Carbon Dots in Photodynamic/Photothermal Antimicrobial Therapy
Abstract
1. Introduction
2. Principles of PDT/PTT for Antimicrobial Therapy
3. Photosensitizers
3.1. Methylene Blue
3.2. Porphyrin-Based Photosensitizers
3.3. Phthalocyanines
3.4. Natural Dyes
3.5. Fullerenes
3.6. Up-Conversion Nanoparticles and Nanoplatforms
4. Carbon Dots
4.1. Photosensitizing Properties of Carbon Dots
4.2. General Synthetic Methods of Carbon Dots
| Approaches | Synthetic Method | Advantages | Disadvantages | Ref. |
|---|---|---|---|---|
| Top-down | Laser ablation | Simple and easy to use The size of the CDs can be controlled via adjusting the input laser pulses | Low QY (4.5–18%) | [127] |
| Arc discharge | Obtained as by-product from the purification of carbon nanotubes | Low QY (2.3–8.7%) Cumbersome to purify | [128] | |
| Chemical oxidation | Easy to control the dimension of the CDs by regulating oxidation time and temperature Simple to setup and easy to operate | Time consuming (up to 12 h) Needs further modification | [129] | |
| Electrochemical oxidation | Economical and environmentally friendly | Time consuming | [130] | |
| Bottom-up | Hydrothermal | Carbon source could be obtained from natural resources or biomass Low-cost apparatus setup Easy to control parameters Large-scale synthesis can be achieved | Product contains impurities such as larger and undissolved particles | [131] |
| Combustion | Easy to operate Carbon source could be easily obtained | Low QY (about 1.6%) Broad particle size distribution | [132] | |
| Microwave irradiation | Rapid and controllable heating | Limitation of large-scale reactions because of small volumes of microwave reactors | [133] | |
| Pyrolysis | Easy and quick to operate and react | Broad particle size distribution High temperature (180–450 °C) | [134] |
5. Carbon Dots Used for PDT/PTT Antimicrobial Therapy
5.1. Carbon Dots Photosensitizer
5.1.1. Singular Nanosystem
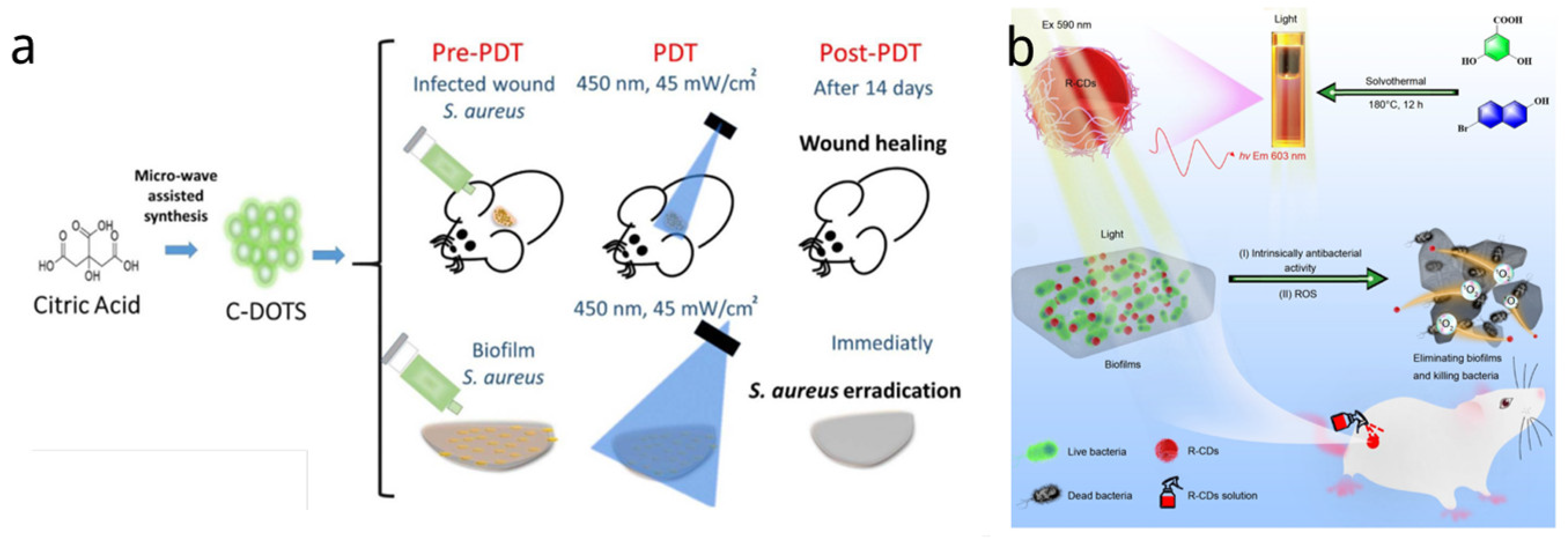
5.1.2. Nanocomposite
5.2. CDs as Booster in Combination with Other Photosensitizing Agents
6. Conclusions and Future Perspectives
Funding
Data Availability Statement
Conflicts of Interest
References
- Salam, M.A.; Al-Amin, M.Y.; Salam, M.T.; Pawar, J.S.; Akhter, N.; Rabaan, A.A.; Alqumber, M.A.A. Antimicrobial Resistance: A Growing Serious Threat for Global Public Health. Healthcare 2023, 11, 1946. [Google Scholar] [CrossRef] [PubMed]
- Aslam, B.; Wang, W.; Arshad, M.I.; Khurshid, M.; Muzammil, S.; Rasool, M.H.; Nisar, M.A.; Alvi, R.F.; Aslam, M.A.; Qamar, M.U.; et al. Antibiotic resistance: A rundown of a global crisis. Infect. Drug Resist. 2018, 11, 1645–1658. [Google Scholar] [CrossRef] [PubMed]
- Celli, J.P.; Spring, B.Q.; Rizvi, I.; Evans, C.L.; Samkoe, K.S.; Verma, S.; Pogue, B.W.; Hasan, T. Imaging and photodynamic therapy: Mechanisms, monitoring, and optimization. Chem. Rev. 2010, 110, 2795–2838. [Google Scholar] [CrossRef] [PubMed]
- Xie, Z.; Fan, T.; An, J.; Choi, W.; Duo, Y.; Ge, Y.; Zhang, B.; Nie, G.; Xie, N.; Zheng, T.; et al. Emerging combination strategies with phototherapy in cancer nanomedicine. Chem. Soc. Rev. 2020, 49, 8065–8087. [Google Scholar] [CrossRef]
- Pham, T.C.; Nguyen, V.-N.; Choi, Y.; Lee, S.; Yoon, J. Recent Strategies to Develop Innovative Photosensitizers for Enhanced Photodynamic Therapy. Chem. Rev. 2021, 121, 13454–13619. [Google Scholar] [CrossRef] [PubMed]
- Kwon, N.; Kim, H.; Li, X.; Yoon, J. Supramolecular agents for combination of photodynamic therapy and other treatments. Chem. Sci. 2021, 12, 7248–7268. [Google Scholar] [CrossRef] [PubMed]
- Abrahamse, H.; Hamblin, M.R. New photosensitizers for photodynamic therapy. Biochem. J. 2016, 473, 347–364. [Google Scholar] [CrossRef]
- Chizenga, E.P.; Abrahamse, H. Nanotechnology in Modern Photodynamic Therapy of Cancer: A Review of Cellular Resistance Patterns Affecting the Therapeutic Response. Pharmaceutics 2020, 12, 632. [Google Scholar] [CrossRef] [PubMed]
- Escudero, A.; Carrillo-Carrión, C.; Castillejos, M.C.; Romero-Ben, E.; Rosales-Barrios, C.; Khiar, N. Photodynamic therapy: Photosensitizers and nanostructures. Mater. Chem. Front. 2021, 5, 3788–3812. [Google Scholar] [CrossRef]
- Hamblin, M.R.; Chiang, L.Y.; Lakshmanan, S.; Huang, Y.Y.; Garcia-Diaz, M.; Karimi, M.; de Souza Rastelli, A.N.; Chandran, R. Nanotechnology for photodynamic therapy: A perspective from the Laboratory of Dr. Michael R. Hamblin in the Wellman Center for Photomedicine at Massachusetts General Hospital and Harvard Medical School. Nanotechnol. Rev. 2015, 4, 359–372. [Google Scholar] [CrossRef]
- Zarrintaj, P.; Ahmadi, Z.; Hosseinnezhad, M.; Saeb, M.R.; Laheurte, P.; Mozafari, M. Photosensitizers in medicine: Does nanotechnology make a difference? Mater. Today Proc. 2018, 5, 15836–15844. [Google Scholar] [CrossRef]
- Vivero-Escoto, J.L.; Vega, D.L. Target-specific porphyrin-loaded hybrid nanoparticles to improve photodynamic therapy for cancer treatment. In Proceedings of the Society of Photo-Optical Instrumentation Engineers (SPIE) Conference Series, San Francisco, CA, USA, 1 February 2017; Kessel, D.H., Hasan, T., Eds.; p. 1004713. [Google Scholar]
- Yang, M.; Wang, H.; Wang, Z.; Han, Z.; Gu, Y. A Nd3+ sensitized upconversion nanosystem with dual photosensitizers for improving photodynamic therapy efficacy. Biomater. Sci. 2019, 7, 1686–1695. [Google Scholar] [CrossRef] [PubMed]
- Jiao, Q.; Zheng, Y.; Xie, Q.; Luo, X.; Zhou, S.; Pei, S.; Zhang, T.; Wu, X.; Xu, K.; Zhong, W. A Dual-Responsive Morphologically-Adaptable Nanoplatform for Targeted Delivery of Activatable Photosensitizers in Precision Photodynamic Therapy. Small 2023, 20, e2309054. [Google Scholar] [CrossRef] [PubMed]
- Wan, S.S.; Tao, J.; Wu, Q.; Liu, W.R.; Ding, X.G.; Zhang, X.Z. Size-Controllable Nanosystem with Double Responsive for Deep Photodynamic Therapy. Pharmaceutics 2023, 15, 940. [Google Scholar] [CrossRef] [PubMed]
