Suppression of Secondary Electron Emission by Vertical Graphene Coating on Ni Microcavity Substrate
Abstract
:1. Introduction
2. Materials and Methods
2.1. Preparation of Sample
2.2. SEE Characteristic Measurement of the Samples
3. Results and Discussion
3.1. Characterization of VG Coating
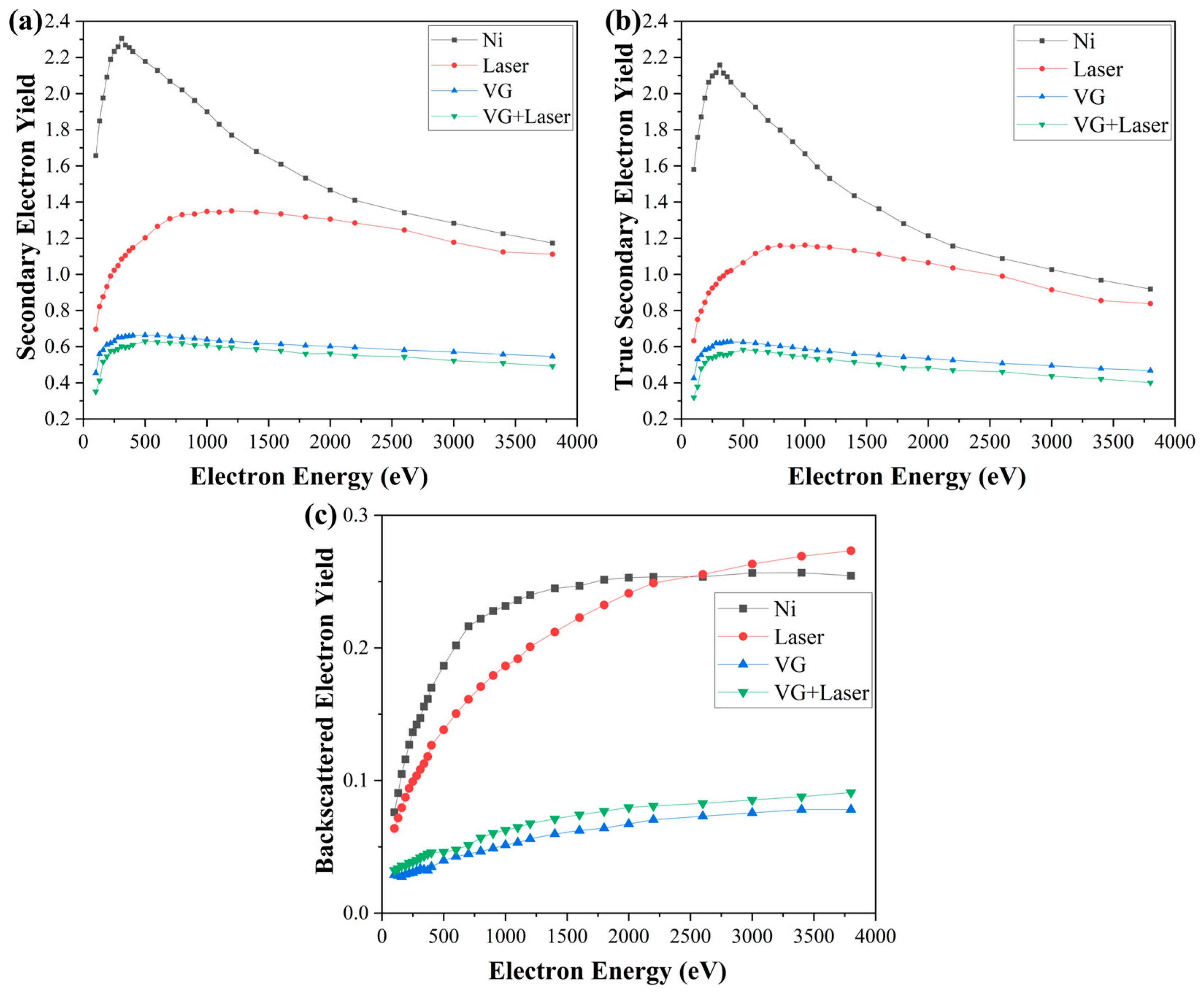
3.2. SEY Measurement Results
3.3. SEE Suppression Mechanism
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Kishek, R.A.; Lau, Y.Y. A novel phase focusing mechanism in multipactor discharge. Phys. Plasmas 1996, 3, 1481–1483. [Google Scholar] [CrossRef]
- Ohmi, K.; Zimmermann, F. Head-tail instability caused by electron clouds in positron storage rings. Phys. Rev. Lett. 2000, 85, 3821–3824. [Google Scholar] [CrossRef] [PubMed]
- Flanagan, J.W.; Ohmi, K.; Fukuma, H.; Hiramatsu, S.; Tobiyama, M.; Perevedentsev, E. Observation of vertical betatron sideband due to electron clouds in the KEKB low energy ring. Phys. Rev. Lett. 2005, 94, 054801. [Google Scholar] [CrossRef] [PubMed]
- Zhu, F.; Proch, D.; Hao, J.K. Multipacting phenomenon at high electric fields of superconducting cavities. Chin. Phys. 2005, 14, 494–499. [Google Scholar] [CrossRef]
- Lai, S.T.; Tautz, M.F. Aspects of spacecraft charging in sunlight. IEEE Trans. Plasma Sci. 2006, 34, 2053–2061. [Google Scholar] [CrossRef]
- Pivi, M.; King, F.K.; Kirby, R.E.; Raubenheimer, T.O.; Stupakov, G.; Le Pimpec, F. Sharp reduction of the secondary electron emission yield from grooved surfaces. J. Appl. Phys. 2008, 104, 104904. [Google Scholar] [CrossRef]
- Ye, M.; He, Y.N.; Hu, S.G.; Wang, R.; Hu, T.C.; Yang, J.; Cui, W.Z. Suppression of secondary electron yield by micro-porous array structure. J. Appl. Phys. 2013, 113, 074904. [Google Scholar] [CrossRef]
- Suharyanto; Michizono, S.; Saito, Y.; Yamano, Y.; Kobayashi, S. Secondary electron emission of TiN-coated alumina ceramics. Vacuum 2007, 81, 799–802. [Google Scholar] [CrossRef]
- Chen, J.; Louis, E.; Verhoeven, J.; Harmsen, R.; Lee, C.J.; Lubomska, M.; van Kampen, M.; van Schaik, W.; Bijkerk, F. Secondary electron yield measurements of carbon covered multilayer optics. Appl. Surf. Sci. 2010, 257, 354–361. [Google Scholar] [CrossRef]
