Ultrasonication-Assisted Green Synthesis and Physicochemical and Cytotoxic Activity Characterization of Protein-Based Nanoparticles from Moringa oleifera Seeds
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Instrumentation
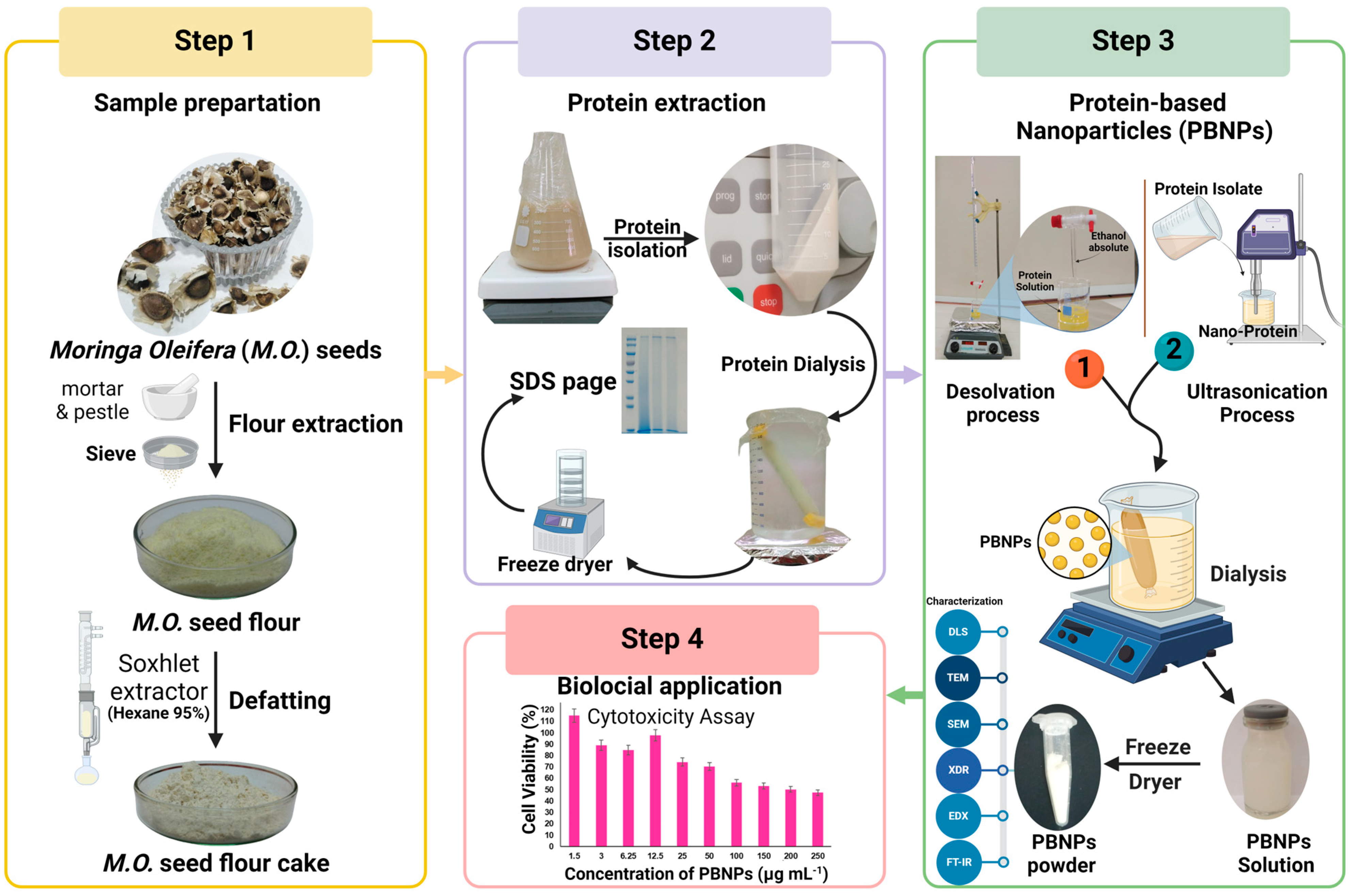
2.3. Preparation of the M. oleifera Seed
2.4. Preparation of Defatted Moringa Seed Flour (DMSF)
2.5. Proximate Composition
2.6. Preparation of Moringa Protein Isolate (MPI)
2.7. Preparation of Protein-Based Nanoparticles (PBNPs)
2.8. Molecular Weight of MPI and PBNPs
2.9. Characterization of MPI and PBNPs
2.9.1. The Morphology and Particle Size
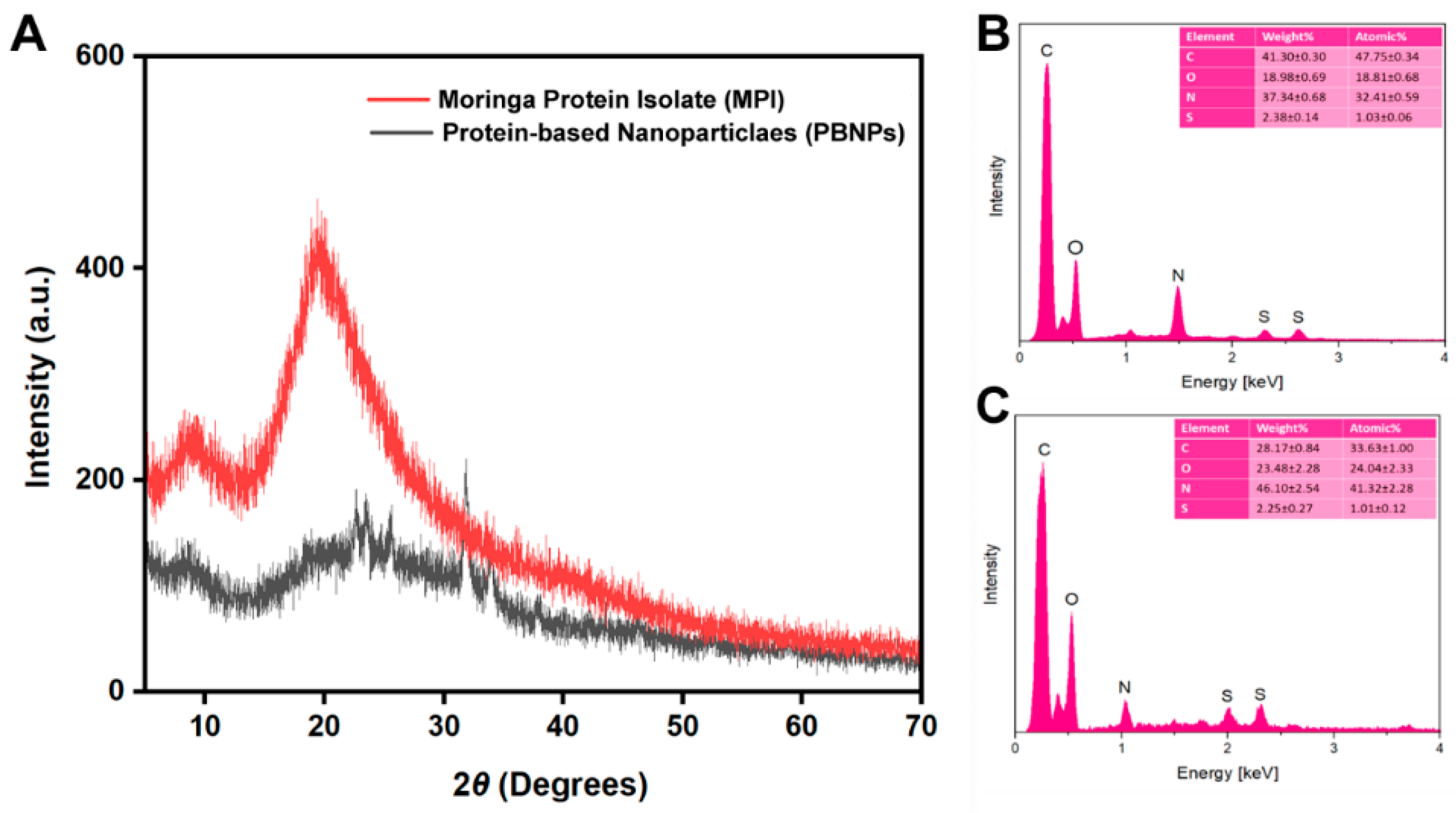
2.9.2. X-ray Diffraction (XRD) Analysis
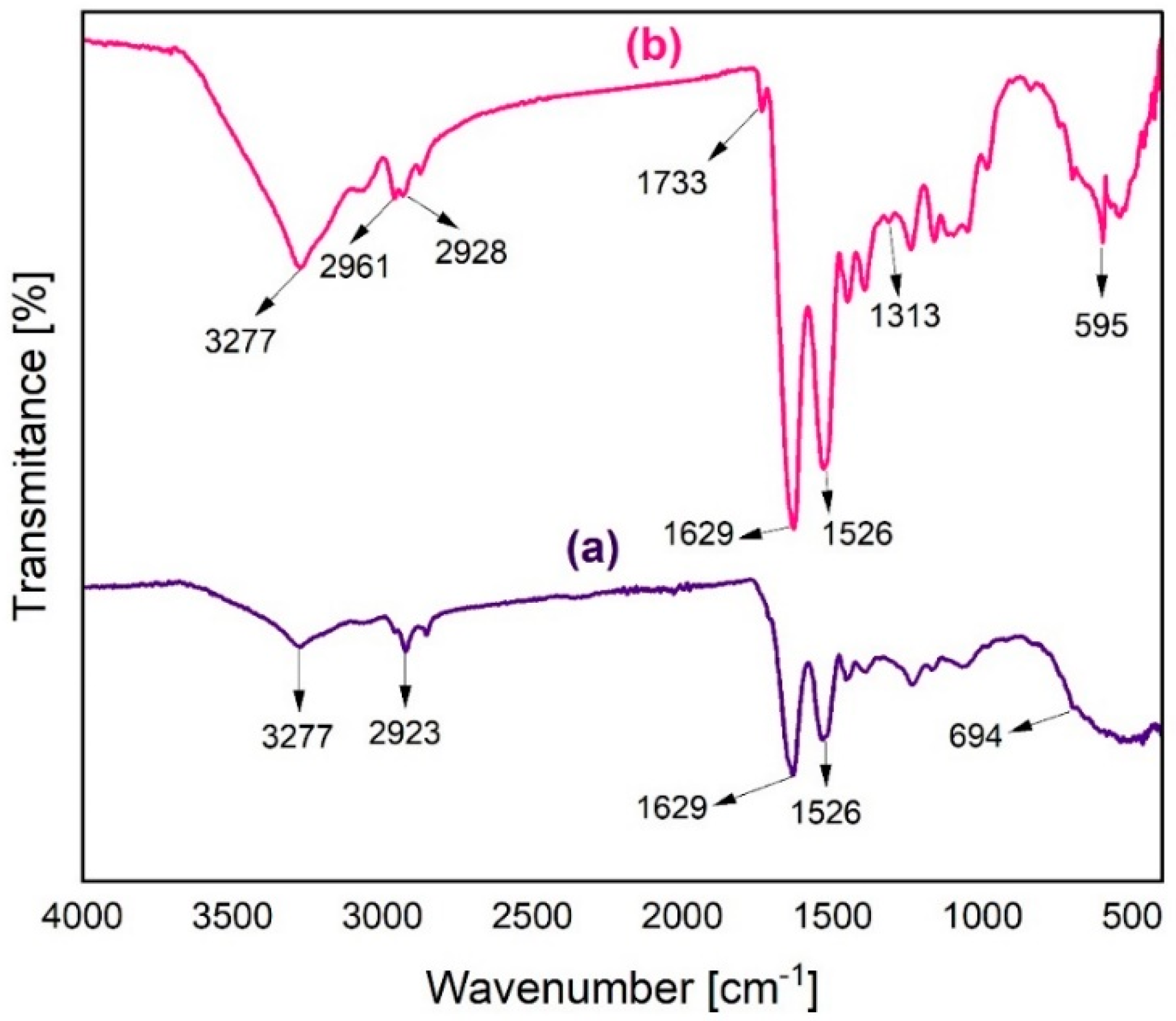
2.9.3. Fourier-Transform Infrared Spectroscopy (FT-IR)
2.10. Cell Culture and In Vitro Cytotoxicity Using MTT Assay
2.10.1. Cell Culture
2.10.2. MTT Assay
2.10.3. Cytokine Assay by ELISA
3. Results and Discussion
3.1. Proximate Composition
3.2. Characterization of MPI and PBNPs Nanoparticles
3.2.1. The Morphology and Particle Size
3.2.2. X-ray Diffraction (XRD) and EDX Analysis
3.2.3. Fourier-Transform Infrared Spectroscopy (FT-IR)
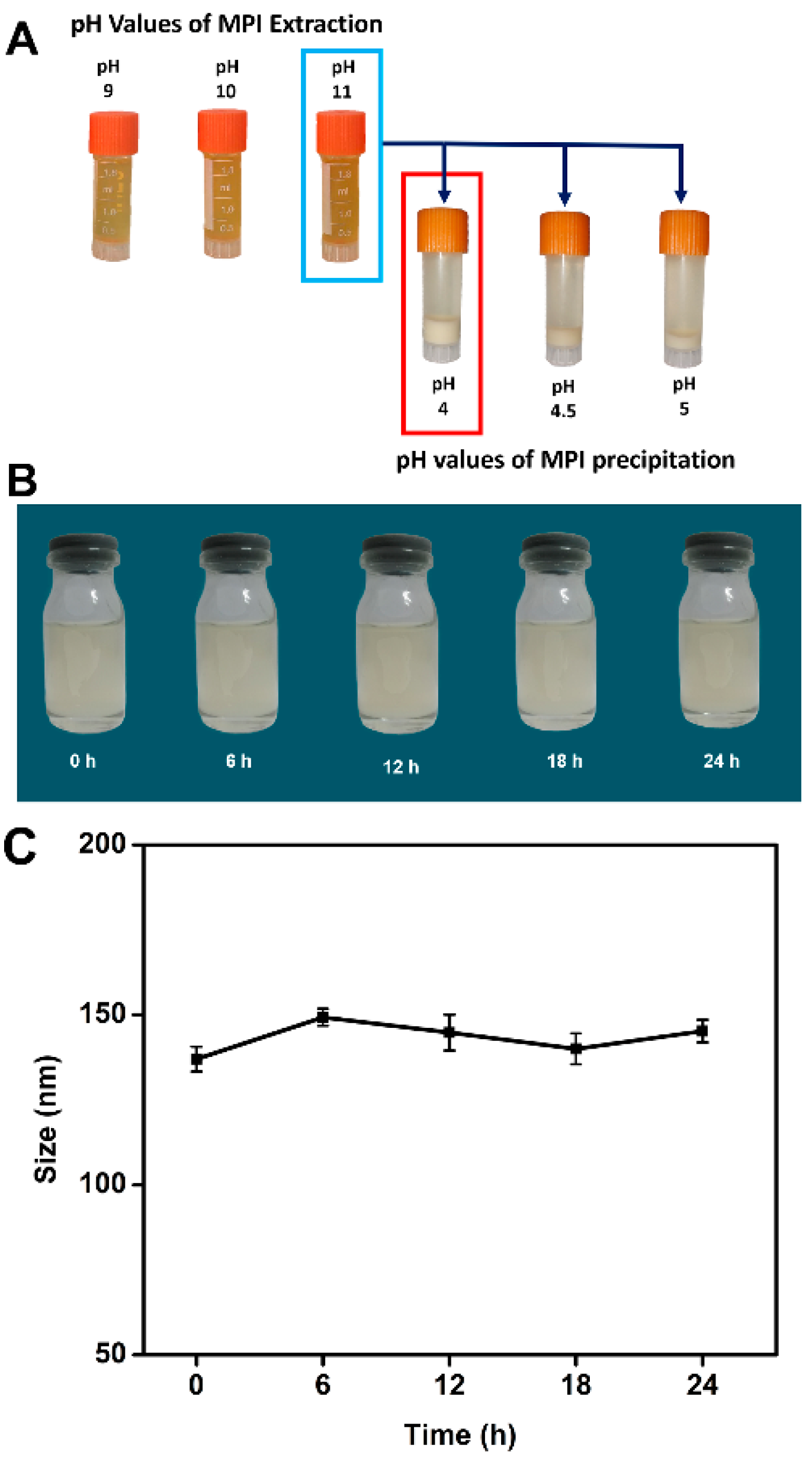
3.3. Influence of pH on MPI Charge, Isolation Efficiency, and Stability of PBNPs
4. Molecular Weight of MPI and PBNPs
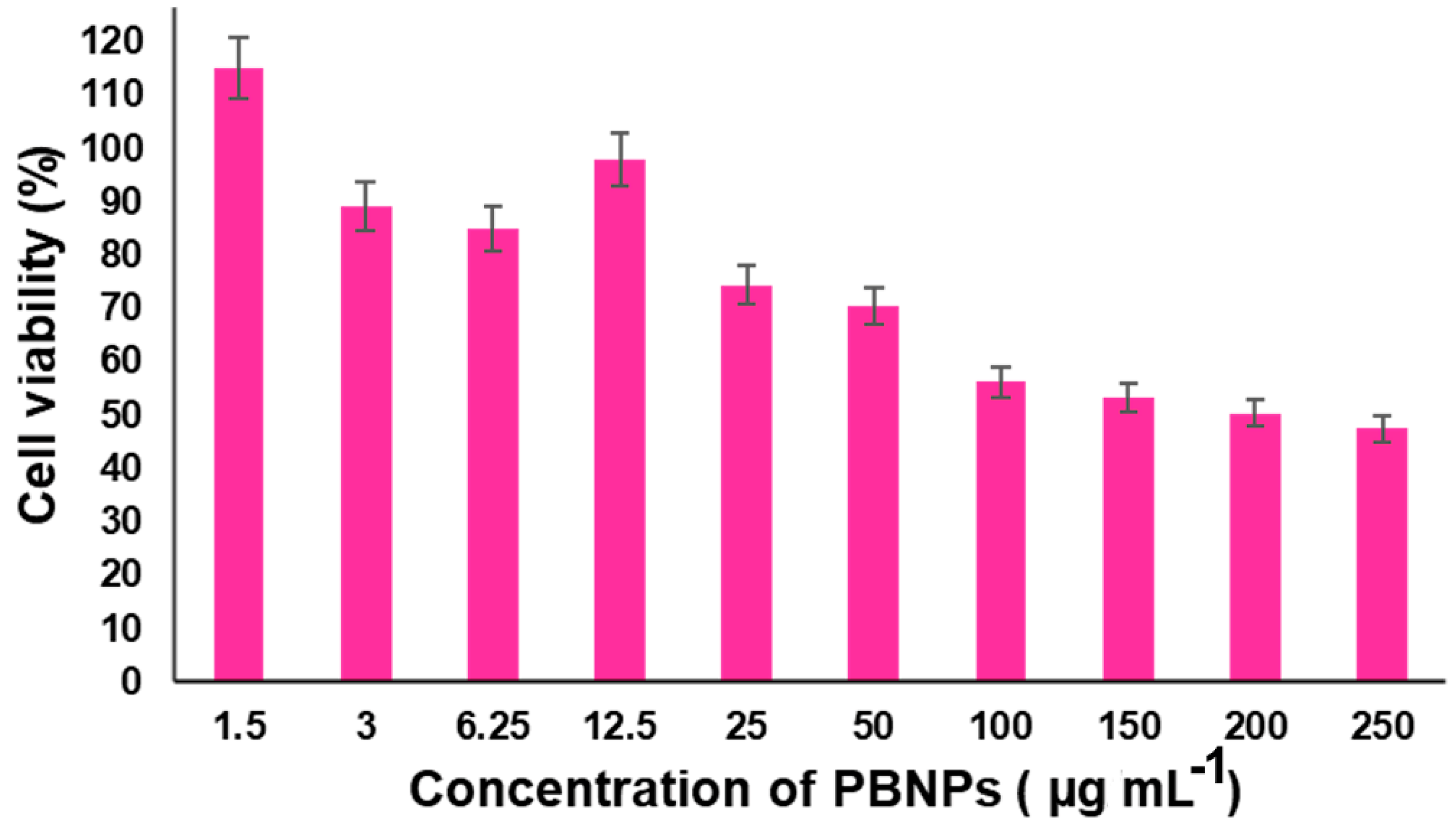
4.1. Cytotoxicity Assay
4.2. Cytokine Expression Levels
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Singh, A.K.; Rana, H.K.; Tshabalala, T.; Kumar, R.; Gupta, A.; Ndhlala, A.R.; Pandey, A.K. Phytochemical, nutraceutical and pharmacological attributes of a functional crop Moringa oleifera Lam: An overview. S. Afr. J. Bot. 2020, 129, 209–220. [Google Scholar] [CrossRef]
- Dzuvor, C.K.; Pan, S.; Amanze, C.; Amuzu, P.; Asakiya, C.; Kubi, F. Bioactive components from Moringa oleifera seeds: Production, functionalities and applications–a critical review. Crit. Rev. Biotechnol. 2022, 42, 271–293. [Google Scholar] [CrossRef]
- Perumalsamy, H.; Balusamy, S.R.; Sukweenadhi, J.; Nag, S.; MubarakAli, D.; El-Agamy Farh, M.; Vijay, H.; Rahimi, S. A comprehensive review on Moringa oleifera nanoparticles: Importance of polyphenols in nanoparticle synthesis, nanoparticle efficacy and their applications. J. Nanobiotechnol. 2024, 22, 71. [Google Scholar] [CrossRef]
- Pareek, A.; Pant, M.; Gupta, M.M.; Kashania, P.; Ratan, Y.; Jain, V.; Pareek, A.; Chuturgoon, A.A. Moringa oleifera: An updated comprehensive review of its pharmacological activities, ethnomedicinal, phytopharmaceutical formulation, clinical, phytochemical, and toxicological aspects. Int. J. Mol. Sci. 2023, 24, 2098. [Google Scholar] [CrossRef]
- Kumar, M.; Selvasekaran, P.; Kapoor, S.; Barbhai, M.D.; Lorenzo, J.M.; Saurabh, V.; Potkule, J.; Changan, S.; ElKelish, A.; Selim, S. Moringa oleifera Lam. seed proteins: Extraction, preparation of protein hydrolysates, bioactivities, functional food properties, and industrial application. Food Hydrocoll. 2022, 131, 107791. [Google Scholar] [CrossRef]
