Hybrid Amino Acid Ligand-Regulated Excited Dynamics of Highly Luminescent Perovskite Quantum Dots for Bright White Light-Emitting Diodes
Abstract
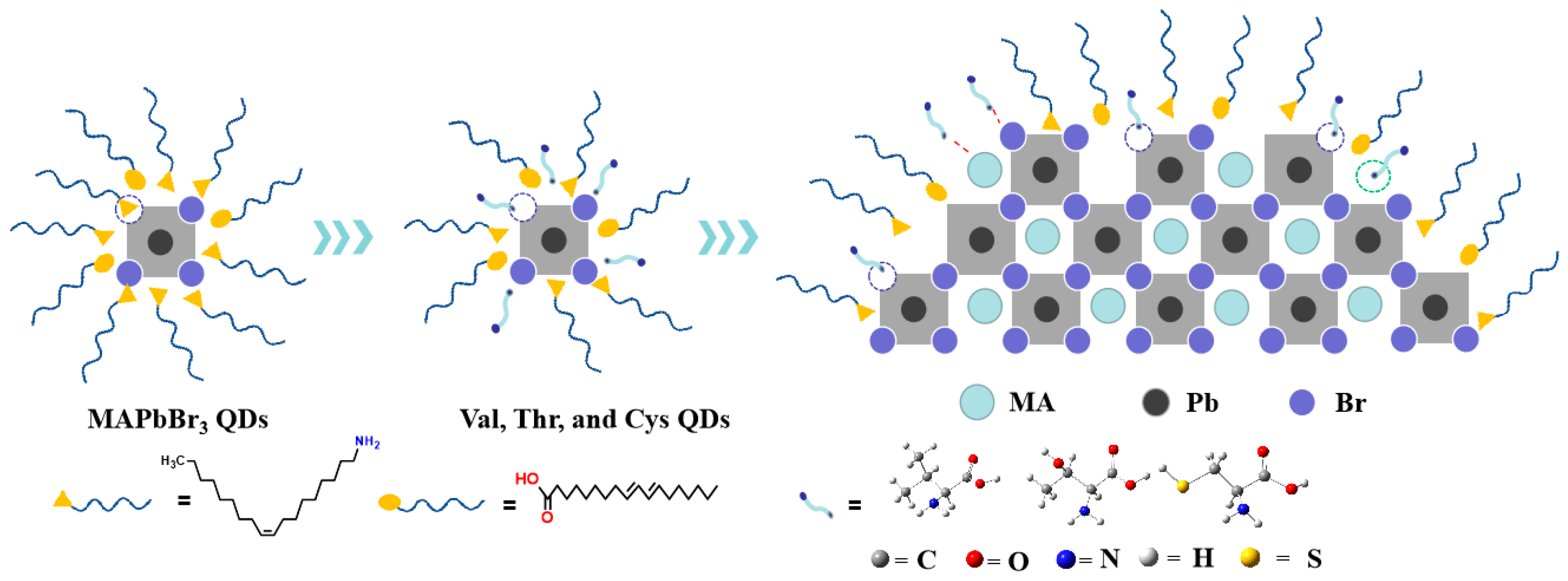
:1. Introduction
2. Experimentation Section
2.1. Materials
2.2. Synthesis Process of CH3NH3Br
2.3. Synthesis Process of CH3NH3PbBr3 QDs
2.4. Fabricating Process of WLED Device
2.5. Characteristic Section
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Wang, Y.X.; Ding, G.L.; Mao, J.Y.; Zhou, Y.; Han, S.T. Recent advances in synthesis and application of perovskite quantum dot based composites for photonics, electronics and sensors. Sci. Technol. Adv. Mater. 2020, 21, 278–302. [Google Scholar] [CrossRef]
- Zhou, X.J.; Zhao, S.C.; Zhang, J.; Xiang, G.T.; Jiang, S.; Li, L.; Wang, Y.J.; Li, Y.H.; Jing, C.; Yao, L.; et al. FA+ and Mn2+ codoped CsPbCl3 perovskite quantum dots with super thermal stability. Ceram. Int. 2023, 49, 1002–1008. [Google Scholar] [CrossRef]
- Zhang, J.; Yang, X.K.; Deng, H.; Qiao, K.K.; Farooq, U.; Ishaq, M.; Yi, F.; Liu, H.; Tang, J.; Song, H.S. Low-dimensional halide perovskites and their advanced optoelectronic applications. Nanomicro Lett. 2017, 9, 36. [Google Scholar] [CrossRef]
- Tian, J.J.; Xue, Q.F.; Yao, Q.; Li, N.; Brabec, C.J.; Yip, H.L. Inorganic halide perovskite solar cells: Progress and challenges. Adv. Energy Mater. 2020, 10, 2000183. [Google Scholar] [CrossRef]
- Liu, X.K.; Xu, W.D.; Bai, S.; Jin, Y.Z.; Wang, J.P.; Friend, R.H.; Gao, F. Metal halide perovskites for light-emitting diodes. Nat. Mater. 2021, 20, 10–21. [Google Scholar] [CrossRef]
- Xing, J.; Yan, F.; Zhao, Y.W.; Chen, S.; Yu, H.K.; Zhang, Q.; Zeng, R.G.; Demir, H.V.; Huan, A.; Xiong, Q.H. High-efficiency light-emitting diodes of organometal halide perovskite amorphous nanoparticles. ACS Nano 2016, 10, 6623–6630. [Google Scholar] [CrossRef]
- Dong, H.Y.; Zhang, C.H.; Liu, X.L.; Yao, J.N.; Zhao, Y.S. Materials chemistry and engineering in metal halide perovskite lasers. Chem. Soc. Rev. 2020, 49, 951–982. [Google Scholar] [CrossRef]
- Fu, Y.P.; Zhu, H.M.; Stoumpos, C.C.; Ding, Q.; Wang, J.; Kanatzidis, M.G.; Zhu, X.Y.; Jin, S. Broad wavelength tunable robust lasing from single-crystal nanowires of cesium lead Halide perovskites (CsPbX3, X = Cl, Br, I). ACS Nano 2016, 10, 7963–7972. [Google Scholar] [CrossRef]