- Jing, C.; Gai, W.; Power, J.G.; Konecny, R.; Liu, W.; Gold, S.H.; Kinkead, A.K.; Tantawi, S.G.; Dolgashev, V.; Kanareykin, A. Progress toward Externally Powered X-Band Dielectric-Loaded Accelerating Structures. IEEE Trans. Plasma Sci. 2010, 38, 1354–1360. [Google Scholar] [CrossRef]
- Burton, T.S.; Back, T.C.; Fairchild, S.B.; Thompson, G.B. Influence of surface roughness on secondary electron emission from graphite. J. Vac. Sci. Technol. A 2017, 35, 041404. [Google Scholar] [CrossRef]
- Suetsugu, Y.; Kanazawa, K.; Shibata, K.; Hisamatsu, H. Continuing study on the photoelectron and secondary electron yield of TiN coating and NEG (Ti-Zr-V) coating under intense photon irradiation at the KEKB positron ring. Nucl. Instrum. Methods Phys. Res. Sect. A Accel. Spectrometers Detect. Assoc. Equip. 2006, 556, 399–409. [Google Scholar] [CrossRef]
- Valizadeh, R.; Malyshev, O.B.; Wang, S.; Zolotovskaya, S.A.; Gillespie, W.A.; Abdolvand, A. Low secondary electron yield engineered surface for electron cloud mitigation. Appl. Phys. Lett. 2014, 105, 231605. [Google Scholar] [CrossRef]
- He, J.; Yang, J.; Zhao, W.; Long, J.; Lan, C.; Liu, E.; Chen, X.; Li, J.; Yang, Z.; Dong, P.; et al. The influence of low energy titanium ion beam irradiation on secondary electron emission of metal materials by electron impact. Appl. Surf. Sci. 2020, 515, 145990. [Google Scholar] [CrossRef]
- Zhang, L.X.; Sun, Z.; Qi, J.L.; Shi, J.M.; Hao, T.D.; Feng, J.C. Understanding the growth mechanism of vertically aligned graphene and control of its wettability. Carbon 2016, 103, 339–345. [Google Scholar] [CrossRef]
- He, J.; Yang, J.; Peng, Y.; Long, J.; Yang, Z.; Wang, T.; Liu, P.; Li, J.; Zheng, L.; Dong, P.; et al. Measurement of yield and spectrum of secondary electron emission and their characteristics under modification of conductive materials. Rev. Sci. Instrum. 2019, 90, 063304. [Google Scholar] [CrossRef]
