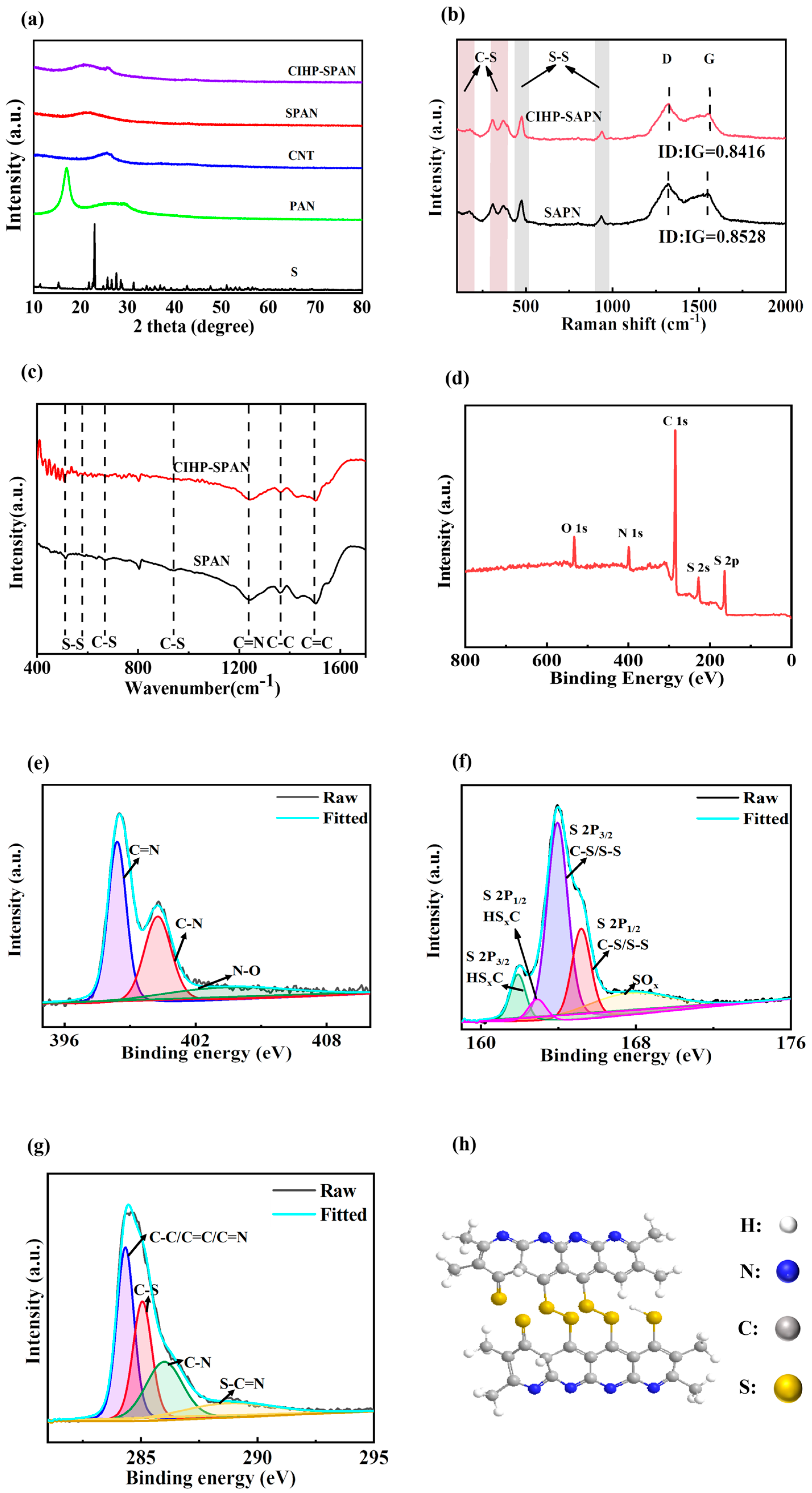
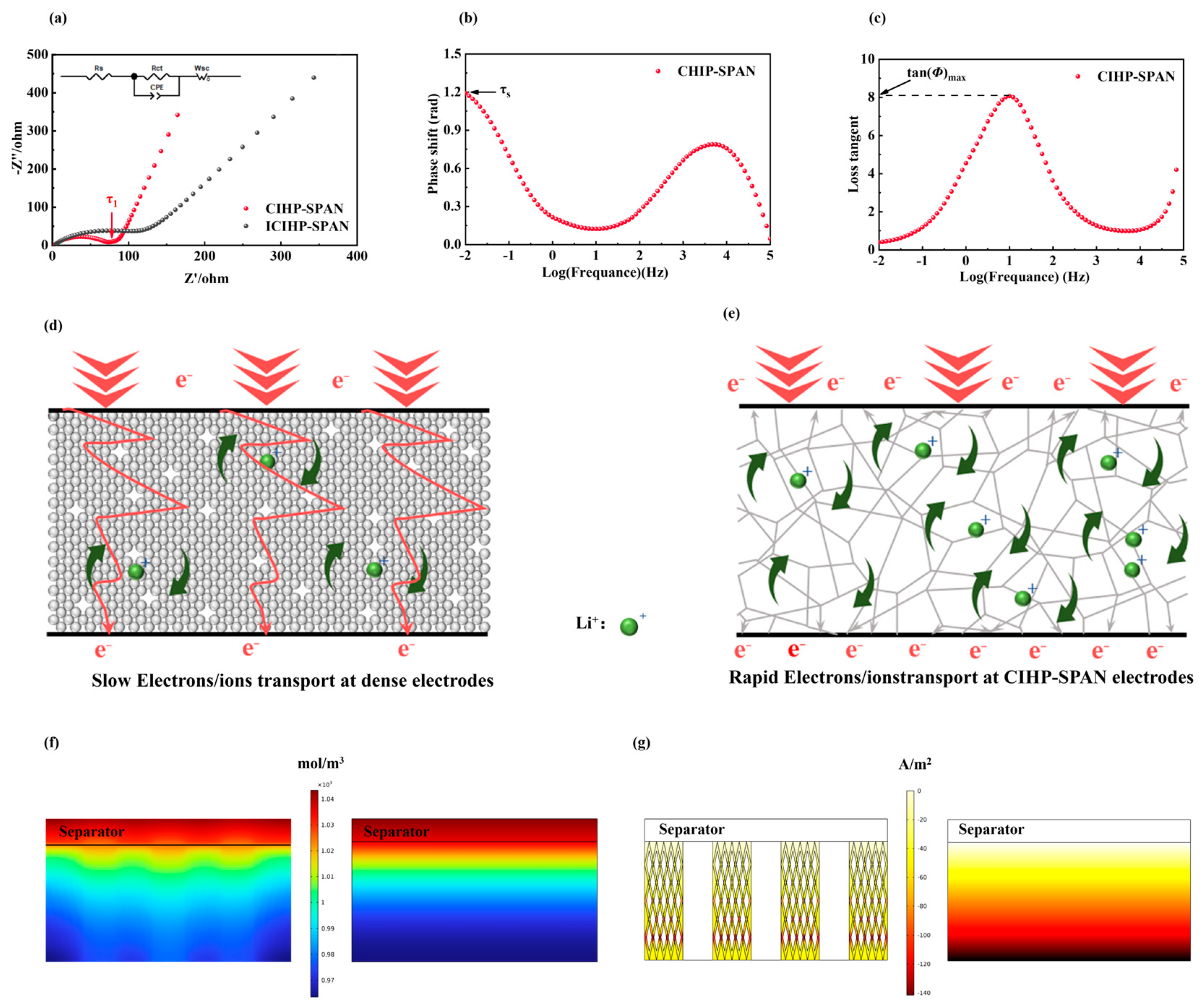
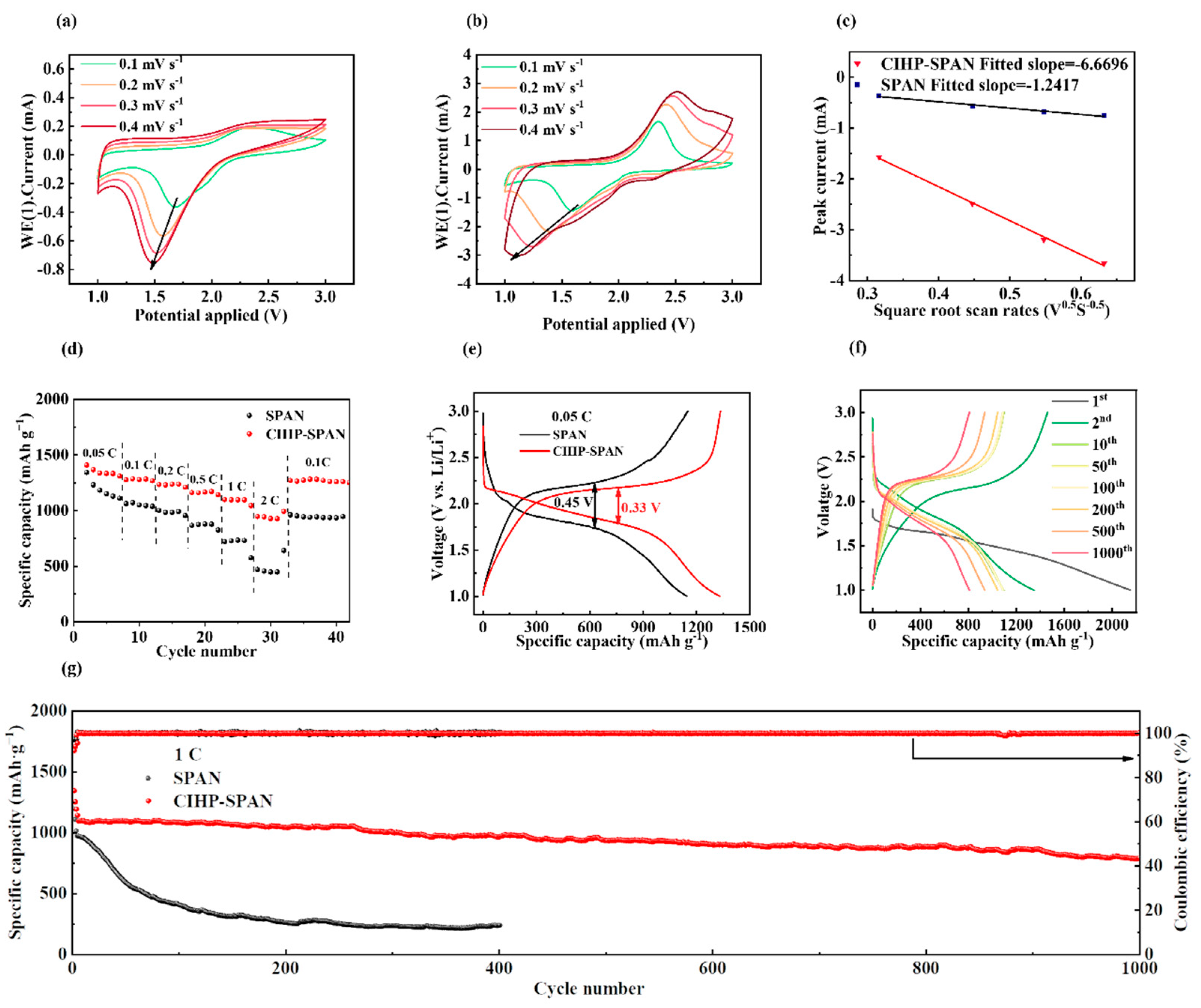
3. Results
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Mallapaty, S.J.N. How China could be carbon neutral by mid-century. Nature 2020, 586, 482–483. [Google Scholar] [CrossRef] [PubMed]
- Benveniste, G.; Rallo, H.; Canals Casals, L.; Merino, A.; Amante, B. Comparison of the state of Lithium-Sulphur and Lithium-ion batteries applied to electromobility. J. Environ. Manag. 2018, 226, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Danuta, H.; Juliusz, U. Electric Dry Cells and Storage Batteries. U.S. Patent No. 3,043,896, 10 July 1962. [Google Scholar]
- Manthiram, A.; Fu, Y.; Su, Y.S. Challenges and prospects of lithium-sulfur batteries. Acc. Chem. Res. 2013, 46, 1125–1134. [Google Scholar] [CrossRef] [PubMed]
- Philipp, A.; Pascal, H.; Bender, C.L.; Martin, B.; Christine, E.; Juergen, J.J. From lithium to sodium: Cell chemistry of room temperature sodium–air and sodium–sulfur batteries. Beilstein J. Nanotechnol. 2015, 6, 1016–1055. [Google Scholar]
- Nakamura, N.; Ahn, S.; Momma, T.; Osaka, T.J. Future Potential for Lithium-Sulfur Batteries. J. Power Sources 2023, 558, 232566. [Google Scholar] [CrossRef]
- Zhu, K.; Wang, C.; Chi, Z.; Ke, F.; Yang, Y.; Wang, A.; Wang, W.; Miao, L. How far away are lithium-sulfur batteries from commercialization? Front. Energy Res. 2019, 7, 123. [Google Scholar] [CrossRef]
- Lv, Z.C.; Wang, P.F.; Wang, J.C.; Tian, S.H.; Yi, T.F. Key challenges, recent advances and future perspectives of rechargeable lithium-sulfur batteries. J. Ind. Eng. Chem. 2023, 124, 68–88. [Google Scholar] [CrossRef]
- Li, J.; Li, K.; Li, M.; Gosselink, D.; Zhang, Y.; Chen, P. A sulfur–polyacrylonitrile/graphene composite cathode for lithium batteries with excellent cyclability. J. Power Sources 2014, 252, 107–112. [Google Scholar] [CrossRef]
- Huang, C.J.; Cheng, J.H.; Su, W.N.; Partovi-Azar, P.; Hwang, B.J. Origin of shuttle-free sulfurized polyacrylonitrile in lithium-sulfur batteries. J. Power Sources 2021, 492, 229508. [Google Scholar] [CrossRef]
