Oxygen-Vacancy-Induced Enhancement of BiVO4 Bifunctional Photoelectrochemical Activity for Overall Water Splitting
Abstract
1. Introduction
2. Computational Model and Methods
3. Results and Discussion
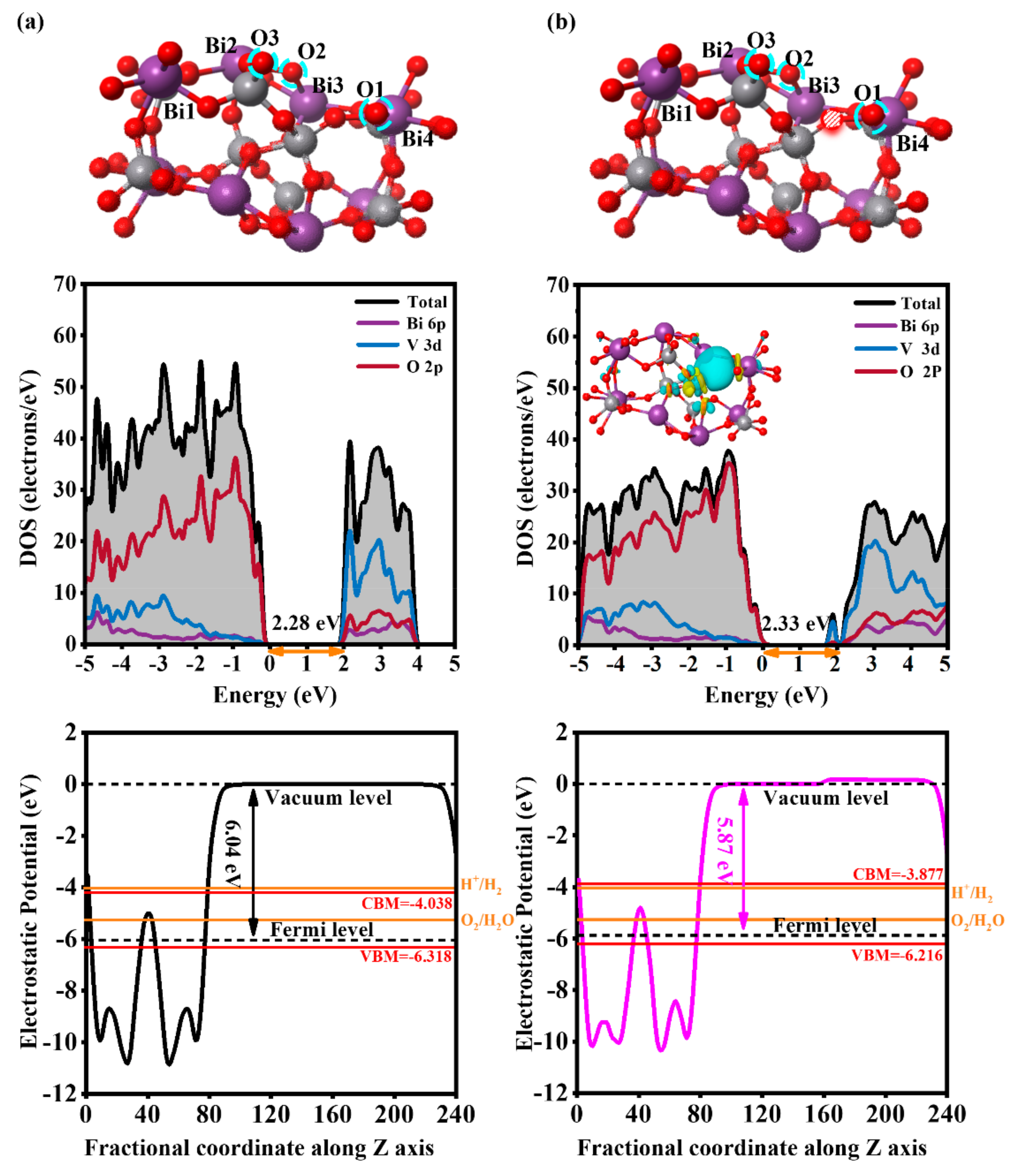
3.1. The Effect of the Ovac on the Electronic Structure of the BiVO4 (110) Facet