- Matlou, G.G.; Abrahamse, H. Nanoscale metal-organic frameworks as photosensitizers and nanocarriers in photodynamic therapy. Front. Chem. 2022, 10, 971747. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Shen, X.; Xu, Q.-H.; Cao, Y. Gold nanorod enhanced conjugated polymer/photosensitizer composite nanoparticles for simultaneous two-photon excitation fluorescence imaging and photodynamic therapy. Nanoscale 2019, 11, 19551–19560. [Google Scholar] [CrossRef] [PubMed]
- Kirar, S.; Thakur, N.S.; Laha, J.K.; Banerjee, U.C. Porphyrin Functionalized Gelatin Nanoparticle-Based Biodegradable Phototheranostics: Potential Tools for Antimicrobial Photodynamic Therapy. ACS Appl. Bio Mater. 2019, 2, 4202–4212. [Google Scholar] [CrossRef]
- Reddy, Y.N.; De, A.; Paul, S.; Pujari, A.K.; Bhaumik, J. In Situ Nanoarchitectonics of a MOF Hydrogel: A Self-Adhesive and pH-Responsive Smart Platform for Phototherapeutic Delivery. Biomacromolecules 2023, 24, 1717–1730. [Google Scholar] [CrossRef]
- Kováčová, M.; Špitalská, E.; Markovic, Z.; Špitálský, Z. Carbon Quantum Dots As Antibacterial Photosensitizers and Their Polymer Nanocomposite Applications. Part. Part. Syst. Charact. 2019, 37, 1900348. [Google Scholar] [CrossRef]
- Liu, H.; Jiang, Y.; Wang, Z.; Zhao, L.; Yin, Q.; Liu, M. Nanomaterials as carriers to improve the photodynamic antibacterial therapy. Front. Chem. 2022, 10, 1044627. [Google Scholar] [CrossRef]
- Wu, X.; Abbas, K.; Yang, Y.; Li, Z.; Tedesco, A.C.; Bi, H. Photodynamic Anti-Bacteria by Carbon Dots and Their Nano-Composites. Pharmaceuticals 2022, 15, 487. [Google Scholar] [CrossRef]
- Karagianni, A.; Tsierkezos, N.G.; Prato, M.; Terrones, M.; Kordatos, K.V. Application of carbon-based quantum dots in photodynamic therapy. Carbon 2023, 203, 273–310. [Google Scholar] [CrossRef]
- Wang, Y.; Guo, G.; Gao, J.; Li, Z.; Yin, X.; Zhu, C.; Xia, Y. Multicenter-Emitting Carbon Dots: Color Tunable Fluorescence and Dynamics Monitoring Oxidative Stress In Vivo. Chem. Mater. 2020, 32, 8146–8157. [Google Scholar] [CrossRef]
- Geng, X.; Sun, Y.; Li, Z.; Yang, R.; Zhao, Y.; Guo, Y.; Xu, J.; Li, F.; Wang, Y.; Lu, S. Retrosynthesis of tunable fluorescent carbon dots for precise long-term mitochondrial tracking. Small 2019, 15, 1901517. [Google Scholar] [CrossRef]
- Wang, Y.; Zhu, Y.; Yu, S.; Jiang, C. Fluorescent carbon dots: Rational synthesis, tunable optical properties and analytical applications. RSC Adv. 2017, 7, 40973–40989. [Google Scholar] [CrossRef]
- Xin, N.; Gao, D.; Su, B.; Zhou, T.; Zhu, Y.; Wu, C.; Wei, D.; Sun, J.; Fan, H. Orange-emissive carbon dots with high photostability for mitochondrial dynamics tracking in living cells. ACS Sens. 2023, 8, 1161–1172. [Google Scholar] [CrossRef]
- Javed, N.; O’Carroll, D.M. Carbon dots and stability of their optical properties. Part. Part. Syst. Charact. 2021, 38, 2000271. [Google Scholar] [CrossRef]
- Cui, F.; Sun, J.; Ji, J.; Yang, X.; Wei, K.; Xu, H.; Gu, Q.; Zhang, Y.; Sun, X. Carbon dots-releasing hydrogels with antibacterial activity, high biocompatibility, and fluorescence performance as candidate materials for wound healing. J. Hazard. Mater. 2021, 406, 124330. [Google Scholar] [CrossRef]
- Zhang, Z.; Yan, K.; Yang, Q.; Liu, Y.; Yan, Z.; Chen, J. One-pot synthesis of fluorescent nitrogen-doped carbon dots with good biocompatibility for cell labeling. Luminescence 2017, 32, 1488–1493. [Google Scholar] [CrossRef]
- Koutsogiannis, P.; Thomou, E.; Stamatis, H.; Gournis, D.; Rudolf, P. Advances in fluorescent carbon dots for biomedical applications. Adv. Phys. X 2020, 5, 1758592. [Google Scholar] [CrossRef]
- Shi, X.; Zhang, C.Y.; Gao, J.; Wang, Z. Recent advances in photodynamic therapy for cancer and infectious diseases. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnology 2019, 11, e1560. [Google Scholar] [CrossRef] [PubMed]
- Luan, X.; Pan, Y.; Gao, Y.; Song, Y. Recent near-infrared light-activated nanomedicine toward precision cancer therapy. J. Mater. Chem. B 2021, 9, 7076–7099. [Google Scholar] [CrossRef] [PubMed]
- Jia, Q.; Song, Q.; Li, P.; Huang, W. Rejuvenated photodynamic therapy for bacterial infections. Adv. Healthc. Mater. 2019, 8, 1900608. [Google Scholar] [CrossRef] [PubMed]
- Wen, F.; Li, P.; Meng, H.; Yan, H.; Huang, X.; Cui, H.; Su, W. Nitrogen-doped carbon dots/curcumin nanocomposite for combined Photodynamic/photothermal dual-mode antibacterial therapy. Photodiagnosis Photodyn. Ther. 2022, 39, 103033. [Google Scholar] [CrossRef] [PubMed]
- Yan, H.; Zhang, B.; Zhang, Y.; Su, R.; Li, P.; Su, W. Fluorescent Carbon Dot–Curcumin Nanocomposites for Remarkable Antibacterial Activity with Synergistic Photodynamic and Photothermal Abilities. ACS Appl. Bio Mater. 2021, 4, 6703–6718. [Google Scholar] [CrossRef] [PubMed]
- Kumari, S.; Rajit Prasad, S.; Mandal, D.; Das, P. Carbon dot-DNA-protoporphyrin hybrid hydrogel for sustained photoinduced antimicrobial activity. J. Colloid Interface Sci. 2019, 553, 228–238. [Google Scholar] [CrossRef] [PubMed]
- Horne, T.K.; Cronjé, M.J. Mechanistics and photo-energetics of macrocycles and photodynamic therapy: An overview of aspects to consider for research. Chem. Biol. Drug Des. 2017, 89, 221–242. [Google Scholar] [CrossRef] [PubMed]
- Martinez De Pinillos Bayona, A.; Mroz, P.; Thunshelle, C.; Hamblin, M.R. Design features for optimization of tetrapyrrole macrocycles as antimicrobial and anticancer photosensitizers. Chem. Biol. Drug Des. 2017, 89, 192–206. [Google Scholar] [CrossRef]
- Yao, Q.; Fan, J.; Long, S.; Zhao, X.; Li, H.; Du, J.; Shao, K.; Peng, X. The concept and examples of type-III photosensitizers for cancer photodynamic therapy. Chem 2022, 8, 197–209. [Google Scholar] [CrossRef]
- Zacharioudaki, D.-E.; Fitilis, I.; Kotti, M. Review of Fluorescence Spectroscopy in Environmental Quality Applications. Molecules 2022, 27, 4801. [Google Scholar] [CrossRef]
- Gao, L.; Giglio, K.M.; Nelson, J.L.; Sondermann, H.; Travis, A.J. Ferromagnetic nanoparticles with peroxidase-like activity enhance the cleavage of biological macromolecules for biofilm elimination. Nanoscale 2014, 6, 2588–2593. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Cheng, Q.; Lei, J.H.; Hao, T.; Deng, C.X.; Tang, Z.; Qu, S. Visible-light-induced strong oxidation capacity of metal-free carbon nanodots through photo-induced surface reduction for photocatalytic antibacterial and tumor therapy. J. Colloid Interface Sci. 2023, 644, 107–115. [Google Scholar] [CrossRef]