- Li, X.; Yuan, L.; Liu, D.; Xiang, J.; Li, Z.; Huang, Y.H. Solid/Quasi-Solid Phase Conversion of Sulfur in Lithium-Sulfur Battery. Small 2022, 18, e2106970. [Google Scholar] [CrossRef]
- Zhao, M.; Li, B.Q.; Peng, H.J.; Yuan, H.; Wei, J.Y.; Huang, J.Q. Lithium–Sulfur Batteries under Lean Electrolyte Conditions: Challenges and Opportunities. Angew. Chem. Int. Ed. 2020, 59, 12636–12652. [Google Scholar] [CrossRef] [PubMed]
- Wu, Z.; Bak, S.M.; Shadike, Z.; Yu, S.; Liu, P. Understanding the Roles of the Electrode/Electrolyte Interface for Enabling Stable Li∥Sulfurized Polyacrylonitrile Batteries. ACS Appl. Mater. Interfaces 2021, 13, 31733–31740. [Google Scholar] [CrossRef] [PubMed]
- Jin, Z.Q.; Liu, Y.G.; Wang, W.K.; Wang, A.B.; Hu, B.W.; Shen, M.; Gao, T.; Zhao, P.C.; Yang, Y.S. A New Insight into the Lithium Storage Mechanism of Sulfurized Polyacrylonitrile with no Soluble Intermediates. Energy Storage Mater. 2018, 14, 272–278. [Google Scholar] [CrossRef]
- Yu, X.; Xie, J.; Li, Y.; Huang, H.; Lai, C.; Wang, K. Stable-cycle and high-capacity conductive sulfur-containing cathode materials for rechargeable lithium batteries. J. Power Sources 2005, 146, 335–339. [Google Scholar] [CrossRef]
- Weret, M.A.; Kuo, C.J.; Su, W.N.; Zeleke, T.S.; Huang, C.J.; Sahalie, N.A.; Zegeye, T.A.; Wondimkun, Z.T.; Fenta, F.W.; Jote, B.A.J.; et al. Fibrous organosulfur cathode materials with high bonded sulfur for high-performance lithium-sulfur batteries. J. Power Sources 2022, 541, 231693. [Google Scholar] [CrossRef]
- Zhao, X.; Wang, C.; Li, Z.; Hu, X.; Razzaq, A.A.; Deng, Z.J. Sulfurized polyacrylonitrile for high-performance lithium sulfur batteries: Advances and prospects. J. Mater. Chem. A 2021, 9, 19282–19297. [Google Scholar] [CrossRef]
- Yang, R.; Gong, S.M.; Zhao, S.Q.; Zhao, C.R.; Zhang, L.J. S@CNT-graphene-TiN multi-dimensional composites with high sulfur content as the high-performance lithium-sulfur battery cathode materials. J. Solid State Electrochem. 2020, 24, 1397–1404. [Google Scholar] [CrossRef]