- Özcan, M.M. Moringa spp: Composition and bioactive properties. S. Afr. J. Bot. 2020, 129, 25–31. [Google Scholar] [CrossRef]
- Wang, X.; He, L.; Zhao, Q.; Chen, H.; Shi, Y.; Fan, J.; Chen, Y.; Huang, A. Protein function analysis of germinated Moringa oleifera seeds, and purification and characterization of their milk-clotting peptidase. Int. J. Biol. Macromol. 2021, 171, 539–549. [Google Scholar] [CrossRef]
- Asif, M.N.; Imran, M.; Ahmad, M.H.; Khan, M.K.; Hailu, G.G. Physicochemical and Functional Properties of Moringa Seed Protein Treated with Ultrasound. ACS Omega 2024, 9, 4102–4110. [Google Scholar] [CrossRef]
- Aderinola, T.A.; Fagbemi, T.N.; Enujiugha, V.N.; Alashi, A.M.; Aluko, R.E. Amino acid composition and antioxidant properties of Moringa oleifera seed protein isolate and enzymatic hydrolysates. Heliyon 2018, 4, e00877. [Google Scholar] [CrossRef]
- Alain Mune Mune, M.; Nyobe, E.C.; Bakwo Bassogog, C.; Minka, S.R.; Yildiz, F. A comparison on the nutritional quality of proteins from Moringa oleifera leaves and seeds. Cogent Food Agric. 2016, 2, 1213618. [Google Scholar] [CrossRef]
- Jain, A.; Subramanian, R.; Manohar, B.; Radha, C. Preparation, characterization and functional properties of Moringa oleifera seed protein isolate. J. Food Sci. Technol. 2019, 56, 2093–2104. [Google Scholar] [CrossRef] [PubMed]
- Bridgemohan, P.; Bridgemohan, R.; Mohamed, M. Chemical composition of a high protein animal supplement from Moringa oleifera. Afr. J. Food Sci. Technol. 2014, 5, 125–128. [Google Scholar]
- Bakwo Bassogog, C.B.; Nyobe, C.E.; Ngui, S.P.; Minka, S.R.; Mune Mune, M.A. Effect of heat treatment on the structure, functional properties and composition of Moringa oleifera seed proteins. Food Chem. 2022, 384, 132546. [Google Scholar] [CrossRef] [PubMed]
- Salem, S.S. A mini review on green nanotechnology and its development in biological effects. Arch. Microbiol. 2023, 205, 128. [Google Scholar] [CrossRef] [PubMed]
- Diaz, D.; Care, A.; Sunna, A. Bioengineering Strategies for Protein-Based Nanoparticles. Genes 2018, 9, 370. [Google Scholar] [CrossRef] [PubMed]
- DeFrates, K.; Markiewicz, T.; Gallo, P.; Rack, A.; Weyhmiller, A.; Jarmusik, B.; Hu, X. Protein Polymer-Based Nanoparticles: Fabrication and Medical Applications. Int. J. Mol. Sci. 2018, 19, 1717. [Google Scholar] [CrossRef] [PubMed]
- Su, S.; Kang, P.M. Systemic Review of Biodegradable Nanomaterials in Nanomedicine. Nanomaterials 2020, 10, 656. [Google Scholar] [CrossRef] [PubMed]
- Sell, M.; Lopes, A.R.; Escudeiro, M.; Esteves, B.; Monteiro, A.R.; Trindade, T.; Cruz-Lopes, L. Application of Nanoparticles in Cancer Treatment: A Concise Review. Nanomaterials 2023, 13, 2887. [Google Scholar] [CrossRef] [PubMed]
- Shabani, L.; Abbasi, M.; Azarnew, Z.; Amani, A.M.; Vaez, A. Neuro-nanotechnology: Diagnostic and therapeutic nano-based strategies in applied neuroscience. Biomed. Eng. Online 2023, 22, 1. [Google Scholar] [CrossRef]