- Hu, X.; Zhang, H.; Wang, Y.; Shiu, B.-C.; Lin, J.-H.; Zhang, S.; Lou, C.-W.; Li, T.-T. Synergistic antibacterial strategy based on photodynamic therapy: Progress and perspectives. Chem. Eng. J. 2022, 450, 138129. [Google Scholar] [CrossRef]
- Li, X.; Lovell, J.F.; Yoon, J.; Chen, X. Clinical development and potential of photothermal and photodynamic therapies for cancer. Nat. Rev. Clin. Oncol. 2020, 17, 657–674. [Google Scholar] [CrossRef] [PubMed]
- Cui, X.; Ruan, Q.; Zhuo, X.; Xia, X.; Hu, J.; Fu, R.; Li, Y.; Wang, J.; Xu, H. Photothermal Nanomaterials: A Powerful Light-to-Heat Converter. Chem. Rev. 2023, 123, 6891–6952. [Google Scholar] [CrossRef]
- Liu, B.; Jiao, J.; Xu, W.; Zhang, M.; Cui, P.; Guo, Z.; Deng, Y.; Chen, H.; Sun, W. Highly Efficient Far-Red/NIR-Absorbing Neutral Ir(III) Complex Micelles for Potent Photodynamic/Photothermal Therapy. Adv. Mater. 2021, 33, 2100795. [Google Scholar] [CrossRef]
- Xu, F.-Z.; Zhu, L.; Han, H.-H.; Zou, J.-W.; Zang, Y.; Li, J.; James, T.D.; He, X.-P.; Wang, C.-Y. Molecularly engineered AIEgens with enhanced quantum and singlet-oxygen yield for mitochondria-targeted imaging and photodynamic therapy. Chem. Sci. 2022, 13, 9373–9380. [Google Scholar] [CrossRef] [PubMed]
- Lepock, J.R.; Frey, H.E.; Ritchie, K.P. Protein denaturation in intact hepatocytes and isolated cellular organelles during heat shock. J. Cell Biol. 1993, 122, 1267–1276. [Google Scholar] [CrossRef]
- Richter, K.; Haslbeck, M.; Buchner, J. The heat shock response: Life on the verge of death. Mol. Cell 2010, 40, 253–266. [Google Scholar] [CrossRef]
- Stapleton, S.; Dunne, M.; Milosevic, M.; Tran, C.W.; Gold, M.J.; Vedadi, A.; Mckee, T.D.; Ohashi, P.S.; Allen, C.; Jaffray, D.A. Radiation and heat improve the delivery and efficacy of nanotherapeutics by modulating intratumoral fluid dynamics. ACS Nano 2018, 12, 7583–7600. [Google Scholar] [CrossRef]
- He, L.; Di, D.; Chu, X.; Liu, X.; Wang, Z.; Lu, J.; Wang, S.; Zhao, Q. Photothermal antibacterial materials to promote wound healing. J. Control. Release 2023, 363, 180–200. [Google Scholar] [CrossRef] [PubMed]
- Yougbaré, S.; Mutalik, C.; Krisnawati, D.I.; Kristanto, H.; Jazidie, A.; Nuh, M.; Cheng, T.-M.; Kuo, T.-R. Nanomaterials for the Photothermal Killing of Bacteria. Nanomaterials 2020, 10, 1123. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.; Chen, C.; Qiu, Y.; Xu, C.; Yao, J. Paying attention to tumor blood vessels: Cancer phototherapy assisted with nano delivery strategies. Biomaterials 2021, 268, 120562. [Google Scholar] [CrossRef] [PubMed]
- Melamed, J.R.; Edelstein, R.S.; Day, E.S. Elucidating the Fundamental Mechanisms of Cell Death Triggered by Photothermal Therapy. ACS Nano 2015, 9, 6–11. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Q.; Wang, J.; Yin, C.; Zhang, P.; Zhang, J.; Shi, M.; Shen, K.; Xiao, Y.; Zhao, Y.; Yang, X.; et al. Near-Infrared Light-Sensitive Nano Neuro-Immune Blocker Capsule Relieves Pain and Enhances the Innate Immune Response for Necrotizing Infection. Nano Lett. 2019, 19, 5904–5914. [Google Scholar] [CrossRef] [PubMed]
- Hou, X.; Tao, Y.; Pang, Y.; Li, X.; Jiang, G.; Liu, Y. Nanoparticle-based photothermal and photodynamic immunotherapy for tumor treatment. Int. J. Cancer 2018, 143, 3050–3060. [Google Scholar] [CrossRef] [PubMed]
- Kessel, D. Photodynamic Therapy: A Brief History. J. Clin. Med. 2019, 8, 1581. [Google Scholar] [CrossRef] [PubMed]
- Kou, J.; Dou, D.; Yang, L. Porphyrin photosensitizers in photodynamic therapy and its applications. Oncotarget 2017, 8, 81591–81603. [Google Scholar] [CrossRef] [PubMed]
- Aires-Fernandes, M.; Botelho Costa, R.; Rochetti do Amaral, S.; Mussagy, C.U.; Santos-Ebinuma, V.C.; Primo, F.L. Development of Biotechnological Photosensitizers for Photodynamic Therapy: Cancer Research and Treatment-From Benchtop to Clinical Practice. Molecules 2022, 27, 6848. [Google Scholar] [CrossRef]
- Lu, G.; Nagbanshi, M.; Goldau, N.; Mendes Jorge, M.; Meissner, P.; Jahn, A.; Mockenhaupt, F.P.; Müller, O. Efficacy and safety of methylene blue in the treatment of malaria: A systematic review. BMC Med. 2018, 16, 59. [Google Scholar] [CrossRef]
- Zolfaghari, P.S.; Packer, S.; Singer, M.; Nair, S.P.; Bennett, J.; Street, C.; Wilson, M. In vivo killing of Staphylococcus aureus using a light-activated antimicrobial agent. BMC Microbiol. 2009, 9, 27. [Google Scholar] [CrossRef] [PubMed]
- Pereira, A.H.C.; Pinto, J.G.; Freitas, M.A.A.; Fontana, L.C.; Soares, C.P.; Ferreira-Strixino, J. Methylene blue internalization and photodynamic action against clinical and ATCC Pseudomonas aeruginosa and Staphyloccocus aureus strains. Photodiagnosis Photodyn. Ther. 2018, 22, 43–50. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.; Hsu, C.-H.; Huang, C.-C.; Chang, P.-Y. Development of therapeutic Au–methylene blue nanoparticles for targeted photodynamic therapy of cervical cancer cells. ACS Appl. Mater. Interfaces 2015, 7, 432–441. [Google Scholar] [CrossRef] [PubMed]
- Kofler, B.; Romani, A.; Pritz, C.; Steinbichler, T.B.; Schartinger, V.H.; Riechelmann, H.; Dudas, J. Photodynamic effect of methylene blue and low level laser radiation in head and neck squamous cell carcinoma cell lines. Int. J. Mol. Sci. 2018, 19, 1107. [Google Scholar] [CrossRef] [PubMed]
- Lim, D.J. Methylene Blue-Based Nano and Microparticles: Fabrication and Applications in Photodynamic Therapy. Polymers 2021, 13, 3955. [Google Scholar] [CrossRef] [PubMed]
- Dougherty, T.J. Hematoporphyrin as a photosensitizer of tumors. Photochem. Photobiol. 1983, 38, 377–379. [Google Scholar] [CrossRef] [PubMed]
- Malik, Z.; Ladan, H.; Hanania, J.; Nitzan, Y. Mesosomal structures and antimicrobial activity induced by hemin oxidation or porphyrin photodynamic sensitization inStaphylococci. Curr. Microbiol. 1988, 16, 321–328. [Google Scholar] [CrossRef]
