Silver Nanocomposites with Enhanced Shelf-Life for Fruit and Vegetable Preservation: Mechanisms, Advances, and Prospects
Abstract
:1. Introduction
2. Preparation of Silver Nanocomposites
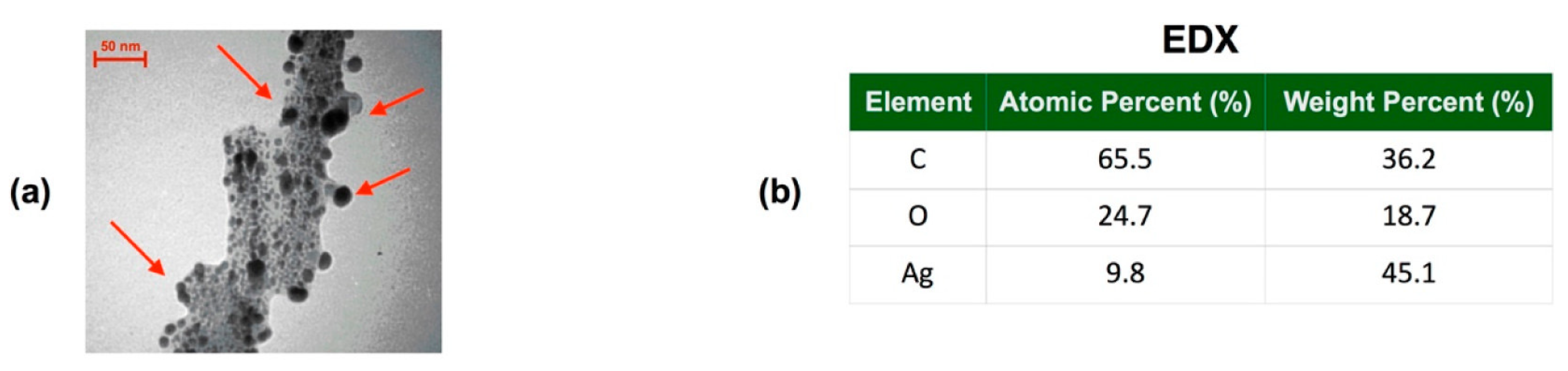
2.1. Preparation of Ag@composites
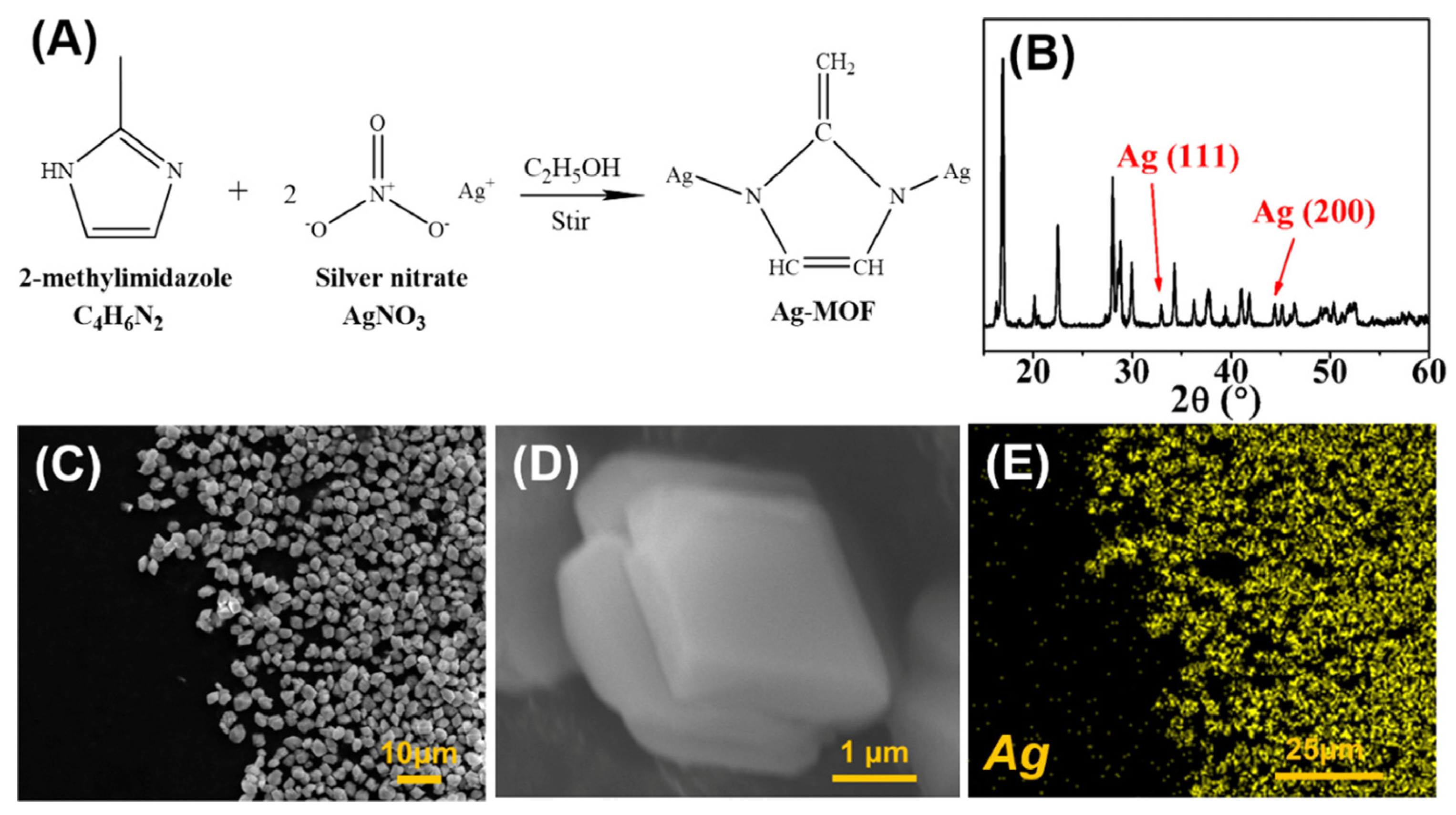
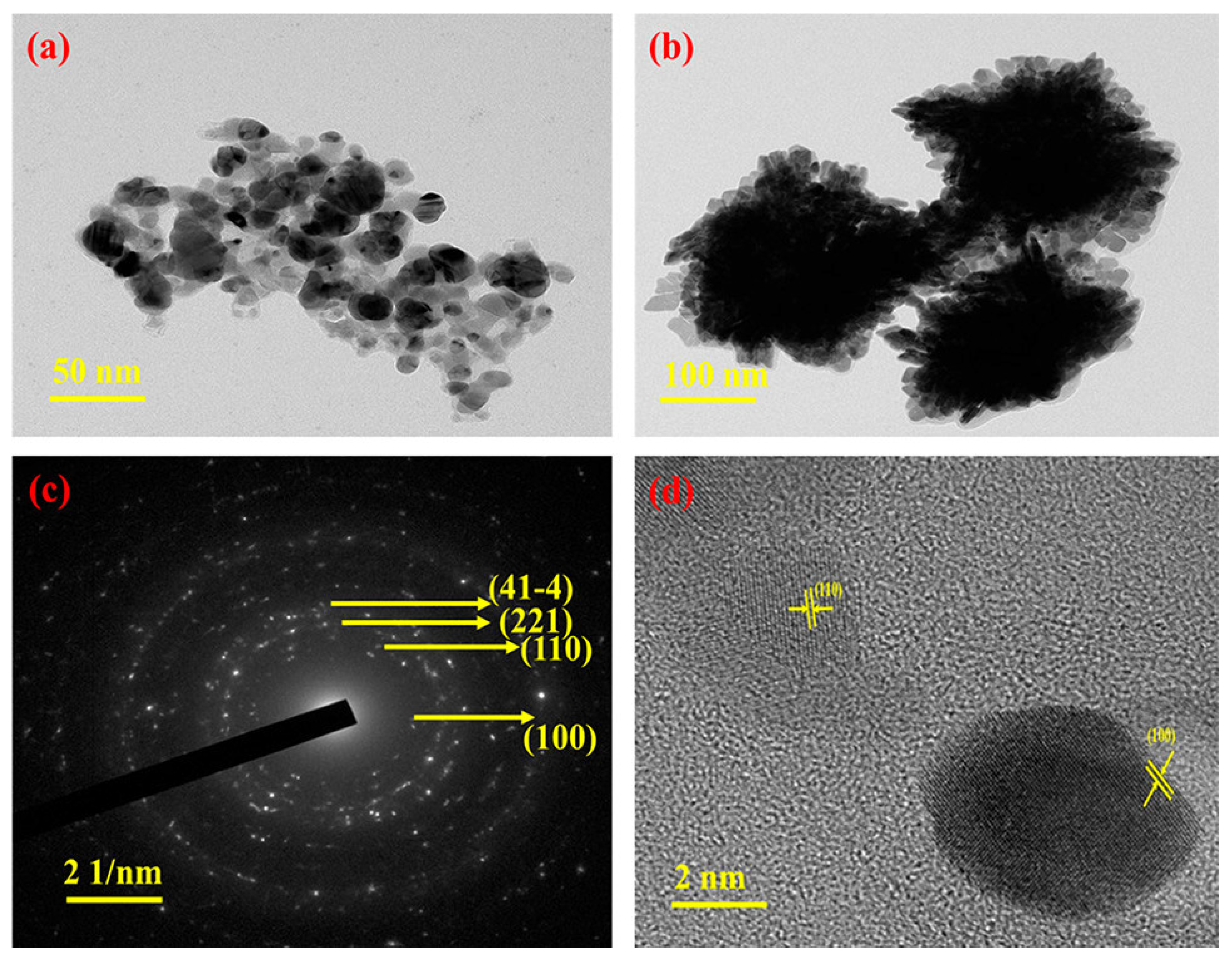
2.2. Preparation of Ag-MOFs
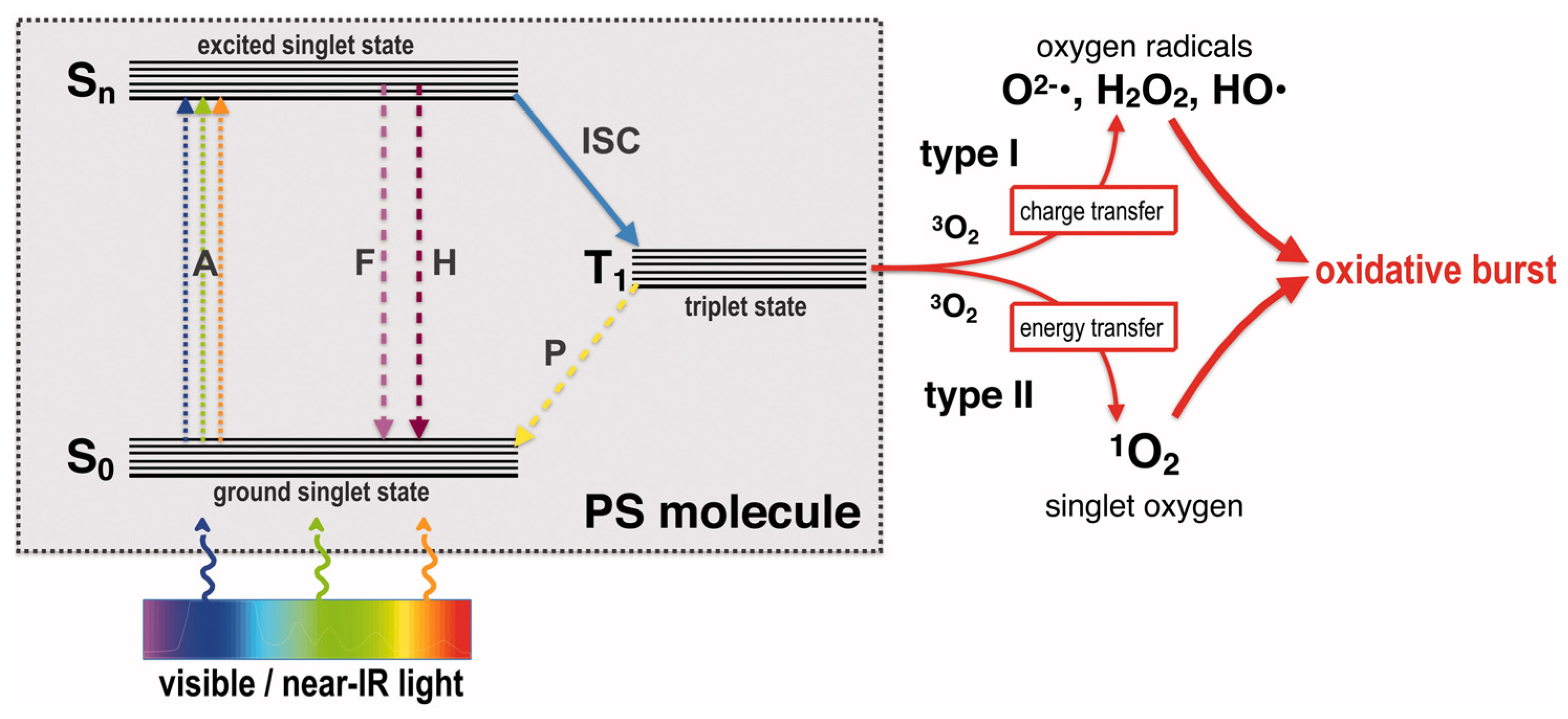
3. Preservation Mechanism of Silver Nanocomposites
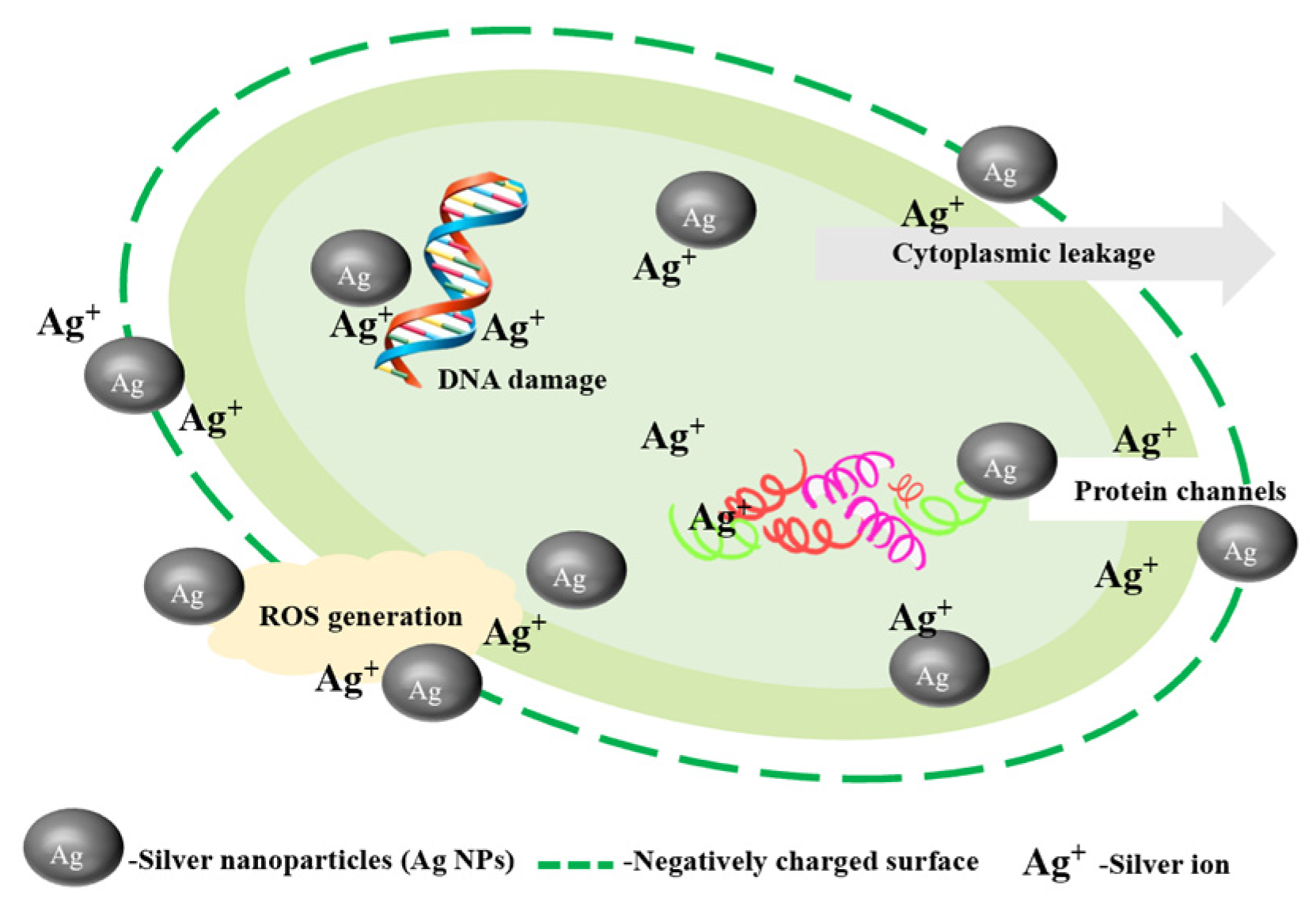
3.1. Ag NPs and Silver Ion Migration
3.2. Synergistic Antimicrobials in Composites
4. Silver Nanocomposites for Freshness Preservation Applications
4.1. Antimicrobial Activity
4.2. UV-Resistant Applications
4.3. Moisture-Resistant Applications
4.4. Antioxidant Applications
4.5. Gas Conditioning Applications
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Rakesh, B.; Mahendran, R. Upcycling of food waste and food loss—A sustainable approach in the food sector. Trends Food Sci. Technol. 2024, 143, 104274. [Google Scholar] [CrossRef]
- Casonato, C.; Garcia-Herrero, L.; Caldeira, C.; Sala, S. What a waste! Evidence of consumer food waste prevention and its effectiveness. Sustain. Prod. Consum. 2023, 41, 305–319. [Google Scholar] [CrossRef]
- Shen, D.; Zhang, M.; Mujumdar, A.S.; Ma, Y. Consumer-oriented smart dynamic detection of fresh food quality: Recent advances and future prospects. Crit. Rev. Food Sci. Nutr. 2023, 1–21. [Google Scholar] [CrossRef] [PubMed]
- Lv, Y.; Ai, Y.; Fang, F.; Liao, H. Development of active packaging films utilized natural colorants derived from plants and their diverse applications in protein-rich food products. Food Innov. Adv. 2023, 2, 203–216. [Google Scholar] [CrossRef]
- Lin, H.; Xu, Y.; Guan, W.; Zhao, S.; Li, X.; Zhang, C.; Blecker, C.; Liu, J. The importance of supercooled stability for food during supercooling preservation: A review of mechanisms, influencing factors, and control methods. Crit. Rev. Food Sci. Nutr. 2023, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.; Ahn, G.; Shin, W.-R.; Choi, J.-W.; Kim, Y.-H.; Ahn, J.-Y. Synergistic outcomes of Chlorella-bacterial cellulose based hydrogel as an ethylene scavenger. Carbohydr. Polym. 2023, 321, 121256. [Google Scholar] [CrossRef] [PubMed]
- Wanli, Z.; Hamed, A.; Zhengke, Z.; Seid Mahdi, J. Role of silica (SiO2) nano/micro-particles in the functionality of degradable packaging films/coatings and their application in food preservation. Trends Food Sci. Technol. 2023, 133, 75–86. [Google Scholar]
- Priyanka, S.; S, K.R.N.; R. S., A.B.; John, A. Biocompatible green technology principles for the fabrication of food packaging material with noteworthy mechanical and antimicrobial properties A sustainable developmental goal towards the effective, safe food preservation strategy. Chemosphere 2023, 336, 139240. [Google Scholar] [CrossRef] [PubMed]
- Wasilewska, A.; Bielicka, M.; Klekotka, U.; Kalska-Szostko, B. Nanoparticle applications in food—A review. Food Funct. 2023, 14, 2544–2567. [Google Scholar] [CrossRef]
- Kumar, S.; Basumatary, I.B.; Sudhani, H.P.K.; Bajpai, V.K.; Chen, L.; Shukla, S.; Mukherjee, A. Plant extract mediated silver nanoparticles and their applications as antimicrobials and in sustainable food packaging: A state-of-the-art review. Trends Food Sci. Technol. 2021, 112, 651–666. [Google Scholar] [CrossRef]
- Shujat, A.; Xiaojing, C.; Shujaat, A.; Wahid, S.; Muhammad, S.; Pramila, C.; Gulam, M.; Amal, A.; Sitah, A. Advancements and challenges in phytochemical-mediated silver nanoparticles for food packaging: Recent review (2021–2023). Trends Food Sci. Technol. 2023, 141, 104197. [Google Scholar]
- Tehri, N.; Vashishth, A.; Gahlaut, A.; Hooda, V. Biosynthesis, antimicrobial spectra and applications of silver nanoparticles: Current progress and future prospects. Inorg. Nano-Met. Chem. 2022, 52, 1–19. [Google Scholar] [CrossRef]
- Trotta, F.; Da Silva, S.; Massironi, A.; Mirpoor, S.F.; Lignou, S.; Ghawi, S.K.; Charalampopoulos, D. Silver Bionanocomposites as Active Food Packaging: Recent Advances & Future Trends Tackling the Food Waste Crisis. Polymers 2023, 15, 4243. [Google Scholar] [CrossRef] [PubMed]
- Krasniewska, K.; Galus, S.; Gniewosz, M. Biopolymers-Based Materials Containing Silver Nanoparticles as Active Packaging for Food Applications—A Review. Int. J. Mol. Sci. 2020, 21, 698. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Cao, C.; Wang, Y.; Xie, L.; Li, W.; Li, B.; Guo, R.; Yan, H. Magnesium oxide/silver nanoparticles reinforced poly(butylene succinate-co-terephthalate) biofilms for food packaging applications. Food Packag. Shelf Life 2021, 30, 100748. [Google Scholar]