- Kianfar, E. Protein nanoparticles in drug delivery: Animal protein, plant proteins and protein cages, albumin nanoparticles. J. Nanobiotechnol. 2021, 19, 159. [Google Scholar] [CrossRef]
- Guan, T.; Zhang, Z.; Li, X.; Cui, S.; McClements, D.J.; Wu, X.; Chen, L.; Long, J.; Jiao, A.; Qiu, C. Preparation, characteristics, and advantages of plant protein-based bioactive molecule delivery systems. Foods 2022, 11, 1562. [Google Scholar] [CrossRef] [PubMed]
- Ruth, M.R.; Field, C.J. The immune modifying effects of amino acids on gut-associated lymphoid tissue. J. Anim. Sci. Biotechnol. 2013, 4, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Reddy, N.; Rapisarda, M. Properties and Applications of Nanoparticles from Plant Proteins. Materials 2021, 14, 3607. [Google Scholar] [CrossRef]
- Gomes, A.; Sobral, P.J.d.A. Plant protein-based delivery systems: An emerging approach for increasing the efficacy of lipophilic bioactive compounds. Molecules 2021, 27, 60. [Google Scholar] [CrossRef]
- Hong, S.; Choi, D.W.; Kim, H.N.; Park, C.G.; Lee, W.; Park, H.H. Protein-Based Nanoparticles as Drug Delivery Systems. Pharmaceutics 2020, 12, 604. [Google Scholar] [CrossRef]
- Khramtsov, P.; Kalashnikova, T.; Bochkova, M.; Kropaneva, M.; Timganova, V.; Zamorina, S.; Rayev, M. Measuring the concentration of protein nanoparticles synthesized by desolvation method: Comparison of Bradford assay, BCA assay, hydrolysis/UV spectroscopy and gravimetric analysis. Int. J. Pharm. 2021, 599, 120422. [Google Scholar] [CrossRef] [PubMed]
- Rahman, M.M.; Lamsal, B.P. Ultrasound-assisted extraction and modification of plant-based proteins: Impact on physicochemical, functional, and nutritional properties. Compr. Rev. Food Sci. Food Saf. 2021, 20, 1457–1480. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Z.; Li, Y.; Xie, M.B. Silk fibroin-based nanoparticles for drug delivery. Int. J. Mol. Sci. 2015, 16, 4880. [Google Scholar] [CrossRef]
- Weber, C.; Coester, C.; Kreuter, J.; Langer, K. Desolvation process and surface characterisation of protein nanoparticles. Int. J. Pharm. 2000, 194, 91–102. [Google Scholar] [CrossRef]
- Sandhya, M.; Ramasamy, D.; Sudhakar, K.; Kadirgama, K.; Harun, W. Ultrasonication an intensifying tool for preparation of stable nanofluids and study the time influence on distinct properties of graphene nanofluids—A systematic overview. Ultrason. Sonochem. 2021, 73, 105479. [Google Scholar]
- Bhangu, S.K.; Baral, A.; Zhu, H.; Ashokkumar, M.; Cavalieri, F. Sound methods for the synthesis of nanoparticles from biological molecules. Nanoscale Adv. 2021, 3, 4907–4917. [Google Scholar] [CrossRef] [PubMed]
- Chabattula, S.C.; Gupta, P.K.; Govarthanan, K.; Varadaraj, S.; Rayala, S.K.; Chakraborty, D.; Verma, R.S. Anti-cancer activity of biogenic nat-zno nanoparticles synthesized using Nyctanthes arbor-tristis (Nat) flower extract. Appl. Biochem. Biotechnol. 2024, 196, 382–399. [Google Scholar] [CrossRef] [PubMed]
