Current and Future Perspectives of Bioactive Glasses as Injectable Material
Abstract
:1. Introduction
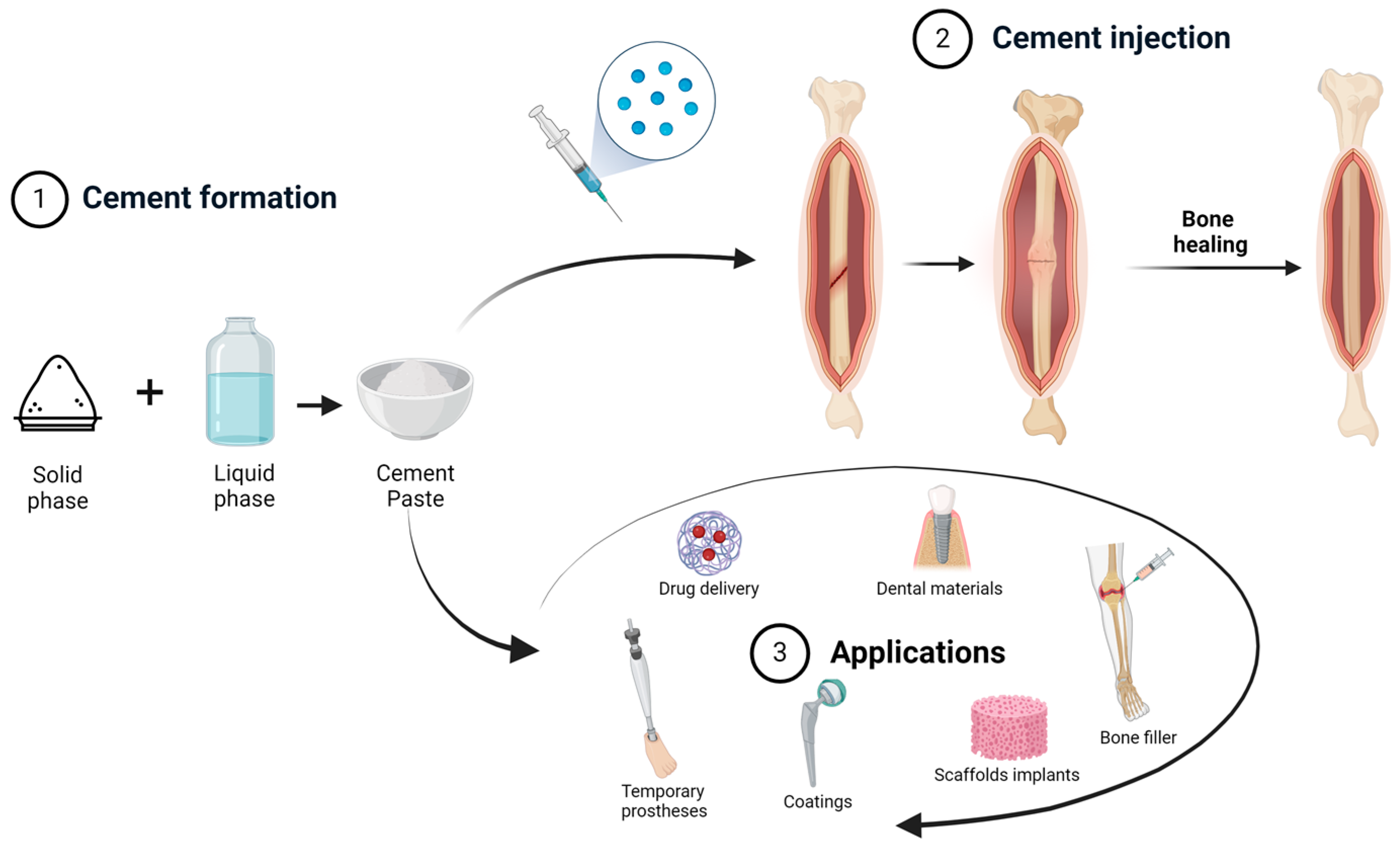
2. Cements
- Be easily injectable with appropriate homogeneity, cohesion and viscosity;
- Have an adequate curing time;
- Have a low risk of inducing necrosis;
- Have proper tensile, compressive and shear strength in accordance with the injection site;
- Have stiffness after curing similar to that of the surrounding bone;
- Have a high radiopacity to be easily distinguishable from surrounding tissues on x-ray imaging;
- Be bioactive;
- Have a resorption rate similar to that of the new tissue formation, such as during this complex process of resorption and new bone formation, the characteristics to be maintained properly;
- Have micro- and macropores to allow nutrient transfer, angiogenesis and osseointegration to occur [20].
2.1. Acrylic Bone Cements (ABCs)
- The liquid component contains three basic ingredients: MMA monomer; N,N-Dimethyl-p-toluidine as an accelerator of the polymerization reaction; and hydroquinone, which acts as an inhibitor, preventing premature polymerization of the monomer. This is a volatile, transparent, low-viscosity component.
- The solid component also contains three basic ingredients: PMMA granules; benzoyl peroxide, which acts as an initiator; and barium sulfate or zirconium dioxide, which is added to obtain radiopacity.
2.2. Calcium Sulphate Cements
2.3. Calcium Phosphate Cements
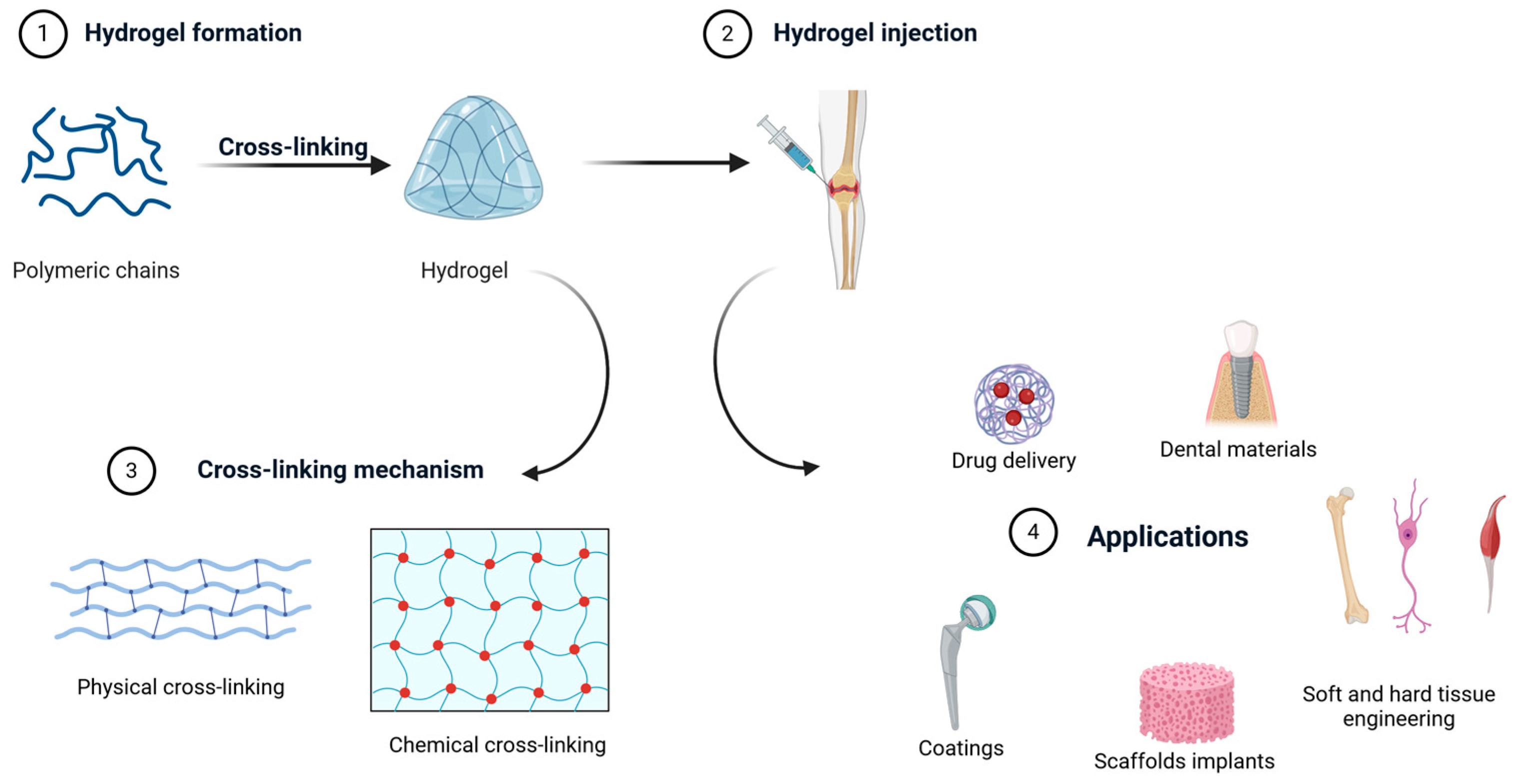
3. Hydrogels
3.1. Alginate
3.2. Chitosan
3.3. Collagen
3.4. Gelatin
3.5. Pectin
3.6. Hyaluronic Acid
3.7. Gellan Gum
4. Bioactive Glass-Based Formulations in Tissue Engineering
5. Conclusions and Future Directions
Funding
Conflicts of Interest
References
- Cannio, M.; Bellucci, D.; Roether, J.A.; Boccaccini, D.N.; Cannillo, V. Bioactive Glass Applications: A Literature Review of Human Clinical Trials. Materials 2021, 14, 5440. [Google Scholar] [CrossRef] [PubMed]
- Anita Lett, J.; Sagadevan, S.; Fatimah, I.; Hoque, M.E.; Lokanathan, Y.; Léonard, E.; Alshahateet, S.F.; Schirhagl, R.; Oh, W.C. Recent Advances in Natural Polymer-Based Hydroxyapatite Scaffolds: Properties and Applications. Eur. Polym. J. 2021, 148, 110360. [Google Scholar] [CrossRef]
- Sergi, R.; Bellucci, D.; Cannillo, V. A Review of Bioactive Glass/Natural Polymer Composites: State of the Art. Materials 2020, 13, 5560. [Google Scholar] [CrossRef] [PubMed]
- Krishnan, V.; Lakshmi, T. Bioglass: A Novel Biocompatible Innovation. J. Adv. Pharm. Technol. Res. 2013, 4, 78–83. [Google Scholar] [CrossRef]
- Hench, L.L. The Story of Bioglass®. J. Mater. Sci. Mater. Med. 2006, 17, 967–978. [Google Scholar] [CrossRef]
- Bellucci, D.; Veronesi, E.; Dominici, M.; Cannillo, V. A New Bioactive Glass with Extremely High Crystallization Temperature and Outstanding Biological Performance. Mater. Sci. Eng. C 2020, 110, 110699. [Google Scholar] [CrossRef]
- Kona, S.; Wadajkar, A.S.; Nguyen, K.T. Tissue Engineering Applications of Injectable Biomaterials. In Injectable Biomaterials; Woodhead Publishing: Sawston, UK, 2011; pp. 142–182. [Google Scholar] [CrossRef]
- Canillas, M.; Pena, P.; De Aza, A.H.; Rodríguez, M.A. Calcium Phosphates for Biomedical Applications. Bol. Soc. Esp. Ceram. Vidr. 2017, 56, 91–112. [Google Scholar] [CrossRef]
- Zhou, H.; Liang, C.; Wei, Z.; Bai, Y.; Bhaduri, S.B.; Webster, T.J.; Bian, L.; Yang, L. Injectable Biomaterials for Translational Medicine. Mater. Today 2019, 28, 81–97. [Google Scholar] [CrossRef]
- McLaren, A.C.; Estes, C.S. Orthopaedic Applications of Injectable Biomaterials. In Injectable Biomaterials; Woodhead Publishing: Sawston, UK, 2011; pp. 202–226. [Google Scholar] [CrossRef]
- Burguera, E.F.; Xu, H.H.; Weir, M.D. Injectable and Rapid-setting Calcium Phosphate Bone Cement with Dicalcium Phosphate. J. Biomed. Mater. Res. 2005, 77, 126–134. [Google Scholar] [CrossRef]
- Qiu, Y.; Hamilton, S.K.; Temenoff, J. Improving Mechanical Properties of Injectable Polymers and Composites. In Injectable Biomaterials; Woodhead Publishing: Sawston, UK, 2011; pp. 61–91. [Google Scholar] [CrossRef]
- Hou, Q.; De Bank, P.A.; Shakesheff, K.M. Injectable Scaffolds for Tissue Regeneration. J. Mater. Chem. 2004, 14, 1915–1923. [Google Scholar] [CrossRef]
- Béduer, A.; Bonini, F.; Verheyen, C.A.; Genta, M.; Martins, M.; Brefie-Guth, J.; Braschler, T. An Injectable Meta-Biomaterial From Design and Simulation to In Vivo Shaping and Tissue Induction. Adv. Mater. 2021, 33, 2102350. [Google Scholar] [CrossRef] [PubMed]
- McLemore, R. Rheological Properties of Injectable Biomaterials. In Injectable Biomaterials; Woodhead Publishing: Sawston, UK, 2011; pp. 46–60. [Google Scholar] [CrossRef]
- Sadiasa, A.; Sarkar, S.K.; Franco, R.A.; Min, Y.K.; Lee, B.T. Bioactive Glass Incorporation in Calcium Phosphate Cement-Based Injectable Bone Substitute for Improved in Vitro Biocompatibility and in Vivo Bone Regeneration. J. Biomater. Appl. 2014, 28, 739–756. [Google Scholar] [CrossRef] [PubMed]
- Motameni, A.; Alshemary, A.Z.; Evis, Z. A Review of Synthesis Methods, Properties and Use of Monetite Cements as Filler for Bone Defects. Ceram. Int. 2021, 47, 13245–13256. [Google Scholar] [CrossRef]
- Zhu, T.; Ren, H.; Li, A.; Liu, B.; Cui, C.; Dong, Y.; Tian, Y.; Qiu, D. Novel Bioactive Glass Based Injectable Bone Cement with Improved Osteoinductivity and Its in Vivo Evaluation. Sci. Rep. 2017, 7, 3622. [Google Scholar] [CrossRef] [PubMed]
- Fernandez de Grado, G.; Keller, L.; Idoux-Gillet, Y.; Wagner, Q.; Musset, A.M.; Benkirane-Jessel, N.; Bornert, F.; Offner, D. Bone Substitutes: A Review of Their Characteristics, Clinical Use, and Perspectives for Large Bone Defects Management. J. Tissue Eng. 2018, 9, 2041731418776819. [Google Scholar] [CrossRef] [PubMed]
- No, Y.J.; Roohani-Esfahani, S.I.; Zreiqat, H. Nanomaterials: The next Step in Injectable Bone Cements. Nanomedicine 2014, 9, 1745–1764. [Google Scholar] [CrossRef] [PubMed]
- Bohner, M. Designing Ceramics for Injectable Bone Graft Substitutes. In Injectable Biomaterials; Woodhead Publishing: Sawston, UK, 2011; pp. 24–45. [Google Scholar] [CrossRef]
- Chang, Y.; Lin, Z.; Xie, X.; Wu, Z.; Yao, A.; Ye, S.; Lin, J.; Wang, D.; Cui, X. An Injectable Composite Bone Cement Based on Mesoporous Borosilicate Bioactive Glass Spheres. Wuji Cailiao Xuebao/J. Inorg. Mater. 2020, 35, 1398–1406. [Google Scholar] [CrossRef]
- Lewis, G. Viscoelastic Properties of Injectable Bone Cements for Orthopaedic Applications: State-of-the-Art Review. J. Biomed. Mater. Res. Part B Appl. Biomater. 2011, 98 B, 171–191. [Google Scholar] [CrossRef]
- Created with BioRender. Available online: https://www.biorender.com (accessed on 16 June 2024).
- Gunatillake, P.A.; Adhikari, R. Nondegradable Synthetic Polymers for Medical Devices and Implants; Elsevier Ltd.: Amsterdam, The Netherlands, 2016; ISBN 9781782421139. [Google Scholar]
- Ginebra, M.P.; Montufar, E.B. Cements as Bone Repair Materials. In Bone Repair Biomaterials: Regeneration and Clinical Applications, 2nd ed.; Elsevier Ltd.: Amsterdam, The Netherlands, 2018; pp. 233–271. ISBN 9780081024515. [Google Scholar]
- Arcos, D.; Ragel, C.V.; Vallet-Regí, M. Bioactivity in Glass/PMMA Composites Used as Drug Delivery System. Biomaterials 2001, 22, 701–708. [Google Scholar] [CrossRef]
- Gad, M.M.; Abu-Rashid, K.; Alkhaldi, A.; Alshehri, O.; Khan, S.Q. Evaluation of the Effectiveness of Bioactive Glass Fillers against Candida Albicans Adhesion to PMMA Denture Base Materials: An In Vitro Study. Saudi Dent. J. 2022, 34, 730–737. [Google Scholar] [CrossRef]
- Rouein, Z.; Jafari, H.; Pishbin, F.; Mohandes, F.; Simchi, A. In Vitro Bioactivity and Biocompatibility of Magnesium Implants Coated with Poly(Methyl Methacrylate)—Bioactive Glass Composite. Mater. Today Commun. 2022, 33, 104872. [Google Scholar] [CrossRef]
