Fluorescent Aromatic Polyether Sulfones: Processable, Scalable, Efficient, and Stable Polymer Emitters and Their Single-Layer Polymer Light-Emitting Diodes
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Instrumentation
3. Results
3.1. Synthesis and Characterization of Monomers
3.2. Synthesis and Characterization of Polymers
3.3. Optical and Electrochemical Characterizations
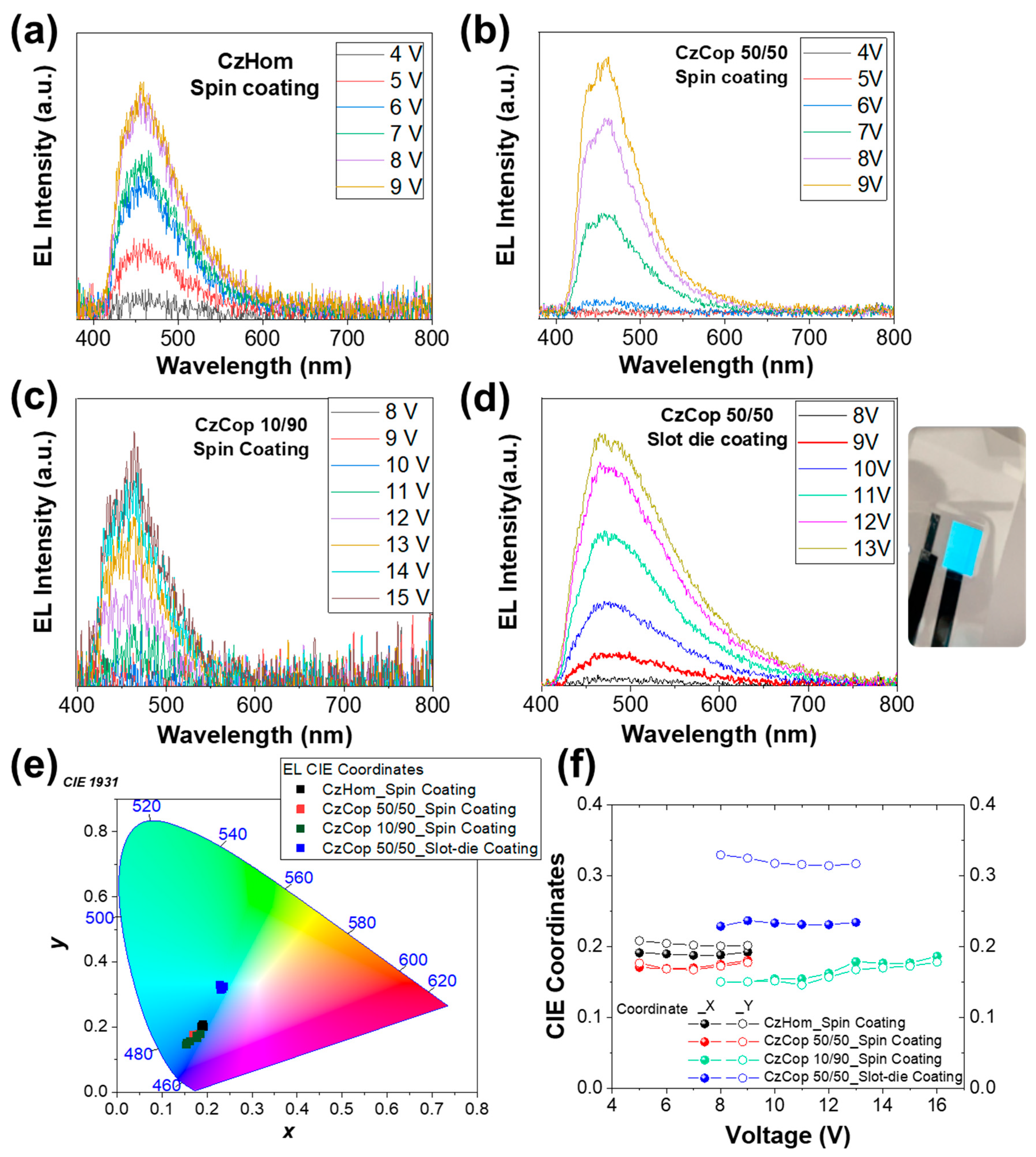
3.4. Device Fabrication and Electroluminescence Characterization
3.4.1. Blue and Yellow PLEDs
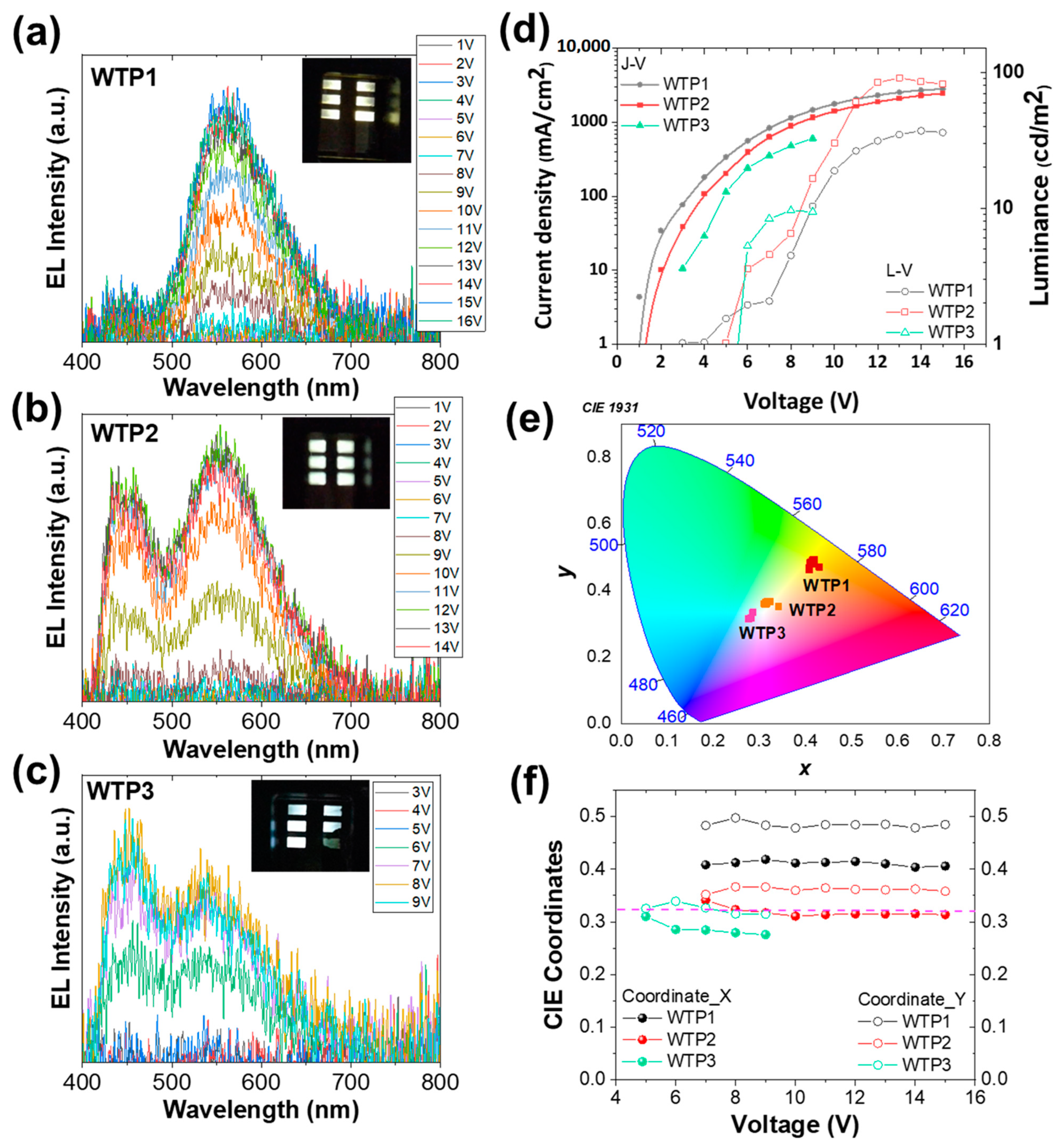
3.4.2. White PLEDs
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zou, S.J.; Shen, Y.; Xie, F.M.; Chen, J.D.E.; Li, Y.Q.; Tang, J.X. Recent Advances in Organic Light-Emitting Diodes: Toward Smart Lighting and Displays. Mater. Chem. Front. 2020, 4, 788–820. [Google Scholar] [CrossRef]
- Andreopoulou, A.K.; Gioti, M.; Kallitsis, J.K. Organic Light-emitting Diodes Based on Solution-Processable Organic Materials. In Solution-Processable Components for Organic Electronic Devices; Ulanski, J., Luszczynska, M., Matyjaszewski, K., Eds.; Wiley-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2019; pp. 413–482. [Google Scholar] [CrossRef]
- Pode, R. Organic Light Emitting Diode Devices: An Energy Efficient Solid State Lighting for Applications. Renew. Sustain. Energy Rev. 2020, 133, 110043. [Google Scholar] [CrossRef]
- Eccher, J.; Zajaczkowski, W.; Faria, G.C.; Bock, H.; Von Seggern, H.; Pisula, W.; Bechtold, I.H. Thermal Evaporation versus Spin-Coating: Electrical Performance in Columnar Liquid Crystal OLEDs. ACS Appl. Mater. Interfaces 2015, 7, 16374–16381. [Google Scholar] [CrossRef] [PubMed]
- Müller, C.D.; Falcou, A.; Reckefuss, N.; Rojahn, M.; Wiederhirn, V.; Rudati, P.; Frohne, H.; Nuyken, O.; Becker, H.; Meerholz, K. Multi-Colour Organic Light-Emitting by Solution Processing. Nature 2003, 421, 829–833. [Google Scholar] [CrossRef] [PubMed]
- Zeng, X.Y.; Tang, Y.Q.; Cai, X.Y.; Tang, J.X.; Li, Y.Q. Solution-Processed OLEDs for Printing Displays. Mater. Chem. Front. 2023, 7, 1166–1196. [Google Scholar] [CrossRef]