- Liu, Z.; Qiu, W.D.; Peng, X.M.; Sun, G.W.; Liu, X.Y.; Liu, D.H.; Li, Z.C.; He, F.G.; Shen, C.Y.; Gu, Q.; et al. Perovskite light-emitting diodes with EQE exceeding 28% through a synergetic dual-additive strategy for defect passivation and nanostructure regulation. Adv. Mater. 2021, 33, e2103268. [Google Scholar] [CrossRef]
- Schmidt, L.C.; Pertegás, A.; González-Carrero, S.; Malinkiewicz, O.; Agouram, S.; Espallargas, G.M.; Bolink, H.J.; Galian, R.E.; Pérez-Prieto, J. Nontemplate synthesis of CH3NH3PbBr3 perovskite nanoparticles. J. Am. Chem. Soc. 2014, 136, 850–853. [Google Scholar] [CrossRef]
- Xu, Y.Q.; Chen, Q.; Zhang, C.F.; Wang, R.; Wu, H.; Zhang, X.Y.; Xing, G.C.; Yu, W.W.; Wang, X.Y.; Zhang, Y.; et al. Two-photon-pumped perovskite semiconductor nanocrystal lasers. J. Am. Chem. Soc. 2016, 138, 3761–3768. [Google Scholar] [CrossRef]
- Huang, C.Y.; Zou, C.; Mao, C.Y.; Corp, K.L.; Yao, Y.C.; Lee, Y.J.; Schlenker, C.W.; Jen, A.K.Y.; Lin, L.Y. CsPbBr3 perovskite quantum dot vertical cavity lasers with low threshold and high stability. ACS Photonics 2017, 4, 2281–2289. [Google Scholar] [CrossRef]
- Sun, C.; Zhang, Y.; Ruan, C.; Yin, C.Y.; Wang, X.Y.; Wang, Y.D.; Yu, W.W. Efficient and stable white LEDs with silica-coated inorganic perovskite quantum dots. Adv. Mater. 2016, 28, 10088–10094. [Google Scholar] [CrossRef] [PubMed]
- Fang, T.; Wang, T.T.; Li, X.S.; Dong, Y.H.; Bai, S.; Song, J.Z. Perovskite QLED with an external quantum efficiency of over 21% by modulating electronic transport. Sci. Bull. 2021, 66, 36–43. [Google Scholar] [CrossRef]
- Hu, L.; Zhao, Q.; Huang, S.J.; Zheng, J.H.; Guan, X.W.; Patterson, R.; Kim, J.Y.; Shi, L.; Lin, C.H.; Lei, Q.; et al. Flexible and efficient perovskite quantum dot solar cells via hybrid interfacial architecture. Nat. Commun. 2021, 12, 466. [Google Scholar] [CrossRef]
- Xiao, M.; Hao, M.M.; Lyu, M.; Moore, E.G.; Zhang, C.; Luo, B.; Hou, J.W.; Lipton-Duffin, J.; Wang, L.Z. Surface ligands stabilized lead halide perovskite quantum dot photocatalyst for visible light-driven hydrogen generation. Adv. Funct. Mater. 2019, 29, 1905683. [Google Scholar] [CrossRef]
- Ma, L.; Wang, F.J. Toward high-performances of halide light-emitting diodes: The importance of ligands engineering. Inorganics 2023, 11, 230. [Google Scholar] [CrossRef]
- Li, Y.M.; Deng, M.; Zhang, X.Y.; Qian, L.; Xiang, C.Y. Proton-Prompted Ligand Exchange to Achieve High-Efficiency CsPbI3 Quantum Dot Light-Emitting Diodes. NanoMicro Lett. 2024, 26, 105. [Google Scholar] [CrossRef]