- García-García, J.M.; Garrido, L.; Quijada-Garrido, I.; Kaschta, J.; Schubert, D.W.; Boccaccini, A.R. Novel Poly(Hydroxyalkanoates)-Based Composites Containing Bioglass® and Calcium Sulfate for Bone Tissue Engineering. Biomed. Mater. 2012, 7, 054105. [Google Scholar] [CrossRef] [PubMed]
- Beuerlein, M.J.S.; McKee, M.D. Calcium Sulfates: What Is the Evidence? J. Orthop. Trauma 2010, 24, 46–51. [Google Scholar] [CrossRef]
- Thomas, M.V.; Puleo, D.A. Calcium Sulfate: Properties and Clinical Applications. J. Biomed. Mater. Res. Part B Appl. Biomater. 2009, 88, 597–610. [Google Scholar] [CrossRef] [PubMed]
- Tay Vikas, B.K.B.; Patel, V.; Bradford, D.S. Calcium Sulfate- and Calcium Phosphate-Based Bone Substitutes Mimicry of the Mineral Phase of Bone. Orthop. Clin. N. Am. 1999, 30, 615–623. [Google Scholar] [CrossRef]
- Kok, J.; Törnquist, E.; Raina, D.B.; Le Cann, S.; Novak, V.; Širka, A.; Lidgren, L.; Grassi, L.; Isaksson, H. Fracture Behavior of a Composite of Bone and Calcium Sulfate/Hydroxyapatite. J. Mech. Behav. Biomed. Mater. 2022, 130, 105201. [Google Scholar] [CrossRef] [PubMed]
- Lewis, G. Injectable Bone Cements for Use in Vertebroplasty and Kyphoplasty State of the Art Review. J. Biomed. Mater. Res. Part B: Appl. Biomater. 2005, 76, 456–468. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, G.; Abhyankar, V.; M O’Dell, J. Calcium Sulfate as a Scaffold for Bone Tissue Engineering: A Descriptive Review. J. Dent. Oral Disord. Ther. 2021, 9, 1–22. [Google Scholar] [CrossRef]
- Tao, R.; Wu, J.; Luo, J.; Hong, L.; Zhou, C.; Cheng, G.; Qin, C. Antibiotic-Impregnated Calcium Sulfate for the Treatment of Pediatric Hematogenous Osteomyelitis. BMC Pediatr. 2022, 22, 732. [Google Scholar] [CrossRef]
- Cui, X.; Zhao, C.; Gu, Y.; Li, L.; Wang, H.; Huang, W.; Zhou, N.; Wang, D.; Zhu, Y.; Xu, J.; et al. A Novel Injectable Borate Bioactive Glass Cement for Local Delivery of Vancomycin to Cure Osteomyelitis and Regenerate Bone. J. Mater. Sci. Mater. Med. 2014, 25, 733–745. [Google Scholar] [CrossRef]
- Huang, K.H.; Wang, C.Y.; Chen, C.Y.; Hsu, T.T.; Lin, C.P. Incorporation of Calcium Sulfate Dihydrate into a Mesoporous Calcium Silicate/Poly-ε-Caprolactone Scaffold to Regulate the Release of Bone Morphogenetic Protein-2 and Accelerate Bone Regeneration. Biomedicines 2021, 9, 128. [Google Scholar] [CrossRef] [PubMed]
- LeGeros, R.Z. Calcium Phosphate Materials in Restorative Dentistry: A Review. Adv. Dent. Res. 1988, 2, 164–180. [Google Scholar] [CrossRef] [PubMed]
- Dorozhkin, S.V. A Detailed History of Calcium Orthophosphates from 1770s till 1950. Mater. Sci. Eng. C 2013, 33, 3085–3110. [Google Scholar] [CrossRef] [PubMed]
- Islam, M.T.; Felfel, R.M.; Abou Neel, E.A.; Grant, D.M.; Ahmed, I.; Hossain, K.M.Z. Bioactive Calcium Phosphate–Based Glasses and Ceramics and Their Biomedical Applications: A Review. J. Tissue Eng. 2017, 8, 2041731417719170. [Google Scholar] [CrossRef] [PubMed]
- Pedro Fernandes Graça, M.; Rodrigues Gavinho, S. Calcium Phosphate Cements in Tissue Engineering. In Contemporary Topics about Phosphorus in Biology and Materials; IntechOpen: London, UK, 2020. [Google Scholar] [CrossRef]
- Schumacher, M.; Reither, L.; Thomas, J.; Kampschulte, M.; Gbureck, U.; Lode, A.; Gelinsky, M. Calcium Phosphate Bone Cement/Mesoporous Bioactive Glass Composites for Controlled Growth Factor Delivery. Biomater. Sci. 2017, 5, 578–588. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.H.K.; Weir, M.D.; Burguera, E.F.; Fraser, A.M. Injectable and Macroporous Calcium Phosphate Cement Scaffold. Biomaterials 2006, 27, 4279–4287. [Google Scholar] [CrossRef] [PubMed]
- Félix Lanao, R.P.; Leeuwenburgh, S.C.G.; Wolke, J.G.C.; Jansen, J.A. Bone Response to Fast-Degrading, Injectable Calcium Phosphate Cements Containing PLGA Microparticles. Biomaterials 2011, 32, 8839–8847. [Google Scholar] [CrossRef]
- Demir-Oğuz, Ö.; Boccaccini, A.R.; Loca, D. Injectable Bone Cements: What Benefits the Combination of Calcium Phosphates and Bioactive Glasses Could Bring? Bioact. Mater. 2023, 19, 217–236. [Google Scholar] [CrossRef] [PubMed]
- Yu, L.; Li, Y.; Zhao, K.; Tang, Y.; Cheng, Z.; Chen, J.; Zang, Y.; Wu, J.; Kong, L.; Liu, S.; et al. A Novel Injectable Calcium Phosphate Cement-Bioactive Glass Composite for Bone Regeneration. PLoS ONE 2013, 8, e62570. [Google Scholar] [CrossRef]
- Vorndran, E.; Geffers, M.; Ewald, A.; Lemm, M.; Nies, B.; Gbureck, U. Ready-to-Use Injectable Calcium Phosphate Bone Cement Paste as Drug Carrier. Acta Biomater. 2013, 9, 9558–9567. [Google Scholar] [CrossRef]
- Brunner, T.J.; Bohner, M.; Dora, C.; Gerber, C.; Stark, W.J. Comparison of Amorphous TCP Nanoparticles to Micron-Sized α-TCP as Starting Materials for Calcium Phosphate Cements. Eur. Cells Mater. 2007, 13, 4. [Google Scholar] [CrossRef]
- Neira, I.S.; Kolen’Ko, Y.V.; Kommareddy, K.P.; Manjubala, I.; Yoshimura, M.; Guitián, F. Reinforcing of a Calcium Phosphate Cement with Hydroxyapatite Crystals of Various Morphologies. ACS Appl. Mater. Interfaces 2010, 2, 3276–3284. [Google Scholar] [CrossRef]
- Xu, H.H.K.; Wang, P.; Wang, L.; Bao, C.; Chen, Q.; Weir, M.D.; Chow, L.C.; Zhao, L.; Zhou, X.; Reynolds, M.A. Calcium Phosphate Cements for Bone Engineering and Their Biological Properties. Bone Res. 2017, 5, 17056. [Google Scholar] [CrossRef]
- Richter, R.F.; Ahlfeld, T.; Gelinsky, M.; Lode, A. Composites Consisting of Calcium Phosphate Cements and Mesoporous Bioactive Glasses as a 3D Plottable Drug Delivery System. Acta Biomater. 2023, 156, 146–157. [Google Scholar] [CrossRef]
- Renno, A.C.M.; Nejadnik, M.R.; Van De Watering, F.C.J.; Crovace, M.C.; Zanotto, E.D.; Hoefnagels, J.P.M.; Wolke, J.G.C.; Jansen, J.A.; Van Den Beucken, J.J.J.P. Incorporation of Bioactive Glass in Calcium Phosphate Cement: Material Characterization and In Vitro Degradation. J. Biomed. Mater. Res. Part A 2013, 101 A, 2365–2373. [Google Scholar] [CrossRef]
- Zhang, J.; Liu, W.; Gauthier, O.; Sourice, S.; Pilet, P.; Rethore, G.; Khairoun, K.; Bouler, J.M.; Tancret, F.; Weiss, P. A Simple and Effective Approach to Prepare Injectable Macroporous Calcium Phosphate Cement for Bone Repair: Syringe-Foaming Using a Viscous Hydrophilic Polymeric Solution. Acta Biomater. 2016, 31, 326–338. [Google Scholar] [CrossRef]
- Wagoner Johnson, A.J.; Herschler, B.A. A Review of the Mechanical Behavior of CaP and CaP/Polymer Composites for Applications in Bone Replacement and Repair. Acta Biomater. 2011, 7, 16–30. [Google Scholar] [CrossRef]
- Lim, H.C.; Sohn, J.Y.; Park, J.C.; Um, Y.J.; Jung, U.W.; Kim, C.S.; Lee, Y.K.; Choi, S.H. Osteoconductive Effects of Calcium Phosphate Glass Cement Grafts in Rabbit Calvarial Defects. J. Biomed. Mater. Res. Part B Appl. Biomater. 2010, 95, 47–52. [Google Scholar] [CrossRef]
- Gao, L.; Zhou, Y.; Peng, J.; Xu, C.; Xu, Q.; Xing, M.; Chang, J. A Novel Dual-Adhesive and Bioactive Hydrogel Activated by Bioglass for Wound Healing. NPG Asia Mater. 2019, 11, 66. [Google Scholar] [CrossRef]
- Grumezescu, A.; Holban, A.-M. Materials for Biomedical Engineering. Hydrogels and Polymer-Based Scaffolds; Elsevier Ltd.: Amsterdam, The Netherlands, 2019; ISBN 9781931971874. [Google Scholar]
- Couto, D.S.; Hong, Z.; Mano, J.F. Development of Bioactive and Biodegradable Chitosan-Based Injectable Systems Containing Bioactive Glass Nanoparticles. Acta Biomater. 2009, 5, 115–123. [Google Scholar] [CrossRef] [PubMed]
- Huang, S.; Hong, X.; Zhao, M.; Liu, N.; Liu, H.; Zhao, J.; Shao, L.; Xue, W.; Zhang, H.; Zhu, P.; et al. Nanocomposite Hydrogels for Biomedical Applications. Bioeng. Transl. Med. 2022, 7, e10315. [Google Scholar] [CrossRef]
- Moreira, C.D.F.; Carvalho, S.M.; Sousa, R.G.; Mansur, H.S.; Pereira, M.M. Nanostructured Chitosan/Gelatin/Bioactive Glass in Situ Forming Hydrogel Composites as a Potential Injectable Matrix for Bone Tissue Engineering. Mater. Chem. Phys. 2018, 218, 304–316. [Google Scholar] [CrossRef]