- Schuitmaker, J.J.; Baas, P.; van Leengoed, H.L.L.M.; van der Meulen, F.W.; Star, W.M.; van Zandwijk, N. Photodynamic therapy: A promising new modality for the treatment of cancer. J. Photochem. Photobiol. B Biol. 1996, 34, 3–12. [Google Scholar] [CrossRef]
- De Rosa, F.S.; Marchetti, J.M.; Thomazini, J.A.; Tedesco, A.C.; Bentley, M.V.L.B. A vehicle for photodynamic therapy of skin cancer: Influence of dimethylsulphoxide on 5-aminolevulinic acid in vitro cutaneous permeation and in vivo protoporphyrin IX accumulation determined by confocal microscopy. J. Control. Release 2000, 65, 359–366. [Google Scholar] [CrossRef]
- Fagadar-Cosma, E.; Cseh, L.; Badea, V.; Fagadar-Cosma, G.; Vlascici, D. Combinatorial synthesis and characterization of new asymmetric porphyrins as potential photosensitizers in photodynamic therapy. Comb. Chem. High Throughput Screen. 2007, 10, 466–472. [Google Scholar] [CrossRef]
- Fu, X.-j.; Fang, Y.; Yao, M. Antimicrobial photodynamic therapy for methicillin-resistant Staphylococcus aureus infection. BioMed Res. Int. 2013, 2013, 3. [Google Scholar] [CrossRef] [PubMed]
- Board-Davies, E.L.; Rhys-Williams, W.; Hynes, D.; Williams, D.; Farnell, D.J.J.; Love, W. Antibacterial and antibiofilm potency of XF drugs, impact of photodynamic activation and synergy with antibiotics. Front. Cell. Infect. Microbiol. 2022, 12, 904465. [Google Scholar] [CrossRef]
- Van Lier, J. Phthalocyanines as sensitizers for PDT of cancer. Photodyn. Ther. Neoplast. Dis. 1990, 1, 279–291. [Google Scholar]
- Xia, L.; Kong, X.; Liu, X.; Tu, L.; Zhang, Y.; Chang, Y.; Liu, K.; Shen, D.; Zhao, H.; Zhang, H. An upconversion nanoparticle–Zinc phthalocyanine based nanophotosensitizer for photodynamic therapy. Biomaterials 2014, 35, 4146–4156. [Google Scholar] [CrossRef] [PubMed]
- Zafar, I.; Arfan, M.; Nasir, R.; Shaikh, A. Aluminum phthalocyanine derivatives: Potential in antimicrobial PDT and photodiagnosis. Austin Biomol. Open Access 2016, 1, 1010. [Google Scholar]
- Mitra, K.; Hartman, M.C. Silicon phthalocyanines: Synthesis and resurgent applications. Org. Biomol. Chem. 2021, 19, 1168–1190. [Google Scholar] [CrossRef]
- Rak, J.; Pouckova, P.; Benes, J.; Vetvicka, D. Drug delivery systems for phthalocyanines for photodynamic therapy. Anticancer. Res. 2019, 39, 3323–3339. [Google Scholar] [CrossRef]
- Miretti, M.; Prucca, C.G.; Tempesti, T.C.; Baumgartner, M.T. Current phthalocyanines delivery systems in photodynamic therapy: An updated review. Curr. Med. Chem. 2021, 28, 5339–5367. [Google Scholar] [CrossRef] [PubMed]
- Moreira, L.M.; dos Santos, F.V.; Lyon, J.P.; Maftoum-Costa, M.; Pacheco-Soares, C.; da Silva, N.S. Photodynamic therapy: Porphyrins and phthalocyanines as photosensitizers. Aust. J. Chem. 2008, 61, 741–754. [Google Scholar] [CrossRef]
- Iqbal, Z.; Chen, J.; Chen, Z.; Huang, M. Phthalocyanine-biomolecule conjugated photosensitizers for targeted photodynamic therapy and imaging. Curr. Drug Metab. 2015, 16, 816–832. [Google Scholar] [CrossRef]
- Jin, Y.; Zhang, X.; Zhang, B.; Kang, H.; Du, L.; Li, M. Nanostructures of an amphiphilic zinc phthalocyanine polymer conjugate for photodynamic therapy of psoriasis. Colloids Surf. B Biointerfaces 2015, 128, 405–409. [Google Scholar] [CrossRef] [PubMed]
- Saxena, S.; Raja, A. Natural dyes: Sources, chemistry, application and sustainability issues. In Roadmap to Sustainable Textiles and Clothing: Eco-Friendly Raw Materials, Technologies, and Processing Methods; Springer: Berlin/Heidelberg, Germany, 2014; pp. 37–80. [Google Scholar]
- Li, N.; Wang, Q.; Zhou, J.; Li, S.; Liu, J.; Chen, H. Insight into the progress on natural dyes: Sources, structural features, health effects, challenges, and potential. Molecules 2022, 27, 3291. [Google Scholar] [CrossRef]
- Dias, L.D.; Blanco, K.C.; Mfouo-Tynga, I.S.; Inada, N.M.; Bagnato, V.S. Curcumin as a photosensitizer: From molecular structure to recent advances in antimicrobial photodynamic therapy. J. Photochem. Photobiol. C Photochem. Rev. 2020, 45, 100384. [Google Scholar] [CrossRef]
- Basnet, P.; Skalko-Basnet, N. Curcumin: An anti-inflammatory molecule from a curry spice on the path to cancer treatment. Molecules 2011, 16, 4567–4598. [Google Scholar] [CrossRef]
- Menon, V.P.; Sudheer, A.R. Antioxidant and anti-inflammatory properties of curcumin. In The Molecular Targets and Therapeutic Uses of Curcumin in Health and Disease; Springer: Berlin/Heidelberg, Germany, 2007; pp. 105–125. [Google Scholar]
- Trigo-Gutierrez, J.K.; Vega-Chacón, Y.; Soares, A.B.; Mima, E.G.d.O. Antimicrobial activity of curcumin in nanoformulations: A comprehensive review. Int. J. Mol. Sci. 2021, 22, 7130. [Google Scholar] [CrossRef]
- Sanchez de Araujo, H.; Ferreira, F. Quantum dots and photodynamic therapy in COVID-19 treatment. Quantum Eng. 2021, 3, e78. [Google Scholar] [CrossRef]
- Pal, T.; Mohiyuddin, S.; Packirisamy, G. Facile and Green Synthesis of Multicolor Fluorescence Carbon Dots from Curcumin: In Vitro and in Vivo Bioimaging and Other Applications. ACS Omega 2018, 3, 831–843. [Google Scholar] [CrossRef]
- Kiesslich, T.; Krammer, B.; Plaetzer, K. Cellular mechanisms and prospective applications of hypericin in photodynamic therapy. Curr. Med. Chem. 2006, 13, 2189–2204. [Google Scholar] [CrossRef] [PubMed]
- Farah, N.; Chin, V.K.; Chong, P.P.; Lim, W.F.; Lim, C.W.; Basir, R.; Chang, S.K.; Lee, T.Y. Riboflavin as a promising antimicrobial agent? A multi-perspective review. Curr. Res. Microb. Sci. 2022, 3, 100111. [Google Scholar] [CrossRef]
- Huang, Y.Y.; Sharma, S.K.; Yin, R.; Agrawal, T.; Chiang, L.Y.; Hamblin, M.R. Functionalized fullerenes in photodynamic therapy. J. Biomed. Nanotechnol. 2014, 10, 1918–1936. [Google Scholar] [CrossRef]
- An, Y.; Xu, D.; Wen, X.; Chen, C.; Liu, G.; Lu, Z. Internal Light Sources-Mediated Photodynamic Therapy Nanoplatforms: Hope for the Resolution of the Traditional Penetration Problem. Adv. Healthc. Mater. 2023, 13, 2301326. [Google Scholar] [CrossRef]