- Verma, D.; Gulati, N.; Kaul, S.; Mukherjee, S.; Nagaich, U. Protein Based Nanostructures for Drug Delivery. J. Pharm. 2018, 2018, 9285854. [Google Scholar] [CrossRef] [PubMed]
- Han, M.; Liu, K.; Liu, X.; Rashid, M.T.; Zhang, H.; Wang, M. Research Progress of Protein-Based Bioactive Substance Nanoparticles. Foods 2023, 12, 2999. [Google Scholar] [CrossRef] [PubMed]
- Miao, Y.; Yang, T.; Yang, S.; Yang, M.; Mao, C. Protein nanoparticles directed cancer imaging and therapy. Nano Converg. 2022, 9, 2. [Google Scholar] [CrossRef] [PubMed]
- Nikzamir, M.; Akbarzadeh, A.; Panahi, Y. An overview on nanoparticles used in biomedicine and their cytotoxicity. J. Drug Deliv. Sci. Technol. 2021, 61, 102316. [Google Scholar] [CrossRef]
- Khatua, S.; Simal-Gandara, J.; Acharya, K. Understanding immune-modulatory efficacy in vitro. Chem. Biol. Interact. 2022, 352, 109776. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez Garza, N.G.; Chuc Koyoc, J.A.; Torres Castillo, J.A.; Garcia Zambrano, E.A.; Betancur Ancona, D.; Chel Guerrero, L.; Sinagawa Garcia, S.R. Biofunctional properties of bioactive peptide fractions from protein isolates of moringa seed (Moringa oleifera). J. Food Sci. Technol. 2017, 54, 4268–4276. [Google Scholar] [CrossRef] [PubMed]
- Aderinola, T.A.; Alashi, A.M.; Nwachukwu, I.D.; Fagbemi, T.N.; Enujiugha, V.N.; Aluko, R.E. In vitro digestibility, structural and functional properties of Moringa oleifera seed proteins. Food Hydrocoll. 2020, 101, 105574. [Google Scholar] [CrossRef]
- Metzger, L.E. Nutrition labeling using a computer program. In Nielsen’s Food Analysis Laboratory Manual; Springer: Cham, Switzerland, 2010; pp. 1–7. [Google Scholar]
- Jahanban-Esfahlan, A.; Dastmalchi, S.; Davaran, S. A simple improved desolvation method for the rapid preparation of albumin nanoparticles. Int. J. Biol. Macromol. 2016, 91, 703–709. [Google Scholar] [CrossRef]
- Vauthier, C.; Cabane, B.; Labarre, D. How to concentrate nanoparticles and avoid aggregation? Eur. J. Pharm. Biopharm. 2008, 69, 466–475. [Google Scholar] [CrossRef]
- Arzeni, C.; Martínez, K.; Zema, P.; Arias, A.; Pérez, O.E.; Pilosof, A.M.R. Comparative study of high intensity ultrasound effects on food proteins functionality. J. Food Eng. 2012, 108, 463–472. [Google Scholar] [CrossRef]
- Fan, Y.; Yi, J.; Zhang, Y.; Yokoyama, W. Fabrication of curcumin-loaded bovine serum albumin (BSA)-dextran nanoparticles and the cellular antioxidant activity. Food Chem. 2018, 239, 1210–1218. [Google Scholar] [CrossRef] [PubMed]
- Dyballa, N.; Metzger, S. Fast and sensitive colloidal coomassie G-250 staining for proteins in polyacrylamide gels. J. Vis. Exp. 2009, 30, e143. [Google Scholar] [CrossRef]
- Fan, Y.; Yi, J.; Zhang, Y.; Wen, Z.; Zhao, L. Physicochemical stability and in vitro bioaccessibility of β-carotene nanoemulsions stabilized with whey protein-dextran conjugates. Food Hydrocoll. 2017, 63, 256–264. [Google Scholar] [CrossRef]