- Auer-Berger, M.; Trattnig, R.; Qin, T.; Schlesinger, R.; Nardi, M.V.; Ligorio, G.; Christodoulou, C.; Koch, N.; Baumgarten, M.; Müllen, K.; et al. All-Solution-Processed Multilayer Polymer/Dendrimer Light Emitting Diodes. Org. Electron. 2016, 35, 164–170. [Google Scholar] [CrossRef]
- Merklein, L.; Mink, M.; Kourkoulos, D.; Ulber, B.; Raupp, S.M.; Meerholz, K.; Scharfer, P.; Schabel, W. Multilayer OLEDs with Four Slot Die-Coated Layers. J. Coat. Technol. Res. 2019, 16, 1643–1652. [Google Scholar] [CrossRef]
- Kinner, L.; List-Kratochvil, E.J.W.; Dimopoulos, T. Gentle Plasma Process for Embedded Silver-Nanowire Flexible Transparent Electrodes on Temperature-Sensitive Polymer Substrates. Nanotechnology 2020, 31, 365303. [Google Scholar] [CrossRef] [PubMed]
- Gioti, M. Optical, Photophysical, and Electrooptical Studies on Slot-Die Polyfluorene-Based Flexible OLED Devices. Opt. Mater. Express 2021, 11, 1442. [Google Scholar] [CrossRef]
- Amruth, C.; Szymański, M.Z.; Łuszczyńska, B.; Ulański, J. Inkjet Printing of Super Yellow: Ink Formulation, Film Optimization, OLEDs Fabrication, and Transient Electroluminescence. Sci. Rep. 2019, 9, 8493. [Google Scholar] [CrossRef]
- Hermerschmidt, F.; Choulis, S.A.; List-Kratochvil, E.J.W. Implementing Inkjet-Printed Transparent Conductive Electrodes in Solution-Processed Organic Electronics. In Advanced Materials Technologies; Wiley-Blackwell: Hoboken, NJ, USA, 2019; Volume 4. [Google Scholar] [CrossRef]
- Grimsdale, A.C.; Chan, K.L.; Martin, R.E.; Jokisz, P.G.; Holmes, A.B. Synthesis of Light-Emitting Conjugated Polymers for Applications in Electroluminescent Devices. Chem. Rev. 2009, 109, 897–1091. [Google Scholar] [CrossRef]
- Kim, D.Y.; Cho, H.N.; Kim, C.Y. Blue Light Emitting Polymers. Prog. Polym. Sci. 2000, 25, 1089–1139. [Google Scholar] [CrossRef]
- Lin, H.Y.; Sher, C.W.; Lin, C.H.; Tu, H.H.; Chen, X.Y.; Lai, Y.C.; Lin, C.C.; Chen, H.M.; Yu, P.; Meng, H.F.; et al. Fabrication of Flexible White Light-Emitting Diodes from Photoluminescent Polymer Materials with Excellent Color Quality. ACS Appl. Mater. Interfaces 2017, 9, 35279–35286. [Google Scholar] [CrossRef] [PubMed]