- Mouzahim, M.E.; Eddarai, E.M.; Eladaoui, S.; Guenbour, A.; Bellaouchou, A.; Zarrouk, A.; Boussen, R. Effect of Kaolin clay and Ficus Carica mediated silver nanoparticles on chitosan food packaging film for fresh apple slice preservation. Food Chem. 2023, 410, 135470. [Google Scholar] [CrossRef] [PubMed]
- Babaei-Ghazvini, A.; Shahabi-Ghahfarrokhi, I.; Goudarzi, V. Preparation of UV-protective starch/kefiran/ZnO nanocomposite as a packaging film: Characterization. Food Packag. Shelf Life 2018, 16, 103–111. [Google Scholar]
- Indumathi, M.P.; Sarojini, K.S.; Rajarajeswari, G.R. Antimicrobial and biodegradable chitosan/cellulose acetate phthalate/ZnO nano composite films with optimal oxygen permeability and hydrophobicity for extending the shelf life of black grape fruits. Int. J. Biol. Macromol. 2019, 132, 1112–1120. [Google Scholar] [PubMed]
- Chowdhury, S.; Teoh, Y.L.; Ong, K.M.; Zaidi NS, R.; Mah, S.-K. Poly(vinyl) alcohol crosslinked composite packaging film containing gold nanoparticles on shelf life extension of banana. Food Packag. Shelf Life 2020, 24, 100463. [Google Scholar] [CrossRef]
- Zhou, W.; Wu, Z.; Xie, F.; Tang, S.; Fang, J.; Wang, X. 3D printed nanocellulose-based label for fruit freshness keeping and visual monitoring. Carbohyd. Polym. 2021, 273, 118545. [Google Scholar] [CrossRef]
- Liu, L.; Wu, W.; Zheng, L.; Yu, J.; Sun, P.; Shao, P. Intelligent packaging films incorporated with anthocyanins-loaded ovalbumin-carboxymethyl cellulose nanocomplexes for food freshness monitoring. Food Chem. 2022, 387, 132908. [Google Scholar] [CrossRef]
- Zhao, Y.; Li, C.; Xia, X.; Tan, M.; Wang, H.; Lv, Y.; Cheng, Y.; Tao, Y.; Lu, J.; Li, D.; et al. Eco-friendly and intelligent cellulosic fibers-based packaging system for real-time visual detection of food freshness. Chem. Eng. J. 2023, 474, 146013. [Google Scholar] [CrossRef]
- Sarwar, M.S.; Niazi MB, K.; Jahan, Z.; Ahmad, T.; Hussain, A. Preparation and characterization of PVA/nanocellulose/Ag nanocomposite films for antimicrobial food packaging. Carbohydr. Polym. 2018, 184, 453–464. [Google Scholar] [CrossRef]
- Biswas, M.C.; Tiimob, B.J.; Abdela, W.; Jeelani, S.; Rangari, V.K. Nano silica-carbon-silver ternary hybrid induced antimicrobial composite films for food packaging application. Food Packag. Shelf Life 2019, 19, 104–113. [Google Scholar] [CrossRef]
- Lan, W.; Li, S.; Shama, S.; Zhao, Y.; Sameen, D.E.; He, L.; Liu, Y. Investigation of Ultrasonic Treatment on Physicochemical, Structural and Morphological Properties of Sodium Alginate/AgNPs/Apple Polyphenol Films and Its Preservation Effect on Strawberry. Polymers 2020, 12, 2096. [Google Scholar] [CrossRef]
- Zhou, Y.; Wu, W.; Wang, L.; Goksen, G.; Shao, P. Multifunctional pectin films based on mussel-inspired modified 2D Ag nanosheets for long-lasting antibacterial and enhanced barrier properties. Food Hydrocoll. 2023, 137, 108331. [Google Scholar] [CrossRef]
- Xiao, Y.; Ahmad, T.; Belwal, T.; Aadil, R.M.; Siddique, M.; Pang, L.; Xu, Y. A review on protein based nanocarriers for polyphenols: Interaction and stabilization mechanisms. Food Innov. Adv. 2023, 2, 193–202. [Google Scholar] [CrossRef]
- Waqas, M.; Campbell, L.; T, P. Polyaniline-Coated Surface-Modified Ag/PANI Nanostructures for Antibacterial and Colorimetric Melamine Sensing in Milk Samples. ACS Omega 2023, 8, 24010–24015. [Google Scholar] [CrossRef] [PubMed]
- Singha, S.K.; Hoque, S.M.; Das, H.; Alim, M.A. Evaluation of chitosan-Ag/TiO2 nanocomposite for the enhancement of shelf life of chili and banana fruits. Heliyon 2023, 9, e21752. [Google Scholar] [CrossRef] [PubMed]
- El-Sherbiny, M.M.; Devassy, R.P.; El-Hefnawy, M.E.; Al-Goul, S.T.; Orif, M.I.; El-Newehy, M.H. Facile Synthesis, Characterization, and Antimicrobial Assessment of a Silver/Montmorillonite Nanocomposite as an Effective Antiseptic against Foodborne Pathogens for Promising Food Protection. Molecules 2023, 28, 3699. [Google Scholar] [CrossRef]
- Sun, L.; Cao, C.; Zhi, Y.; Shan, Y.; Zhang, H.; Dou, B.; Zhang, L.; Huang, W. Au–Ag Nanoparticles with Controllable Morphologies for the Surface-Enhanced Raman Scattering Detection of Trace Thiram. ACS Appl. Nano Mater. 2023, 6, 4253–4261. [Google Scholar] [CrossRef]
- Zhang, Y.; Ling, S.; Chen, Y.; Qin, X.; Wang, K.; Zhu, Q.; Liu, Y. Application of Ag/Tannic acid-FeIII nanocomposite as multifunctional bacteriostatic to enhance the performance of chitosan/gelatin/polyvinyl alcohol complex films. Food Hydrocoll. 2023, 147, 109302. [Google Scholar] [CrossRef]
- Ali, S.; Sharma, A.S.; Ahmad, W.; Zareef, M.; Hassan, M.M.; Viswadevarayalu, A.; Jiao, T.; Li, H.; Chen, Q. Noble Metals Based Bimetallic and Trimetallic Nanoparticles: Controlled Synthesis, Antimicrobial and Anticancer Applications. Crit. Rev. Anal. Chem. 2021, 51, 454–481. [Google Scholar] [CrossRef]