- Zhao, X.; Zhu, H.; Zhang, B.; Chen, J.; Ao, Q.; Wang, X. XRD, SEM, and XPS Analysis of Soybean Protein Powders Obtained Through Extraction Involving Reverse Micelles. J. Am. Oil Chem. Soc. 2015, 92, 975–983. [Google Scholar] [CrossRef]
- Su, J.-F.; Huang, Z.; Yuan, X.-Y.; Wang, X.-Y.; Li, M. Structure and properties of carboxymethyl cellulose/soy protein isolate blend edible films crosslinked by Maillard reactions. Carbohydr. Polym. 2010, 79, 145–153. [Google Scholar] [CrossRef]
- Salem, M.A.; Aborehab, N.M.; Al-Karmalawy, A.A.; Fernie, A.R.; Alseekh, S.; Ezzat, S.M. Potential Valorization of Edible Nuts By-Products: Exploring the Immune-Modulatory and Antioxidants Effects of Selected Nut Shells Extracts in Relation to Their Metabolic Profiles. Antioxidants 2022, 11, 462. [Google Scholar] [CrossRef]
- Guo, C.; Bi, J.; Li, X.; Lyu, J.; Liu, X.; Wu, X.; Liu, J. Immunomodulation effects of polyphenols from thinned peach treated by different drying methods on RAW264.7 cells through the NF-kappaB and Nrf2 pathways. Food Chem. 2021, 340, 127931. [Google Scholar] [CrossRef]
- Hernandez-Santos, B.; Santiago-Adame, R.; Navarro-Cortez, R.O.; Gomez-Aldapa, C.A.; Castro-Rosas, J.; Martinez-Sanchez, C.E.; Vivar-Vera, M.A.; Herman-Lara, E.; Rodriguez-Miranda, J. Physical properties of ebony seed (Pithecellobium flexicaule) and functional properties of whole and defatted ebony seed meal. J. Food Sci. Technol. 2015, 52, 4483–4490. [Google Scholar] [CrossRef]
- Saa, R.W.; Fombang Nig, E.; Radha, C.; Ndjantou, E.B.; Njintang Yanou, N. Effect of soaking, germination, and roasting on the proximate composition, antinutrient content, and some physicochemical properties of defatted Moringa oleifera seed flour. J. Food Process. Preserv. 2022, 46, e16329. [Google Scholar] [CrossRef]
- Cattan, Y.; Patil, D.; Vaknin, Y.; Rytwo, G.; Lakemond, C.; Benjamin, O. Characterization of Moringa oleifera leaf and seed protein extract functionality in emulsion model system. Innov. Food Sci. Emerg. Technol. 2022, 75, 102903. [Google Scholar] [CrossRef]
- Chang, R.; Yang, J.; Ge, S.; Zhao, M.; Liang, C.; Xiong, L.; Sun, Q. Synthesis and self-assembly of octenyl succinic anhydride modified short glucan chains based amphiphilic biopolymer: Micelles, ultrasmall micelles, vesicles, and lutein encapsulation/release. Food Hydrocoll. 2017, 67, 14–26. [Google Scholar] [CrossRef]
- Gharibzahedi, S.M.T.; Smith, B. The functional modification of legume proteins by ultrasonication: A review. Trends Food Sci. Technol. 2020, 98, 107–116. [Google Scholar] [CrossRef]
- Xiong, T.; Xiong, W.; Ge, M.; Xia, J.; Li, B.; Chen, Y. Effect of high intensity ultrasound on structure and foaming properties of pea protein isolate. Food Res. Int. 2018, 109, 260–267. [Google Scholar] [CrossRef] [PubMed]
- Shamsara, O.; Muhidinov, Z.K.; Jafari, S.M.; Bobokalonov, J.; Jonmurodov, A.; Taghvaei, M.; Kumpugdee-Vollrath, M. Effect of ultrasonication, pH and heating on stability of apricot gum-lactoglobuline two layer nanoemulsions. Int. J. Biol. Macromol. 2015, 81, 1019–1025. [Google Scholar] [CrossRef] [PubMed]