- Hilborn, J. In Vivo Injectable Gels for Tissue Repair. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnology 2011, 3, 589–606. [Google Scholar] [CrossRef]
- Zhu, S.; Li, Y.; He, Z.; Ji, L.; Zhang, W.; Tong, Y.; Luo, J.; Yu, D.; Zhang, Q.; Bi, Q. Advanced Injectable Hydrogels for Cartilage Tissue Engineering. Front. Bioeng. Biotechnol. 2022, 10, 954501. [Google Scholar] [CrossRef]
- Rimmer, S. Biomedical Hydrogels: Biochemistry, Manufacture and Medical Applications; Woodhead Publishing: Sawston, UK, 2011; ISBN 9781845695903. [Google Scholar]
- Rezakhani, S.; Gjorevski, N.; Lutolf, M.P. Low-Defect Thiol-Michael Addition Hydrogels as Matrigel Substitutes for Epithelial. Adv. Funct. Mater. 2020, 30, 2000761. [Google Scholar] [CrossRef]
- Gopinathan, J.; Noh, I. Click Chemistry-Based Injectable Hydrogels and Bioprinting Inks for Tissue Engineering Applications. Tissue Eng. Regen. Med. 2018, 15, 531–546. [Google Scholar] [CrossRef]
- Song, W.; Ko, J.; Choi, Y.H.; Hwang, N.S. Recent Advancements in Enzyme-Mediated Crosslinkable Hydrogels: In Vivo-Mimicking Strategies. APL Bioeng. 2021, 5, 021502. [Google Scholar] [CrossRef]
- Nguyen, K.T.; West, J.L. Photopolymerizable Hydrogels for Tissue Engineering Applications. Biomaterials 2002, 23, 4307–4314. [Google Scholar] [CrossRef]
- Sharifi, E.; Bigham, A.; Yousefiasl, S.; Trovato, M.; Ghomi, M.; Esmaeili, Y.; Samadi, P.; Zarrabi, A.; Ashrafizadeh, M.; Sharifi, S.; et al. Mesoporous Bioactive Glasses in Cancer Diagnosis and Therapy Stimuli-Responsive, Toxicity, Immunogenicity, and Clinical Translation. Adv. Sci. 2022, 9, 2102678. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Seles, M.A.; Rajan, M. Role of Bioglass Derivatives in Tissue Regeneration and Repair: A Review. Rev. Adv. Mater. Sci. 2023, 62, 20220318. [Google Scholar] [CrossRef]
- Murab, S.; Gupta, A.; Włodarczyk-Biegun, M.K.; Kumar, A.; van Rijn, P.; Whitlock, P.; Han, S.S.; Agrawal, G. Alginate Based Hydrogel Inks for 3D Bioprinting of Engineered Orthopedic Tissues. Carbohydr. Polym. 2022, 296, 119964. [Google Scholar] [CrossRef] [PubMed]
- Motelica, L.; Ficai, D.; Oprea, O.C.; Ficai, A.; Ene, V.L.; Vasile, B.S.; Andronescu, E.; Holban, A.M. Antibacterial Biodegradable Films Based on Alginate with Silver Nanoparticles and Lemongrass Essential Oil–Innovative Packaging for Cheese. Nanomaterials 2021, 11, 2377. [Google Scholar] [CrossRef] [PubMed]
- Paredes Juárez, G.A.; Spasojevic, M.; Faas, M.M.; de Vos, P. Immunological and Technical Considerations in Application of Alginate-Based Microencapsulation Systems. Front. Bioeng. Biotechnol. 2014, 2, 26. [Google Scholar] [CrossRef] [PubMed]
- Angra, V.; Sehgal, R.; Kaur, M.; Gupta, R. Commercialization of Bionanocomposites. In Bionanocomposites in Tissue Engineering and Regenerative Medicine; Woodhead Publishing: Sawston, UK, 2021; pp. 587–610. [Google Scholar]
- Venkatesan, J.; Bhatnagar, I.; Manivasagan, P.; Kang, K.H.; Kim, S.K. Alginate Composites for Bone Tissue Engineering: A Review. Int. J. Biol. Macromol. 2015, 72, 269–281. [Google Scholar] [CrossRef] [PubMed]
- van Damme, L.; Blondeel, P.; van Vlierberghe, S. Injectable Biomaterials as Minimal Invasive Strategy towards Soft Tissue Regeneration—An Overview. J. Phys. Mater. 2021, 4, 022001. [Google Scholar] [CrossRef]
- Zeng, Q.; Han, Y.; Li, H.; Chang, J. Bioglass/Alginate Composite Hydrogel Beads as Cell Carriers for Bone Regeneration. J. Biomed. Mater. Res. Part B Appl. Biomater. 2014, 102, 42–51. [Google Scholar] [CrossRef] [PubMed]
- Preman, N.K.; Jain, S.; Sanjeeva, S.G.; Johnson, R.P. Alginate Derived Nanoassemblies in Drug Delivery and Tissue Engineering. In Polysaccharide Nanoparticles. Preparation and Biomedical Applications; Elsevier Ltd.: Amsterdam, The Netherlands, 2022; pp. 247–280. [Google Scholar]
- Motelica, L.; Ficai, D.; Oprea, O.; Ficai, A.; Trusca, R.D.; Andronescu, E.; Holban, A.M. Biodegradable Alginate Films with Zno Nanoparticles and Citronella Essential Oil—A Novel Antimicrobial Structure. Pharmaceutics 2021, 13, 1020. [Google Scholar] [CrossRef] [PubMed]
- Hernández-González, A.C.; Téllez-Jurado, L.; Rodríguez-Lorenzo, L.M. Alginate Hydrogels for Bone Tissue Engineering, from Injectables to Bioprinting: A Review. Carbohydr. Polym. 2020, 229, 115514. [Google Scholar] [CrossRef] [PubMed]
- Rowley, J.A.; Madlambayan, G.; Mooney, D.J. Alginate Hydrogels as Synthetic Extracellular Matrix Materials. Biomaterials 1999, 20, 45–53. [Google Scholar] [CrossRef]
- Tariverdian, T.; Navaei, T.; Milan, P.B.; Samadikuchaksaraei, A.; Mozafari, M. Functionalized Polymers for Tissue Engineering and Regenerative Medicines; Elsevier Inc.: Amsterdam, The Netherlands, 2019; ISBN 9780128163498. [Google Scholar]
- Motelica, L.; Ficai, D.; Ficai, A.; Ilie, C.; Oprea, O.; Andronescu, E. Innovative Antimicrobial Chitosan/ZnO/Ag NPs/Citronella Essential Oil Nanocomposite—Potential Coating for Grape. Foods 2020, 9, 1801. [Google Scholar] [CrossRef]
- Kumar, A.; SooHan, S. Bioactive Glass-Based Composites in Bone Tissue Engineering: Synthesis, Processing, and Cellular Responses; Elsevier Inc.: Amsterdam, The Netherlands, 2019; ISBN 9780128169094. [Google Scholar]
- Szymańska, E.; Winnicka, K. Stability of Chitosan—A Challenge for Pharmaceutical and Biomedical Applications. Mar. Drugs 2015, 13, 1819–1846. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Hou, X.; Wang, H.; Kong, D.; Zhou, Y. Preparation of Chitosan-Sodium Alginate/Bioactive Glass Composite Cartilage Scaffolds with High Cell Activity and Bioactivity. Ceram. Int. 2023, 49, 1987–1996. [Google Scholar] [CrossRef]
- Bharathi, R.; Ganesh, S.S.; Harini, G.; Vatsala, K.; Anushikaa, R.; Aravind, S.; Abinaya, S.; Selvamurugan, N. Chitosan-Based Scaffolds as Drug Delivery Systems in Bone Tissue Engineering. Int. J. Biol. Macromol. 2022, 222, 132–153. [Google Scholar] [CrossRef] [PubMed]
- Sohrabi, M.; Yekta, B.E.; Rezaie, H.; Naimi-Jamal, M.R.; Kumar, A.; Cochis, A.; Miola, M.; Rimondini, L. Enhancing Mechanical Properties and Biological Performances of Injectable Bioactive Glass by Gelatin and Chitosan for Bone Small Defect Repair. Biomedicines 2020, 8, 616. [Google Scholar] [CrossRef] [PubMed]
- Rahimnejad, M.; Charbonneau, C.; He, Z.; Lerouge, S. Injectable Cell-Laden Hybrid Bioactive Scaffold Containing Bioactive Glass Microspheres. J. Biomed. Mater. Res. 2022, 111, 1031–1043. [Google Scholar] [CrossRef] [PubMed]
- Levengood, S.K.L.; Zhang, M. Chitosan-Based Scaffolds for Bone Tissue Engineering. J. Mater. Chem. B 2014, 2, 3161–3184. [Google Scholar] [CrossRef] [PubMed]
- Fermani, M.; Platania, V.; Kavasi, R.; Karavasili, C.; Zgouro, P.; Fatouros, D.; Chatzinikolaidou, M.; Bouropoulos, N. 3D-Printed Scaffolds from Alginate/Methyl Cellulose/Trimethyl Chitosan/Silicate Glasses for Bone Tissue Engineering. Appl. Sci. 2021, 11, 8677. [Google Scholar] [CrossRef]
- Kertmen, A.; Dziedzic, I.; Ehrlich, H. Patentology of Chitinous Biomaterials. Part II: Chitosan; Elsevier Ltd.: Amsterdam, The Netherlands, 2023; Volume 301, ISBN 2018104349. [Google Scholar]
- Yao, Q.; Nooeaid, P.; Detsch, R.; Roether, J.A.; Dong, Y.; Goudouri, O.M.; Schubert, D.W.; Boccaccini, A.R. Bioglass®/Chitosan-Polycaprolactone Bilayered Composite Scaffolds Intended for Osteochondral Tissue Engineering. J. Biomed. Mater. Res. Part A 2014, 102, 4510–4518. [Google Scholar] [CrossRef] [PubMed]
- Ciraldo, F.E.; Boccardi, E.; Melli, V.; Westhauser, F.; Boccaccini, A.R. Tackling Bioactive Glass Excessive in Vitro Bioreactivity: Preconditioning Approaches for Cell Culture Tests. Acta Biomater. 2018, 75, 3–10. [Google Scholar] [CrossRef]
- Hamman, J.H.; Kotzé, A.F. Effect of the Type of Base and Number of Reaction Steps on the Degree of Quaternization and Molecular Weight of N-Trimethyl Chitosan Chloride. Drug Dev. Ind. Pharm. 2001, 27, 373–380. [Google Scholar] [CrossRef]