- León-Valencia, A.; Briceño, S.; Reinoso, C.; Vizuete, K.; Debut, A.; Caetano, M.; González, G. Photochemical Reduction of Silver Nanoparticles on Diatoms. Mar. Drugs 2023, 21, 185. [Google Scholar] [CrossRef]
- Fakher, S.N.; Kashi, F.J. Microbial Synthesized Ag/AgCl Nanoparticles Using Staphylococcus pasteuri sp. nov., ZAR1: Antimutagenicity, Antimicrobial Agent. J. Inorg. Organomet. Polym. Mater. 2021, 31, 1688–1703. [Google Scholar] [CrossRef]
- Islam, M.J.; Khatun, N.; Bhuiyan, R.H.; Sultana, S.; Ali Shaikh, M.A.; Amin Bitu, M.N.; Chowdhury, F.; Islam, S. Psidium guajava leaf extract mediated green synthesis of silver nanoparticles and its application in antibacterial coatings. RSC Adv. 2023, 13, 19164–19172. [Google Scholar] [CrossRef] [PubMed]
- Bushra Hafeez, K.; Irshad, A.; Sodha, N.; Mohammed, K.O.; Taghreed, N.A.; Wahidah, H.A.-Q.; Mostafa, A.A.-M. Evaluation of Biogenic Silver Nanoparticles Synthesized from Vegetable Waste. Int. J. Nanomed. 2023, 18, 6527–6544. [Google Scholar]
- Hassan, G.E.G.; Ibrahim, A.A.; Talal, F.Q.; Ahmed, N.A.-H.; Abdu, S.; Fouad, A.; Eida, M.A.; Asnag, G.M. Reinforcement of structural, thermal and electrical properties and antibacterial activity of PVA/SA blend filled with hybrid nanoparticles (Ag and TiO2 NPs): Nanodielectric for energy storage and food packaging industries. Ceram. Int. 2023, 49, 20174–20184. [Google Scholar]
- Asmaa, N.M.A.; Nadia, A.A.; Seenaa, I.H.; Hakamy, A.; Bahaaudin, R.; Ayman, S.A.; Alaa, M.A.E. Nanoarchitectonics of Silver/Poly (Methyl Methacrylate) Films: Structure, Optical Characteristics, Antibacterial Activity, and Wettability. J. Inorg. Organomet. Polym. Mater. 2023, 33, 694–706. [Google Scholar]
- Khalil, I.E.; Fonseca, J.; Reithofer, M.R.; Eder, T.; Chin, J.M. Tackling orientation of metal-organic frameworks (MOFs): The quest to enhance MOF performance. Coord. Chem. Rev. 2023, 481, 215043. [Google Scholar]
- Seyedpour, S.F.; Arabi Shamsabadi, A.; Khoshhal Salestan, S.; Dadashi Firouzjaei, M.; Sharifian Gh, M.; Rahimpour, A.; Akbari Afkhami, F.; Shirzad Kebria, M.R.; Elliott, M.A.; Tiraferri, A.; et al. Tailoring the Biocidal Activity of Novel Silver-Based Metal Azolate Frameworks. ACS Sustain. Chem. Eng. 2020, 8, 7588–7599. [Google Scholar] [CrossRef]
- Yuan, G.; Tian, Y.; Wang, B.; You, X.; Liao, Y. Mitigation of membrane biofouling via immobilizing Ag-MOFs on composite membrane surface for extractive membrane bioreactor. Water Res. 2021, 209, 117940. [Google Scholar] [CrossRef]
- Shalini, S.S.; Balamurugan, R.; Velmathi, S.; Bose, A.C. Systematic Investigation on the Electrochemical Performance of Pristine Silver Metal–Organic Framework as the Efficient Electrode Material for Supercapacitor Application. Energy Fuels 2022, 36, 7104–7114. [Google Scholar] [CrossRef]
- Song, Y.; Liu, X.; Gao, Z.; Wang, Z.; Hu, Y.; Yang, K.; Zhao, Z.; Lan, D.; Wu, G. Core-shell Ag@C spheres derived from Ag-MOFs with tunable ligand exchanging phase inversion for electromagnetic wave absorption. J. Colloid Interface Sci. 2022, 620, 263–272. [Google Scholar] [CrossRef]
- Tan, Z.-K.; Gong, J.-L.; Fang, S.-Y.; Li, J.; Cao, W.-C.; Chen, Z.-P. Outstanding anti-bacterial thin-film composite membrane prepared by incorporating silver-based metal–organic framework (Ag-MOF) for water treatment. Appl. Surf. Sci. 2022, 590, 153059. [Google Scholar] [CrossRef]
- Meshari, M.A.; Salhah, D.A.-Q.; Mubark, A.; Mohamed, G.E.-D.; Ashraf, A.E.-B.; Nashwa, M.E.-M.; Mohamed, A.E.-B. Highly efficient adsorption and removal bio-staining dye from industrial wastewater onto mesoporous Ag-MOFs. Process Saf. Environ. Prot. 2023, 172, 395–407. [Google Scholar]
- Zirehpour, A.; Rahimpour, A.; Arabi Shamsabadi, A.; Sharifian Gh, M.; Soroush, M. Mitigation of Thin-Film Composite Membrane Biofouling via Immobilizing Nano-Sized Biocidal Reservoirs in the Membrane Active Layer. Environ. Sci. Technol. 2017, 51, 5511–5522. [Google Scholar] [CrossRef]
- Medha, K.; Tin, L.; Adrienne, T.; Milad Rabbani, E. Silver Metal Organic Frameworks and Copper Metal Organic Frameworks Immobilized on Graphene oxide for Enhanced Adsorption in Water Treatment. Chem. Eng. J. 2022, 439, 135542. [Google Scholar]
- Zhao, X.; Gong, L.; Wang, C.; Wang, C.; Yu, K.; Zhou, B. Facile grinding method synthesis of 3D Ag-MOFs containing Ag6Mo7O24 for high-performance supercapacitors. Chem. Eur. J. 2020, 26, 4613–4619. [Google Scholar] [CrossRef]
- Singh, R.; Dutt, S.; Sharma, P.; Sundramoorthy, A.K.; Dubey, A.; Singh, A.; Arya, S. Future of Nanotechnology in Food Industry: Challenges in Processing, Packaging, and Food Safety. Glob. Chall. 2023, 7, 2200209. [Google Scholar] [CrossRef]