- Luo, X.; Lai, R.C.; Li, Y.L.; Han, Y.Y.; Liang, G.J.; Liu, X.; Ding, T.; Wang, J.H.; Wu, K.F. Triplet energy transfer from CsPbBr3 nanocrystals enabled by quantum confinement. J. Am. Chem. Soc. 2019, 141, 4186–4190. [Google Scholar] [CrossRef]
- Xu, Y.F.; Yang, M.Z.; Chen, B.X.; Wang, X.D.; Chen, H.Y.; Kuang, D.B.; Su, C.Y. A CsPbBr3 perovskite quantum dot/graphene oxide composite for photocatalytic CO2 reduction. J. Am. Chem. Soc. 2017, 139, 5660–5663. [Google Scholar] [CrossRef]
- Su, Y.; Chen, X.J.; Ji, W.Y.; Zeng, Q.H.; Ren, Z.Y.; Su, Z.S.; Liu, L. Highly controllable and efficient synthesis of mixed-halide CsPbX3 (X = Cl, Br, I) perovskite QDs toward the tunability of entire visible light. ACS Appl. Mater. Interfaces 2017, 9, 33020–33028. [Google Scholar] [CrossRef] [PubMed]
- Hills-Kimball, K.; Yang, H.J.; Cai, T.; Wang, J.Y.; Chen, O. Recent advances in ligand design and engineering in lead halide perovskite nanocrystals. Adv. Sci. 2021, 8, 2100214. [Google Scholar] [CrossRef] [PubMed]
- Hills-Kimball, K.; Nagaoka, Y.; Cao, C.; Chaykovsky, E.; Chen, O. Synthesis of formamidinium lead halide perovskite nanocrystals through solid-liquid-solid cation exchange. J. Mater. Chem. C 2017, 5, 5680–5684. [Google Scholar] [CrossRef]
- Linaburg, M.R.; Mcclure, E.T.; Majher, J.D.; Woodward, P.M. Cs1-xRbxPbCl3 and Cs1-xRbxPbBr3 solid solutions: Understanding octahedral tilting in lead halide perovskites. Chem. Mater. 2017, 29, 3507–3514. [Google Scholar] [CrossRef]
- Baek, S.; Kim, S.; Noh, J.Y.; Heo, J.H.; Im, S.H.; Hong, K.H.; Kim, S.W. Development of mixed-cation CsxRb1-xPbX3 perovskite quantum dots and their full-color film with high stability and wide color gamut. Adv. Opt. Mater. 2018, 6, 1800295. [Google Scholar] [CrossRef]
- Liu, Y.; Pan, G.; Wang, R.; Shao, H.; Wang, H.; Xu, W.; Cui, H.N.; Song, H.W. Considerably enhanced exciton emission of CsPbCl3 perovskite quantum dots by the introduction of potassium and lanthanide ions. Nanoscale 2018, 10, 14067–14072. [Google Scholar] [CrossRef] [PubMed]
- Wu, Q.; Liu, X.; Du, Y.S.; Teng, C.L.; Liang, F. Abnormal bandgap enlargement resulted in a promising mid-infrared nonlinear optical material Rb2CdBrI3 with an ultrahigh laser damage threshold. J. Mater. Chem. C 2020, 8, 9005–9011. [Google Scholar] [CrossRef]
- Xie, G.C.; Jiang, C.B.; Wang, J.H.; Mai, C.H.; Huang, G.H.; Ma, Y.W.; Wang, J.; Peng, J.B.; Cao, Y. Stable mixed-cation perovskite light-emitting diodes. Org. Electron. 2019, 71, 58–64. [Google Scholar] [CrossRef]