- Wang, K.; Zhao, T.; Zhang, N.; Feng, T.; Li, L.; Wu, F.; Chen, R. Powering lithium–sulfur batteries by ultrathin sulfurized polyacrylonitrile nanosheets. Nanoscale 2021, 13, 16690–16695. [Google Scholar] [CrossRef]
- Liu, Y.; Haridas, A.K.; Lee, Y.; Cho, K.K.; Ahn, J.H. Freestanding porous sulfurized polyacrylonitrile fiber as a cathode material for advanced lithium sulfur batteries. Appl. Surf. Sci. 2019, 472, 135–142. [Google Scholar] [CrossRef]
- Luo, F.; Feng, X.; Zeng, L.; Lin, L.; Li, X.Y.; Kang, B.Y.; Xiao, L.R.; Chen, Q.H.; Wei, M.D.; Qian, Q.R. In situ simultaneous encapsulation of defective MoS2 nanolayers and sulfur nanodots into SPAN fibers for high rate sodium-ion batteries. Chem. Eng. J. 2020, 404, 126430. [Google Scholar] [CrossRef]
- Yang, H.; Wang, L.; Geng, C.; Zhao, Y.; Li, Q.; Jiang, X.; Tian, Z.; Wang, M.; Jiang, C.; Sun, Z.; et al. Catalytic Solid-State Sulfur Conversion Confined in Micropores toward Superhigh Coulombic Efficiency Lithium-Sulfur Batteries. Adv. Energy Mater. 2024, 14, 2400249. [Google Scholar] [CrossRef]
- Yu, L.; Yang, J.F.; Lou, X.W. Formation of CoS2 Nanobubble Hollow Prisms for Highly Reversible Lithium Storage. Angew. Chem. Int. Ed. 2016, 55, 13422–13426. [Google Scholar] [CrossRef] [PubMed]
- Xu, Z.Q.; Zou, R.; Liu, W.W.; Liu, G.L. Design of atomic cobalt selenide-doped sulfurized polyacrylonitrile cathode with enhanced electrochemical kinetics for high performance lithium-SPAN batteries. Chem. Eng. J. 2023, 471, 144581. [Google Scholar] [CrossRef]
- Wang, J.; Wu, N.; Han, L.; Liao, C.; Mu, X.; Kan, Y.; Hu, Y. Polyacrylonitrile@metal organic frameworks composite-derived heteroatoms doped carbon@encapsulated cobalt sulfide as superb sodium ion batteries anode. J. Colloid Interface Sci. 2021, 581, 552–565. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Wu, G.; Ji, H.; Chen, D.; Xia, D.; Gao, K.; Xu, J.; Mao, B.; Yi, S.; Zhang, L.Y.; et al. 2D/1D V2O5 Nanoplates Anchored Carbon Nanofibers as Efficient Separator Interlayer for Highly Stable Lithium-Sulfur Battery. Nanomaterials 2020, 10, 705. [Google Scholar] [CrossRef] [PubMed]