- Tihăuan, B.M.; Pircalabioru, G.G.; Axinie, M.; Marinaș, I.C.; Nicoară, A.C.; Măruțescu, L.; Oprea, O.; Matei, E.; Maier, S.S. Crosslinked Collagenic Scaffold Behavior Evaluation by Physico-Chemical, Mechanical and Biological Assessments in an In Vitro Microenvironment. Polymers 2022, 14, 2430. [Google Scholar] [CrossRef] [PubMed]
- Rusu, L.C.; Nedelcu, I.A.; Georgiana Albu, M.; Sonmez, M.; Voicu, G.; Radulescu, M.; Ficai, D.; Ficai, A.; Negrutiu, M.L.; Sinescu, C. Tetracycline Loaded Collagen/Hydroxyapatite Composite Materials for Biomedical Applications. J. Nanomater. 2015, 2015, 361969. [Google Scholar] [CrossRef]
- Păunica-Panea, G.; Ficai, A.; Marin, M.M.; Marin, Ș.; Albu, M.G.; Constantin, V.D.; Dinu-Pîrvu, C.; Vuluga, Z.; Corobea, M.C.; Ghica, M.V. New Collagen-Dextran-Zinc Oxide Composites for Wound Dressing. J. Nanomater. 2016, 2016, 5805034. [Google Scholar] [CrossRef]
- Hum, J.; Boccaccini, A.R. Collagen as Coating Material for 45S5 Bioactive Glass-Based Scaffolds for Bone Tissue Engineering. Int. J. Mol. Sci. 2018, 19, 1807. [Google Scholar] [CrossRef] [PubMed]
- Sarker, B.; Hum, J.; Nazhat, S.N.; Boccaccini, A.R. Combining Collagen and Bioactive Glasses for Bone Tissue Engineering: A Review. Adv. Healthc. Mater. 2015, 4, 176–194. [Google Scholar] [CrossRef] [PubMed]
- Erol-Taygun, M.; Unalan, I.; Idris, M.I.B.; Mano, J.F.; Boccaccini, A.R. Bioactive Glass-Polymer Nanocomposites for Bone Tissue Regeneration Applications: A Review. Adv. Eng. Mater. 2019, 21, 1900287. [Google Scholar] [CrossRef]
- Shoulders, M.D.; Raines, R.T. Collagen Structure and Stability. Annu. Rev. Biochem. 2009, 78, 929–958. [Google Scholar] [CrossRef]
- Moreira, C.D.F.; Carvalho, S.M.; Mansur, H.S.; Pereira, M.M. Thermogelling Chitosan-Collagen-Bioactive Glass Nanoparticle Hybrids as Potential Injectable Systems for Tissue Engineering. Mater. Sci. Eng. C 2016, 58, 1207–1216. [Google Scholar] [CrossRef] [PubMed]
- Nakagawa, T.; Tagawa, T. Ultrastructural Study of Direct Bone Formation Induced by BMPs-collagen Complex Implanted into an Ectopic Site. Oral Dis. 2006, 6, 172–179. [Google Scholar] [CrossRef]
- Miri, A.K.; Muja, N.; Kamranpour, N.O.; Lepry, W.C.; Boccaccini, A.R.; Clarke, S.A.; Nazhat, S.N. Ectopic Bone Formation in Rapidly Fabricated Acellular Injectable Dense Collagen-Bioglass Hybrid Scaffolds via Gel Aspiration-Ejection. Biomaterials 2016, 85, 128–141. [Google Scholar] [CrossRef]
- Andrade, A.L.; Andrade, S.P.; Domingues, R.Z. In Vivo Performance of a Sol–Gel Glass-Coated Collagen. J. Biomed. Mater. Res. 2006, 79, 122–128. [Google Scholar] [CrossRef]
- Zeimaran, E.; Pourshahrestani, S.; Fathi, A.; Razak, N.A.b.A.; Kadri, N.A.; Sheikhi, A.; Baino, F. Advances in Bioactive Glass-Containing Injectable Hydrogel Biomaterials for Tissue Regeneration. Acta Biomater. 2021, 136, 1–36. [Google Scholar] [CrossRef] [PubMed]
- Unal, S.; Arslan, S.; Yilmaz, B.K.; Oktar, F.N.; Ficai, D.; Ficai, A.; Gunduz, O. Polycaprolactone/Gelatin/Hyaluronic Acid Electrospun Scaffolds to Mimic Glioblastoma Extracellular Matrix. Materials 2020, 13, 2661. [Google Scholar] [CrossRef]
- Kumbar, S.G.; Laurencin, C.T.; Deng, M. Natural and Synthetic Biomedical Polymers; Elsevier Ltd.: Amsterdam, The Netherlands, 2014; ISBN 9780123969835. [Google Scholar]
- Kwon, S.; Lee, S.S.; Sivashanmugam, A.; Kwon, J.; Kim, S.H.L.; Noh, M.Y.; Kwon, S.K.; Jayakumar, R.; Hwang, N.S. Bioglass-Incorporated Methacrylated Gelatin Cryogel for Regeneration of Bone Defects. Polymers 2018, 10, 914. [Google Scholar] [CrossRef]
- Alipal, J.; Mohd Pu’ad, N.A.S.; Lee, T.C.; Nayan, N.H.M.; Sahari, N.; Basri, H.; Idris, M.I.; Abdullah, H.Z. A Review of Gelatin: Properties, Sources, Process, Applications, and Commercialisation. Mater. Today Proc. 2019, 42, 240–250. [Google Scholar] [CrossRef]
- Raja Nhari, R.M.H.; Che Man, Y.; Ismail, A.; Anuar, N. Chemical and Functional Properties of Bovine and Porcine Skin Gelatin. Int. Food Res. J. 2011, 817, 813–817. [Google Scholar]
- Barreto, M.E.V.; Medeiros, R.P.; Shearer, A.; Fook, M.V.L.; Montazerian, M.; Mauro, J.C. Gelatin and Bioactive Glass Composites for Tissue Engineering: A Review. J. Funct. Biomater. 2023, 14, 23. [Google Scholar] [CrossRef]
- Sohrabi, M.; Yekta, B.E.; Rezaie, H.; Naimi-Jamal, M.R.; Kumar, A.; Cochis, A.; Miola, M.; Rimondini, L. The Effect of Magnesium on Bioactivity, Rheology and Biology Behaviors of Injectable Bioactive Glass-Gelatin-3-Glycidyloxypropyl Trimethoxysilane Nanocomposite-Paste for Small Bone Defects Repair. Ceram. Int. 2021, 47, 12526–12536. [Google Scholar] [CrossRef]
- Wang, T.; Chen, L.; Shen, T.; Wu, D. Preparation and Properties of a Novel Thermo-Sensitive Hydrogel Based on Chitosan/Hydroxypropyl Methylcellulose/Glycerol. Int. J. Biol. Macromol. 2016, 93, 775–782. [Google Scholar] [CrossRef]
- Moreira, C.D.F.; Carvalho, S.M.; Florentino, R.M.; França, A.; Okano, B.S.; Rezende, C.M.F.; Mansur, H.S.; Pereira, M.M. Injectable Chitosan/Gelatin/Bioactive Glass Nanocomposite Hydrogels for Potential Bone Regeneration: In Vitro and in Vivo Analyses. Int. J. Biol. Macromol. 2019, 132, 811–821. [Google Scholar] [CrossRef]
- May, C.D. Industrial Pectins: Sources, Production and Applications. Carbohydr. Polym. 1990, 12, 79–99. [Google Scholar] [CrossRef]
- Munarin, F.; Guerreiro, S.G.; Grellier, M.A.; Tanzi, M.C.; Barbosa, M.A.; Petrini, P.; Granja, P.L. Pectin-Based Injectable Biomaterials for Bone Tissue Engineering. Biomacromolecules 2011, 12, 568–577. [Google Scholar] [CrossRef] [PubMed]
- Motelica, L.; Ficai, D.; Oprea, O.C.; Ficai, A.; Andronescu, E. Smart Food Packaging Designed by Nanotechnological and Drug Delivery Approaches. Coatings 2020, 10, 806. [Google Scholar] [CrossRef]
- Munarin, F.; Petrini, P.; Barcellona, G.; Roversi, T.; Piazza, L.; Visai, L.; Tanzi, M.C. Reactive Hydroxyapatite Fillers for Pectin Biocomposites. Mater. Sci. Eng. C 2014, 45, 154–161. [Google Scholar] [CrossRef] [PubMed]
- Douglas, T.E.L.; Dziadek, M.; Schietse, J.; Boone, M.; Declercq, H.A.; Coenye, T.; Vanhoorne, V.; Vervaet, C.; Balcaen, L.; Buchweitz, M.; et al. Pectin-Bioactive Glass Self-Gelling, Injectable Composites with High Antibacterial Activity. Carbohydr. Polym. 2019, 205, 427–436. [Google Scholar] [CrossRef] [PubMed]
- Bostanci, N.S.; Büyüksungur, S.; Hasirci, N.; Tezcaner, A. Potential of Pectin for Biomedical Applications: A Comprehensive Review. J. Biomater. Sci. Polym. Ed. 2022, 33, 1866–1900. [Google Scholar] [CrossRef] [PubMed]
- Moreira, H.R.; Munarin, F.; Gentilini, R.; Visai, L.; Granja, P.L.; Tanzi, M.C.; Petrini, P. Injectable Pectin Hydrogels Produced by Internal Gelation: PH Dependence of Gelling and Rheological Properties. Carbohydr. Polym. 2014, 103, 339–347. [Google Scholar] [CrossRef] [PubMed]
- Necas, J.; Bartosikova, L.; Brauner, P.; Kolar, J. Hyaluronic Acid (Hyaluronan): A Review. Vet. Med. 2008, 53, 397–411. [Google Scholar] [CrossRef]
- Sze, J.H.; Brownlie, J.C.; Love, C.A. Biotechnological Production of Hyaluronic Acid: A Mini Review. 3 Biotech 2016, 6, 67. [Google Scholar] [CrossRef]
- Sohrabi, M.; Hesaraki, S.; Kazemzadeh, A. The Influence of Polymeric Component of Bioactive Glass-Based Nanocomposite Paste on Its Rheological Behaviors and in Vitro Responses: Hyaluronic Acid versus Sodium Alginate. J. Biomed. Mater. Res. Part B 2014, 102, 561–573. [Google Scholar] [CrossRef]
- Diba, M.; An, J.; Schmidt, S.; Hembury, M.; Ossipov, D.; Boccaccini, A.R.; Leeuwenburgh, S.C.G. Exploiting Bisphosphonate–Bioactive-Glass Interactions for the Development of Self- Healing and Bioactive Composite Hydrogels. Macromol. Rapid Commun. 2016, 37, 1952–1959. [Google Scholar] [CrossRef] [PubMed]