- Dong, D.; Cui, B. Fabrication, characterization and emulsifying properties of potato starch/soy protein complexes in acidic conditions. Food Hydrocoll. 2021, 115, 106600. [Google Scholar] [CrossRef]
- Han, Y.; Li, K.; Chen, H.; Li, J. Properties of Soy Protein Isolate Biopolymer Film Modified by Graphene. Polymers 2017, 9, 312. [Google Scholar] [CrossRef]
- Gu, W.; Liu, X.; Ye, Q.; Gao, Q.; Gong, S.; Li, J.; Shi, S.Q. Bio-inspired co-deposition strategy of aramid fibers to improve performance of soy protein isolate-based adhesive. Ind. Crops Prod. 2020, 150, 112424. [Google Scholar] [CrossRef]
- Mehra, S.; Nisar, S.; Chauhan, S.; Singh, V.; Rattan, S. Soy Protein-Based Hydrogel under Microwave-Induced Grafting of Acrylic Acid and 4-(4-Hydroxyphenyl)butanoic Acid: A Potential Vehicle for Controlled Drug Delivery in Oral Cavity Bacterial Infections. ACS Omega 2020, 5, 21610–21622. [Google Scholar] [CrossRef]
- Jia, Z.; Yang, C.; Zhao, F.; Chao, X.; Li, Y.; Xing, H. One-Step Reinforcement and Deacidification of Paper Documents: Application of Lewis Base—Chitosan Nanoparticle Coatings and Analytical Characterization. Coatings 2020, 10, 1226. [Google Scholar] [CrossRef]
- Qin, Z.; Mo, L.; Liao, M.; He, H.; Sun, J. Preparation and Characterization of Soy Protein Isolate-Based Nanocomposite Films with Cellulose Nanofibers and Nano-Silica via Silane Grafting. Polymers 2019, 11, 1835. [Google Scholar] [CrossRef] [PubMed]
- Prasetyo, T.; Soegijono, B. Effect of sonication process on natural zeolite at ferric chloride hexahydrate solution. J. Phys. Conf. Ser. 2017, 817, 012032. [Google Scholar] [CrossRef]
- Riaz, T.; Zeeshan, R.; Zarif, F.; Ilyas, K.; Muhammad, N.; Safi, S.Z.; Rahim, A.; Rizvi, S.A.A.; Rehman, I.U. FTIR analysis of natural and synthetic collagen. Appl. Spectrosc. Rev. 2018, 53, 703–746. [Google Scholar] [CrossRef]
- Ji, Y.; Yang, X.; Ji, Z.; Zhu, L.; Ma, N.; Chen, D.; Jia, X.; Tang, J.; Cao, Y. DFT-Calculated IR Spectrum Amide I, II, and III Band Contributions of N-Methylacetamide Fine Components. ACS Omega 2020, 5, 8572–8578. [Google Scholar] [CrossRef] [PubMed]
- Kebede, T.G.; Dube, S.; Nindi, M.M. Removal of non-steroidal anti-inflammatory drugs (NSAIDs) and carbamazepine from wastewater using water-soluble protein extracted from Moringa stenopetala seeds. J. Environ. Chem. Eng. 2018, 6, 3095–3103. [Google Scholar] [CrossRef]
- Li, T.; Lu, X.M.; Zhang, M.R.; Hu, K.; Li, Z. Peptide-based nanomaterials: Self-assembly, properties and applications. Bioact. Mater. 2022, 11, 268–282. [Google Scholar] [CrossRef] [PubMed]
- Saito, K.; Xu, T.; Ishikita, H. Correlation between C horizontal lineO Stretching Vibrational Frequency and pK(a) Shift of Carboxylic Acids. J. Phys. Chem. B 2022, 126, 4999–5006. [Google Scholar] [CrossRef] [PubMed]
- Gupta, R.; Pancholi, K.; Prabhu, R.; Pancholi, M.; Huo, D.; Jha, V.; Latto, J. Integrated self-healing of the composite offshore structures. In Proceedings of the OCEANS 2017, Aberdeen, UK, 19–22 June 2017; pp. 1–4. [Google Scholar]