- Jiang, D.H.; Ree, B.J.; Isono, T.; Xia, X.C.; Hsu, L.C.; Kobayashi, S.; Hoon Ngoi, K.; Chen, W.C.; Jao, C.C.; Veeramuthu, L.; et al. Facile One-Pot Synthesis of Rod-Coil Bio-Block Copolymers and Uncovering Their Role in Forming the Efficient Stretchable Touch-Responsive Light Emitting Diodes. Chem. Eng. J. 2021, 418, 129421. [Google Scholar] [CrossRef]
- Jou, J.H.; Sahoo, S.; Dubey, D.K.; Yadav RA, K.; Swayamprabha, S.S.; Chavhan, S.D. Molecule-Based Monochromatic and Polychromatic OLEDs with Wet-Process Feasibility. J. Mater. Chem. C Mater. 2018, 6, 11492–11518. [Google Scholar] [CrossRef]
- Müllen, K.; Scherf, U. Conjugated Polymers: Where We Come From, Where We Stand, and Where We Might Go. Macromol. Chem. Phys. 2023, 224, 2200337. [Google Scholar] [CrossRef]
- Han, Y.; Bai, L.; Lin, J.; Ding, X.; Xie, L.; Huang, W. Diarylfluorene-Based Organic Semiconductor Materials toward Optoelectronic Applications. Adv. Funct. Mater. 2021, 31, 2105092. [Google Scholar] [CrossRef]
- Li, H.; Yu, M.; Gu, J.; Bao, Q.; Wang, Y.; Li, Y.; Ma, Y.; Bai, L.; Zhuo, Z.; Zhang, Y.; et al. Intrinsically Flexible and Aging Resistant Fluorene-Based Rod-Coil Copolymer for Bendable Deep-Blue PLEDs. Adv. Funct. Mater. 2023, 33, 2303947. [Google Scholar] [CrossRef]
- Burns, S.; Macleod, J.; Trang Do, T.; Sonar, P.; Yambem, S.D. Effect of Thermal Annealing Super Yellow Emissive Layer on Efficiency of OLEDs. Sci. Rep. 2017, 7, 40805. [Google Scholar] [CrossRef]
- Karl, M.; Glackin JM, E.; Schubert, M.; Kronenberg, N.M.; Turnbull, G.A.; Samuel ID, W.; Gather, M.C. Flexible and Ultra-Lightweight Polymer Membrane Lasers. Nat. Commun. 2018, 9, 1525. [Google Scholar] [CrossRef]
- Hengge, M.; Hänsch, P.; Ehjeij, D.; Benneckendorf, F.S.; Freudenberg, J.; Bunz, U.H.F.; Müllen, K.; List-Kratochvil, E.J.W.; Hermerschmidt, F. Crosslinking Super Yellow to Produce Super OLEDs: Crosslinking with Azides Enables Improved Performance. J. Polym. Sci. 2022, 60, 1878–1886. [Google Scholar] [CrossRef]