- Samal, D.; Khandayataray, P.; Sravani, M.; Murthy, M.K. Silver nanoparticle ecotoxicity and phytoremediation: A critical review of current research and future prospects. Environ. Sci. Pollut. 2024, 31, 8400–8428. [Google Scholar] [CrossRef] [PubMed]
- Ali Alharbi, A.; Alghamdi, A.M.; Talal Al-Goul, S.; Allohibi, A.; Baty, R.S.; Qahl, S.H.; Beyari, E.A. Valorizing pomegranate wastes by producing functional silver nanoparticles with antioxidant, anticancer, antiviral, and antimicrobial activities and its potential in food preservation. Saudi J. Biol. Sci. 2024, 31, 103880. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Xu, M.; Guo, Y.; Xiao, Y.; Wang, H.; Gao, X.; Lian, X.; Niu, B.; Li, W. Fabrication of an antibacterial system of arginine-modified chitosan with AgNPs-loaded montmorillonite for food preservation. Food Hydrocoll. 2024, 156, 110258. [Google Scholar] [CrossRef]
- Yin, C.; Ding, X.; Lin, Z.; Cao, J.; Shi, W.; Wang, J.; Xu, D.; Xu, D.; Liu, Y.; Liu, G. Preparation and characterization of quercetin@ZIF-L/GO@AgNPs nanocomposite film for room-temperature strawberry preservation. Food Chem. 2024, 450, 139411. [Google Scholar] [CrossRef] [PubMed]
- Manikandan, N.A.; McCann, R.; Kakavas, D.; Rochfort, K.D.; Sreenilayam, S.P.; Alkan, G.; Stornetta, T.; McGivern, A.R.; Grintzalis, K.; Friedrich, B.; et al. Production of Silver Nano-Inks and Surface Coatings for Anti-Microbial Food Packaging and Its Ecological Impact. Int. J. Mol. Sci. 2023, 24, 5341. [Google Scholar] [CrossRef]
- Azizi-Lalabadi, M.; Garavand, F.; Jafari, S.M. Incorporation of silver nanoparticles into active antimicrobial nanocomposites: Release behavior, analyzing techniques, applications and safety issues. Adv. Colloid Interface Sci. 2021, 293, 102440. [Google Scholar] [CrossRef] [PubMed]
- Amr, M.; Abu-Hussien, S.H.; Ismail, R.; Aboubakr, A.; Wael, R.; Yasser, M.; Hemdan, B.; El-Sayed, S.M.; Bakry, A.; Ebeed, N.M.; et al. Utilization of biosynthesized silver nanoparticles from Agaricus bisporus extract for food safety application: Synthesis, characterization, antimicrobial efficacy, and toxicological assessment. Sci. Rep. 2023, 13, 15048. [Google Scholar] [CrossRef] [PubMed]
- Ouda, S.M. Antifungal activity of silver and copper nanoparticles on two plant pathogens, Alternaria alternata and Botrytis cinerea. Res. J. Microbiol. 2014, 9, 34–42. [Google Scholar] [CrossRef]
- Narayanan, K.B.; Park, H.H. Antifungal activity of silver nanoparticles synthesized using turnip leaf extract (Brassica rapa L.) against wood rotting pathogens. Eur. J. Plant Pathol. 2014, 140, 185–192. [Google Scholar] [CrossRef]
- Zhang, W.; Ye, G.; Liao, D.; Chen, X.; Lu, C.; Nezamzadeh-Ejhieh, A.; Khan, M.S.; Liu, J.; Pan, Y.; Dai, Z. Recent Advances of Silver-Based Coordination Polymers on Antibacterial Applications. Molecules 2022, 27, 7166. [Google Scholar] [CrossRef]
- Pejman, M.; Dadashi Firouzjaei, M.; Aghapour Aktij, S.; Zolghadr, E.; Das, P.; Elliott, M.; Sadrzadeh, M.; Sangermano, M.; Rahimpour, A.; Tiraferri, A. Effective strategy for UV-mediated grafting of biocidal Ag-MOFs on polymeric membranes aimed at enhanced water ultrafiltration. Chem. Eng. J. 2021, 426, 130704. [Google Scholar] [CrossRef]
- Liao, C.; Li, Y.; Tjong, S. Bactericidal and Cytotoxic Properties of Silver Nanoparticles. Int. J. Mol. Sci. 2019, 20, 449. [Google Scholar] [CrossRef]
- Verduzco-Chavira, K.; Vallejo-Cardona, A.A.; González-Garibay, A.S.; Torres-González, O.R.; Sánchez-Hernández, I.M.; Flores-Fernández, J.M.; Padilla-Camberos, E. Antibacterial and Antibiofilm Activity of Chemically and Biologically Synthesized Silver Nanoparticles. Antibiotics 2023, 12, 1084. [Google Scholar] [CrossRef] [PubMed]
- Fouad, K.A.; Mengshi, L.; Azlin, M. Green synthesis of silver nanomaterials and evaluation of their antibacterial and antioxidant effectiveness in chicken meat. Food Biosci. 2023, 56, 103332. [Google Scholar]
- Ibraheem, S.A.; Audu, E.A.; Atabat, A.J.; Jaafar, M.; Tanimu, B.F.; Yahaya, J.Y.; Barminas, J.T. Pectin-stabilized silver nanoparticles: Synthesis, optical and antimicrobial activity against E. coli. Inorg. Chem. Commun. 2023, 158, 111500. [Google Scholar] [CrossRef]
- Yang, W.; Xu, F.; Ma, X.; Guo, J.; Li, C.; Shen, S.; Puglia, D.; Chen, J.; Xu, P.; Kenny, J.; et al. Highly-toughened PVA/nanocellulose hydrogels with anti-oxidative and antibacterial properties triggered by lignin-Ag nanoparticles. Mater. Sci. Eng. C Mater. Biol. Appl. 2021, 129, 112385. [Google Scholar] [CrossRef] [PubMed]