- Sun, L.; Wei, R.; Feng, J.; Zhang, H. Tailored lanthanide-doped upconversion nanoparticles and their promising bioapplication prospects. Coord. Chem. Rev. 2018, 364, 10–32. [Google Scholar] [CrossRef]
- Li, K.; Hong, E.; Wang, B.; Wang, Z.; Zhang, L.; Hu, R.; Wang, B. Advances in the application of upconversion nanoparticles for detecting and treating cancers. Photodiagnosis Photodyn. Ther. 2019, 25, 177–192. [Google Scholar] [CrossRef]
- Santhosh, K.; Modak, M.D.; Paik, P. Graphene oxide for biomedical applications. J. Nanomed Res. 2017, 5, 136. [Google Scholar]
- Fan, H.-y.; Yu, X.-h.; Wang, K.; Yin, Y.-j.; Tang, Y.-j.; Tang, Y.-l.; Liang, X.-h. Graphene quantum dots (GQDs)-based nanomaterials for improving photodynamic therapy in cancer treatment. Eur. J. Med. Chem. 2019, 182, 111620. [Google Scholar] [CrossRef]
- Li, H.; Wang, X.; Ohulchanskyy, T.Y.; Chen, G. Lanthanide-doped near-infrared nanoparticles for biophotonics. Adv. Mater. 2021, 33, 2000678. [Google Scholar] [CrossRef]
- Jain, P.K.; Huang, X.; El-Sayed, I.H.; El-Sayed, M.A. Noble metals on the nanoscale: Optical and photothermal properties and some applications in imaging, sensing, biology, and medicine. Acc. Chem. Res. 2008, 41, 1578–1586. [Google Scholar] [CrossRef]
- Wang, F.; Wang, J.; Liu, X. Direct Evidence of a Surface Quenching Effect on Size-Dependent Luminescence of Upconversion Nanoparticles. Angew. Chem. Int. Ed. 2010, 49, 7456–7460. [Google Scholar] [CrossRef]
- Lee, H.P.; Gaharwar, A.K. Light-responsive inorganic biomaterials for biomedical applications. Adv. Sci. 2020, 7, 2000863. [Google Scholar] [CrossRef]
- Qiu, H.; Tan, M.; Ohulchanskyy, T.Y.; Lovell, J.F.; Chen, G. Recent progress in upconversion photodynamic therapy. Nanomaterials 2018, 8, 344. [Google Scholar] [CrossRef]
- Karakoti, A.S.; Shukla, R.; Shanker, R.; Singh, S. Surface functionalization of quantum dots for biological applications. Adv. Colloid Interface Sci. 2015, 215, 28–45. [Google Scholar] [CrossRef]
- Samia, A.C.; Dayal, S.; Burda, C. Quantum dot-based energy transfer: Perspectives and potential for applications in photodynamic therapy. Photochem. Photobiol. 2006, 82, 617–625. [Google Scholar] [CrossRef]
- Guo, T.; Tang, Q.; Guo, Y.; Qiu, H.; Dai, J.; Xing, C.; Zhuang, S.; Huang, G. Boron Quantum Dots for Photoacoustic Imaging-Guided Photothermal Therapy. ACS Appl. Mater. Interfaces 2021, 13, 306–311. [Google Scholar] [CrossRef]
- Rakovich, A.; Savateeva, D.; Rakovich, T.; Donegan, J.F.; Rakovich, Y.P.; Kelly, V.; Lesnyak, V.; Eychmüller, A. CdTe Quantum Dot/Dye Hybrid System as Photosensitizer for Photodynamic Therapy. Nanoscale Res. Lett. 2010, 5, 753. [Google Scholar] [CrossRef]
- Sennaroglu, A.; Khan, M.; Hashemkhani, M.; Yağci Acar, H. Determination of the Wavelength-Dependent Photothermal Conversion Efficiency of Photosensitizers for Photothermal Therapy: Application to Ag2S-Glutathione Quantum Dots. J. Phys. Chem. B 2021, 125, 11650–11659. [Google Scholar] [CrossRef]
- Zhang, W.; Yu, L.; Jiang, Y.; Guo, C. Phycocyanin-functionalized black phosphorus quantum dots enhance PDT/PTT therapy by inducing ROS and irreparable DNA damage. Biomater. Sci. 2021, 9, 5302–5318. [Google Scholar] [CrossRef]
- Yang, S.T.; Cao, L.; Luo, P.G.; Lu, F.; Wang, X.; Wang, H.; Meziani, M.J.; Liu, Y.; Qi, G.; Sun, Y.P. Carbon dots for optical imaging in vivo. J. Am. Chem. Soc. 2009, 131, 11308–11309. [Google Scholar] [CrossRef]
- He, H.; Wang, X.; Feng, Z.; Cheng, T.; Sun, X.; Sun, Y.; Xia, Y.; Wang, S.; Wang, J.; Zhang, X. Rapid microwave-assisted synthesis of ultra-bright fluorescent carbon dots for live cell staining, cell-specific targeting and in vivo imaging. J. Mater. Chem. B 2015, 3, 4786–4789. [Google Scholar] [CrossRef]
- Edison, T.N.; Atchudan, R.; Sethuraman, M.G.; Shim, J.J.; Lee, Y.R. Microwave assisted green synthesis of fluorescent N-doped carbon dots: Cytotoxicity and bio-imaging applications. J. Photochem. Photobiol. B 2016, 161, 154–161. [Google Scholar] [CrossRef]
- Chang, D.; Shi, L.; Zhang, Y.; Zhang, G.; Zhang, C.; Dong, C.; Shuang, S. Smilax China-derived yellow-fluorescent carbon dots for temperature sensing, Cu2+ detection and cell imaging. Analyst 2020, 145, 2176–2183. [Google Scholar] [CrossRef]
- Liu, Y.; Chen, P.; Yu, K.; Wu, Z.; Wang, N.; Yu, X. Nitrogen and sulfur co-doped carbon dots: Facile synthesis and multifunctional applications for pH sensing, temperature sensing and RNA-selective imaging. Microchem. J. 2021, 168, 106248. [Google Scholar] [CrossRef]
- Ghataty, D.S.; Amer, R.I.; Amer, M.A.; Abdel Rahman, M.F.; Shamma, R.N. Green Synthesis of Highly Fluorescent Carbon Dots from Bovine Serum Albumin for Linezolid Drug Delivery as Potential Wound Healing Biomaterial: Bio-Synergistic Approach, Antibacterial Activity, and In Vitro and Ex Vivo Evaluation. Pharmaceutics 2023, 15, 234. [Google Scholar] [CrossRef]
- Devi, N.; Wangoo, N. Tuning the Luminescence of Microwave-Assisted N-Doped Fluorescent Carbon Dots: Bioimaging Applications and Label-Free Anti-Cancer Drug Delivery. ACS Appl. Bio Mater. 2023, 6, 999–1010. [Google Scholar] [CrossRef]
- Chen, Z.; Liu, Y.; Kang, Z. Diversity and tailorability of photoelectrochemical properties of carbon dots. Acc. Chem. Res. 2022, 55, 3110–3124. [Google Scholar] [CrossRef]
- Ding, H.; Zhou, X.-X.; Wei, J.-S.; Li, X.-B.; Qin, B.-T.; Chen, X.-B.; Xiong, H.-M. Carbon dots with red/near-infrared emissions and their intrinsic merits for biomedical applications. Carbon 2020, 167, 322–344. [Google Scholar] [CrossRef]
- Redondo-Fernandez, G.; Canga, J.C.; Soldado, A.; Encinar, J.R.; Costa-Fernandez, J.M. Functionalized heteroatom-doped carbon dots for biomedical applications: A review. Anal. Chim. Acta 2023, 1284, 341874. [Google Scholar] [CrossRef]
- Kaushal, N.; Jain, A.; Kumar, A.; Sarraf, S.; Basu, A.K.; Raje, C.I.; Saha, A. Solvent-Free Synthesis of S, N-Doped Carbon Dots for Extended Visible-Light-Induced Oxidase-Mimicking Activities and Antimicrobial Applications. ChemPlusChem 2023, 88, e202300125. [Google Scholar] [CrossRef]
- Huang, S.; Yan, L.; Chen, X.; Zhu, J.J. Deep-Red to Near-Infrared Carbon Dots in Biosensing and Bio-medical Applications. Chin. J. Chem. 2023, 41, 2354–2370. [Google Scholar] [CrossRef]