- Obraztsov, A.N.; Tyurnina, A.V.; Obraztsova, E.A.; Zolotukhin, A.A.; Liu, B.H.; Chin, K.C.; Wee, A.T.S. Raman scattering characterization of CVD graphite films. Carbon 2008, 46, 963–968. [Google Scholar] [CrossRef]
- Katkar, P.K.; Kadam, A.N.; Jerng, S.-K.; Chun, S.-H.; Lee, S.-W. Rational design of redox active amorphous Ni-Mn phosphate anchored on vertical graphene nanohills (VGNHs) for solid-state energy storage device. J. Alloys Compd. 2023, 968, 171935. [Google Scholar] [CrossRef]
- Zhang, X.; Gui, H.; He, J.; Wang, R.; Zhao, H.; Zhao, W.; Yang, J.; Tang, B.; Liu, F.; Li, X.; et al. Low secondary electron emission characteristics of carbon nano-onion coating via plasma enhanced chemical vapor deposition. Carbon 2024, 219, 118852. [Google Scholar] [CrossRef]
- Li, J.; Hu, W.; Hao, L.; Wu, S.; Zhang, J. Influence of the substrate temperature on the microstructure and electron induced secondary electron emission properties of MgO/Au composite film. Mater. Res. Bull. 2018, 100, 308–312. [Google Scholar] [CrossRef]
- Uetani, K.; Kajiyama, H.; Kato, A.; Takagi, A.; Hori, T.; Tokomoto, I.; Koizumi, Y.; Nose, K.; Ihara, Y.; Onisawa, K.; et al. Secondary electron emission characteristics of MgO thin films prepared by an advanced ion-plating method. Mater. Trans. 2001, 42, 1870–1873. [Google Scholar] [CrossRef]
- Capece, A.M.; Patino, M.I.; Raitses, Y.; Koel, B.E. Secondary electron emission from lithium and lithium compounds. Appl. Phys. Lett. 2016, 109, 067901. [Google Scholar] [CrossRef]
- Santos, A.; Bundaleski, N.; Shaw, B.-J.; Silva, A.G.; Teodoro, O.M.N.D. Increase of secondary electron yield of amorphous carbon coatings under high vacuum conditions. Vacuum 2013, 98, 37–40. [Google Scholar] [CrossRef]
- Pivi, M.; Collet, G.; King, F.; Kirby, R.; Markiewicz, T.; Raubenheimer, T.; Seeman, J.; Wang, L.; Le Pimpec, F. Secondary electron yield and groove chamber tests in PEP-II. In Proceedings of the IEEE Particle Accelerator Conference, Albuquerque, NM, USA, 25–29 June 2007; p. 3739. [Google Scholar]
- Seiler, H. Secondary-Electron Emission in the Scanning Electron-Microscope. J. Appl. Phys. 1983, 54, R1–R18. [Google Scholar] [CrossRef]