- Wang, T.; Li, K.; Yao, B.; Chen, Y.; Zhan, H.; Xie, G.; Xie, Z.; Cheng, Y. Effect of Molecular Weight on Photoluminescence and Electroluminescence Properties of Thermally Activated Delayed Fluorescence Conjugated Polymers. Chem. Eng. J. 2023, 452, 139123. [Google Scholar] [CrossRef]
- Li, C.; Li, Z.; Liang, J.; Luo, H.; Liu, Y.; Wei, J.; Wang, Y. A Twisted Phenanthroimidazole Based Molecule with High Triplet Energy as a Host Material for High Efficiency Phosphorescent OLEDs. J. Mater. Chem. C Mater. 2018, 6, 12888–12895. [Google Scholar] [CrossRef]
- Li, Z.; Cheng, Z.; Lin, J.; Xie, N.; Li, C.; Yang, G.; Wang, Y. Isomer Dependent Molecular Packing and Carrier Mobility of: N -Phenylcarbazole-Phenanthro [9,10-d] Imidazole Based Materials as Hosts for Efficient Electrophosphorescence Devices. J. Mater. Chem. C Mater. 2019, 7, 13486–13492. [Google Scholar] [CrossRef]
- Shao, S.Y.; Ding, J.Q.; Wang, L.X. New Applications of Poly(Arylene Ether)s in Organic Light-Emitting Diodes. Chin. Chem. Lett. 2016, 27, 1201–1208. [Google Scholar] [CrossRef]
- Shao, S.; Ding, J.; Wang, L.; Jing, X.; Wang, F. Highly Efficient Blue Electrophosphorescent Polymers with Fluorinated Poly(Arylene Ether Phosphine Oxide) as Backbone. J. Am. Chem. Soc. 2012, 134, 15189–15192. [Google Scholar] [CrossRef] [PubMed]
- Chen, T.; Chen, Z.; Ni, F.; Xie, G.; Yang, C. Sky-Blue Thermally Activated Delayed Fluorescence Polymers by Using a Conjugation-Confined Poly(Aryl Ether) Main Chain. Polym. Chem. 2021, 12, 2490–2497. [Google Scholar] [CrossRef]
- Gioti, M.; Kokkinos, D.; Stavrou, K.; Simitzi, K.; Andreopoulou, A.; Laskarakis, A.; Kallitsis, J.; Logothetidis, S. Fabrication and Study of White-Light OLEDs Based on Novel Copolymers with Blue, Yellow, and Red Chromophores. Phys. Status Solidi-Rapid Res. Lett. 2019, 13, 1800419. [Google Scholar] [CrossRef]