- Sohrabi, M.; Hesaraki, S.; Kazemzadeh, A.; Alizadeh, M. Development of Injectable Biocomposites from Hyaluronic Acid and Bioactive Glass Nano-Particles Obtained from Different Sol-Gel Routes. Mater. Sci. Eng. C 2013, 33, 3730–3744. [Google Scholar] [CrossRef] [PubMed]
- Douglas, T.E.L.; Piwowarczyk, W.; Pamula, E.; Liskova, J.; Schaubroeck, D.; Leeuwenburgh, S.C.G.; Brackman, G.; Balcaen, L.; Detsch, R.; Declercq, H.; et al. Injectable Self-Gelling Composites for Bone Tissue Engineering Based on Gellan Gum Hydrogel Enriched with Different Bioglasses. Biomed. Mater. 2014, 9, 045014. [Google Scholar] [CrossRef]
- Gadziński, P.; Froelich, A.; Jadach, B.; Wojtyłko, M.; Tatarek, A.; Białek, A.; Krysztofiak, J.; Gackowski, M.; Otto, F.; Osmałek, T. Ionotropic Gelation and Chemical Crosslinking as Methods for Fabrication of Modified-Release Gellan Gum-Based Drug Delivery Systems. Pharmaceutics 2023, 15, 108. [Google Scholar] [CrossRef] [PubMed]
- Gantar, A.; Da Silva, L.P.; Oliveira, J.M.; Marques, A.P.; Correlo, V.M.; Novak, S.; Reis, R.L. Nanoparticulate Bioactive-Glass-Reinforced Gellan-Gum Hydrogels for Bone-Tissue Engineering. Mater. Sci. Eng. C 2014, 43, 27–36. [Google Scholar] [CrossRef] [PubMed]
- Giavasis, I.; Harvey, L.M.; McNeil, B. Gellan Gum. Crit. Rev. Biotechnol. 2000, 20, 177–211. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.U.A.; Raza, M.A.; Haider, S.; Shah, S.A.; Arshed, M.; Razak, S.I.A.; Haider, A. Medical Applications of Polymer/Functionalized Nanoparticle Composite Systems, Renewable Polymers, and Polymer–Metal Oxide Composites. In Renewable Polymers and Polymer-Metal Oxide Composites. Synthesis, Properties, and Applications; Elsevier Ltd.: Amsterdam, The Netherlands, 2022. [Google Scholar]
- Gong, Y.; Wang, C.; Lai, R.C.; Su, K.; Zhang, F.; Wang, D.A. An Improved Injectable Polysaccharide Hydrogel: Modified Gellan Gum for Long-Term Cartilage Regeneration in Vitro. J. Mater. Chem. 2009, 19, 1968–1977. [Google Scholar] [CrossRef]
- Oliveira, J.T.; Martins, L.; Picciochi, R.; Malafaya, P.B.; Sousa, R.A.; Neves, N.M.; Mano, J.F.; Reis, R.L. Gellan Gum: A New Biomaterial for Cartilage Tissue Engineering Applications. J. Biomed. Mater. Res. Part A 2010, 93, 852–863. [Google Scholar] [CrossRef]
- Karvinen, J.; Koivisto, J.T.; Jönkkäri, I.; Kellomäki, M. The Production of Injectable Hydrazone Crosslinked Gellan Gum-Hyaluronan-Hydrogels with Tunable Mechanical and Physical Properties. J. Mech. Behav. Biomed. Mater. 2017, 71, 383–391. [Google Scholar] [CrossRef] [PubMed]
- Vuornos, K.; Ojansivu, M.; Koivisto, J.T.; Häkkänen, H.; Belay, B.; Montonen, T.; Huhtala, H.; Kääriäinen, M.; Hupa, L.; Kellomäki, M.; et al. Bioactive Glass Ions Induce Efficient Osteogenic Differentiation of Human Adipose Stem Cells Encapsulated in Gellan Gum and Collagen Type I Hydrogels. Mater. Sci. Eng. C 2019, 99, 905–918. [Google Scholar] [CrossRef]
- Oliveira, M.B.; Custódio, C.A.; Gasperini, L.; Reis, R.L.; Mano, J.F. Autonomous Osteogenic Differentiation of HASCs Encapsulated in Methacrylated Gellan-Gum Hydrogels. Acta Biomater. 2016, 41, 119–132. [Google Scholar] [CrossRef] [PubMed]
- Stoica, A.E.; Bîrcă, A.C.; Gherasim, O.; Ficai, A.; Grumezescu, A.M.; Oprea, O.C.; Vasile, B.Ș.; Balta, C.; Andronescu, E.; Hermenean, A.O. Electrospun Fibrous Silica for Bone Tissue Engineering Applications. Pharmaceutics 2023, 15, 1728. [Google Scholar] [CrossRef]
- Busuioc, C.; Isopencu, G.; Banciu, A.; Banciu, D.D.; Oprea, O.; Mocanu, A.; Deleanu, I.; Zăuleţ, M.; Popescu, L.; Tănăsuică, R.; et al. Bacterial Cellulose Hybrid Composites with Calcium Phosphate for Bone Tissue Regeneration. Int. J. Mol. Sci. 2022, 23, 16180. [Google Scholar] [CrossRef] [PubMed]
- Nechifor, G.; Totu, E.E.; Nechifor, A.C.; Isildak, I.; Oprea, O.; Cristache, C.M. Non-Resorbable Nanocomposite Membranes for Guided Bone Regeneration Based on Polysulfone-Quartz Fiber Grafted with Nano-TiO2. Nanomaterials 2019, 9, 985. [Google Scholar] [CrossRef] [PubMed]
- Angioni, D.; Orrù, R.; Cao, G.; Garroni, S.; Bellucci, D.; Cannillo, V. Recent Advances on Innovative Bioactive Glass-Hydroxyapatite Composites for Bone Tissue Applications: Processing, Mechanical Properties, and Biological Performance. J. Eur. Ceram. Soc. 2023, 43, 7688–7696. [Google Scholar] [CrossRef]
- John, A.; Shetty, A.M.; Salian, K.; Sequeria, S.N.; Sumukh, P.R.; Sukmawati, D.; Menon, G.; Abraham, S.; Venkatesan, J.; Narayanan, V.A. Ceramic Nanomaterials: Preparation and Applications in Osteoporosis and Bone Tissue Regeneration. J. Mater. Res. 2023, 38, 4023–4041. [Google Scholar] [CrossRef]
- Rahaman, M.N.; Day, D.E.; Sonny Bal, B.; Fu, Q.; Jung, S.B.; Bonewald, L.F.; Tomsia, A.P. Bioactive Glass in Tissue Engineering. Acta Biomater. 2011, 7, 2355–2373. [Google Scholar] [CrossRef]
- Gunduz, O.; Egles, C.; Pérez, R.A.; Ficai, D.; Ustundag, C.B. Biomaterials and Tissue Engineering; Springer: Cham, Switzerland, 2023; ISBN 9789811027239. [Google Scholar]
- Liu, D.; Cui, C.; Chen, W.; Shi, J.; Li, B.; Chen, S. Biodegradable Cements for Bone Regeneration. J. Funct. Biomater. 2023, 14, 134. [Google Scholar] [CrossRef] [PubMed]
- Lodoso-Torrecilla, I.; van den Beucken, J.J.J.P.; Jansen, J.A. Calcium Phosphate Cements: Optimization toward Biodegradability. Acta Biomater. 2021, 119, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Cole, K.A.; Funk, G.A.; Rahaman, M.N.; McIff, T.E. Characterization of the Conversion of Bone Cement and Borate Bioactive Glass Composites. J. Biomed. Mater. Res. Part B Appl. Biomater. 2020, 108, 1580–1591. [Google Scholar] [CrossRef]
- Tamer, M. Hamdy Novel Bioactive Acrylic-Based Bone Cement Reinforced with Bioglass Nanofillers: Chemical and Mechanical Assessment. Int. J. Dent. Med. Sci. Res. 2021, 3, 1048–1053. [Google Scholar] [CrossRef]
- Lopes, P.P.; Garcia, M.P.; Fernandes, M.H.; Fernandes, M.H.V. Acrylic Formulations Containing Bioactive and Biodegradable Fillers to Be Used as Bone Cements: Properties and Biocompatibility Assessment. Mater. Sci. Eng. C 2013, 33, 1289–1299. [Google Scholar] [CrossRef] [PubMed]
- Miola, M.; Lucchetta, G.; Verné, E. Physical, Mechanical, and Biological Properties of PMMA-Based Composite Bone Cement Containing Silver-Doped Bioactive and Antibacterial Glass Particles with Different Particles Sizes. Materials 2023, 16, 4499. [Google Scholar] [CrossRef] [PubMed]
- Tan, T.; Song, D.; Hu, S.; Li, X.; Li, M.; Wang, L.; Feng, H. Structure and Properties of Bioactive Glass-Modified Calcium Phosphate/Calcium Sulfate Biphasic Porous Self-Curing Bone Repair Materials and Preliminary Research on Their Osteogenic Effect. Materials 2022, 15, 7898. [Google Scholar] [CrossRef] [PubMed]
- Ruan, Z.; Yao, D.; Xu, Q.; Liu, L.; Tian, Z.; Zhu, Y. Effects of Mesoporous Bioglass on Physicochemical and Biological Properties of Calcium Sulfate Bone Cements. Appl. Mater. Today 2017, 9, 612–621. [Google Scholar] [CrossRef]
- Andre, R.; Rachid, Z. Bone Substitute Containig Porous Bio-Glass and Calcium Sulphate. U.S. Patent Application 13/060,958, 4 August 2011. [Google Scholar]
- Mansoori-Kermani, A.; Mashayekhan, S.; Kermani, F.; Abdekhodaie, M.J. The Effect of Tricalcium Silicate Incorporation on Bioactivity, Injectability, and Mechanical Properties of Calcium Sulfate/Bioactive Glass Bone Cement. Ceram. Int. 2023, 49, 15003–15014. [Google Scholar] [CrossRef]
- Chen, X.Y.; Xu, S.Z.; Wang, X.W.; Yang, X.Y.; Ma, L.; Zhang, L.; Yang, G.J.; Yang, F.; Wang, L.H.; Zhang, X.L.; et al. Systematic Comparison of Biologically Active Foreign Ionscodoped Calcium Phosphate Microparticles on Osteogenic Differentiation in Rat Osteoporotic and Normal Mesenchymal Stem Cells. Oncotarget 2017, 8, 36578–36590. [Google Scholar] [CrossRef] [PubMed]
- Hasan, M.L.; Kim, B.; Padalhin, A.R.; Faruq, O.; Sultana, T.; Lee, B.T. In Vitro and in Vivo Evaluation of Bioglass Microspheres Incorporated Brushite Cement for Bone Regeneration. Mater. Sci. Eng. C 2019, 103, 109775. [Google Scholar] [CrossRef]