- Cela, E.M.; Urquiza, D.; Gómez, M.I.; Gonzalez, C.D. New Weapons to Fight against Staphylococcus aureus Skin Infections. Antibiotics 2023, 12, 1477. [Google Scholar] [CrossRef]
- Ozyurt, D.; Al Kobaisi, M.; Hocking, R.K.; Fox, B. Properties, synthesis, and applications of carbon dots: A review. Carbon Trends 2023, 12, 100276. [Google Scholar] [CrossRef]
- Fauziyah, N.A. A brief review of C-Dots preparation using top-down and bottom-up approaches. Int. J. Eco-Innov. Sci. Eng. (IJEISE) 2022, 3, 25–29. [Google Scholar] [CrossRef]
- Nagarajan, D.; Gangadharan, D.; Venkatanarasimhan, S. Synthetic strategies toward developing carbon dots via top-down approach. In Carbon Dots in Analytical Chemistry; Elsevier: Amsterdam, The Netherlands, 2023; pp. 1–13. [Google Scholar]
- Modi, P.D.; Mehta, V.N.; Prajapati, V.S.; Patel, S.; Rohit, J.V. Bottom-up approaches for the preparation of carbon dots. In Carbon Dots in Analytical Chemistry; Elsevier: Amsterdam, The Netherlands, 2023; pp. 15–29. [Google Scholar]
- Gayen, B.; Palchoudhury, S.; Chowdhury, J. Carbon dots: A mystic star in the world of nanoscience. J. Nanomater. 2019, 2019, 3451307. [Google Scholar] [CrossRef]
- Arora, N.; Sharma, N. Arc discharge synthesis of carbon nanotubes: Comprehensive review. Diam. Relat. Mater. 2014, 50, 135–150. [Google Scholar] [CrossRef]
- Ge, G.; Li, L.; Wang, D.; Chen, M.; Zeng, Z.; Xiong, W.; Wu, X.; Guo, C. Carbon dots: Synthesis, properties and biomedical applications. J. Mater. Chem. B 2021, 9, 6553–6575. [Google Scholar] [CrossRef]
- Chen, W.; Lv, G.; Hu, W.; Li, D.; Chen, S.; Dai, Z. Synthesis and applications of graphene quantum dots: A review. Nanotechnol. Rev. 2018, 7, 157–185. [Google Scholar] [CrossRef]
- Ndlwana, L.; Raleie, N.; Dimpe, K.M.; Ogutu, H.F.; Oseghe, E.O.; Motsa, M.M.; Msagati, T.A.; Mamba, B.B. Sustainable Hydrothermal and Solvothermal Synthesis of Advanced Carbon Materials in Multidimensional Applications: A Review. Materials 2021, 14, 5094. [Google Scholar] [CrossRef] [PubMed]
- Rong, M.-C.; Zhang, K.-X.; Wang, Y.-R.; Chen, X. The synthesis of B, N-carbon dots by a combustion method and the application of fluorescence detection for Cu2+. Chin. Chem. Lett. 2017, 28, 1119–1124. [Google Scholar] [CrossRef]
- de Medeiros, T.V.; Manioudakis, J.; Noun, F.; Macairan, J.-R.; Victoria, F.; Naccache, R. Microwave-assisted synthesis of carbon dots and their applications. J. Mater. Chem. C 2019, 7, 7175–7195. [Google Scholar] [CrossRef]
- Nizamuddin, S.; Siddiqui, M.T.H.; Mubarak, N.; Baloch, H.A.; Mazari, S.A.; Tunio, M.; Griffin, G.; Srinivasan, M.; Tanksale, A.; Riaz, S. Advanced nanomaterials synthesis from pyrolysis and hydrothermal carbonization: A review. Curr. Org. Chem. 2018, 22, 446–461. [Google Scholar] [CrossRef]
- Romero, M.P.; Alves, F.; Stringasci, M.D.; Buzza, H.H.; Ciol, H.; Inada, N.M.; Bagnato, V.S. One-Pot Microwave-Assisted Synthesis of Carbon Dots and in vivo and in vitro Antimicrobial Photodynamic Applications. Front. Microbiol. 2021, 12, 662149. [Google Scholar] [CrossRef]
- Lu, S.; Sui, L.; Liu, J.; Zhu, S.; Chen, A.; Jin, M.; Yang, B. Near-Infrared Photoluminescent Polymer-Carbon Nanodots with Two-Photon Fluorescence. Adv. Mater. 2017, 29, 1603443. [Google Scholar] [CrossRef] [PubMed]
- Liu, K.K.; Song, S.Y.; Sui, L.Z.; Wu, S.X.; Jing, P.T.; Wang, R.Q.; Li, Q.Y.; Wu, G.R.; Zhang, Z.Z.; Yuan, K.J.; et al. Efficient Red/Near-Infrared-Emissive Carbon Nanodots with Multiphoton Excited Upconversion Fluorescence. Adv. Sci. 2019, 6, 1900766. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Z.; Zhai, Y.; Li, Z.; Zhu, P.; Mao, S.; Zhu, C.; Du, D.; Belfiore, L.A.; Tang, J.; Lin, Y. Red carbon dots: Optical property regulations and applications. Mater. Today 2019, 30, 52–79. [Google Scholar] [CrossRef]
- Shuang, E.; Mao, Q.X.; Wang, J.H.; Chen, X.W. Carbon dots with tunable dual emissions: From the mechanism to the specific imaging of endoplasmic reticulum polarity. Nanoscale 2020, 12, 6852–6860. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Gu, H.; Ran, B.; Liu, W.; Sun, W.; Wang, D.; Du, J.; Fan, J.; Peng, X. Accelerated antibacterial red-carbon dots with photodynamic therapy against multidrug-resistant Acinetobacter baumannii. Sci. China Mater. 2022, 65, 845–854. [Google Scholar] [CrossRef] [PubMed]
- Sattarahmady, N.; Rezaie-Yazdi, M.; Tondro, G.H.; Akbari, N. Bactericidal laser ablation of carbon dots: An in vitro study on wild-type and antibiotic-resistant Staphylococcus aureus. J. Photochem. Photobiol. B 2017, 166, 323–332. [Google Scholar] [CrossRef] [PubMed]
- Chu, X.; Zhang, P.; Wang, Y.; Sun, B.; Liu, Y.; Zhang, Q.; Feng, W.; Li, Z.; Li, K.; Zhou, N.; et al. Near-infrared carbon dot-based platform for bioimaging and photothermal/photodynamic/quaternary ammonium triple synergistic sterilization triggered by single NIR light source. Carbon 2021, 176, 126–138. [Google Scholar] [CrossRef]
- Jijie, R.; Barras, A.; Bouckaert, J.; Dumitrascu, N.; Szunerits, S.; Boukherroub, R. Enhanced antibacterial activity of carbon dots functionalized with ampicillin combined with visible light triggered photodynamic effects. Colloids Surf. B Biointerfaces 2018, 170, 347–354. [Google Scholar] [CrossRef] [PubMed]
- Mandal, S.; Prasad, S.R.; Mandal, D.; Das, P. Bovine Serum Albumin Amplified Reactive Oxygen Species Generation from Anthrarufin-Derived Carbon Dot and Concomitant Nanoassembly for Combination Antibiotic–Photodynamic Therapy Application. ACS Appl. Mater. Interfaces 2019, 11, 33273–33284. [Google Scholar] [CrossRef]
- Wu, F.; Su, H.; Cai, Y.; Wong, W.-K.; Jiang, W.; Zhu, X. Porphyrin-Implanted Carbon Nanodots for Photoacoustic Imaging and in Vivo Breast Cancer Ablation. ACS Appl. Bio Mater. 2018, 1, 110–117. [Google Scholar] [CrossRef]
- Jiang, X.; Luo, Z.; Zhang, B.; Li, P.; Xiao, J.a.; Su, W. Moderate microwave-assisted preparation of phthalocyanine-based carbon quantum dots for improved photo-inactivation of bacteria. Inorg. Chem. Commun. 2022, 142, 109543. [Google Scholar] [CrossRef]