- Razzaq, A.A.; Yao, Y.; Shah, R.; Qi, P.; Miao, L.; Chen, M.; Zhao, X.; Peng, Y.; Deng, Z. High-Performance Lithium Sulfur Batteries Enabled by a Synergy between Sulfur and Carbon Nanotubes. Energy Storage Mater. 2018, 16, 194–202. [Google Scholar] [CrossRef]
- Wang, L.; He, X.; Sun, W.; Li, J.; Gao, J.; Tian, G.; Wang, J.; Fan, S. Organic polymer material with a multi-electron process redox reaction: Towards ultra-high reversible lithium storage capacity. RSC Adv. 2013, 3, 3227–3231. [Google Scholar] [CrossRef]
- Wang, T.; Zhang, Q.; Zhong, J.; Chen, M.; Lu, B.J. 3D Holey Graphene/Polyacrylonitrile Sulfur Composite Architecture for High Loading Lithium Sulfur Batteries. Adv. Energy Mater. 2021, 11, 2100448. [Google Scholar] [CrossRef]
- Yu, X.G.; Xie, J.Y.; Yang, J.; Huang, H.J.; Wang, K.; Wen, Z.S. Lithium storage in conductive sulfur-containing polymers. J. Electroanal. Chem. 2004, 573, 121–128. [Google Scholar]
- Wang, X.; Qian, Y.; Wang, L.; Yang, H.; Liu, T. Sulfurized Polyacrylonitrile Cathodes with High Compatibility in Both Ether and Carbonate Electrolytes for Ultrastable Lithium–Sulfur Batteries. Adv. Funct. Mater. 2019, 29, 1902929. [Google Scholar] [CrossRef]
- Kim, J.S.; Hwang, T.H.; Kim, B.G.; Min, J.; Choi, J.W. A Lithium-Sulfur Battery with a High Areal Energy Density. Adv. Funct. Mater. 2014, 24, 5359–5367. [Google Scholar] [CrossRef]
- Kuo, C.F.; Weret, M.A.; Hung, H.Y.; Tsai, M.C.; Huang, C.J.; Su, W.N.; Huang, B.J. Sulfurized–poly(acrylonitrile) wrapped carbonsulfur composite cathode material for high performance rechargeable lithiumsulfur batteries. J. Power Sources 2019, 412, 670–676. [Google Scholar] [CrossRef]
- Dementjev, A.P.; De Graaf, A.; Van de Sanden, M.C.M.; Maslakov, K.I.; Naumkin, A.V.; Serov, A.A. X-ray photoelectron spectroscopy reference data for identification of the C3N4 phase in carbon-nitrogen films. Diam. Relat. Mater. 2000, 9, 1904–1907. [Google Scholar] [CrossRef]