- Tselekidou, D.; Papadopoulos, K.; Kyriazopoulos, V.; Andrikopoulos, K.C.; Andreopoulou, A.K.; Kallitsis, J.K.; Laskarakis, A.; Logothetidis, S.; Gioti, M. Photophysical and Electro-optical Properties of Copolymers Bearing Blue and Red Chromophores for Single-layer White Oleds. Nanomaterials 2021, 11, 2629. [Google Scholar] [CrossRef] [PubMed]
- Gioti, M.; Kokkinos, D.; Chaidou, C.I.; Laskarakis, A.; Andreopoulou, A.K.; Kallitsis, J.K.; Logothetidis, S. A Comprehensive Study of the Optical Properties of Emitting Polymers for Efficient Flexible OLED Devices. Phys. Status Solidi (A) Appl. Mater. Sci. 2016, 213, 2947–2953. [Google Scholar] [CrossRef]
- Sasabe, H.; Seino, Y.; Kimura, M.; Kido, J. A m -Terphenyl-Modifed Sulfone Derivative as a Host Material for High-Efficiency Blue and Green Phosphorescent OLEDs. Chem. Mater. 2012, 24, 1404–1406. [Google Scholar] [CrossRef]
- Li, H.; Hong, M.; Scarpaci, A.; He, X.; Risko, C.; Sears, J.S.; Barlow, S.; Winget, P.; Marder, S.R.; Kim, D.; et al. Chemical Stabilities of the Lowest Triplet State in Aryl Sulfones and Aryl Phosphine Oxides Relevant to OLED Applications. Chem. Mater. 2019, 31, 1507–1519. [Google Scholar] [CrossRef]
- Xiang, H.; Wang, R.; Chen, J.; Li, F.; Zeng, H. Research Progress of Full Electroluminescent White Light-Emitting Diodes Based on a Single Emissive Layer. Light Sci. Appl. 2021, 10, 206. [Google Scholar] [CrossRef] [PubMed]
- Yin, Y.; Ali, M.U.; Xie, W.; Yang, H.; Meng, H. Evolution of White Organic Light-Emitting Devices: From Academic Research to Lighting and Display Applications. Mater. Chem. Front. 2019, 3, 970–1031. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, K.; Dai, F.; Zhang, K.; Tang, H.; Wang, L.; Xing, J. A Warm-White Light-Emitting Diode Based on Single-Component Emitter Aromatic Carbon Nitride. Nat. Commun. 2022, 13, 6495. [Google Scholar] [CrossRef] [PubMed]
- Andrikopoulos, K.; Anastasopoulos, C.; Kallitsis, J.K.; Andreopoulou, A.K. Bis-Tridendate Ir(III) Polymer-Metallocomplexes: Hybrid, Main-Chain Polymer Phosphors for Orange–Red Light Emission. Polymers 2020, 12, 2976. [Google Scholar] [CrossRef] [PubMed]
- Jones, S.; Atherton, J.C.C. An Improved Procedure for the Preparation of 9,10-Dibromoanthracene. Synth. Commun. 2001, 31, 1799–1802. [Google Scholar] [CrossRef]