- Ferreira, R.L.; Jr, W.M.; Souza, L.E.A.; Navarro, H.M.C.; de Mello, L.R.; Mastelaro, V.R.; Sales, T.O.; Barbosa, C.; Ribeiro, A.S.; da Silva, E.R.; et al. Harnessing Efficient ROS Generation in Carbon Dots Derived from Methyl Red for Antimicrobial Photodynamic Therapy. ACS Appl. Bio Mater. 2023, 6, 4345–4357. [Google Scholar] [CrossRef]
- Qie, X.; Zan, M.; Gui, P.; Chen, H.; Wang, J.; Lin, K.; Mei, Q.; Ge, M.; Zhang, Z.; Tang, Y.; et al. Design, Synthesis, and Application of Carbon Dots With Synergistic Antibacterial Activity. Front. Bioeng. Biotechnol. 2022, 10, 894100. [Google Scholar] [CrossRef]
- Venkateswarlu, S.; Viswanath, B.; Reddy, A.S.; Yoon, M. Fungus-derived photoluminescent carbon nanodots for ultrasensitive detection of Hg2+ ions and photoinduced bactericidal activity. Sens. Actuators B Chem. 2018, 258, 172–183. [Google Scholar] [CrossRef]
- Zhang, J.; Lu, X.; Tang, D.; Wu, S.; Hou, X.; Liu, J.; Wu, P. Phosphorescent Carbon Dots for Highly Efficient Oxygen Photosensitization and as Photo-oxidative Nanozymes. ACS Appl. Mater. Interfaces 2018, 10, 40808–40814. [Google Scholar] [CrossRef] [PubMed]
- Knoblauch, R.; Harvey, A.; Ra, E.; Greenberg, K.M.; Lau, J.; Hawkins, E.; Geddes, C.D. Antimicrobial carbon nanodots: Photodynamic inactivation and dark antimicrobial effects on bacteria by brominated carbon nanodots. Nanoscale 2021, 13, 85–99. [Google Scholar] [CrossRef] [PubMed]
- Abu Rabe, D.I.; Al Awak, M.M.; Yang, F.; Okonjo, P.A.; Dong, X.; Teisl, L.R.; Wang, P.; Tang, Y.; Pan, N.; Sun, Y.-P.; et al. The dominant role of surface functionalization in carbon dots’ photo-activated antibacterial activity. Int. J. Nanomed. 2019, 14, 2655–2665. [Google Scholar] [CrossRef] [PubMed]
- Samphire, J.; Takebayashi, Y.; Hill, S.A.; Hill, N.; Heesom, K.J.; Lewis, P.A.; Alibhai, D.; Bragginton, E.C.; Dorh, J.; Dorh, N.; et al. Green fluorescent carbon dots as targeting probes for LED-dependent bacterial killing. Nano Sel. 2021, 3, 662–672. [Google Scholar] [CrossRef]
- Su, R.; Yan, H.; Jiang, X.; Zhang, Y.; Li, P.; Su, W. Orange-red to NIR emissive carbon dots for antimicrobial, bioimaging and bacteria diagnosis. J. Mater. Chem. B 2022, 10, 1250–1264. [Google Scholar] [CrossRef]
- Yan, H.; Li, P.; Wen, F.; Xu, Q.; Guo, Q.; Su, W. Green synthesis of carbon quantum dots from plant turmeric holds promise as novel photosensitizer for in vitro photodynamic antimicrobial activity. J. Mater. Res. Technol. 2023, 22, 17–34. [Google Scholar] [CrossRef]
- Marković, Z.M.; Kováčová, M.; Jeremić, S.R.; Nagy, Š.; Milivojević, D.D.; Kubat, P.; Kleinová, A.; Budimir, M.D.; Mojsin, M.M.; Stevanović, M.J.; et al. Highly Efficient Antibacterial Polymer Composites Based on Hydrophobic Riboflavin Carbon Polymerized Dots. Nanomaterials 2022, 12, 4070. [Google Scholar] [CrossRef] [PubMed]
- Nie, X.; Wu, S.; Mensah, A.; Lu, K.; Wei, Q. Carbon quantum dots embedded electrospun nanofibers for efficient antibacterial photodynamic inactivation. Mater. Sci. Eng. C Mater. Biol. Appl. 2020, 108, 110377. [Google Scholar] [CrossRef]
- Lu, Y.; Li, L.; Li, M.; Lin, Z.; Wang, L.; Zhang, Y.; Yin, Q.; Xia, H.; Han, G. Zero-Dimensional Carbon Dots Enhance Bone Regeneration, Osteosarcoma Ablation, and Clinical Bacterial Eradication. Bioconjug. Chem. 2018, 29, 2982–2993. [Google Scholar] [CrossRef]
- Liu, B.; Su, Y.; Wu, S.; Shen, J. Local photothermal/photodynamic synergistic antibacterial therapy based on two-dimensional BP@CQDs triggered by single NIR light source. Photodiagnosis Photodyn. Ther. 2022, 39, 102905. [Google Scholar] [CrossRef]
- Dong, X.; Bond, A.E.; Pan, N.; Coleman, M.; Tang, Y.; Sun, Y.P.; Yang, L. Synergistic photoactivated antimicrobial effects of carbon dots combined with dye photosensitizers. Int. J. Nanomed. 2018, 13, 8025–8035. [Google Scholar] [CrossRef]
- Mushtaq, S.; Yasin, T.; Saleem, M.; Dai, T.; Yameen, M.A. Potentiation of Antimicrobial Photodynamic Therapy by Curcumin-loaded Graphene Quantum Dots. Photochem. Photobiol. 2021, 98, 202–210. [Google Scholar] [CrossRef]
- Al Awak, M.M.; Wang, P.; Wang, S.; Tang, Y.; Sun, Y.P.; Yang, L. Correlation of Carbon Dots’ Light-Activated Antimicrobial Activities and Fluorescence Quantum Yield. RSC Adv. 2017, 7, 30177–30184. [Google Scholar] [CrossRef]
- Wu, M.; Wang, Y.; Wu, W.; Hu, C.; Wang, X.; Zheng, J.; Li, Z.; Jiang, B.; Qiu, J. Preparation of functionalized water-soluble photoluminescent carbon quantum dots from petroleum coke. Carbon 2014, 78, 480–489. [Google Scholar] [CrossRef]
- Zhang, S.; Sui, L.; Dong, H.; He, W.; Dong, L.; Yu, L. High-Performance Supercapacitor of Graphene Quantum Dots with Uniform Sizes. ACS Appl. Mater. Interfaces 2018, 10, 12983–12991. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Li, F.; Zhang, S.; An, Y.; Sun, S. Preparation of N-doped yellow carbon dots and N, P co-doped red carbon dots for bioimaging and photodynamic therapy of tumors. New J. Chem. 2019, 43, 6332–6342. [Google Scholar] [CrossRef]
- Wu, X.; Xu, M.; Wang, S.; Abbas, K.; Huang, X.; Zhang, R.; Tedesco, A.C.; Bi, H. F, N-Doped carbon dots as efficient Type I photosensitizers for photodynamic therapy. Dalton Trans. 2022, 51, 2296–2303. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Jiang, W.; Qiu, L.; Jiang, X.; Zuo, D.; Wang, D.; Yang, L. One pot synthesis of highly luminescent polyethylene glycol anchored carbon dots functionalized with a nuclear localization signal peptide for cell nucleus imaging. Nanoscale 2015, 7, 6104–6113. [Google Scholar] [CrossRef]
- Peng, Z.; Ji, C.; Zhou, Y.; Zhao, T.; Leblanc, R.M. Polyethylene glycol (PEG) derived carbon dots: Preparation and applications. Appl. Mater. Today 2020, 20, 100677. [Google Scholar] [CrossRef]
- Chu, X.; Liu, Y.; Zhang, P.; Li, K.; Feng, W.; Sun, B.; Zhou, N.; Shen, J. Silica-supported near-infrared carbon dots and bicarbonate nanoplatform for triple synergistic sterilization and wound healing promotion therapy. J. Colloid Interface Sci. 2022, 608, 1308–1322. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.Y.; Yu, N.Y.; Fang, W.D.; Tan, Q.G.; Ji, R.; Yang, L.Y.; Wei, S.; Zhang, X.W.; Miao, A.J. Photodegradation of carbon dots cause cytotoxicity. Nat. Commun. 2021, 12, 812. [Google Scholar] [CrossRef] [PubMed]