- Fanous, J.; Wegner, M.; Grimminger, J.; Andresen, Ä.; Buchmeiser, M.R. Structure-Related Electrochemistry of Sulfur-Poly(acrylonitrile) Composite Cathode Materials for Rechargeable Lithium Batteries. Chem. Mater. 2012, 23, 5024–5028. [Google Scholar] [CrossRef]
- Weret, M.A.; Kuo, C.F.J.; Zeleke, T.S.; Beyene, T.T.; Hwang, B.J.J. Mechanistic Understanding of the Sulfurized-Poly(acrylonitrile) Cathode for Lithium-Sulfur Batteries. Energy Storage Mater. 2019, 26, 483–493. [Google Scholar] [CrossRef]
- Kim, S.; Cho, M.; Chanthad, C.; Lee, Y. New redox-mediating polymer binder for enhancing performance of Li-S batteries. J. Energy Chem. 2020, 29, 8. [Google Scholar] [CrossRef]
- Wang, Y.X.; Huang, L.; Sun, L.C.; Xie, S.Y.; Xu, G.L.; Chen, S.R.; Xu, Y.F.; Li, J.T.; Chou, S.L.; Dou, S.X. Facile synthesis of a interleaved expanded graphite-embedded sulphur nanocomposite as cathode of Li-S batteries with excellent lithium storage performance. J. Mater. Chem. 2012, 22, 4744–4750. [Google Scholar] [CrossRef]
- Aurbach, D.; Gamolsky, K.; Markovsky, B.; Gofer, Y.; Heider, U. On the use of vinylene carbonate (VC) as an additive to electrolyte solutions for Li-ion batteries. Electrochim. Acta 2003, 47, 1423–1439. [Google Scholar] [CrossRef]
- Nguyen, T.Q.; Breitkopf, C. Determination of Diffusion Coefficients Using Impedance Spectroscopy Data. J. Electrochem. Soc. 2018, 165, E826–E831. [Google Scholar] [CrossRef]
- Hu, X.R.; Jiang, H.L.; Hou, Q.; Yu, M.; Jiang, X.B.; He, G.H.; Li, X.C. Scalable SPAN Membrane Cathode with High Conductivity and Hierarchically Porous Framework for Enhanced Ion Transfer and Cycling Stability in Li–S Batteries. ACS Mater. Lett. 2023, 5, 2047–2057. [Google Scholar] [CrossRef]
- Li, S.P.; Tian, G.L.; Xiong, R.Y.; He, R.J.; Chen, S.Q.; Zhou, H.M.; Wu, Y.K.; Han, Z.L.; Yu, C.; Chen, S.J.; et al. Enhanced homogeneity of electrochemical reaction via low tortuosity enabling high-voltage nickel-rich layered oxide thick-electrode–ScienceDirect. Energy Storage Mater. 2022, 46, 443–451. [Google Scholar] [CrossRef]