- Sariciftci, N.; Smilowitz, L.; Heeger, A.J.; Wudl, F. Photoinduced Electron Transfer from a Conducting Polymer to Buckminsterfullerene. Science (1979) 1992, 258, 1474–1476. [Google Scholar] [CrossRef] [PubMed]
- Raj, M.R.; Anandan, S.; Solomon, R.V.; Venuvanalingam, P.; Iyer, S.S.K.; Ashokkumar, M. Synthesis of Conjugated Perylene Diimide-Based Copolymer with 5,5′-Bis(4-Aminophenyl)-2-2′-Bifuryl Moiety as an Active Material for Organic Photovoltaics. J. Photochem. Photobiol. A Chem. 2012, 247, 52–62. [Google Scholar] [CrossRef]
- Liu, Q.; Chavhan, S.; Zhang, H.; Sun, H.; Brock, A.J.; Manzhos, S.; Chen, Y.; Feron, K.; Bottle, S.E.; McMurtrie, J.C.; et al. Short Alkyl Chain Engineering Modulation on Naphthalene Flanked Diketopyrrolopyrrole toward High-Performance Single Crystal Transistors and Organic Thin Film Displays. Adv. Electron. Mater. 2021, 7, 2000804. [Google Scholar] [CrossRef]
- Samanta, S.K.; Song, I.; Yoo, J.H.; Oh, J.H. Organic N-Channel Transistors Based on [1]Benzothieno [3,2-b]Benzothiophene-Rylene Diimide Donor-Acceptor Conjugated Polymers. ACS Appl. Mater. Interfaces 2018, 10, 32444–32453. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Gorohmaru, H.; Kadowaki, M.; Kobayashi, T.; Ishi-i, T.; Thlemann, T.; Mataka, S. Benzo-2,1,3-Thiadiazole-Based, Highly Dichroic Fluorescent Dyes for Fluorescent Host-Guest Liquid Crystal Displays. J. Mater. Chem. 2004, 14, 1901–1904. [Google Scholar] [CrossRef]
- Davis, A. Thermal Stability of Polysulphone. Die Makromol. Chem. 1969, 128, 242–251. [Google Scholar] [CrossRef]
- Lee, J.H.; Chen, C.H.; Lee, P.H.; Lin, H.Y.; Leung, M.K.; Chiu, T.L.; Lin, C.F. Blue Organic Light-Emitting Diodes: Current Status, Challenges, and Future Outlook. J. Mater. Chem. C Mater. 2019, 7, 5874–5888. [Google Scholar] [CrossRef]
- Das, D.; Gopikrishna, P.; Singh, A.; Dey, A.; Iyer, P.K. Efficient Blue and White Polymer Light Emitting Diodes Based on a Well Charge Balanced, Core Modified Polyfluorene Derivative. Phys. Chem. Chem. Phys. 2016, 18, 7389–7394. [Google Scholar] [CrossRef]