- Lopes, J.H.; Magalhães, J.A.; Gouveia, R.F.; Bertran, C.A.; Motisuke, M.; Camargo, S.E.A.; Trichês, E. de S. Hierarchical Structures of β-TCP/45S5 Bioglass Hybrid Scaffolds Prepared by Gelcasting. J. Mech. Behav. Biomed. Mater. 2016, 62, 10–23. [Google Scholar] [CrossRef]
- Bernhardt, A.; Lode, A.; Peters, F.; Gelinsky, M. Comparative Evaluation of Different Calcium Phosphate-Based Bone Graft Granules—An in Vitro Study with Osteoblast-like Cells. Clin. Oral Imp. Res. 2013, 24, 441–449. [Google Scholar] [CrossRef]
- Keshaw, H.; Forbes, A.; Day, R.M. Release of Angiogenic Growth Factors from Cells Encapsulated in Alginate Beads with Bioactive Glass. Biomaterials 2005, 26, 4171–4179. [Google Scholar] [CrossRef]
- Shi, C.; Hou, X.; Zhao, D.; Wang, H.; Guo, R.; Zhou, Y. Preparation of the Bioglass/Chitosan-Alginate Composite Scaffolds with High Bioactivity and Mechanical Properties as Bone Graft Materials. J. Mech. Behav. Biomed. Mater. 2022, 126, 105062. [Google Scholar] [CrossRef] [PubMed]
- Yao, H.; Luo, J.; Deng, Y.; Li, Z.; Wei, J. Alginate-Modified Mesoporous Bioactive Glass and Its Drug Delivery, Bioactivity, and Osteogenic Properties. Front. Bioeng. Biotechnol. 2022, 10, 994925. [Google Scholar] [CrossRef] [PubMed]
- Fu, S.; Du, X.; Zhu, M.; Tian, Z.; Wei, D.; Zhu, Y. 3D Printing of Layered Mesoporous Bioactive Glass/Sodium Alginatesodium Alginate Scaffolds with Controllable Dual-Drug Release Behaviors. Biomed. Mater. 2019, 14, 065011. [Google Scholar] [CrossRef] [PubMed]
- Kong, L.; Wu, Z.; Zhao, H.; Cui, H.; Shen, J.; Chang, J.; Li, H.; He, Y. Bioactive Injectable Hydrogels Containing Desferrioxamine and Bioglass for Diabetic Wound Healing. ACS Appl. Mater. Interfaces 2018, 10, 30103–30114. [Google Scholar] [CrossRef] [PubMed]
- Hatton, J.; Davis, G.R.; Mourad, A.H.I.; Cherupurakal, N.; Hill, R.G.; Mohsin, S. Fabrication of Porous Bone Scaffolds Using Alginate and Bioactive Glass. J. Funct. Biomater. 2019, 10, 15. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Aguinagalde, O.; Lejardi, A.; Meaurio, E.; Hernández, R.; Mijangos, C.; Sarasua, J.R. Novel Hydrogels of Chitosan and Poly (Vinyl Alcohol) Reinforced with Inorganic Particles of Bioactive Glass. Polymers 2021, 13, 691. [Google Scholar] [CrossRef] [PubMed]
- Zhu, N.; Chatzistavrou, X.; Papagerakis, P.; Ge, L.; Qin, M.; Wang, Y. Silver-Doped Bioactive Glass/Chitosan Hydrogel with Potential Application in Dental Pulp Repair. ACS Biomater. Sci. Eng. 2019, 5, 4624–4633. [Google Scholar] [CrossRef] [PubMed]
- Chatzistavrou, X.; Tsigkou, O.; Amin, H.D.; Paraskevopoulos, K.M.; Salih, V.; Boccaccini, A.R. Sol-Gel Based Fabrication and Characterization of New Bioactive Glass-Ceramic Composites for Dental Applications. J. Eur. Ceram. Soc. 2012, 32, 3051–3061. [Google Scholar] [CrossRef]
- Fathi, A.; Ravarian, R.; Dehghani, F. Fabrication of Interpenetrate Chitosan: Bioactive Glass, Using Dense Gas CO2. In Proceedings of the 2011 Annual International Conference of the IEEE Engineering in Medicine and Biology Society, Boston, MA, USA, 30 August–3 September 2011; Volume 2, pp. 2459–2463. [Google Scholar] [CrossRef]
- Manoochehri, H.; Ghorbani, M.; Moosazadeh Moghaddam, M.; Nourani, M.R.; Makvandi, P.; Sharifi, E. Strontium Doped Bioglass Incorporated Hydrogel-Based Scaffold for Amplified Bone Tissue Regeneration. Sci. Rep. 2022, 12, 10160. [Google Scholar] [CrossRef]
- Pádua, A.S.; Figueiredo, L.; Silva, J.C.; Borges, J.P. Chitosan Scaffolds with Mesoporous Hydroxyapatite and Mesoporous Bioactive Glass. Prog. Biomater. 2023, 12, 137–153. [Google Scholar] [CrossRef] [PubMed]
- Peter, M.; Binulal, N.S.; Nair, S.V.; Selvamurugan, N.; Tamura, H.; Jayakumar, R. Novel Biodegradable Chitosan-Gelatin/Nano-Bioactive Glass Ceramic Composite Scaffolds for Alveolar Bone Tissue Engineering. Chem. Eng. J. 2010, 158, 353–361. [Google Scholar] [CrossRef]
- Maji, K.; Dasgupta, S.; Pramanik, K.; Bissoyi, A. Preparation and Evaluation of Gelatin-Chitosan-Nanobioglass 3D Porous Scaffold for Bone Tissue Engineering. Int. J. Biomater. 2016, 2016, 9825659. [Google Scholar] [CrossRef]
- Kajave, N.S.; Schmitt, T.; Nguyen, T.U.; Gaharwar, A.K.; Kishore, V. Bioglass Incorporated Methacrylated Collagen Bioactive Ink for 3D Printing of Bone Tissue. Biomed. Mater. 2021, 16, 035003. [Google Scholar] [CrossRef] [PubMed]
- El-Fiqi, A.; Lee, J.H.; Lee, E.J.; Kim, H.W. Collagen Hydrogels Incorporated with Surface-Aminated Mesoporous Nanobioactive Glass: Improvement of Physicochemical Stability and Mechanical Properties Is Effective for Hard Tissue Engineering. Acta Biomater. 2013, 9, 9508–9521. [Google Scholar] [CrossRef] [PubMed]
- Eglin, D.; Maalheem, S.; Livage, J.; Coradin, T. In Vitro Apatite Forming Ability of Type I Collagen Hydrogels Containing Bioactive Glass and Silica Sol-Gel Particles. J. Mater. Sci. Mater. Med. 2006, 17, 161–167. [Google Scholar] [CrossRef] [PubMed]
- Xu, C.; Su, P.; Chen, X.; Meng, Y.; Yu, W.; Xiang, A.P.; Wang, Y. Biocompatibility and Osteogenesis of Biomimetic Bioglass-Collagen-Phosphatidylserine Composite Scaffolds for Bone Tissue Engineering. Biomaterials 2011, 32, 1051–1058. [Google Scholar] [CrossRef] [PubMed]
- Abd El-Aziz, A.M.; Abd El-Fattah, A.; El-Maghraby, A.; Ghareeb, D.A.; Kandil, S. Viscoelasticity, Mechanical Properties, and in Vitro Bioactivity of Gelatin/Borosilicate Bioactive Glass Nanocomposite Hydrogels as Potential Scaffolds for Bone Regeneration. Polymers 2021, 13, 2014. [Google Scholar] [CrossRef] [PubMed]
- Foroutan Koudehi, M.; Imani Fooladi, A.A.; Aghozbeni, E.A.H.; Nourani, M.R. Nano Bioglass/Gelatin Scaffold Enhanced by Nanosilver as an Antibacterial Conduit for Peripheral Nerve Regeneration. Mater. Technol. 2019, 34, 776–784. [Google Scholar] [CrossRef]
- Sadeghian, A.; Kharaziha, M.; Khoroushi, M. Osteoconductive Visible Light-Crosslinkable Nanocomposite for Hard Tissue Engineering. Colloids Surf. A Physicochem. Eng. Asp. 2022, 632, 127761. [Google Scholar] [CrossRef]
- Pajares-Chamorro, N.; Wagley, Y.; Maduka, C.V.; Youngstrom, D.W.; Yeger, A.; Badylak, S.F.; Hammer, N.D.; Hankenson, K.; Chatzistavrou, X. Silver-Doped Bioactive Glass Particles for In Vivo Bone Tissue Regeneration and Enhanced Methicillin-Resistant Staphylococcus Aureus (MRSA) Inhibition. Mater. Sci. Eng. C 2021, 120, 111693. [Google Scholar] [CrossRef] [PubMed]
- Huang, G.; Xu, L.; Wu, J.; Wang, S.; Dong, Y. Gelatin/Bioactive Glass Composite Scaffold for Promoting the Migration and Odontogenic Differentiation of Bone Marrow Mesenchymal Stem Cells. Polym. Test. 2021, 93, 106915. [Google Scholar] [CrossRef]
- Reiter, T.; Panick, T.; Schuhladen, K.; Roether, J.A.; Hum, J.; Boccaccini, A.R. Bioactive Glass Based Scaffolds Coated with Gelatin for the Sustained Release of Icariin. Bioact. Mater. 2019, 4, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Houaoui, A.; Szczodra, A.; Lallukka, M.; El-Guermah, L.; Agniel, R.; Pauthe, E.; Massera, J.; Boissiere, M. New Generation of Hybrid Materials Based on Gelatin and Bioactive Glass Particles for Bone Tissue Regeneration. Biomolecules 2021, 11, 444. [Google Scholar] [CrossRef] [PubMed]
- Goel, H.; Gupta, N.; Santhiya, D.; Dey, N.; Bohidar, H.B.; Bhattacharya, A. Bioactivity Reinforced Surface Patch Bound Collagen-Pectin Hydrogel. Int. J. Biol. Macromol. 2021, 174, 240–253. [Google Scholar] [CrossRef] [PubMed]
- Gupta, N.; Goel, H.; Santhiya, D.; Srivastava, C.M.; Mishra, S.; Rai, P. Aqueous-Phased Electrospun Bioactive Glass Mineralized Gelatin-Pectin Hybrid Composite Fiber Matrix For 7- Dehydrocholesterol Delivery. ChemistrySelect 2020, 5, 4364–4370. [Google Scholar] [CrossRef]