- Li, Y.N.; Tong, W.S.; Yang, J.; Wang, Z.H.; Wang, D.L.; An, Q.; Zhang, Y. Electrode-free piezoelectric nanogenerator based on carbon black/polyvinylidene fluoride–hexafluoropropylene composite achieved via interface polarization effect. Chem. Eng. J. 2011, 457, 141356. [Google Scholar] [CrossRef]
- Zhang, Y.; Yermukhambetova, A.; Bakenov, Z.; Chen, P. Ternary sulfur/polyacrylonitrile/Mg0.6Ni0.4O composite cathodes for high performance lithium/sulfur batteries. J. Mater. Chem. A 2013, 1, 295–301. [Google Scholar] [CrossRef]
- Mentbayeva, A.; Belgibayeva, A.; Umirov, N.; Zhang, Y.; Taniguchi, I.; Kurmanbayeva, I.; Bakenov, Z. High performance freestanding composite cathode for lithium-sulfur batteries. Electrochim. Acta 2016, 217, 242–248. [Google Scholar] [CrossRef]
- Tao, X.; Wang, J.; Liu, C.; Wang, H.; Yao, H.; Zheng, G.; Seh, Z.W.; Cai, Q.; Li, W.; Zhou, G.J. Balancing surface adsorption and diffusion of lithium-polysulfides on nonconductive oxides for lithium–sulfur battery design. Nat. Commun. 2016, 7, 11203. [Google Scholar] [CrossRef] [PubMed]
- Zhou, G.; Tian, H.; Jin, Y.; Tao, X.; Cui, Y.J. Catalytic oxidation of Li2S on the surface of metal sulfides for LiS batteries. Proc. Natl. Acad. Sci. USA 2017, 114, 840–845. [Google Scholar] [CrossRef]
- Zhao, M.; Peng, H.J.; Li, B.Q.; Chen, X.; Xie, J.; Liu, X.; Zhang, Q.; Huang, J.Q. Electrochemical Phase Evolution of Metal-Based Pre-Catalysts for High-Rate Polysulfide Conversion. Angew. Chem. Int. Ed. 2020, 59, 9011–9017. [Google Scholar] [CrossRef]
- Yan, D.; Bazant, M.Z.; Biesheuvel, P.M.; Pugh, M.C.; Dawson, F.P. Theory of linear sweep voltammetry with diffuse charge: Unsupported electrolytes, thin films, and leaky membranes. Phys. Rev. E 2016, 95, 033303. [Google Scholar] [CrossRef]
- Patel, M.U.; Dominko, R. Application of in operando UV/vis spectroscopy in lithium–sulfur batteries. ChemSusChem 2014, 7, 2167–2175. [Google Scholar] [CrossRef]