- Gather, M.C.; Alle, R.; Becker, H.; Meerholz, K. On the Origin of the Color Shift in White-Emitting OLEDs. Adv. Mater. 2007, 19, 4460–4465. [Google Scholar] [CrossRef]
- Huang, C.W.; Lin, T.A.; Lee, W.K.; Lu, C.H.; Chatterjee, T.; Chou, C.H.; Wong, K.T.; Wu, C.C. Analyses of Emission Efficiencies of White Organic Light-Emitting Diodes Having Multiple Emitters in Single Emitting Layer. Org. Electron. 2022, 104, 106474. [Google Scholar] [CrossRef]
- Chen, Z.; Ho, C.L.; Wang, L.; Wong, W.Y. Single-Molecular White-Light Emitters and Their Potential WOLED Applications. Adv. Mater. 2020, 32, 1903269. [Google Scholar] [CrossRef] [PubMed]
- Guo, T.-F.; Wen, T.-C.; Huang, Y.-S.; Lin, M.-W.; Tsou, C.-C.; Chung, C.-T. White-Emissive Tandem-Type Hybrid Organic/Polymer Diodes with (0.33, 0.33) Chromaticity Coordinates. Opt. Express 2009, 17, 21205–21215. [Google Scholar] [CrossRef] [PubMed]
- Dubey, D.K.; Sahoo, S.; Wang, C.W.; Jou, J.H. Solution Process Feasible Highly Efficient White Organic Light Emitting Diode. Org. Electron. 2019, 69, 232–240. [Google Scholar] [CrossRef]
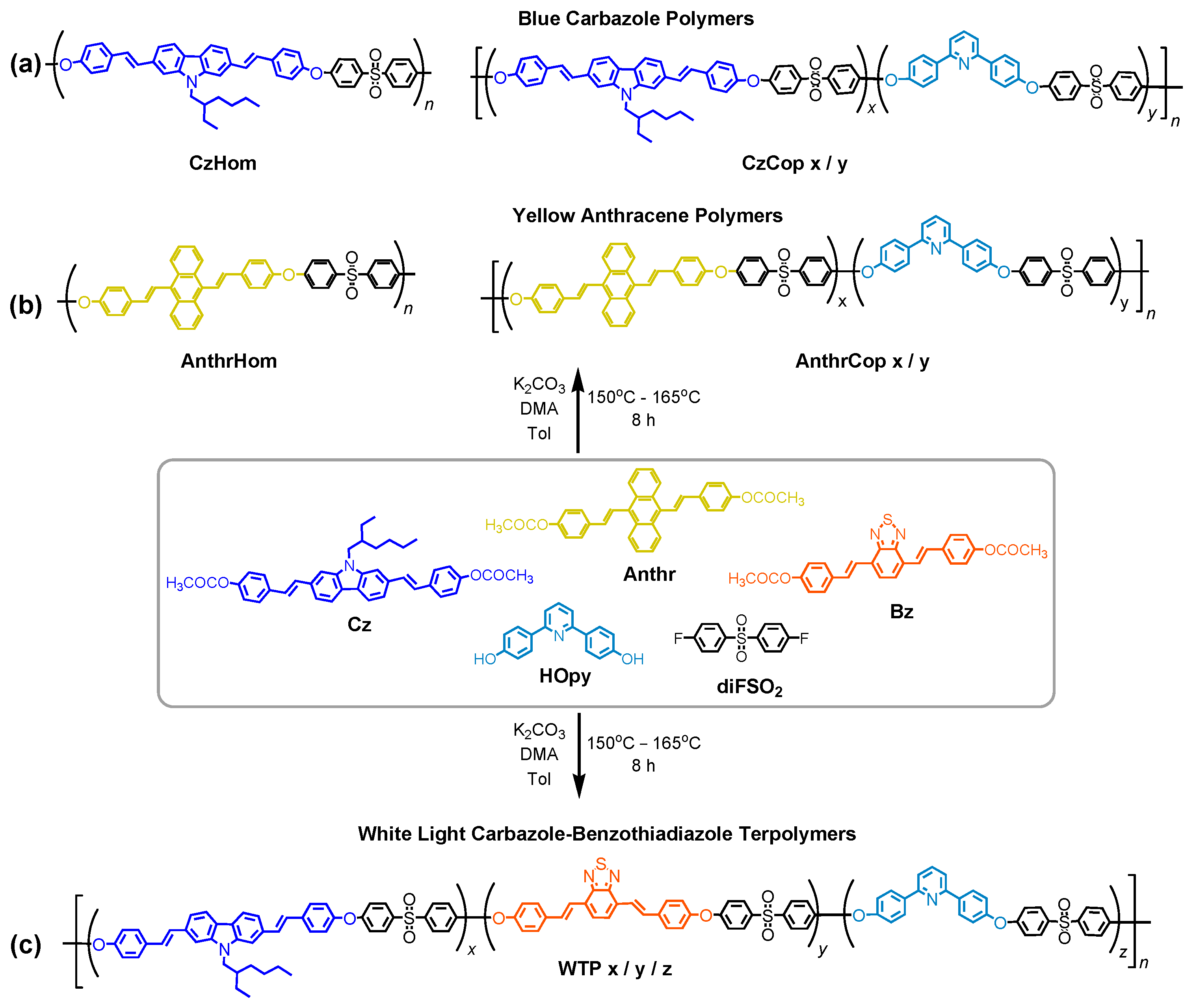
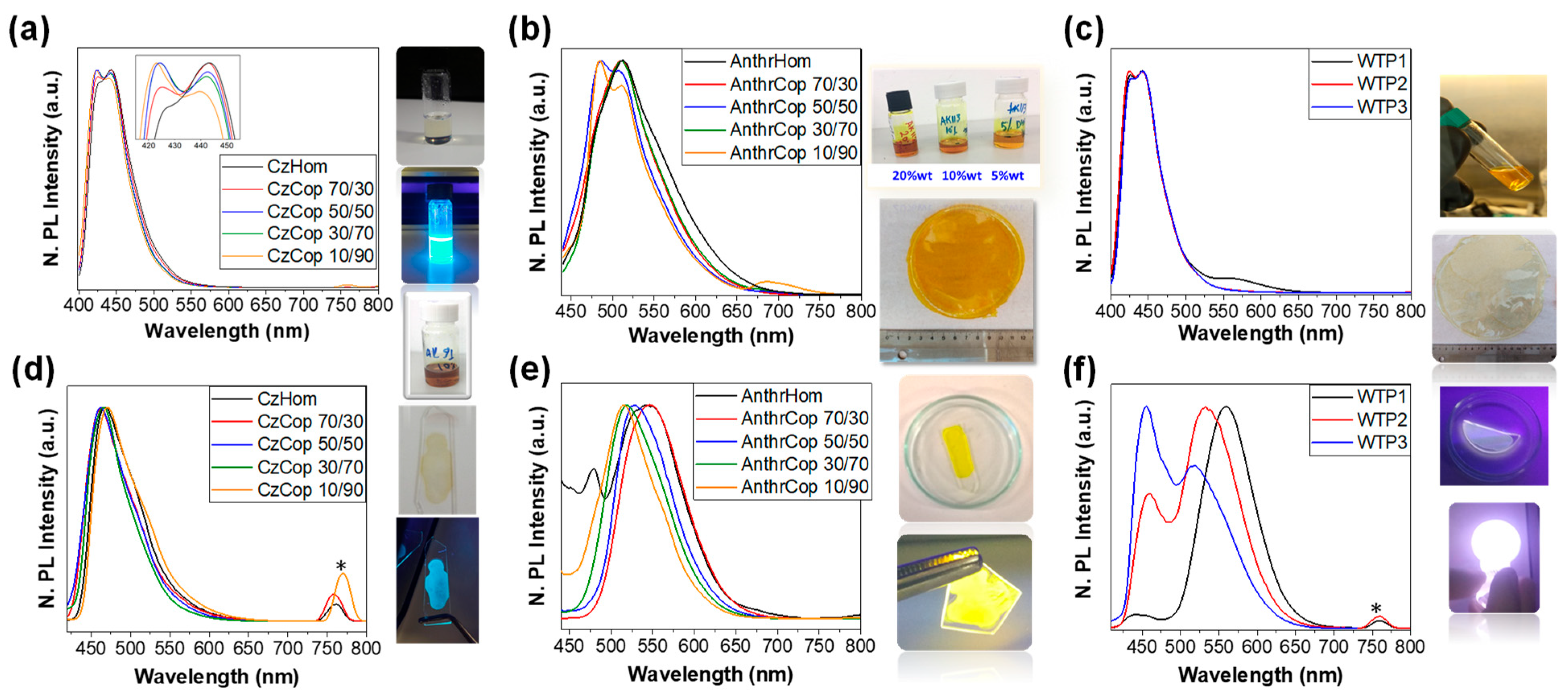
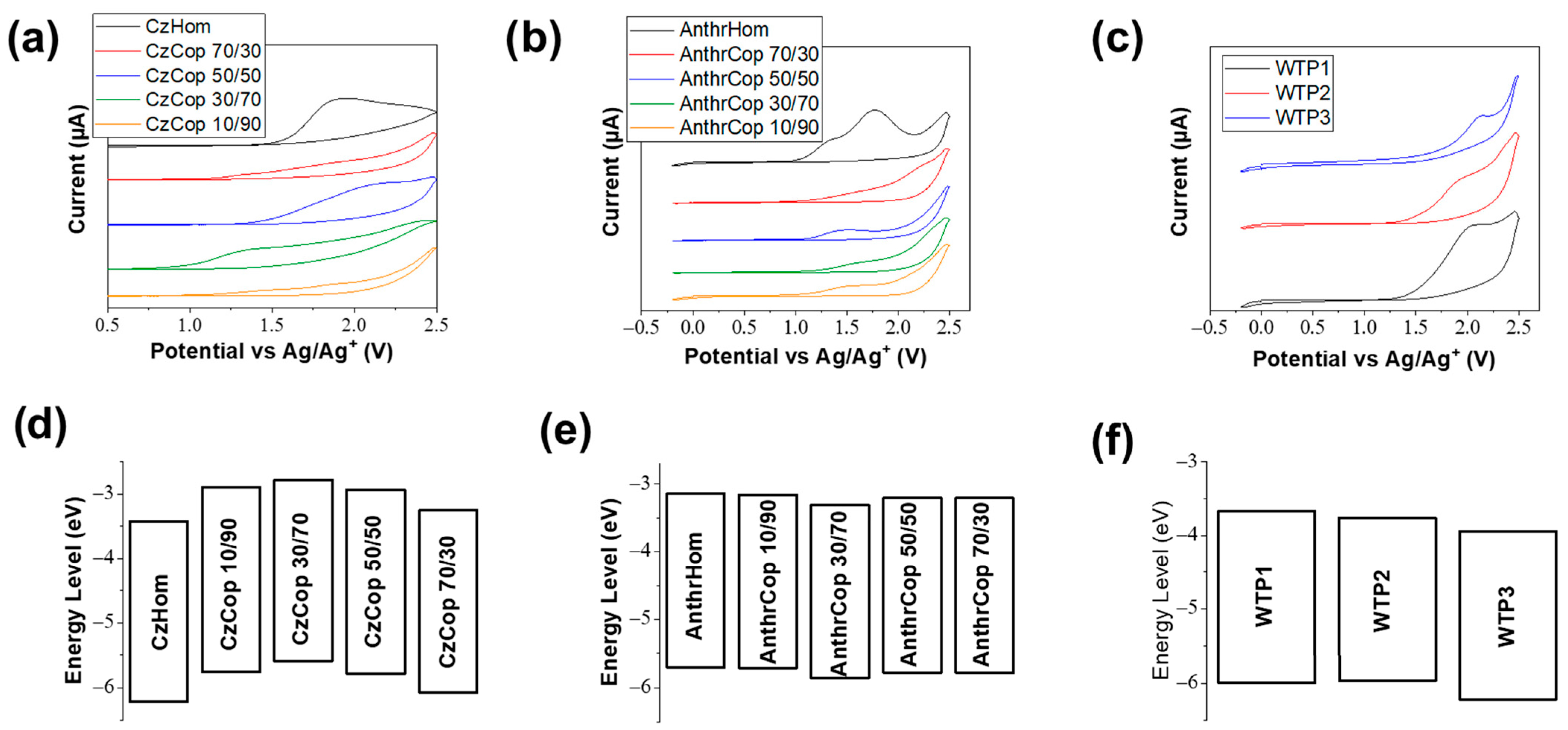

| Code | Chromophore x (%) | Hopy y (%) | Mp a, Mn b (kDa) | Disp c | Quantity |
|---|---|---|---|---|---|
| Carbazole (Cz)-bearing polyethersulfones | |||||
| CzHom | 100 | 0 | - | - | 3 |
| CzCop 70/30 | 70 | 30 | 16.20, 7.00 | 2.50 | 1 |
| CzCop 50/50 | 50 | 50 | 63.30, 45.50 | 1.50 | 10 |
| CzCop 30/70 | 30 | 70 | 92.00, 59.40 | 1.80 | 2 |
| CzCop 10/90 | 10 | 90 | 92.40, 67.70 | 1.60 | 1 |
| Anthracene (Anthr)-bearing polyethersulfones | |||||
| AnthrHom | 100 | 0 | 21.80, 17.20 | 1.46 | 3 |
| AnthrCop 70/30 | 70 | 30 | 52.10, 33.80 | 1.65 | 1.7 |
| AnthrCop 50/50 | 50 | 50 | 101.40. 67.80 | 2.15 | 25 |
| AnthrCop 30/70 | 30 | 70 | 99.80, 60.10 | 1.95 | 1 |
| AnthrCop 10/90 | 10 | 90 | 90.20, 70.10 | 1.60 | 1 |
| Cz x (%) | Bz y (%) | HOpy z (%) | Mp a, Mn b (kDa) | Disp c | Quantity | |