- Wang, D.; Ye, M.; Feng, P.; He, Y.-N.; Cui, W.-Z. An effective reduction on secondary electron emission yield of gold coated surfaces by laser etching. Acta Phys. Sin. 2019, 68, 067901. [Google Scholar] [CrossRef]
- Larciprete, R.; Grosso, D.R.; Di Trolio, A.; Cimino, R. Evolution of the secondary electron emission during the graphitization of thin C films. Appl. Surf. Sci. 2015, 328, 356–360. [Google Scholar] [CrossRef]
- Jin, X.L.; Ji, P.Y.; Zhuge, L.J.; Wu, X.M.; Jin, C.G. Secondary electron emission yield from vertical graphene nanosheets by helicon plasma deposition. Chin. Phys. B 2022, 31, 027901. [Google Scholar] [CrossRef]

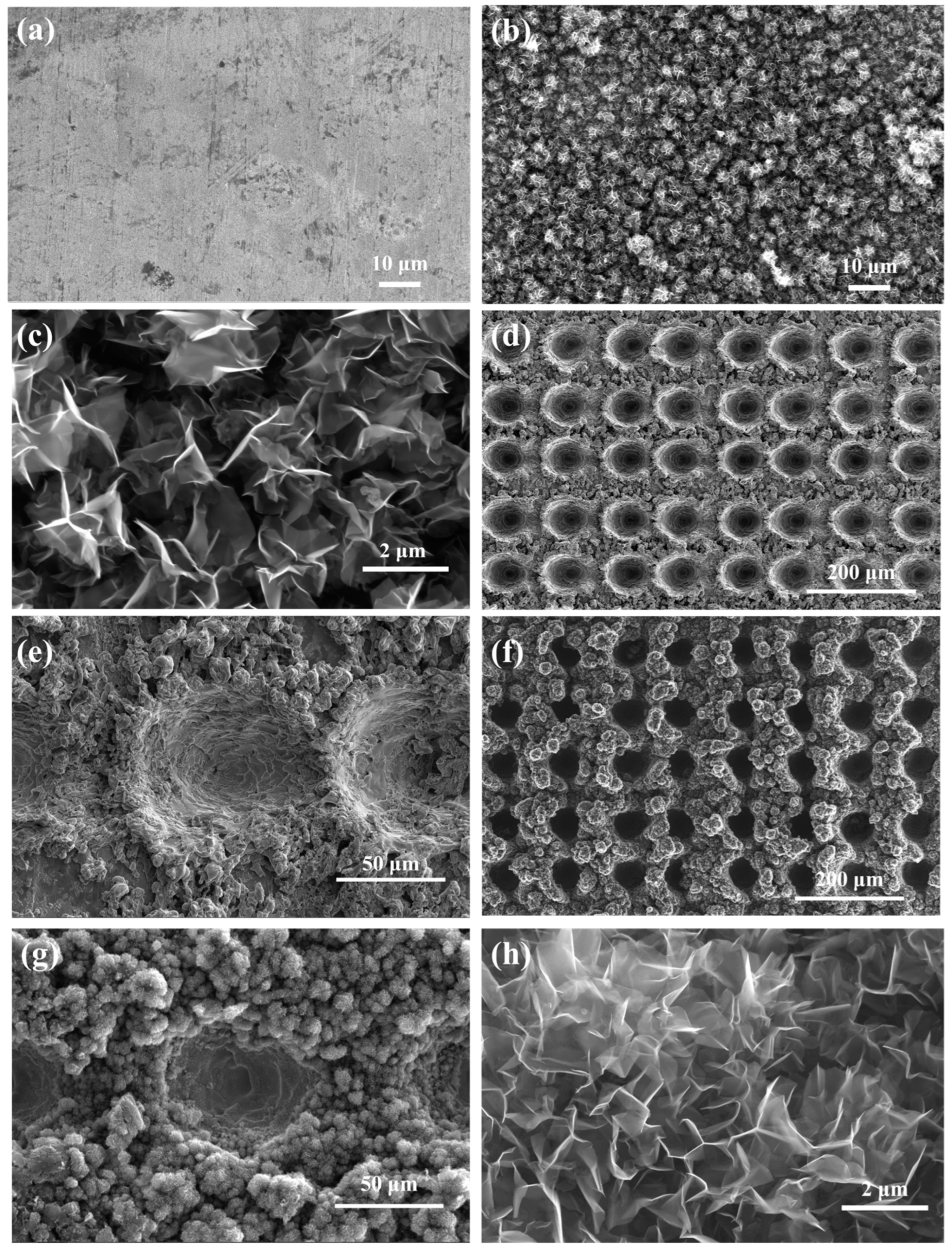

| Parameters | Flow Rate of H2 and CH4 | Pressure (Torr) | Plasma Power (kW) | Temperature (°C) | Time (min) |
|---|---|---|---|---|---|
| Value | 25:2 | 70 | 4.2 | 650 | 30 |
| Value | 10:1 | 70 | 4 | 850 | 100 |
| Sample | δmax | δmax Decreases Relative to Ni | σmax | σmax Decreases Relative to Ni | BEY with Maximum δmax |
|---|---|---|---|---|---|
| Ni | 2.30 | - | 2.1 | - | 0.23 |
| LE | 1.34 | 42% | 1.24 | 41% | 0.20 |
| VG | 0.66 | 71% | 0.62 | 70% | 0.04 |
| LE + VG | 0.62 | 73% | 0.57 | 73% | 0.04 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, X.; Tang, B.; He, J.; Zhao, H.; Wang, R.; Gui, H.; Li, X.; Liu, K.; Shi, J.; Chang, G. Suppression of Secondary Electron Emission by Vertical Graphene Coating on Ni Microcavity Substrate. Nanomaterials 2024, 14, 1268. https://doi.org/10.3390/nano14151268
Zhang X, Tang B, He J, Zhao H, Wang R, Gui H, Li X, Liu K, Shi J, Chang G. Suppression of Secondary Electron Emission by Vertical Graphene Coating on Ni Microcavity Substrate. Nanomaterials. 2024; 14(15):1268. https://doi.org/10.3390/nano14151268
Chicago/Turabian StyleZhang, Xiaoning, Bin Tang, Jialong He, Hui Zhao, Ronghua Wang, Hao Gui, Xinlu Li, Kefu Liu, Jinshui Shi, and Guomei Chang. 2024. "Suppression of Secondary Electron Emission by Vertical Graphene Coating on Ni Microcavity Substrate" Nanomaterials 14, no. 15: 1268. https://doi.org/10.3390/nano14151268