- Parobek, D.; Roman, B.J.; Dong, Y.T.; Jin, H.; Lee, E.; Sheldon, M.; Son, D.H. Exciton-to-dopant energy transfer in Mn-doped cesium lead halide perovskite nanocrystals. Nano Lett. 2016, 16, 7376–7380. [Google Scholar] [CrossRef]
- Adhikari, G.C.; Zhu, H.Y.; Vargas, P.A.; Zhu, P.F. UV-Green Emission from Organolead Bromide Perovskite Nanocrystals. J. Phys. Chem. C 2018, 122, 15041–15046. [Google Scholar] [CrossRef]
- Begum, R.; Chin, X.Y.; Li, M.; Damodaran, B.; Sum, T.C.; Mhaisalkar, S.; Mathews, N. Stable Sn2+ doped FAPbI3 nanocrystals for near-infrared LEDs. Chem. Commun. 2019, 55, 5451–5454. [Google Scholar] [CrossRef]
- Yan, D.D.; Mo, Q.H.; Zhao, S.Y.; Cai, W.S.; Zang, Z.G. Room temperature synthesis of Sn2+ doped highly luminescent CsPbBr3 quantum dots for high CRI white light-emitting diodes. Nanoscale 2021, 13, 9740–9746. [Google Scholar] [CrossRef]
- Zhang, Z.; Ren, L.X.; Yan, H.; Guo, S.J.; Wang, S.H.; Wang, M.; Jin, K.X. Bandgap narrowing in Bi-doped CH3NH3PbCl3 perovskite single crystals and thin films. J. Phys. Chem. C 2017, 121, 17436–17441. [Google Scholar] [CrossRef]
- Tan, Z.; Li, J.H.; Zhang, C.; Hu, Q.S.; Xiao, Z.W.; Kamiya, T.; Hosono, H.; Niu, G.D.; Lifshitz, E.; Cheng, Y.B.; et al. Highly efficient blue-emitting Bi-doped Cs2SnCl6 perovskite variant: Photoluminescence induced by impurity doping. Adv. Funct. Mater. 2018, 28, 1801131. [Google Scholar] [CrossRef]
- Singh, H.; Dey, P.; Chatterjee, S.; Sen, P.; Maiti, T. Formamidinium containing tetra cation organic-inorganic hybrid perovskite solar cell. Sol. Energy 2021, 220, 258–268. [Google Scholar] [CrossRef]
- Zou, S.H.; Liu, Y.S.; Li, J.H.; Liu, C.P.; Feng, R.; Jiang, F.L.; Li, Y.X.; Song, J.Z.; Zeng, H.B.; Hong, M.C.; et al. Stabilizing cesium lead halide perovskite lattice through Mn(II) substitution for air-stable light-emitting diodes. J. Am. Chem. Soc. 2017, 139, 11443–11450. [Google Scholar] [CrossRef]
- Liu, F.; Zhang, Y.H.; Ding, C.; Kobayashi, S.; Izuishi, T.; Nakazawa, N.; Toyoda, T.; Ohta, T.; Hayase, S.; Minemoto, T.; et al. Highly luminescent phase-stable CsPbI3 perovskite quantum dots achieving near 100% absolute photoluminescence quantum yield. ACS Nano 2017, 11, 10373–10383. [Google Scholar] [CrossRef] [PubMed]
- Zou, X.S.; Wen, G.Z.; Hu, R.; Dong, G.; Zhang, C.Y.; Zhang, W.; Huang, H.; Dang, W. An insight into the excitation states of small molecular semiconductor Y6. Molecules 2020, 25, 4118. [Google Scholar] [CrossRef]