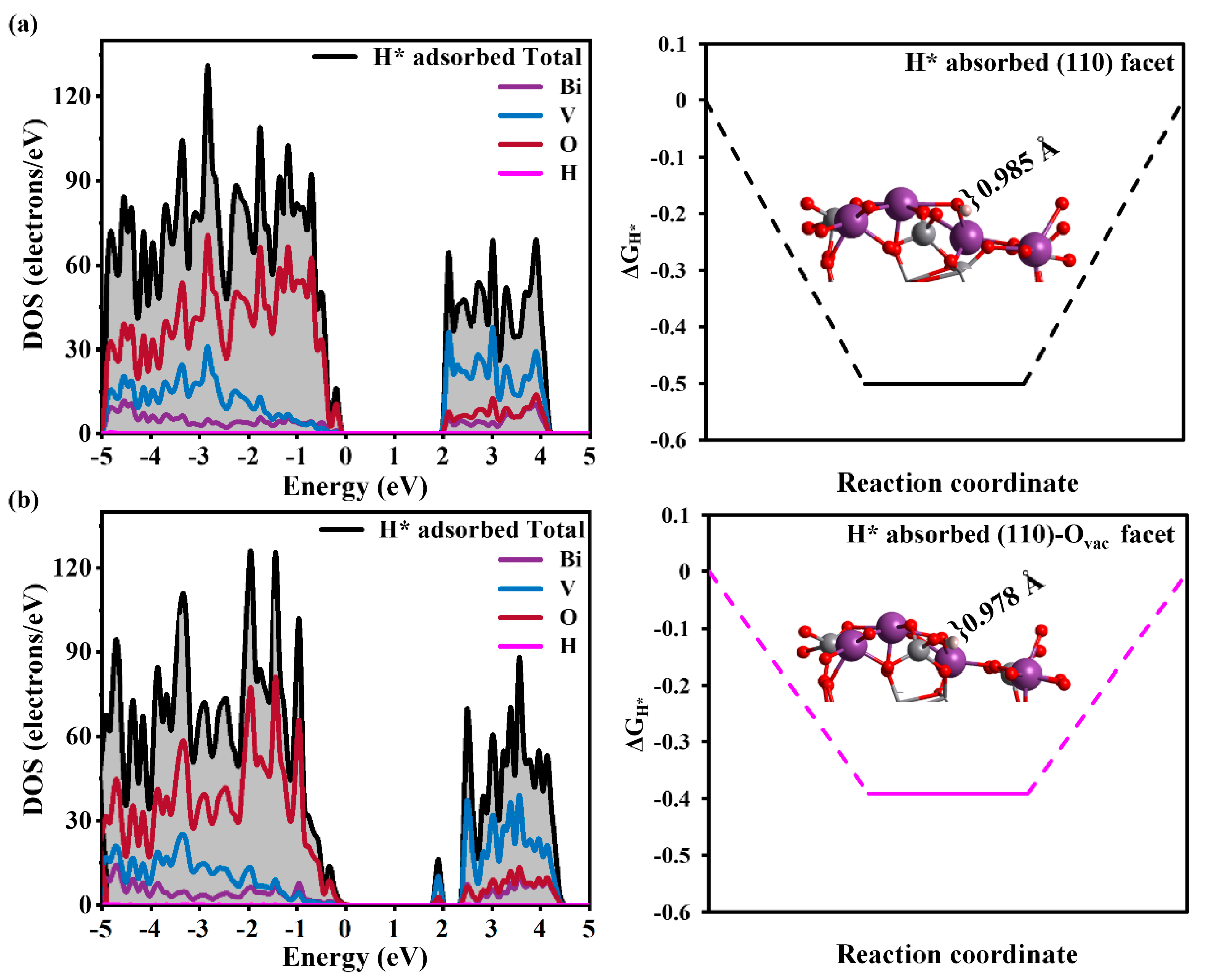
3.2. The Effect of the Ovac on the HER of the BiVO4 (110) Facet