- Fadlelmoula, A.; Pinho, D.; Carvalho, V.H.; Catarino, S.O.; Minas, G. Fourier Transform Infrared (FTIR) Spectroscopy to Analyse Human Blood over the Last 20 Years: A Review towards Lab-on-a-Chip Devices. Micromachines 2022, 13, 187. [Google Scholar] [CrossRef]
- Liu, Q.; Cheng, J.; Sun, X.; Guo, M. Preparation, characterization, and antioxidant activity of zein nanoparticles stabilized by whey protein nanofibrils. Int. J. Biol. Macromol. 2021, 167, 862–870. [Google Scholar] [CrossRef]
- Wei, Y.; Zhan, X.; Dai, L.; Zhang, L.; Mao, L.; Yuan, F.; Liu, J.; Gao, Y. Formation mechanism and environmental stability of whey protein isolate-zein core-shell complex nanoparticles using the pH-shifting method. LWT 2021, 139, 110605. [Google Scholar] [CrossRef]
- Tang, Q.; Roos, Y.H.; Miao, S. Plant Protein versus Dairy Proteins: A pH-Dependency Investigation on Their Structure and Functional Properties. Foods 2023, 12, 368. [Google Scholar] [CrossRef] [PubMed]
- Ahlstrom, C.; Thuvander, J.; Rayner, M.; Matos, M.; Gutierrez, G.; Ostbring, K. The Effect of Precipitation pH on Protein Recovery Yield and Emulsifying Properties in the Extraction of Protein from Cold-Pressed Rapeseed Press Cake. Molecules 2022, 27, 2957. [Google Scholar] [CrossRef] [PubMed]
- Saif, A.; Anjum, L.; Faisal, Z.; Akram, N.; Shah, Y.A.; Irfan, R.; Saeed, F.; Afzaal, M.; Asif Shah, M. Recent advances in protein-based nanoparticles and their applications in the delivery of bioactive compounds. Int. J. Food Prop. 2023, 26, 2866–2880. [Google Scholar] [CrossRef]
- Chandrashekar, S.; Vijayakumar, R.; Chelliah, R.; Oh, D.H. Identification and Purification of Potential Bioactive Peptide of Moringa oleifera Seed Extracts. Plants 2020, 9, 1445. [Google Scholar] [CrossRef] [PubMed]
- Huang, Z.; Huang, X.; Zhou, W.; Zhang, L.; Liu, F.; Li, J.; Peng, S.; Cao, Y.; Li, Y.; Li, R.; et al. Fabrication and stability of Pickering emulsions using moringa seed residue protein: Effect of pH and ionic strength. Int. J. Food Sci. Technol. 2021, 56, 3484–3494. [Google Scholar] [CrossRef]
- Chen, R.; Wang, X.J.; Zhang, Y.Y.; Xing, Y.; Yang, L.; Ni, H.; Li, H.H. Simultaneous extraction and separation of oil, proteins, and glucosinolates from Moringa oleifera seeds. Food Chem. 2019, 300, 125162. [Google Scholar] [CrossRef] [PubMed]
- Yadav, P.; Yadav, A.B. Preparation and characterization of BSA as a model protein loaded chitosan nanoparticles for the development of protein-/peptide-based drug delivery system. Future J. Pharm. Sci. 2021, 7, 200. [Google Scholar] [CrossRef]
- Rodrigues, S.; Dionisio, M.; Lopez, C.R.; Grenha, A. Biocompatibility of chitosan carriers with application in drug delivery. J. Funct. Biomater. 2012, 3, 615–641. [Google Scholar] [CrossRef]
- Yao, W.; Zha, Q.; Cheng, X.; Wang, X.; Wang, J.; Tang, R. Folic acid-conjugated soybean protein-based nanoparticles mediate efficient antitumor ability in vitro. J. Biomater. Appl. 2017, 31, 832–843. [Google Scholar] [CrossRef]