|---|---|---|---|---|---|---|
| WTP1 | 45 | 5 | 50 | 75.00, 49.80 | 1.80 | 5 |
| WTP2 | 49.5 | 0.5 | 50 | 104.30, 78.80 | 1.60 | 10 |
| WTP3 | 49.75 | 0.25 | 50 | 95.00, 69.00 | 1.56 | 5 |
| Code | (nm) | Von (V) | Luminance (cd/m2) |
|---|---|---|---|
| CzHom_Spin Coating | 457 | 4.8 | 13 |
| CzCop 50/50_Spin Coating | 456 | 3.4 | 29 |
| CzCop 10/90_Spin Coating | 456 | 11 | 91 |
| CzCop 50/50_Slot-die Coating | 466 | 8 | 128 |
| AnthrHom_Spin coating | 560 | 4 | 88 |
| AnthrCop 70/30_Spin coating | 567 | 5 | 415 |
| AnthrCop 50/50_Spin coating | 573 | 6 | 328 |
| AnthrCop 50/50_Slot-die coating | 577 | 7 | 333 |
| Code | Von (V) | Luminance (cd/m2) | CRI | CCT (K) |
|---|---|---|---|---|
| WTP1 | 4 | 37 | 60 | 4063 |
| WTP2 | 5 | 92 | 70 | 5498 |
| WTP3 | 5.5 | 10 | 83 | 8000 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Andrikopoulos, K.C.; Tselekidou, D.; Anastasopoulos, C.; Papadopoulos, K.; Kyriazopoulos, V.; Logothetidis, S.; Kallitsis, J.K.; Gioti, M.; Andreopoulou, A.K. Fluorescent Aromatic Polyether Sulfones: Processable, Scalable, Efficient, and Stable Polymer Emitters and Their Single-Layer Polymer Light-Emitting Diodes. Nanomaterials 2024, 14, 1246. https://doi.org/10.3390/nano14151246
Andrikopoulos KC, Tselekidou D, Anastasopoulos C, Papadopoulos K, Kyriazopoulos V, Logothetidis S, Kallitsis JK, Gioti M, Andreopoulou AK. Fluorescent Aromatic Polyether Sulfones: Processable, Scalable, Efficient, and Stable Polymer Emitters and Their Single-Layer Polymer Light-Emitting Diodes. Nanomaterials. 2024; 14(15):1246. https://doi.org/10.3390/nano14151246
Chicago/Turabian StyleAndrikopoulos, Konstantinos C., Despoina Tselekidou, Charalampos Anastasopoulos, Kyparisis Papadopoulos, Vasileios Kyriazopoulos, Stergios Logothetidis, Joannis K. Kallitsis, Maria Gioti, and Aikaterini K. Andreopoulou. 2024. "Fluorescent Aromatic Polyether Sulfones: Processable, Scalable, Efficient, and Stable Polymer Emitters and Their Single-Layer Polymer Light-Emitting Diodes" Nanomaterials 14, no. 15: 1246. https://doi.org/10.3390/nano14151246