- Yang, Z.; Yang, Z.; Ding, L.; Zhang, P.; Liu, C.; Chen, D.; Zhao, F.; Wang, G.; Chen, X. Self-Adhesive Hydrogel Biomimetic Periosteum to Promote Critical-Size Bone Defect Repair via Synergistic Osteogenesis and Angiogenesis. ACS Appl. Mater. Interfaces 2022, 14, 36395–36410. [Google Scholar] [CrossRef] [PubMed]
- Zurita-Méndez, N.N.; Carbajal-De la Torre, G.; Flores-Merino, M.V.; Espinosa-Medina, M.A. Development of Bioactive Glass-Collagen-Hyaluronic Acid-Polycaprolactone Scaffolds for Tissue Engineering Applications. Front. Bioeng. Biotechnol. 2022, 10, 825903. [Google Scholar] [CrossRef] [PubMed]
- De Luca, S.; Verdoliva, V.; Kargozar, S.; Baino, F. Bioactive Glass-Ceramic Scaffolds Coated with Hyaluronic Acid–Fatty Acid Conjugates: A Feasibility Study †. J. Funct. Biomater. 2023, 14, 26. [Google Scholar] [CrossRef]
- Riveiro, A.; Amorim, S.; Solanki, A.; Costa, D.S.; Pires, R.A.; Quintero, F.; del Val, J.; Comesaña, R.; Badaoui, A.; Lusquiños, F.; et al. Hyaluronic Acid Hydrogels Reinforced with Laser Spun Bioactive Glass Micro- and Nanofibres Doped with Lithium. Mater. Sci. Eng. C 2021, 126, 112124. [Google Scholar] [CrossRef]
- Chen, X.; Meng, Y.; Wang, Y.; Du, C.; Yang, C. A Biomimetic Material with a High Bio-Responsibility for Bone Reconstruction and Tissue Engineering. J. Biomater. Sci. Polym. Ed. 2011, 22, 153–163. [Google Scholar] [CrossRef] [PubMed]
- Almeida, A.C.; Vale, A.C.; Reis, R.L.; Alves, N.M. Bioactive and Adhesive Properties of Multilayered Coatings Based on Catechol-Functionalized Chitosan/Hyaluronic Acid and Bioactive Glass Nanoparticles. Int. J. Biol. Macromol. 2020, 157, 119–134. [Google Scholar] [CrossRef] [PubMed]
- Im, S.B.; Tripathi, G.; Le, T.T.T.; Lee, B.T. Early-Stage Bone Regeneration of Hyaluronic Acid Supplemented with Porous 45s5 Bioglass-Derived Granules: An Injectable System. Biomed. Mater. 2021, 16, 045034. [Google Scholar] [CrossRef] [PubMed]
- Douglas, T.E.L.; Dziadek, M.; Gorodzha, S.; Lišková, J.; Brackman, G.; Vanhoorne, V.; Vervaet, C.; Balcaen, L.; del Garcia, M.R.F.; Boccaccini, A.R.; et al. Novel Injectable Gellan Gum Hydrogel Composites Incorporating Zn- and Sr-enriched Bioactive Glass Microparticles: High- Resolution X-ray Microcomputed Tomography, Antibacterial and in Vitro Testing. J. Tissue Eng. Regen. Med. 2018, 12, 1313–1326. [Google Scholar] [CrossRef] [PubMed]
- Zvicer, J.; Medic, A.; Veljovic, D.; Jevtic, S.; Novak, S.; Obradovic, B. Biomimetic Characterization Reveals Enhancement of Hydroxyapatite Formation by Fluid Flow in Gellan Gum and Bioactive Glass Composite Scaffolds. Polym. Test. 2019, 76, 464–472. [Google Scholar] [CrossRef]
- Astanina, A.; Koivisto, J.T.; Hannula, M.; Salminen, T.; Kellomäki, M.; Massera, J. Chemical Interactions in Composites of Gellan Gum and Bioactive Glass: Self-Crosslinking and in Vitro Dissolution. Front. Chem. 2023, 11, 1133374. [Google Scholar] [CrossRef] [PubMed]
- Low, K.L.; Tan, S.H.; Zein, S.H.S.; Roether, J.A.; Mourino, V.; Boccaccini, A.R. Calcium Phosphate-Based Composites as Injectable Bone Substitute Materials. J. Biomed. Mater. Res. B Appl. Biomater. 2010, 94, 273–286. [Google Scholar] [CrossRef] [PubMed]
- Al-Harbi, N.; Mohammed, H.; Al-Hadeethi, Y.; Bakry, A.S.; Umar, A.; Hussein, M.A.; Abbassy, M.A.; Vaidya, K.G.; Al Berakdar, G.; Mkawi, E.M.; et al. Silica-Based Bioactive Glasses and Their Applications in Hard Tissue Regeneration: A Review. Pharmaceuticals 2021, 14, 75. [Google Scholar] [CrossRef] [PubMed]
- Dong, X.; Chang, J.; Li, H. Bioglass Promotes Wound Healing through Modulating the Paracrine Effects between Macrophages and Repairing Cells. J. Mater. Chem. B 2017, 5, 5240–5250. [Google Scholar] [CrossRef]
- Kaou, M.H.; Furkó, M.; Balázsi, K.; Balázsi, C. Advanced Bioactive Glasses: The Newest Achievements and Breakthroughs in the Area. Nanomaterials 2023, 13, 2287. [Google Scholar] [CrossRef]
- Batool, S.; Hussain, Z.; Liaqat, U. In Vitro and In Vivo Studies of Bioactive Glasses. In Bioactive Glasses and Glass-Cermics; Baino, F., Saeid Kargozar, Eds.; John Wiley & Sons: Hoboken, NJ, USA, 2022; pp. 397–425. [Google Scholar]
- Ege, D.; Lu, H.H.; Boccaccini, A.R. Bioactive Glass and Silica Particles for Skeletal and Cardiac Muscle Tissue Regeneration. Tissue Eng. Part B Rev. 2024; 1–14, ahead of print. [Google Scholar] [CrossRef]
- Wen, L.; Rüssel, C.; Day, D.E.; Völksch, G. Bioactive Comparison of a Borate, Phosphate and Silicate Glass. J. Mater. Res. 2006, 21, 125–131. [Google Scholar] [CrossRef]
- Abodunrin, O.D.; Semlali, A.; El Mabrouk, K.; Bricha, M. The Effect of Borate on Acellular Bioactivity of Novel Mesoporous Borosilicate Bioactive Glasses for Tissue Engineering. Ceram. Int. 2024, 50, 2303–2318. [Google Scholar] [CrossRef]
- Fu, Q.; Rahaman, M.N.; Fu, H.; Liu, X. Silicate, Borosilicate, and Borate Bioactive Glass Scaffolds with Controllable Degradation Rate for Bone Tissue Engineering Applications. I. Preparation and in Vitro Degradation. J. Biomed. Mater. Res. Part A 2010, 95, 164–171. [Google Scholar] [CrossRef] [PubMed]
- Erasmus, E.P.; Sule, R.; Johnson, O.T.; Massera, J.; Sigalas, I.; Africa, S. In Vitro Evaluation of Porous Borosilicate, Borophosphate and Phosphate Bioactive Glasses Scaffolds Fabricated Using Foaming Agent for Bone Regeneration African Material Science and Engineering Network (A Carnegie-IAS RISE Network) OPEN. Sci. Rep. 2018, 8, 3699. [Google Scholar] [CrossRef] [PubMed]
- Jia, W.; Hu, H.; Li, A.; Deng, H.; Hogue, C.L.; Mauro, J.C.; Zhang, C.; Fu, Q. Glass-Activated Regeneration of Volumetric Muscle Loss. Acta Biomater. 2020, 103, 306–317. [Google Scholar] [CrossRef] [PubMed]
- Taye, M.B.; Ningsih, H.S.; Shih, S.J. Exploring the Advancements in Surface-Modified Bioactive Glass: Enhancing Antibacterial Activity, Promoting Angiogenesis, and Modulating Bioactivity. J. Nanoparticle Res. 2024, 26, 28. [Google Scholar] [CrossRef]
- Ryu, J.H.; Mangal, U.; Lee, M.J.; Seo, J.Y.; Jeong, I.J.; Park, J.Y.; Na, J.Y.; Lee, K.J.; Yu, H.S.; Cha, J.K.; et al. Effect of Strontium Substitution on Functional Activity of Phosphate-Based Glass. Biomater. Sci. 2023, 11, 6299–6310. [Google Scholar] [CrossRef] [PubMed]
- Keskin-Erdogan, Z.; Patel, K.D.; Chau, D.Y.S.; Day, R.M.; Kim, H.-W.; Knowles, J.C. Utilization of GelMA with Phosphate Glass Fibers for Glial Cell Alignment. J. Biomed. Mater. Res. Part A 2021, 109, 2212–2224. [Google Scholar] [CrossRef]
- Carta, D.; Foroutan, F.; Kyffin, B.A.; Abrahams, I.; Corrias, A.; Gupta, P.; Velliou, E.; Knowles, J.C. Mesoporous Phosphate-Based Glasses Prepared via Sol-Gel. ACS Biomater. Sci. Eng. 2020, 6, 1428–1437. [Google Scholar] [CrossRef]
- Vaid, C.; Murugavel, S. New Trends in Bioactive Glasses: The Importance of Mesostructure. Trans. Indian Ceram. Soc. 2013, 72, 1–4. [Google Scholar] [CrossRef]
- McLaren, J.S.; Macri-Pellizzeri, L.; Hossain, K.M.Z.; Patel, U.; Grant, D.M.; Scammell, B.E.; Ahmed, I.; Sottile, V. Porous Phosphate-Based Glass Microspheres Show Biocompatibility, Tissue Infiltration, and Osteogenic Onset in an Ovine Bone Defect Model. ACS Appl. Mater. Interfaces 2019, 11, 15436–15446. [Google Scholar] [CrossRef] [PubMed]
- Nakanishi, A.; Ningsih, H.S.; Putra, D.F.A.; Moriga, T.; Shih, S.-J. Fabrication and Characterization of Granulated β-Tricalcium Phosphate and Bioactive Glass Powders by Spray Drying. J. Compos. Sci. 2024, 8, 111. [Google Scholar] [CrossRef]
- Sujon, M.K.; Mohd Noor, S.N.F.; Zabidi, M.A.; Shariff, K.A. Combined sol–gel bioactive glass and β-tricalcium phosphate for potential dental tissue engineering: A preliminary study. J. Aust. Ceram. Soc. 2023, 59, 415–424. [Google Scholar] [CrossRef]
- Park, J.; Park, S.M.; Ham, D.W.; Hong, J.Y.; Kim, H.J.; Yeom, J.S. Anterior Cervical Discectomy and Fusion Performed Using a CaO-SiO2-P2O5-B2O3 Bioactive Glass Ceramic or Polyetheretherketone Cage Filled with Hydroxyapatite/β-Tricalcium Phosphate: A Prospective Randomized Controlled Trial. J. Clin. Med. 2023, 12, 4069. [Google Scholar] [CrossRef]