- Liu, M.; Li, Q.; Liang, G.M.; Han, W.J.; Zhou, D.; He, Y.B.; Li, B.H.; Kang, F.Y. Suppressing self-discharge and shuttle effect of lithium–sulfur batteries with V2O5-decorated carbon nanofiber interlayer. Small 2017, 13, 1602539. [Google Scholar] [CrossRef]
- Chen, K.; Zhang, G.D.; Xiao, L.P.; Li, P.W.; Li, W.L.; Xu, Q.C.; Xu, J. Polyaniline Encapsulated Amorphous V2O5 Nanowire-Modified Multi-Functional Separators for Lithium–Sulfur Batteries. Small Methods 2021, 5, 2001056. [Google Scholar] [CrossRef] [PubMed]
- Xu, R.; Shao, J.S.; Gao, K.K.; Chen, Y.X.; Li, J.; Liu, Y.F.; Hou, X.H.; Ji, H.P.; Yi, S.S.; Zhang, L.Y.; et al. Highly stable lithium sulfur batteries enhanced by flocculation and solidification of soluble polysulfides in routine ether electrolyte. J. Colloid Interface Sci. 2023, 649, 223–233. [Google Scholar] [CrossRef]
- Xue, W.; Miao, L.; Qie, L.; Wang, C.; Li, S.; Wang, J.; Li, J. Gravimetric and volumetric energy densities of lithium-sulfur batteries. Curr. Opin. Electrochem. 2019, 6, 92–99. [Google Scholar] [CrossRef]
- Yi, Y.; Hai, F.; Guo, J.; Gao, X.; Chen, W.; Tian, X.; Tang, W.; Hua, W.; Li, M. Electrochemical Enhancement of Lithium-Ion Diffusion in Polypyrrole-Modified Sulfurized Polyacrylonitrile Nanotubes for Solid-to-Solid Free-Standing Lithium–Sulfur Cathodes. Small 2023, 19, e2303781. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Peng, L.; Wang, L.; Yang, J.; Hao, Z.; Xiang, J.; Yuan, K.; Huang, Y.; Shan, B.; Yuan, L.; et al. Ether-compatible sulfurized polyacrylonitrile cathode with excellent performance enabled by fast kinetics via selenium doping. Nat. Commun. 2019, 10, 1021. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.; Hwang, J.Y.; Bang, S.; Jung, H.G.; Sun, Y.K. Geometrical engineering of a SPAN–graphene composite cathode for practical Li–S batteries. J. Mater. Chem. A. 2022, 10, 10844–10853. [Google Scholar] [CrossRef]
- Wang, L.; Chen, X.; Li, S.; Yang, J.; Sun, Y.; Peng, L.; Shan, B.; Xie, J. Effect of eutectic accelerator in selenium-doped sulfu-rized polyacrylonitrile for high performance room temperature sodium-sulfur batteries. J. Mater. Chem. A 2019, 7, 12732–12739. [Google Scholar] [CrossRef]