- Alireza, D.; Kamran, A.; Mohammadreza, A. Prolonged release of silver and iodine from ZIF-7 carrier with great antibacterial activity. CrystEngComm 2023, 25, 3931–3942. [Google Scholar]
- Singh, A.; Goswami, A.; Nain, S. Enhanced antibacterial activity and photo-remediation of toxic dyes using Ag/SWCNT/PPy based nanocomposite with core–shell structure. Appl. Nanosci. 2020, 10, 2255–2268. [Google Scholar] [CrossRef]
- Xu, Z.; Wang, X.; Liu, X.; Cui, Z.; Yang, X.; Yeung, K.W.K.; Chung, J.C.; Chu, P.K.; Wu, S. Tannic Acid/Fe3+/Ag Nanofilm Exhibiting Superior Photodynamic and Physical Antibacterial Activity. ACS Appl. Mater. Interfaces 2017, 9, 39657–39671. [Google Scholar] [CrossRef]
- Liu, S.; Zhao, Y.; Xu, M.; Wen, J.; Wang, H.; Yan, H.; Gao, X.; Niu, B.; Li, W. Antibacterial photodynamic properties of silver nanoparticles-loaded curcumin composite material in chitosan-based films. Int. J. Biol. Macromol. 2023, 256 Pt 1, 128014. [Google Scholar] [CrossRef]
- Ren, W.; Tang, Q.; Cao, H.; Wang, L.; Zheng, X. Biological Preparation of Chitosan-Loaded Silver Nanoparticles: Study of Methylene Blue Adsorption as Well as Antibacterial Properties under Light. ACS Omega 2023, 8, 22998–23007. [Google Scholar] [CrossRef] [PubMed]
- Cieplik, F.; Deng, D.; Crielaard, W.; Buchalla, W.; Hellwig, E.; Al-Ahmad, A.; Maisch, T. Antimicrobial photodynamic therapy—What we know and what we don’t. Crit. Rev. Microbiol. 2018, 44, 571–589. [Google Scholar] [CrossRef]
- Khan, S.; Hashim, S.B.H.; Arslan, M.; Zhang, K.; Siman, L.; Mukhtar, A.; Zhihua, L.; Tahir, H.E.; Zhai, X.; Shishir, M.R.I.; et al. Development of an active biogenic silver nanoparticles composite film based on berry wax and chitosan for rabbit meat preservation. Int. J. Biol. Macromol. 2024, 275 Pt 1, 133128. [Google Scholar] [CrossRef] [PubMed]
- Ge, J.; Lu, W.; Zhang, H.; Gong, Y.; Wang, J.; Xie, Y.; Chang, Q.; Deng, X. Exploring sustainable food packaging: Nanocellulose composite films with enhanced mechanical strength, antibacterial performance, and biodegradability. Int. J. Biol. Macromol. 2024, 259 Pt 2, 129200. [Google Scholar] [CrossRef] [PubMed]
- Fareeha, A.; Muhammedin, D.; Pavithra, S.; Mohammad, A.Q.; James Salveo, O.; Gil Nonato, S.; Vipul, B.; Minhaz Uddin, A. Recent developments and applications of nanomaterial-based lab-on-a-chip devices for sustainable agri-food industries. Trends Food Sci. Technol. 2023, 136, 145–158. [Google Scholar]
- Moreno-Gordaliza, E.; Dolores Marazuela, M.; Milagros Gómez-Gómez, M. Risk assessment of silver and microplastics release from antibacterial food containers under conventional use and microwave heating. Food Chem. 2023, 420, 136097. [Google Scholar] [CrossRef] [PubMed]
- Hamouda, R.A.; Almaghrabi, F.Q.; Alharbi, O.M.; Al-Harbi, A.D.M.; Alsulami, R.M.; Alhumairi, A.M. Antifungal Activities of Biogenic Silver Nanoparticles Mediated by Marine Algae: In Vitro and In Vivo Insights of Coating Tomato Fruit to Protect against Penicillium italicum Blue Mold. Mar. Drugs 2024, 22, 225. [Google Scholar] [CrossRef] [PubMed]
- Busolo, M.A.; Lagaron, J.M. Antimicrobial biocomposites of melt-compounded polylactide films containing silver-based engineered clays. J. Plast. Film Sheeting 2013, 29, 290–305. [Google Scholar] [CrossRef]
- Martinez-Abad, A.; Lagaron, J.M.; Ocio, M.J. Development and Characterization of Silver-Based Antimicrobial Ethylene-Vinyl Alcohol Copolymer (EVOH) Films for Food-Packaging Applications. J. Agric. Food Chem. 2012, 60, 5350–5359. [Google Scholar]
- das Neves, M.d.S.; Scandorieiro, S.; Pereira, G.N.; Ribeiro, J.M.; Seabra, A.B.; Dias, A.P.; Yamashita, F.; Martinez, C.B.d.R.; Kobayashi, R.K.T.; Nakazato, G. Antibacterial Activity of Biodegradable Films Incorporated with Biologically-Synthesized Silver Nanoparticles and the Evaluation of Their Migration to Chicken Meat. Antibiotics 2023, 12, 178. [Google Scholar] [CrossRef]
- Huang, P.; Meng, L.; Pang, J.; Huang, H.; Ma, J.; He, L.; Amani, P. Development of a high-performance label-free electrochemical immunosensor for early cancer diagnosis using anti-CEA/Ag-MOF/GO/GCE nanocomposite. Environ. Res. 2023, 238 Pt 2, 117178. [Google Scholar] [CrossRef]
- Zhang, Y.; Jiang, F.; Zhao, W.; Fu, L.; Xu, C.; Lin, B. One-Pot Synthesis of Degradable and Renewable Cellulose-Based Packaging Films. ACS Sustain. Chem. Eng. 2022, 10, 16871–16881. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, H.; Yao, Y.; Shen, X.; Xu, C.; Fu, L.; Lin, B. Multifunctional flexible Ag-MOFs@CMFP composite paper for fruit preservation and real-time wireless monitoring of fruit quality during storage and transportation. Food Chem. 2022, 395, 133614. [Google Scholar] [CrossRef]