- Stanković, N.K.; Bodik, M.; Šiffalovič, P.; Kotlar, M.; Mičušik, M.; Špitalsky, Z.; Danko, M.; Milivojević, D.D.; Kleinova, A.; Kubat, P.; et al. Antibacterial and Antibiofouling Properties of Light Triggered Fluorescent Hydrophobic Carbon Quantum Dots Langmuir–Blodgett Thin Films. ACS Sustain. Chem. Eng. 2018, 6, 4154–4163. [Google Scholar] [CrossRef]
- Xu, N.; Du, J.; Yao, Q.; Ge, H.; Shi, C.; Xu, F.; Xian, L.; Fan, J.; Peng, X. Carbon dots inspired by structure-inherent targeting for nucleic acid imaging and localized photodynamic therapy. Sens. Actuators B Chem. 2021, 344, 130322. [Google Scholar] [CrossRef]
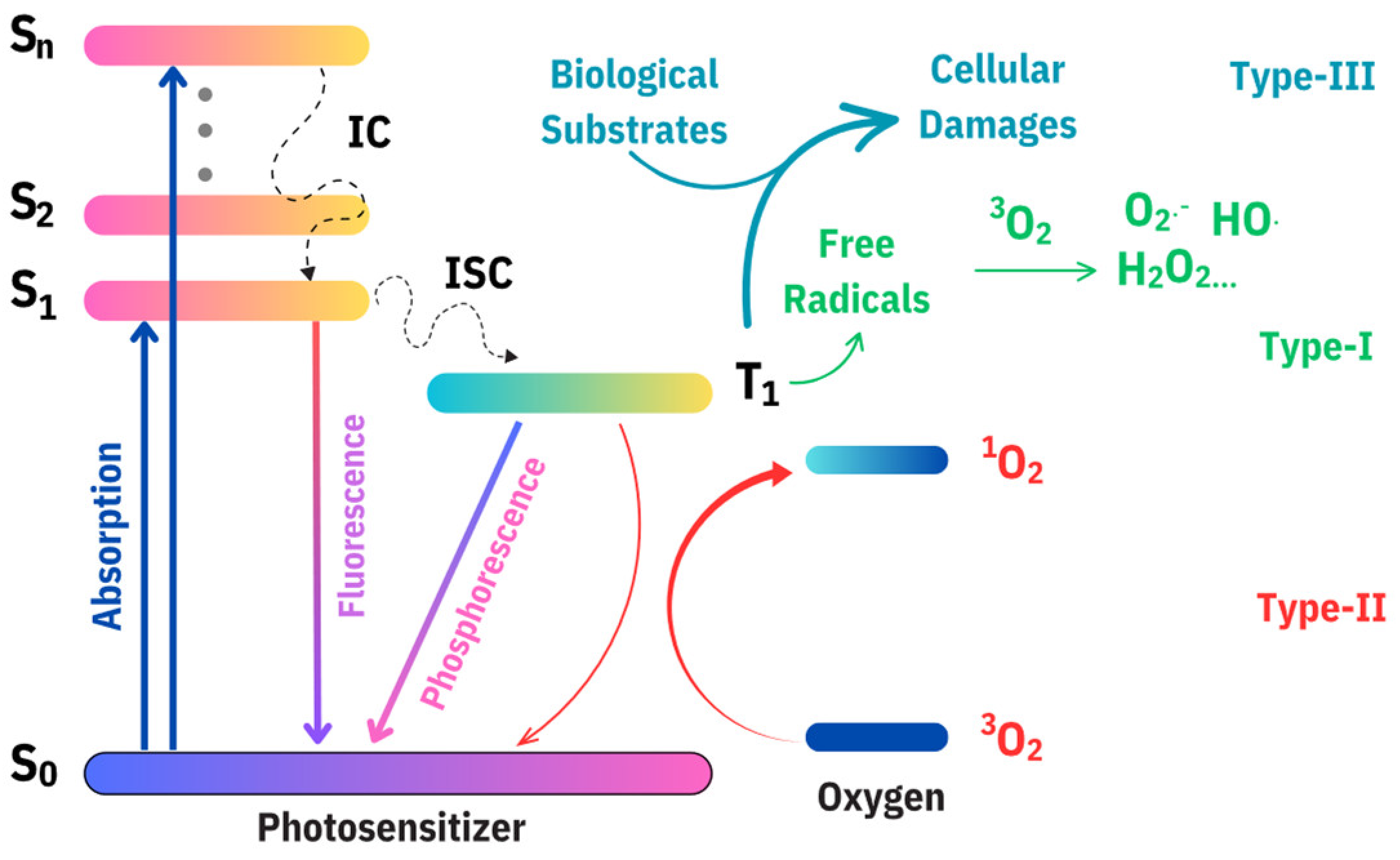
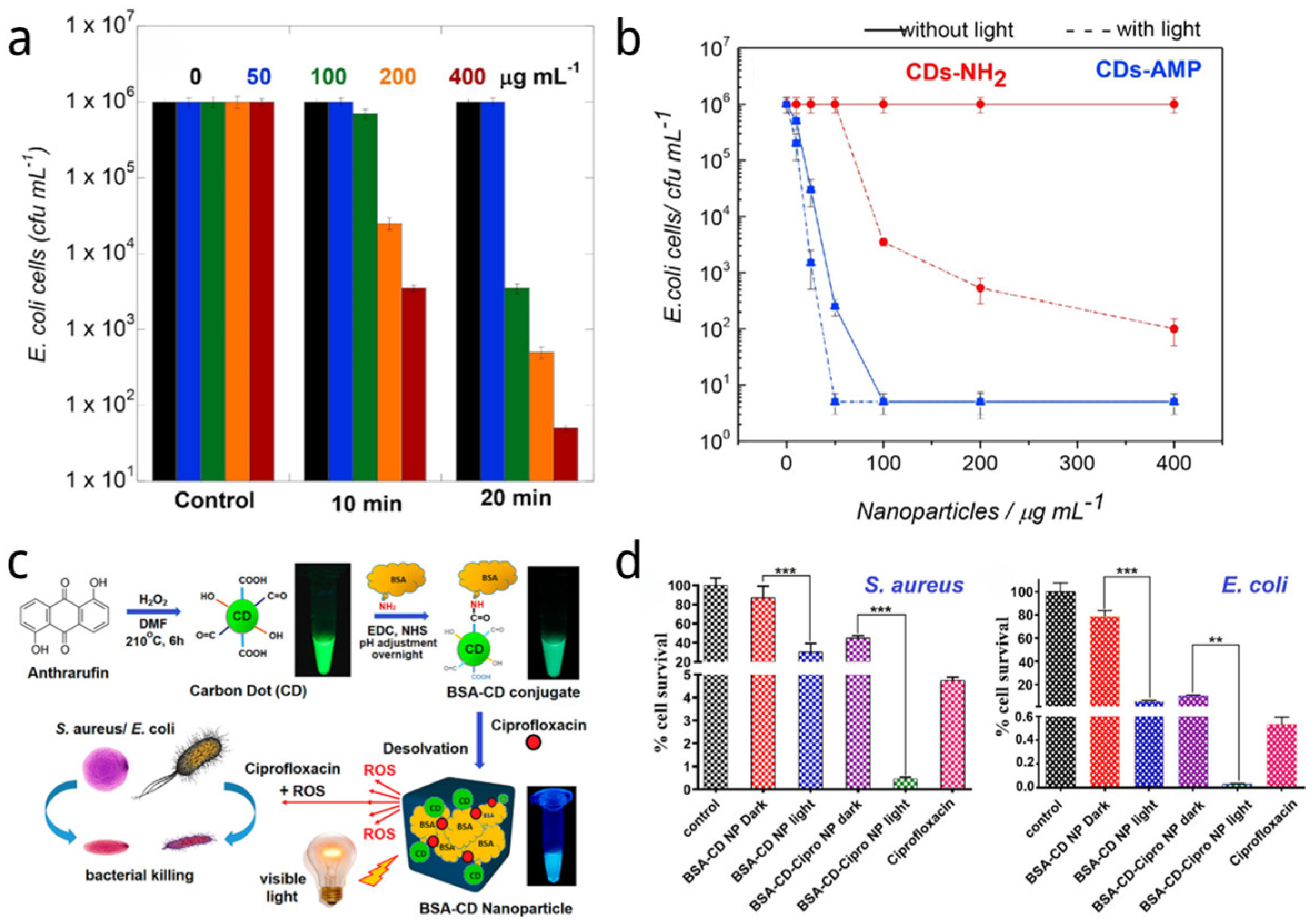
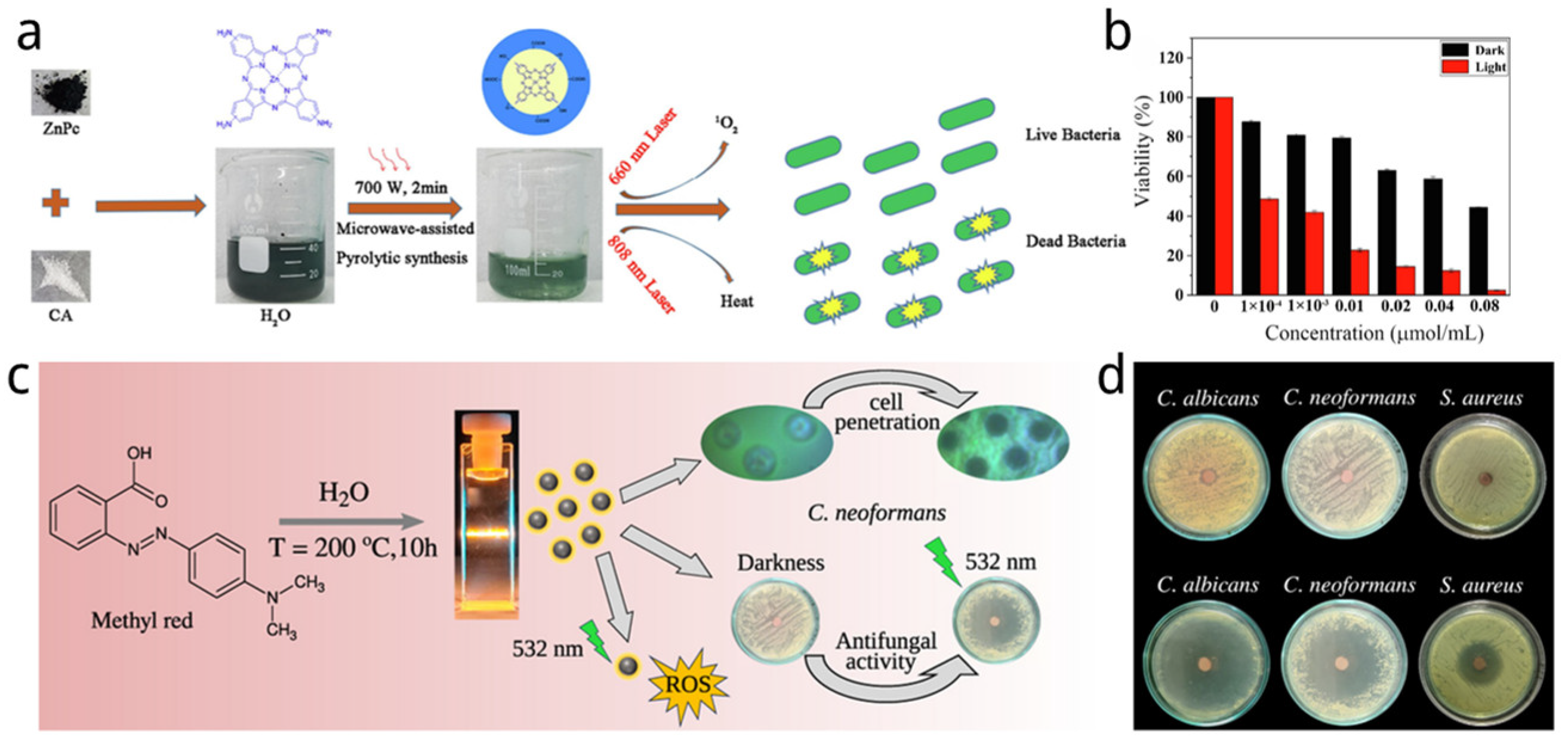
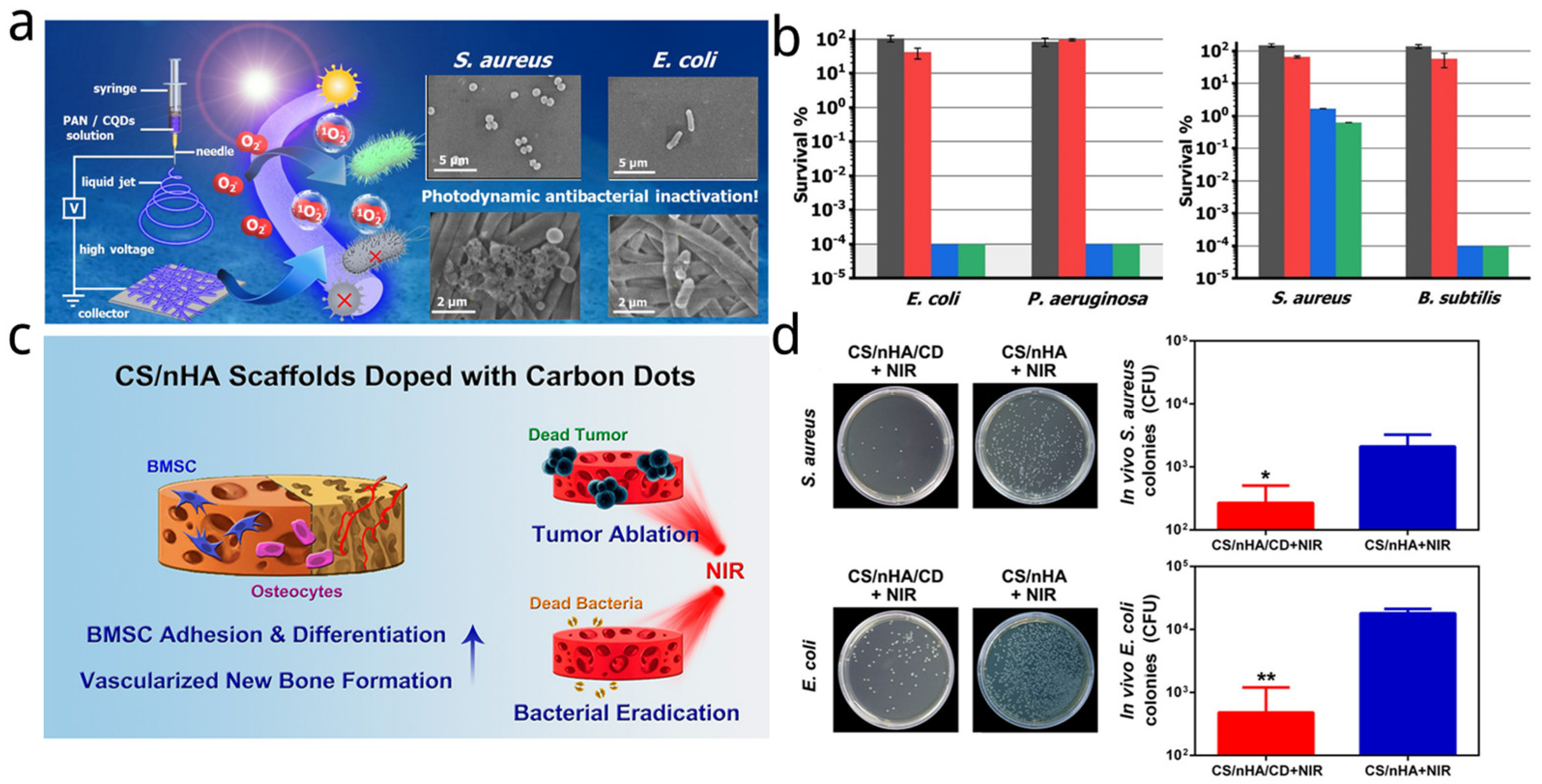
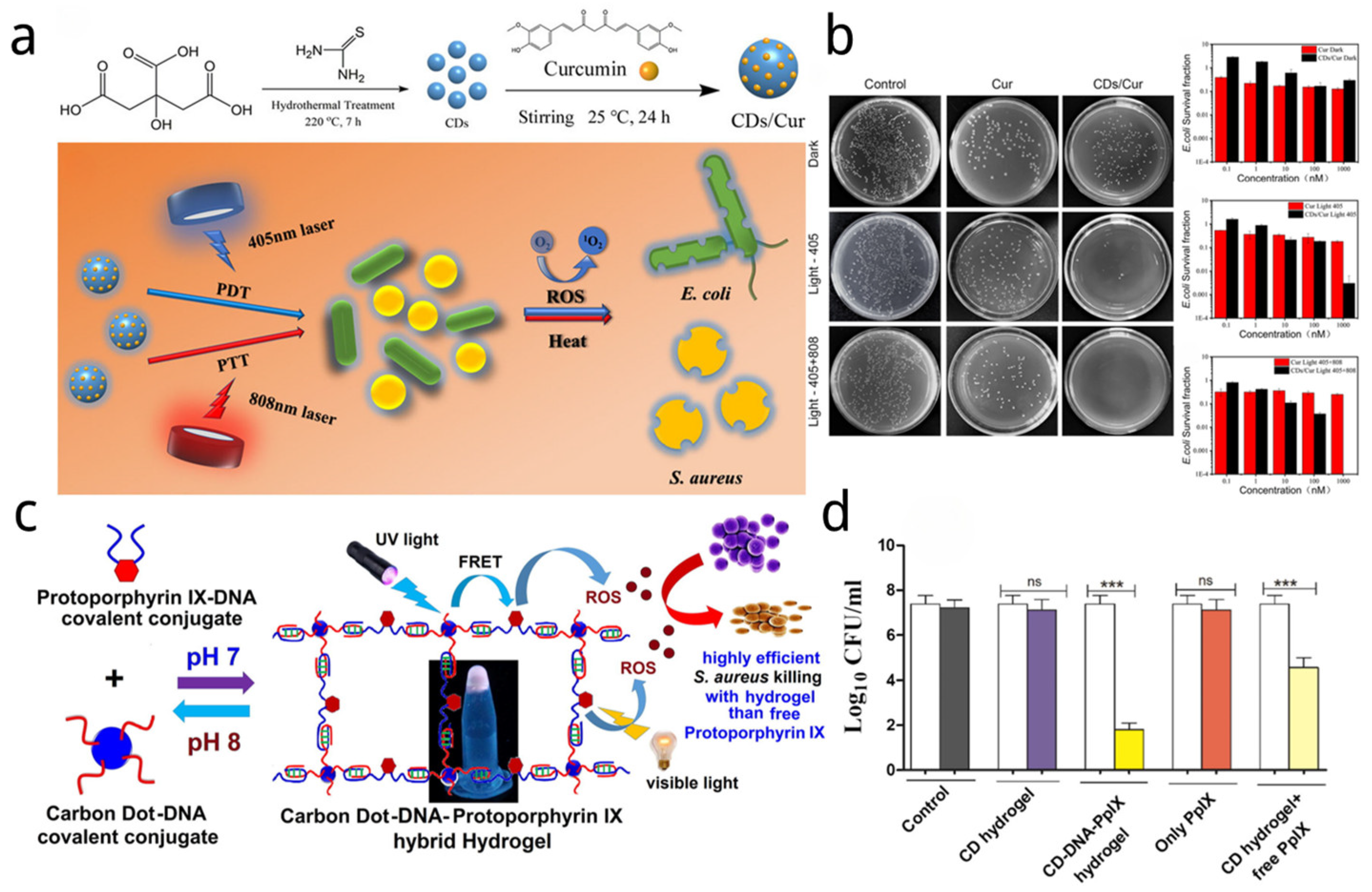
| Name | Size of CDs (nm) | Sources of CDs | Synthetic Method of CDs | Light Source and Power and Time | Application | Strain and Dosage | Ref. |
|---|---|---|---|---|---|---|---|
| C-DOTS | 1.5–4.5 | Citric acid | Microwave | 450 nm; 40 mW/cm2 | PDT | S. aureus 13.8 mg/mL | [135] |
| R-CDs | 3.31 | 2,4-dihydroxybenzoic acid and 6bromo-2-naphthol | Solvothermal | 590 nm, 30 mW/cm2 | PDT | MRAB and MRSA 30 µg/mL−1 | [140] |
| C-dots | 12 ± 1 | Ascorbic acid and copper acetate hydrate | Hydrothermal | 808 nm diode laser 0.36–0.84 KJ/cm2 | PTT | S. aureus and MRSA 7.0 μg/mL−1 | [141] |
| Cu-RCDs-C35 | 8.8 | Citric acid, urea, and cupric chloride dihydrate (CuCl2 2H2O) | Solvothermal | 808 nm irradiation (2.0 W/cm2, 10 min) | PDT and PTT | E. coli and S. aureu 800 μg/mL | [142] |
| BSA-CDs | 5 | 1,5-dihydroxyanthraquinone | Hydrothermal | Visible light (100 W, 1 h, 30 cm distance) | PDT | S. aureus and E. coli | [144] |
| ZnPc-CQDs | 5.2 ± 1.2 | ZnPc, citric acid | Microwave-assisted | 660 + 808 nm, 12 min, 0.5 W/cm2 | PDT and PTT | E. coli and S. aureu, 0.08 μM | [146] |
| CD-MR | 3.3 | Methyl red azo dye | Hydrothermal method | LED 532 nm, 10 mW | PDT | C. albicans, C. neoformans, and S. aureus | [147] |
| BAPTCDs | 3–5 | O-phenylenediamine and D-Glu | Hydrothermal | 808 nm, 1.5 W/cm2 for 10 min | PTT | S. aureus and E. coli 200 μg/ml | [148] |
| DHLA@MCDs | 2–6 | Edible mushroom | Oven | Visible LED light 2.70 mW/cm2 | PDT | E. coli | [149] |
| CDs-AMP | 2 | Citric acid and ethylenediamine | Hydrothermal | LED light (365 nm) 3 V/3 W, 1 h | PDT | E. coli and Salmonella 5 µM | [150] |
| BrCDs | 0.7 | Natural gas, HBr | Halogenation | Ultraviolet lamp (365 nm) 3 mW | PDT | Listeria monocytogenes, S. aureus and E. coli. | [151] |
| EDA-CDs/EPA-CDs | 4–5 | Carbon—nano-powders | Hydrothermal | 400–800 nm light bulb 36 W, 12 V | PDT | Bacillus subtilis | [152] |
| FCDs | 1–10 | Glucosamine hydrochloride and mphenylenediamine | Microwave-assisted | Blue-LED strip lights (460 nm) 24 W, 12 V | PDT | Klebsiella pneumoniae, Pseudomonas aeruginosa, E. coli and S. aureus 200 µg/mL | [153] |
| Cur-NRCQDs | 3.83 | Ctric acid, neutral red, curcumin | Hydrothermal method | Xenon light (400–450 nm) | PDT | S. aureus and E. coli, 10 mM and 15 mM | [154] |
| ST-JHCQDs | 10.7 | Turmeric extract | Hydrothermal method | 405 nm (20 mW/cm2 for 30 min) | PDT | S. aureus and E. coli 5 mg/mL | [155] |
| Name | Size of CDs (nm) | Sources of CDs | Synthetic Method of CDs | Light Source and Power and Time | Application | Strain and Dosage | Ref. |
|---|---|---|---|---|---|---|---|
| RF-CPDs/PU | 35.2 ± 2.1 | Riboflavin, ethylenediamine | Hydrothermal method | Blue light (power 3 W) for 30, 60, and 120 min | PDT | E. coli and S. aureus. | [156] |
| PAN-CQD | - | Citric acid and 1,5-diaminonaphthalene | Solvothermal | Xe lamp, λ ≥ 420 nm, 12 cm sample distance, 500 W for 1.5 h | PDT | Escherichia coli and Pseudomonas aeruginosa | [157] |
| CS/nHA/CD scaffolds | 5 | Citric acid and glycine | Domestic microwave oven (800 W) | 808 nm, 1 W/cm2 for 10 min | PTT | S. aureus and E. coli, 1 mg/mL | [158] |
| BPs@CQDs | 1–10 | Tellurocystine | Solvothermal | 808 nm, 1.5 W/cm2 | PDT and PTT | S. aureus and E. coli | [159] |
| Name | Sources for CDs | Photosensitizers | Synthetic Method | Light Source and Power and Time | Application | Strain and Dosage | Ref. |
|---|---|---|---|---|---|---|---|
| CDs-Cur | Citric acid and ethylenediamine | Curcumin | Microwave | 800 nm (500 mW/cm2) 405 nm (200 mW/cm2) | PDT/PTT | S. aureus/E. coli 1 µM | [35] |
| CDs/Cur | Citric acid and thiourea | Curcumin | Hydrothermal | 800 nm (500 mW/cm2) 405 nm (200 mW/cm2) | PDT/PTT | 1 µM and 0.1 nM CD/Cur for E. coli and S. aureus respectively | [36] |
| CD-DNA-PpIX hybrid hydrogel | Citric acid and Branched Polyethylenimine | PpIX | - | UV lamp (302 nm) | PDT | S. aureus | [37] |
| cur-GQDs | Coal and curcumin | Curcumin | Solvothermal method | 405 nm LEDs 30 J/cm2 | PDT | Pseudomonas aeruginosa, MRSA, E. coli, and Candida albicans. | [161] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, S.; McCoy, C.P.; Li, P.; Li, Y.; Zhao, Y.; Andrews, G.P.; Wylie, M.P.; Ge, Y. Carbon Dots in Photodynamic/Photothermal Antimicrobial Therapy. Nanomaterials 2024, 14, 1250. https://doi.org/10.3390/nano14151250
Wang S, McCoy CP, Li P, Li Y, Zhao Y, Andrews GP, Wylie MP, Ge Y. Carbon Dots in Photodynamic/Photothermal Antimicrobial Therapy. Nanomaterials. 2024; 14(15):1250. https://doi.org/10.3390/nano14151250
Chicago/Turabian StyleWang, Siqi, Colin P. McCoy, Peifeng Li, Yining Li, Yinghan Zhao, Gavin P. Andrews, Matthew P. Wylie, and Yi Ge. 2024. "Carbon Dots in Photodynamic/Photothermal Antimicrobial Therapy" Nanomaterials 14, no. 15: 1250. https://doi.org/10.3390/nano14151250
APA StyleWang, S., McCoy, C. P., Li, P., Li, Y., Zhao, Y., Andrews, G. P., Wylie, M. P., & Ge, Y. (2024). Carbon Dots in Photodynamic/Photothermal Antimicrobial Therapy. Nanomaterials, 14(15), 1250. https://doi.org/10.3390/nano14151250