- Song, J.Z.; Li, J.H.; Xu, L.M.; Li, J.H.; Zhang, F.J.; Han, B.N.; Shan, Q.S.; Zeng, H.B. Room-temperature triple-ligand surface engineering synergistically boosts ink stability, recombination dynamics, and charge injection toward EQE-11.6% perovskite QLEDs. Adv. Mater. 2018, 30, e1800764. [Google Scholar] [CrossRef]
- Yang, D.D.; Li, X.M.; Zhou, W.H.; Zhang, S.L.; Meng, C.F.; Wu, Y.; Wang, Y.; Zeng, H.B. CsPbBr3 quantum dots 2.0: Benzenesulfonic acid equivalent ligand awakens complete purification. Adv. Mater. 2019, 31, 1900767. [Google Scholar] [CrossRef]
- Adhikari, G.C.; Vargas, P.A.; Zhu, H.; Zhu, P. Saponification precipitation method for CsPbBr3 nanocrystals with blue-green tunable emission. J. Phys. Chem. C 2019, 123, 1406–1412. [Google Scholar] [CrossRef]
- Kumar, G.; Lin, C.C.; Kuo, H.C.; Chen, F.C. Enhancing photoluminescence performance of perovskite quantum dots with plasmonic nanoparticles: Insights into mechanisms and light-emitting applications. Nanoscale Adv. 2024, 6, 782–791. [Google Scholar] [CrossRef] [PubMed]
- Yang, M.; Peng, H.S.; Zeng, F.L.; Teng, F.; Qu, Z.; Yang, D.; Wang, Y.Q.; Chen, G.X.; Wang, D.W. In situ silica coating-directed synthesis of orthorhombic methylammonium lead bromide perovskite quantum dots with high stability. J. Colloid Interface Sci. 2018, 509, 32–38. [Google Scholar] [CrossRef] [PubMed]
- Xu, Z.Q.; Chen, L.H.; Zhang, L.Q.; Jing, S.L.; Zhuang, B.; Xu, W.H.; Chen, D.Q. Yb/Er: Cs2Ag(In/Bi)Cl6 lead-free double perovskite for dual-modal optical temperature sensing. J. Lumin. 2022, 248, 118996. [Google Scholar] [CrossRef]
- Adhikari, G.C.; Vargas, P.A.; Zhu, H.; Grigoriev, A.; Zhu, P. Tetradic phosphor white light with variable CCT and superlative CRI through organolead halide perovskite nanocrystals. Nanoscale Adv. 2019, 1, 1791–1798. [Google Scholar] [CrossRef]
- Li, Z.J.; Hofman, E.; Li, J.; Davis, A.H.; Tung, C.H.; Wu, L.Z.; Zheng, W.W. Photoelectrochemically active and environmentally stable CsPbBr3/TiO2 core/shell nanocrystals. Adv. Funct. Mater. 2017, 28, 1704288. [Google Scholar] [CrossRef]
- Ren, J.J.; Li, T.R.; Zhou, X.P.; Dong, X.; Shorokhov, A.V.; Semenov, M.B.; Krevchik, V.D.; Wang, Y.H. Encapsulating all-inorganic perovskite quantum dots into mesoporous metal organic frameworks with significantly enhanced stability for optoelectronic applications. Chem. Eng. J. 2019, 358, 30–39. [Google Scholar] [CrossRef]
- Wan, S.P.; Ou, M.; Zhong, Q.; Wang, X.M. Perovskite-type CsPbBr3 quantum dots/UiO-66(NH2) nanojunction as efficient visible-light-driven photocatalyst for CO2 reduction. Chem. Eng. J. 2019, 358, 1287–1295. [Google Scholar] [CrossRef]