- Deng, X.; Li, J.; Zhao, B.; Li, Z. Design of a novel Ag-MOF@GO composite with a high specific surface area and structural stability for the efficient removal of malachite green. New J. Chem. 2023, 47, 16022–16029. [Google Scholar] [CrossRef]
- Alkhamis, K.M.; Aljohani, M.M.; Ibarhiam, S.F.; Hameed, Y.A.S.; Abumelha, H.M.; Habeebullah, T.M.; El-Metwaly, N.M. Application of Metal-Organic Frameworks for Efficient Removal of Doxorubicin Hydrochloride: Removal Process Optimization and Biological Activity. ACS Omega 2023, 8, 30374–30388. [Google Scholar] [CrossRef] [PubMed]
- Firouzjaei, M.D.; Shamsabadi, A.A.; Aktij, S.A.; Seyedfour, S.F.; Sharifian Gh, M.; Rahimpour, A.; Esfahani, M.R.; Ulbricht, M.; Soroush, M. Exploiting Synergetic Effects of Graphene Oxide and a Silver-Based Metal-Organic Framework To Enhance Antifouling and Anti-Biofouling Properties of Thin-Film Nanocomposite Membranes. ACS Appl. Mater. Interfaces 2018, 10, 42967–42978. [Google Scholar] [CrossRef] [PubMed]
- El-Metwaly, N.M.; Katouah, H.A.; El-Desouky, M.G.; El-Bindary, A.A.; El-Bindary, M.A. Fabricating of Fe3O4@Ag-MOF nanocomposite and evaluating its adsorption activity for removal of doxorubicin. J. Environ. Sci. Health Part A 2022, 57, 1099–1115. [Google Scholar] [CrossRef] [PubMed]
- Shefali, T.; Lokesh, K.; Ram Kumar, D.; Kirtiraj, K.G. Ultraviolet Blocking Films for Food Packaging Applications. Food Bioprocess Technol. 2023, 17, 1563–1582. [Google Scholar]
- Sarmast, E.; Shankar, S.; Salmieri, S.; Rahmouni, S.A.; Mahmud, J.; Lacroix, M. Cross-linked gelatin–riboflavin-based film incorporated with essential oils and silver nanoparticle by gamma-irradiation: A novel approach for extending the shelf life of meat. Food Hydrocoll. 2024, 147, 109330. [Google Scholar] [CrossRef]
- Feng, Q.; Fan, B.; He, Y.-C.; Ma, C. Antibacterial, antioxidant and fruit packaging ability of biochar-based silver nanoparticles-polyvinyl alcohol-chitosan composite film. Int. J. Biol. Macromol. 2024, 256 Pt 2, 128297. [Google Scholar]
- Yin, Y.; Zhang, J.; Fan, R.; Zhu, K.; Jiang, X.; Ji, C.; Jia, W.; Wu, J.; Tao, H.; Yang, Y. Terbium-functionalized silver-based metal-organic frameworks for efficient antibacterial and simultaneous monitoring of bacterial spores. J. Hazard. Mater. 2023, 446, 130753. [Google Scholar] [CrossRef]
- Chen, N.; Wang, C.; Kong, F.; Wang, S. In situ facile synthesis and antibacterial activity of Ag-MOFs/cellulose filter paper composites for fruit fresh-keeping. Int. J. Biol. Macromol. 2023, 256 Pt 1, 128424. [Google Scholar] [CrossRef] [PubMed]
- Shilpa, K.; Asha, K.; Rahul, S. Safe and sustainable food packaging: Argemone albiflora mediated green synthesized silver-carrageenan nanocomposite films. Int. J. Biol. Macromol. 2024, 264 Pt 1, 130626. [Google Scholar]
- Zeng, J.; Ma, Y.; Li, P.; Zhang, X.; Gao, W.; Wang, B.; Xu, J.; Chen, K. Development of high-barrier composite films for sustainable reduction of non-biodegradable materials in food packaging application. Carbohydr. Polym. 2024, 330, 121824. [Google Scholar] [CrossRef] [PubMed]
- Majumder, S.; Huang, S.; Zhou, J.; Wang, Y.; George, S. Tannic acid-loaded halloysite clay grafted with silver nanoparticles enhanced the mechanical and antimicrobial properties of soy protein isolate films for food-packaging applications. Food Packag. Shelf Life 2023, 39, 101142. [Google Scholar] [CrossRef]
- Rozo, D.F.; Alvarado, J.F.; Chaparro, L.M.; Medina, J.A.; Salcedo, F. Modeling oxidation kinetics of linseed oil in oxygen scavenger nanocapsules to be potentially used in active food packaging. Food Packag. Shelf Life 2024, 42, 101256. [Google Scholar] [CrossRef]
- Hayat, Z.; Manel, D.; Ali, H.B.; Mona, M.K.; Talaat, H.H.; Khaled, D.K. Multifunctional Ag2O/chitosan nanocomposites synthesized via sol-gel with enhanced antimicrobial, and antioxidant properties: A novel food packaging material. Int. J. Biol. Macromol. 2024, 264 Pt 1, 129990. [Google Scholar]
- Zhang, M.; Zheng, Y.; Jin, Y.; Wang, D.; Wang, G.; Zhang, X.; Li, Y.; Lee, S. Ag@MOF-loaded p-coumaric acid modified chitosan/chitosan nanoparticle and polyvinyl alcohol/starch bilayer films for food packing applications. Int. J. Biol. Macromol. 2022, 202, 80–90. [Google Scholar] [CrossRef]
- Goncalves, A.A.; de Oliveira, A.R.M. Combinate effect of antimelanosic agents (acerola fruit extract and sodium metabisulphite) with the modified atmosphere packaging on the quality of white shrimp (Litopenaeus vannamei) stored under refrigeration. Food Innov. Adv. 2023, 2, 233–246. [Google Scholar] [CrossRef]
- Shouket, S.; Khurshid, S.; Khan, J.; Nadeem, A.A.; Sarwar, A.; Aziz, T.; Alotaibi, N.M.; Alamri, A.S.; Alhormani, M.; Sameeh, M.Y. Biosynthetically produced glucose oxidase immobilized silver nanoparticle bioconjugate treatment improves the shelf life of mango fruit: An innovative method towards food safety and sustainability. Biomass Convers. Biorefinery 2023. [Google Scholar] [CrossRef]