- Aslam, A.A.; Akram, J.; Mehmood, R.A.; Mubarak, A.; Khatoon, A.; Akbar, U.; Ahmad, S.A.; Atif, M. Boron-Based Bioactive Glasses: Properties, Processing, Characterization and Applications. Ceram. Int. 2023, 49, 19595–19605. [Google Scholar] [CrossRef]
- Mishra, A.; Rocherulle, J.; Massera, J. Ag-Doped Phosphate Bioactive Glasses: Thermal, Structural and in-Vitro Dissolution Properties. Biomed. Glas. 2016, 2, 38–48. [Google Scholar] [CrossRef]
- Chethan, M.; Rajiv, A. Examination of Bioactivity Studies of Phosphate Glasses Doped with Strontium. Mater. Today Commun. 2023, 37, 107424. [Google Scholar]
- Kitagawa, H.; Kohno, T.; Deng, F.; Abe, G.L.; Sakai, H.; Fan, Y.-S.; Wu, T.; Sasaki, J.; Imazato, S. Metal-Doped Silicate and Phosphate Glasses for Antibacterial Dental Biomaterials. Biomater. Investig. Dent. 2023, 10, 2284372. [Google Scholar] [CrossRef] [PubMed]
- Taye, M.B. Biomedical Applications of Ion-Doped Bioactive Glass: A Review. Appl. Nanosci. 2022, 12, 3797–3812. [Google Scholar] [CrossRef]
- Balasubramanian, P.; Strobel, L.A.; Kneser, U.; Boccaccini, A.R. Zinc-Containing Bioactive Glasses for Bone Regeneration, Dental and Orthopedic Applications. Biomed. Glas. 2015, 1, 51–69. [Google Scholar] [CrossRef]
- Shendage, S.S.; Gaikwad, K.; Kachare, K.; Kashte, S.; Ghule, A.V. In Vitro and in Vivo Study of Copper-Doped Bioactive Glass for Bone Regeneration Application. Mater. Chem. Phys. 2024, 313, 128789. [Google Scholar] [CrossRef]
- Özel, C.; Çevlik, C.B.; Özarslan, A.C.; Emir, C.; Elalmis, Y.B.; Yücel, S. Evaluation of Biocomposite Putty with Strontium and Zinc Co-Doped 45S5 Bioactive Glass and Sodium Hyaluronate. Int. J. Biol. Macromol. 2023, 242, 124901. [Google Scholar] [CrossRef] [PubMed]
- Hoppe, A.; Güldal, N.S.; Boccaccini, A.R. A Review of the Biological Response to Ionic Dissolution Products from Bioactive Glasses and Glass-Ceramics. Biomaterials 2011, 32, 2757–2774. [Google Scholar] [CrossRef] [PubMed]
- Pantulap, U.; Arango-Ospina, M.; Boccaccini, A.R. Bioactive Glasses Incorporating Less-Common Ions to Improve Biological and Physical Properties. J. Mater. Sci. Mater. Med. 2022, 33, 3. [Google Scholar] [CrossRef] [PubMed]
- Rabiee, S.M.; Nazparvar, N.; Azizian, M.; Vashaee, D.; Tayebi, L. Effect of Ion Substitution on Properties of Bioactive Glasses: A Review. Ceram. Int. 2015, 41, 7241–7251. [Google Scholar] [CrossRef]
- Pal, A.; Das Karmakar, P.; Vel, R.; Bodhak, S. Synthesis and Characterizations of Bioactive Glass Nanoparticle-Incorporated Triblock Copolymeric Injectable Hydrogel for Bone Tissue Engineering. ACS Appl. Bio Mater. 2023, 6, 445–457. [Google Scholar] [CrossRef]

| Composition | Glass Composition (Molar Ratio) | Applications | References |
|---|---|---|---|
| Acrylic Bone Cements | 58SiO2-36CaO-6P2O5 | Drug delivery system | [27] |
| 45SiO2-24.5Na2O-24.5CaO-6P2O5 | Denture base materials | [28] | |
| 45SiO2-24.5Na2O-24.5CaO-6P2O5 | Coatings | [29] | |
| 5.5 Na2O-11.1 K2O-4.6MgO-18.5CaO-56.6B2O3-3.7P2O5 | Bone regeneration | [149] | |
| 45SiO2-24.5Na2O-24.5CaO-6P2O5 | Bone regeneration | [150] | |
| 24.5 SiO2-38CaO-12.7P2O5-24.8MgO | Bone regeneration | [151] | |
| Ag doped 48SiO2-18Na2O-30CaO-3P2O5-0.43B2O3-0.57Al2O3 | Temporary prostheses | [152] | |
| Calcium Sulphate Cements | 60SiO2-35CaO-5P2O5 | Tissue graft | [39] |
| SiO2-CaO-P2O5-B2O3-MgO | Polymorphic bone defect repair | [153] | |
| 80SiO2-15CaO-5P2O5 | Bone repair and drug release | [154] | |
| 45SiO2-24.5Na2O-24.5CaO-6P2O5 | Bone substitute | [155] | |
| 45SiO2-30CaO-5P2O5-2B2O3-15CaCl2-3MgO | Orthopaedics-vertebroplasty and kyphoplasty | [156] | |
| Calcium Phosphate Cements | 45SiO2-24.5Na2O-24.5CaO-6P2O5 | Cell proliferation and osteogenic differentiation | [157] |
| 45SiO2-24.5Na2O-24.5CaO-6P2O5 | Bone regeneration | [158,159] | |
| 30.67CaO-43.14P2O5-9.42Na2O-14.32K2O-2.45MgO | Bone implant | [160] |
| Composition | Glass Composition (Molar Ratio) | Applications | References |
|---|---|---|---|
| Alginate | 45SiO2-24.5Na2O-24.5CaO-6P2O5 | Therapeutic angiogenesis | [161] |
| SiO2-CaO-P2O5 | Scaffolds in bone tissue engineering | [162,163] | |
| 80SiO2-15CaO-5P2O5 | Scaffolds in bone tissue engineering | [164] | |
| 45SiO2-24.5Na2O-24.5CaO-6P2O5 | Skin repair-promoting biomaterials | [165] | |
| 49.46SiO2-6.6Na2O-27.27CaO-1.07P2O5-3SrO-6.6K2O-3MgO-3ZnO | Scaffolds in bone tissue engineering | [166] | |
| Chitosan | 60SiO2-36CaO-4P2O5 | Bone regeneration | [167] |
| Ag-60SiO2-36CaO-4P2O5 | Dental pulp repair | [168,169] | |
| 64SiO2-31CaO-5P2O5 | Scaffolds in bone tissue engineering | [170] | |
| Sr-45SiO2-24.5Na2O-24.5CaO-6P2O5 | Repairing large bone injuries | [171] | |
| 85SiO2-10CaO-5P2O5 | Scaffolds in bone tissue engineering | [104,172] | |
| 55SiO2-40CaO-5P2O5 | Alveolar bone tissue engineering | [173] | |
| 58SiO2-33CaO-9P2O5 | Bone regeneration | [174] | |
| Collagen | 45SiO2-24.5Na2O-24.5CaO-6P2O5 | Scaffolds in bone tissue engineering | [175] |
| 85SiO2-15CaO | Stem cell culture for bone tissue engineering | [176] | |
| 53SiO2-23Na2O-20CaO-4P2O5 | Bone tissue engineering | [177] | |
| 58SiO2-33CaO-9P2O5 | Scaffolds in bone tissue engineering | [178] | |
| Gelatin | 55SiO2-24CaO-6P2O5-15B2O3 | Scaffolds in bone tissue engineering | [179] |
| 64SiO2-5P2O5-26CaO-5MgO | Scaffolds for nerve regeneration | [180] | |
| 45SiO2-24.5Na2O-24.5CaO-6P2O5 | Hydrogel in hard tissue engineering | [181] | |
| 64SiO2-27CaO-4MgO-5P2O5 | Small bone defect | [89] | |
| Ag-58SiO2-33CaO-9P2O5 | Bone tissue engineering | [182] | |
| 54.2SiO2-35CaO-10.8P2O5 | Pulp regeneration | [183] | |
| 45SiO2-24.5Na2O-24.5CaO-6P2O5 | Drug delivery as 3D sponge-like scaffolds | [184] | |
| SiO2-B2O3-CaO-K2O-MgO-Na2O-P2O5 | Bone tissue regeneration | [185] | |
| Pectin | 40SiO2-54CaO-6P2O5 | Injectable hydrogel in bone tissue engineering | [122] |
| 45SiO2-24.5Na2O-24.5CaO-6P2O5 | Soft and hard tissue engineering | [186] | |
| 45SiO2-24.5Na2O-24.5CaO-6P2O5 | Fiber construct in bone regeneration | [187] | |
| Hyaluronic acid | 60SiO2-36CaO-4P2O5 | Critical-size bone defect repair | [188] |
| SiO2-CaO-P2O5 | Scaffolds or coating in tissue engineering | [189] | |
| 47.5SiO2-2.5P2O5-20CaO-20MgO-10Na2O-10K2O | Scaffolds in bone tissue engineering | [190] | |
| SiO2-Na2O-K2O-CaO-MgO-P2O5 | Soft tissue engineering | [191] | |
| 58SiO2-33CaO-9P2O5 | Bone tissue engineering | [192] | |
| 50SiO2-45CaO-5P2O5 | Coating in orthopaedic implants | [193] | |
| 45SiO2-24.5Na2O-24.5CaO-6P2O5 | Injectable bone substitute | [194] | |
| Gellan gum | 54SiO2-40CaO-6P2O5 | Injectable bone substitute | [195] |
| 70SiO2-30CaO | Bone tissue engineering | [196] | |
| 66SiO2-10Na2O-22CaO-2P2O5 70SiO2-30CaO | Scaffolds in bone tissue engineering | [132] | |
| 43.7SiO2-10.9B2O3-22.1CaO-7.9K2O-7.7MgO-6.0Na2O-1.7P2O5 | Bone tissue engineering | [197] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mîrț, A.-L.; Ficai, D.; Oprea, O.-C.; Vasilievici, G.; Ficai, A. Current and Future Perspectives of Bioactive Glasses as Injectable Material. Nanomaterials 2024, 14, 1196. https://doi.org/10.3390/nano14141196
Mîrț A-L, Ficai D, Oprea O-C, Vasilievici G, Ficai A. Current and Future Perspectives of Bioactive Glasses as Injectable Material. Nanomaterials. 2024; 14(14):1196. https://doi.org/10.3390/nano14141196
Chicago/Turabian StyleMîrț, Andreea-Luiza, Denisa Ficai, Ovidiu-Cristian Oprea, Gabriel Vasilievici, and Anton Ficai. 2024. "Current and Future Perspectives of Bioactive Glasses as Injectable Material" Nanomaterials 14, no. 14: 1196. https://doi.org/10.3390/nano14141196
APA StyleMîrț, A.-L., Ficai, D., Oprea, O.-C., Vasilievici, G., & Ficai, A. (2024). Current and Future Perspectives of Bioactive Glasses as Injectable Material. Nanomaterials, 14(14), 1196. https://doi.org/10.3390/nano14141196