- Verma, M.L.; Dhanya, B.S.; Sukriti; Rani, V.; Thakur, M.; Jeslin, J.; Kushwaha, R. Carbohydrate and protein based biopolymeric nanoparticles: Current status and biotechnological applications. Int. J. Biol. Macromol. 2020, 154, 390–412. [Google Scholar] [CrossRef] [PubMed]
- Kaltbeitzel, J.; Wich, P.R. Protein-based Nanoparticles: From Drug Delivery to Imaging, Nanocatalysis and Protein Therapy. Angew. Chem. Int. Ed. Engl. 2023, 62, e202216097. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.; Kim, H.; Suh, S.-Y.; Park, H.; Lee, H. Association between inflammatory cytokines and caregiving distress in family caregivers of cancer patients. Support. Care Cancer 2022, 30, 1715–1722. [Google Scholar] [CrossRef] [PubMed]
- Shen, J.; Xiao, Z.; Zhao, Q.; Li, M.; Wu, X.; Zhang, L.; Hu, W.; Cho, C.H. Anti-cancer therapy with TNF α and IFN γ: A comprehensive review. Cell Prolif. 2018, 51, e12441. [Google Scholar] [CrossRef] [PubMed]
- Hirano, T. IL-6 in inflammation, autoimmunity and cancer. Int. Immunol. 2021, 33, 127–148. [Google Scholar] [CrossRef]
- Zhang, L.; Reddy, N.; Khoo, C.S.; Koyyalamudi, S.R. Structural Characterization and In-Vitro Antioxidant and Immunomodulatory Activities of Polysaccharide Fractions Isolated from Artemisia annua L. Molecules 2022, 27, 3643. [Google Scholar] [CrossRef]
- Groysman, L.; Carlsen, L.; Huntington, K.E.; Shen, W.H.; Zhou, L.; El-Deiry, W.S. Chemotherapy-induced cytokines and prognostic gene signatures vary across breast and colorectal cancer. Am. J. Cancer Res. 2021, 11, 6086. [Google Scholar]

| Parameter | Moringa Seed Flour | Moringa Seed Cake Flour |
|---|---|---|
| Moisture | 5.35 ± 0.15 | 6.93 ± 0.08 |
| Ash | 3.55 ± 0.05 | 4.72 ± 0.08 |
| Protein | 32.00 ± 0.20 | 54.20 ± 0.10 |
| Lipids | 36.20 ± 0.30 | 3.17 ± 0.17 |
| Fibers | 7.00 ± 0.20 | 9.12 ± 0.08 |
| Carbohydrates | 15.90 ± 0.10 | 20.87 ± 0.83 |
| Test | IL-6 (pg mL−1) | TNF-α (pg mL−1) |
|---|---|---|
| Control | 113 ± 3.40 | 78 ± 1.78 |
| Treated | 88 ± 0.92 | 89.23 ± 0.76 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nafeh, A.A.E.-S.A.E.-K.; Mohamed, I.M.A.E.-A.; Foda, M.F. Ultrasonication-Assisted Green Synthesis and Physicochemical and Cytotoxic Activity Characterization of Protein-Based Nanoparticles from Moringa oleifera Seeds. Nanomaterials 2024, 14, 1254. https://doi.org/10.3390/nano14151254
Nafeh AAE-SAE-K, Mohamed IMAE-A, Foda MF. Ultrasonication-Assisted Green Synthesis and Physicochemical and Cytotoxic Activity Characterization of Protein-Based Nanoparticles from Moringa oleifera Seeds. Nanomaterials. 2024; 14(15):1254. https://doi.org/10.3390/nano14151254
Chicago/Turabian StyleNafeh, Amany Abd El-Shafy Abd El-Kader, Ibrahim Mohamed Abd El-Aleem Mohamed, and Mohamed Frahat Foda. 2024. "Ultrasonication-Assisted Green Synthesis and Physicochemical and Cytotoxic Activity Characterization of Protein-Based Nanoparticles from Moringa oleifera Seeds" Nanomaterials 14, no. 15: 1254. https://doi.org/10.3390/nano14151254