- Sabet, S.M.; Sapkota, N.; Chiluwal, S.; Zheng, T.; Clemons, C.M.; Rao, A.M.; Pilla, S. Sulfurized Polyacrylonitrile Impregnated Delignified Wood-Based 3D Carbon Framework for High-Performance Lithium–Sulfur Batteries. ACS Sustain. Chem. Eng. 2023, 11, 2314–2323. [Google Scholar] [CrossRef]
- Li, H.; Xue, W.; Xu, W.; Wang, L.; Liu, T. Controllable synthesis of sulfurized polyacrylonitrile nanofibers for high performance lithium–sulfur batteries. Compos. Commun. 2021, 24, 100675. [Google Scholar] [CrossRef]
- Wang, K.; Ju, S.; Gao, Q.; Xia, G.; Yu, X. Porous sulfurized poly(acrylonitrile) nanofiber as a long-life and high-capacity cathode for lithium–sulfur batteries. J. Alloys Compd. 2020, 860, 158445. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shao, J.; Huang, C.; Zhu, Q.; Sun, N.; Zhang, J.; Wang, R.; Chen, Y.; Zhang, Z. Flexible CNT-Interpenetrating Hierarchically Porous Sulfurized Polyacrylonitrile (CIHP-SPAN) Electrodes for High-Rate Lithium-Sulfur (Li-S) Batteries. Nanomaterials 2024, 14, 1155. https://doi.org/10.3390/nano14131155
Shao J, Huang C, Zhu Q, Sun N, Zhang J, Wang R, Chen Y, Zhang Z. Flexible CNT-Interpenetrating Hierarchically Porous Sulfurized Polyacrylonitrile (CIHP-SPAN) Electrodes for High-Rate Lithium-Sulfur (Li-S) Batteries. Nanomaterials. 2024; 14(13):1155. https://doi.org/10.3390/nano14131155
Chicago/Turabian StyleShao, Jiashuo, Cheng Huang, Qi Zhu, Nan Sun, Junning Zhang, Rihui Wang, Yunxiang Chen, and Zongtao Zhang. 2024. "Flexible CNT-Interpenetrating Hierarchically Porous Sulfurized Polyacrylonitrile (CIHP-SPAN) Electrodes for High-Rate Lithium-Sulfur (Li-S) Batteries" Nanomaterials 14, no. 13: 1155. https://doi.org/10.3390/nano14131155
APA StyleShao, J., Huang, C., Zhu, Q., Sun, N., Zhang, J., Wang, R., Chen, Y., & Zhang, Z. (2024). Flexible CNT-Interpenetrating Hierarchically Porous Sulfurized Polyacrylonitrile (CIHP-SPAN) Electrodes for High-Rate Lithium-Sulfur (Li-S) Batteries. Nanomaterials, 14(13), 1155. https://doi.org/10.3390/nano14131155