- Thu Hoai, P.T.; Lam, T.D.; Mai Huong, N.T.; Van Anh, M.T. Removal of ethylene by synthesized Ag/TiO2 photocatalyst under visible light irradiation. Chemosphere 2023, 329, 138607. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Zhang, J.; Wang, B.; Li, W.; Wang, H.; Guo, R.; Yu, W.; Xie, L.; Zheng, Q. Modified magnesium oxide/silver nanoparticles reinforced poly (butylene succinate-co-terephthalate) composite biofilms for food packaging application. Food Chem. 2023, 435, 137492. [Google Scholar] [CrossRef] [PubMed]
- Ananthi, P.; Hemkumar, K.; Subasini, S.; Anitha, P. Development of biodegradable films reinforced with silver functionalized cow milk carbon dots for active food packaging applications. Mater. Today Sustain. 2023, 24, 100609. [Google Scholar] [CrossRef]
- Hassan, G.E.G.; Asnag, G.M.; Tarabiah, A.E.; Talal, F.Q.; Abdelrazek, E.M.; Emre, C.; Ahmed, N.A.-H.; Abdulwahed, J.A.M.; Aysh, Y.M.; Fawziah, A.; et al. Modification and development of optical, thermal, dielectric properties and antibacterial activity of PVA/SA blend by Ag/Se nanofillers: Nanocomposites for energy storage devices and food packaging applications. Polym. Test. 2023, 129, 108258. [Google Scholar]
- Shashank, S.; Vijay, C.; Bhasha, S.; Purnima, J.; Amit, K.; Ajay Kumar, B.; Mahendra Kumar, M. Bioinspired molecular modeling and antibacterial efficacy of silver/graphene oxide grafted chitosan nanocomposite for food packaging applications. Biomass Convers. Biorefinery 2023. [Google Scholar] [CrossRef]
- Gumber, S.; Kanwar, S.; Mazumder, K. Properties and antimicrobial activity of wheat-straw nanocellulose-arabinoxylan acetate composite films incorporated with silver nanoparticles. Int. J. Biol. Macromol. 2023, 246, 125480. [Google Scholar] [CrossRef] [PubMed]
- Nazia, Y.; Arooj, Z.; Shagufta, K.; Maheen, A.; Saima, R. Development of multifunctional bioactive food packaging based on silver nanoparticles/grape fruit peel extract reinforced PVA composites. Mater. Today Commun. 2023, 37, 107529. [Google Scholar]
- Xia, Y.; Wang, S.; Meng, F.; Xu, Z.; Fang, Q.; Gu, Z.; Zhang, C.; Li, P.; Kong, F. Eco-friendly food packaging based on paper coated with a bio-based antibacterial coating composed of carbamate starch, calcium lignosulfonate, cellulose nanofibrils, and silver nanoparticles. Int. J. Biol. Macromol. 2023, 254 Pt 1, 127659. [Google Scholar] [CrossRef] [PubMed]
- Ma, Z.; Xing, Z.; Zhao, Y.; Zhang, H.; Xin, Q.; Liu, J.; Qin, M.; Ding, C.; Li, J. Lotus leaf inspired sustainable and multifunctional Janus film for food packaging. Chem. Eng. J. 2023, 457, 141279. [Google Scholar] [CrossRef]
- M’Sakni, N.H.; Alsufyani, T. Part B: Improvement of the Optical Properties of Cellulose Nanocrystals Reinforced Thermoplastic Starch Bio-Composite Films by Ex Situ Incorporation of Green Silver Nanoparticles from Chaetomorpha linum. Polymers 2023, 15, 2148. [Google Scholar] [CrossRef]
- Nguyen, Q.L.; Le, D.V.; Phan, A.N.; Nguyen, V.D. Synthesis of Biodegradable and Antimicrobial Nanocomposite Films Reinforced for Coffee and Agri-Food Product Preservation. ACS Omega 2023, 8, 42177–42185. [Google Scholar] [CrossRef] [PubMed]
- Vimalasruthi, N.; Chandramohan, G.; Esakkimuthu, S.; Vigneshkumar, G.; Rajaram, R.; Stalin, T. Preparation of gallic acid functionalized polyvinyl alcohol/β-cyclodextrin-silver nanoparticles cast film as an antimicrobial and antioxidant agent. J. Appl. Polym. Sci. 2023, 140, e54652. [Google Scholar]
- Yang, Z.; Li, M.; Li, Y.; Huang, X.; Li, Z.; Zhai, X.; Shi, J.; Zou, X.; Xiao, J.; Sun, Y.; et al. Sodium alginate/guar gum based nanocomposite film incorporating β-Cyclodextrin/persimmon pectin-stabilized baobab seed oil Pickering emulsion for mushroom preservation. Food Chem. 2023, 437 Pt 1, 137891. [Google Scholar] [CrossRef] [PubMed]
- Hira, N.; Tahir, I. Experimental and theoretical analysis for Ag doped ZnO photocatalyst for its novel application to improve shelf life of peach. Opt. Quantum Electron. 2023, 55, 471. [Google Scholar]
- Zhang, Y.; Lin, Z.; He, Q.; Deng, Y.; Wei, F.; Xu, C.; Fu, L.; Lin, B. Enhanced aqueous stability and long-acting antibacterial of silver-based MOFs via chitosan-crosslinked for fruit fresh-keeping. Appl. Surf. Sci. 2021, 571, 151351. [Google Scholar] [CrossRef]
| Type of Ag NPs | Preservation Principle | Antibacterial Species | Application | References |
|---|---|---|---|---|
| PBST/MgO/Ag complex film | bacteriostatic, waterproof | E. coli, S. aureus | tomato preservation | [102] |