- Krishnakanth, K.N.; Chandu, B.; Bharathi, M.S.S.; Raavi, S.S.K.; Rao, S.V. Ultrafast excited state dynamics and femtosecond nonlinear optical properties of laser fabricated Au and Ag50Au50 nanoparticles. Opt. Mater. 2019, 95, 109239. [Google Scholar] [CrossRef]
- Chen, K.Q.; Zhong, Q.H.; Chen, W.; Sang, B.H.; Wang, Y.W.; Yang, T.Q.; Liu, Y.L.; Zhang, Y.P.; Zhang, H. Short-chain ligand-passivated stable α-CsPbI3 quantum dot for all-inorganic perovskite solar cells. Adv. Funct. Mater. 2019, 29, 1900991. [Google Scholar] [CrossRef]
- Wang, J.T.; Xu, Y.S.; Zou, S.H.; Pang, C.; Cao, R.M.; Pan, Z.X.; Guo, C.; Hu, S.B.; Liu, J.C.; Xie, Z.Y.; et al. Effective defect passivation of CsPbBr3 quantum dots using galliumbcations toward the fabrication of bright perovskite LEDs. J. Mater. Chem. C 2021, 9, 11324–11330. [Google Scholar] [CrossRef]
- Yin, Y.M.; Hu, Z.P.; Ali, M.U.; Duan, M.; Gao, L.; Liu, M.; Peng, W.X.; Geng, J.; Pan, S.; Wu, Y.W.; et al. Full-color micro-LED display with CsPbBr3 perovskite and CdSe quantum dots as color conversion layers. Adv. Mater. Technol. 2020, 5, 2000251. [Google Scholar] [CrossRef]






| MAPbBr3 QDs | Val QDs | Thr QDs | Cys QDs | |
|---|---|---|---|---|
| Absorption wavelength (nm) | 490 | 480 | 470 | 460 |
| Emission wavelength (nm) | 550 | 534 | 515 | 500 |
| Sample | (ns) | (ns) | A1 (%) | A2 (%) | (ns) |
|---|---|---|---|---|---|
| MAPbBr3 QDs | 25.37 | 541.26 | 12.8 | 87.2 | 475.04 |
| Val-QDs | 29.41 | 580.23 | 11.2 | 88.8 | 518.28 |
| Thr-QDs | 26.95 | 547.55 | 12.3 | 87.7 | 483.84 |
| Cys-QDs | 32.28 | 616.29 | 10.4 | 89.6 | 555.49 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hu, B.; Zhang, W.; Chu, Y. Hybrid Amino Acid Ligand-Regulated Excited Dynamics of Highly Luminescent Perovskite Quantum Dots for Bright White Light-Emitting Diodes. Nanomaterials 2024, 14, 1266. https://doi.org/10.3390/nano14151266
Hu B, Zhang W, Chu Y. Hybrid Amino Acid Ligand-Regulated Excited Dynamics of Highly Luminescent Perovskite Quantum Dots for Bright White Light-Emitting Diodes. Nanomaterials. 2024; 14(15):1266. https://doi.org/10.3390/nano14151266
Chicago/Turabian StyleHu, Baoye, Weiqiang Zhang, and Ya Chu. 2024. "Hybrid Amino Acid Ligand-Regulated Excited Dynamics of Highly Luminescent Perovskite Quantum Dots for Bright White Light-Emitting Diodes" Nanomaterials 14, no. 15: 1266. https://doi.org/10.3390/nano14151266