| PVA/SA/Ag–TiO2 NPs complex film | bacteriostatic | E. coli, S. aureus | food packaging | [38] |
| agar/Ag-carbon dots complex film | bacteriostatic, UV resistant | E. coli, S. aureus | tomato preservation | [103] |
| Ag/PMMA complex film | bacteriostatic, waterproof, UV resistant | S. aureus | food packaging | [39] |
| PVA/SA/Ag–Se NPs complex film | bacteriostatic, UV resistant | E. coli, S. aureus | food packaging | [104] |
| PVA/CS/GO/Ag NPs complex film | bacteriostatic | E. coli, S. aureus | food packaging | [105] |
| Nanocellulose/arabinoxylan/ Ag NPs complex film | bacteriostatic, waterproof, UV resistant | Shigella flexneri, P. aeruginosa, aspergillus brasiliensis | food packaging | [106] |
| Ag/TiO2 powder | bacteriostatic, controlled ethylene | E. coli, S. aureus | fruit preservation | [101] |
| CS-Ag/TiO2 fiber complex film | bacteriostatic, waterproof, UV resistant | E. coli | pepper, and banana preservation | [29] |
| Ag@PVA/CS complex film | bacteriostatic, waterproof, UV resistant, antioxidative | E. coli, S. aureus, bacillus subtilis, P. aeruginosa | blueberry preservation | [90] |
| Ag NPs/montmorillonite powder | bacteriostatic | E. coli, S. aureus, salmonella, P. aeruginosa, listeria monocytogenes | food packaging | [30] |
| Ag/PMMA complex film | bacteriostatic, waterproof, UV resistant | S. aureus | food packaging | [39] |
| GOx modified Ag NPs liquid | bacteriostatic, oxygen removal | mango preservation | [100] | |
| Ag NPs synthesized by Agaricus bisporus | bacteriostatic | E. coli, S. aureus | food packaging | [57] |
| CS/Ag NPs complex film | bacteriostatic, waterproof, UV resistant, antioxidative | E. coli, S. aureus | apple preservation | [16] |
| Ag NPs- grape peel extract/PVA complex film | bacteriostatic, antioxidative, maintain moisture | E. coli, B. subtilis | food packaging | [107] |
| Ag NPs synthesized from pomegranate extract | bacteriostatic, antioxidative, antiviral | E. coli, S. aureus | orange preservation | [52] |
| carbamate starch/lignite/cellulose microfibril/Ag NPs masking liquid | bacteriostatic, waterproof, UV resistant, antioxidative, blocking oxygen | E. coli, S. aureus | tomato preservation | [108] |
| soybean polysaccharide /Ag NPs Janus film | bacteriostatic, waterproof, UV resistant, antioxidative | E. coli, S. aureus | grape preservation | [109] |
| cellulose/starch/Ag NPs complex film | UV resistant | food packaging | [110] | |
| PVA/cassava starch/CS/Ag NPs complex film | bacteriostatic | Bacillus perfringens, S. aureus, B. subtilis, aspergillus flavus | tomato, orange, coffee bean preservation | [111] |
| PVA/beta-cyclodextrin/Gallic acid functionalized Ag NPs complex film | bacteriostatic, waterproof, UV resistant, antioxidative | E. coli, S. aureus, A. flavus, candida albicans | food packaging | [112] |
| SA/guar gum/anthocyanin extract of wolfberry /Ag NPs/BO Pickering emulsion-complex film | bacteriostatic, waterproof, UV resistant, antioxidative, blocking oxygen | E. coli, S. aureus | mushroom preservation | [113] |
| Ag/ZnO NPs powder | bacteriostatic, maintains moisture, inhibiting metabolism | peach preservation | [114] | |
| Ag-MOF@CMC | E. coli, S. aureus, A. niger | strawberry preservation | [82] | |
| Ag-MOF/CMFP | bacteriostatic, UV resistant | E. coli, S. aureus | tomato, peach preservation | [92] |
| Ag-MOFs@CS suspension liquid | bacteriostatic | E. coli, S. aureus | strawberry, pitaya preservation | [115] |
| PVA/starch/CS/Ag@MOF complex film | bacteriostatic, waterproof, UV resistant, antioxidative, isolation oil | E. coli, S. aureus | food packaging | [98] |
| Ag-MOFs@CMFP | bacteriostatic, humidity response, quality monitoring | E. coli, S. aureus, A. niger | strawberry, blueberry, plum preservation | [83] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ding, X.; Lin, H.; Zhou, J.; Lin, Z.; Huang, Y.; Chen, G.; Zhang, Y.; Lv, J.; Chen, J.; Liu, G.; et al. Silver Nanocomposites with Enhanced Shelf-Life for Fruit and Vegetable Preservation: Mechanisms, Advances, and Prospects. Nanomaterials 2024, 14, 1244. https://doi.org/10.3390/nano14151244
Ding X, Lin H, Zhou J, Lin Z, Huang Y, Chen G, Zhang Y, Lv J, Chen J, Liu G, et al. Silver Nanocomposites with Enhanced Shelf-Life for Fruit and Vegetable Preservation: Mechanisms, Advances, and Prospects. Nanomaterials. 2024; 14(15):1244. https://doi.org/10.3390/nano14151244
Chicago/Turabian StyleDing, Xin, Huan Lin, Jie Zhou, Zhihao Lin, Yanyan Huang, Ge Chen, Yanguo Zhang, Jun Lv, Jing Chen, Guangyang Liu, and et al. 2024. "Silver Nanocomposites with Enhanced Shelf-Life for Fruit and Vegetable Preservation: Mechanisms, Advances, and Prospects" Nanomaterials 14, no. 15: 1244. https://doi.org/10.3390/nano14151244