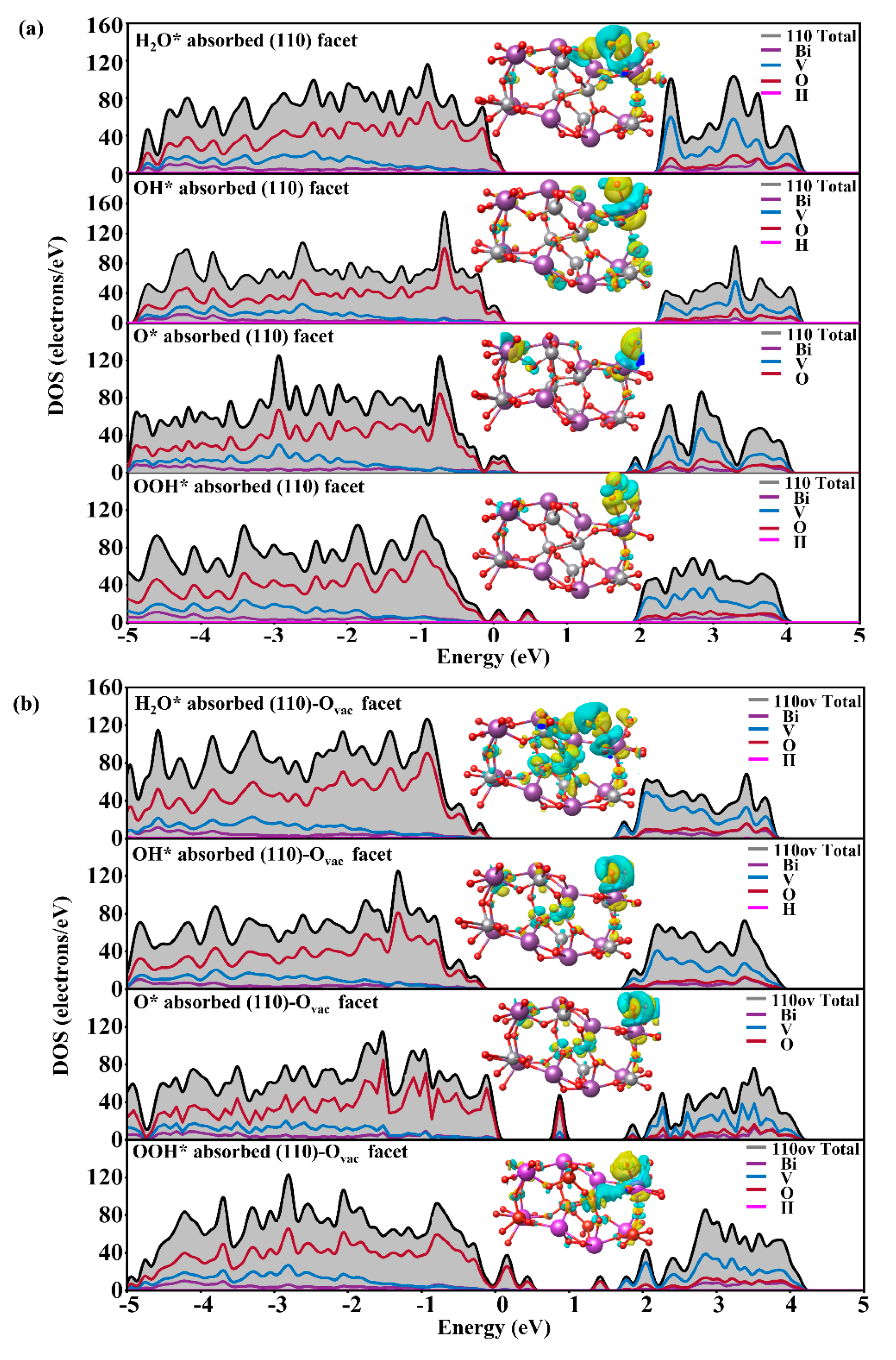
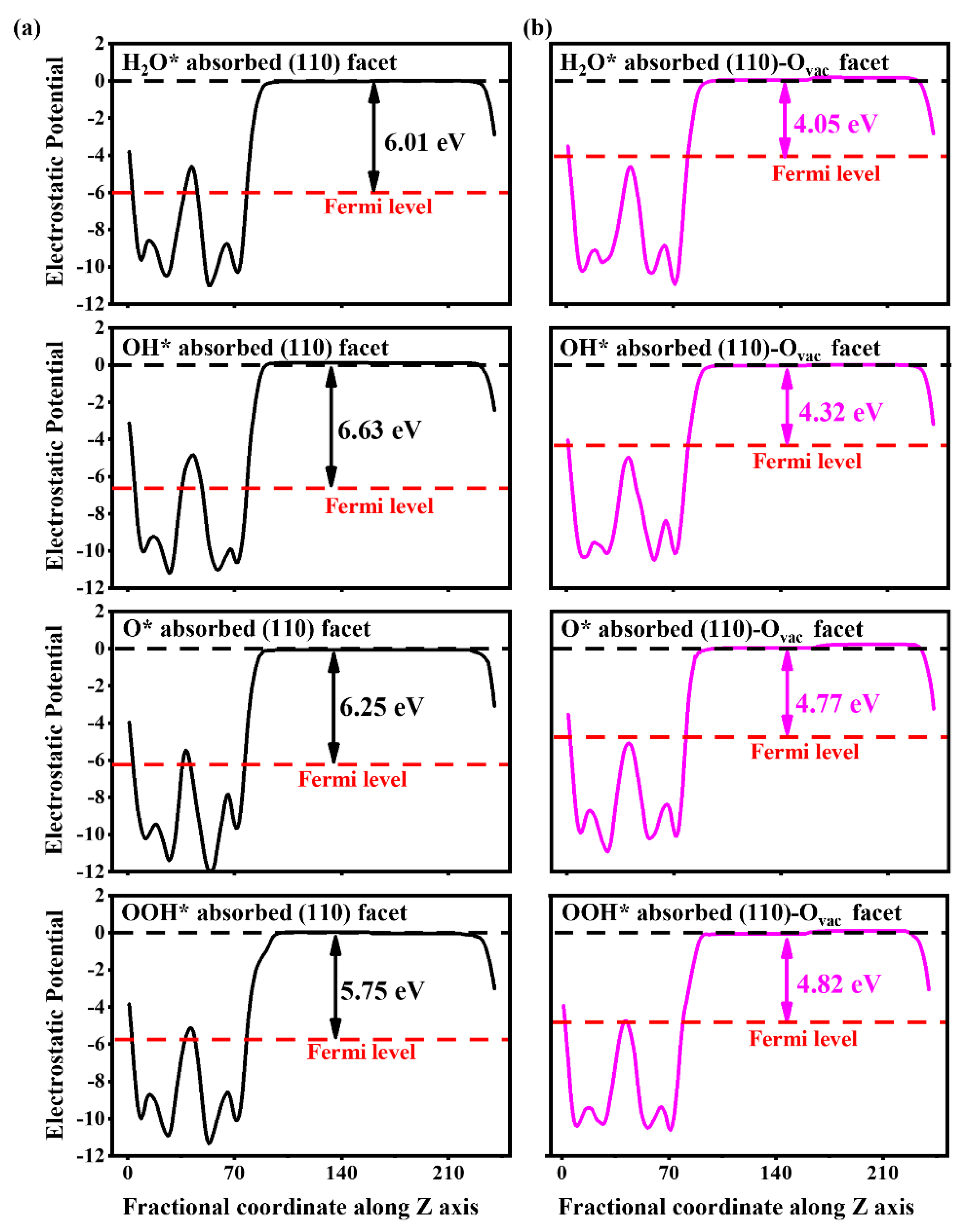
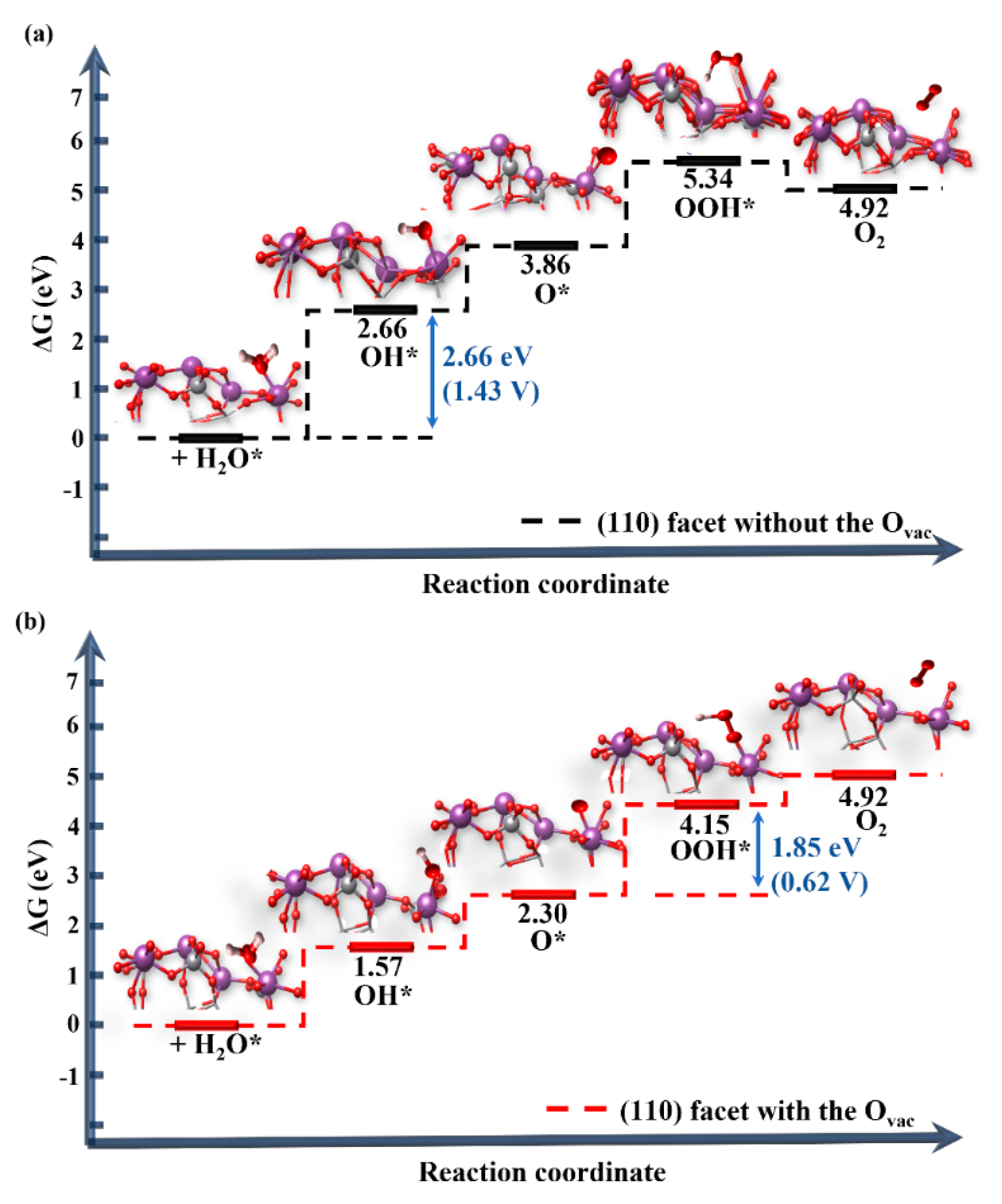
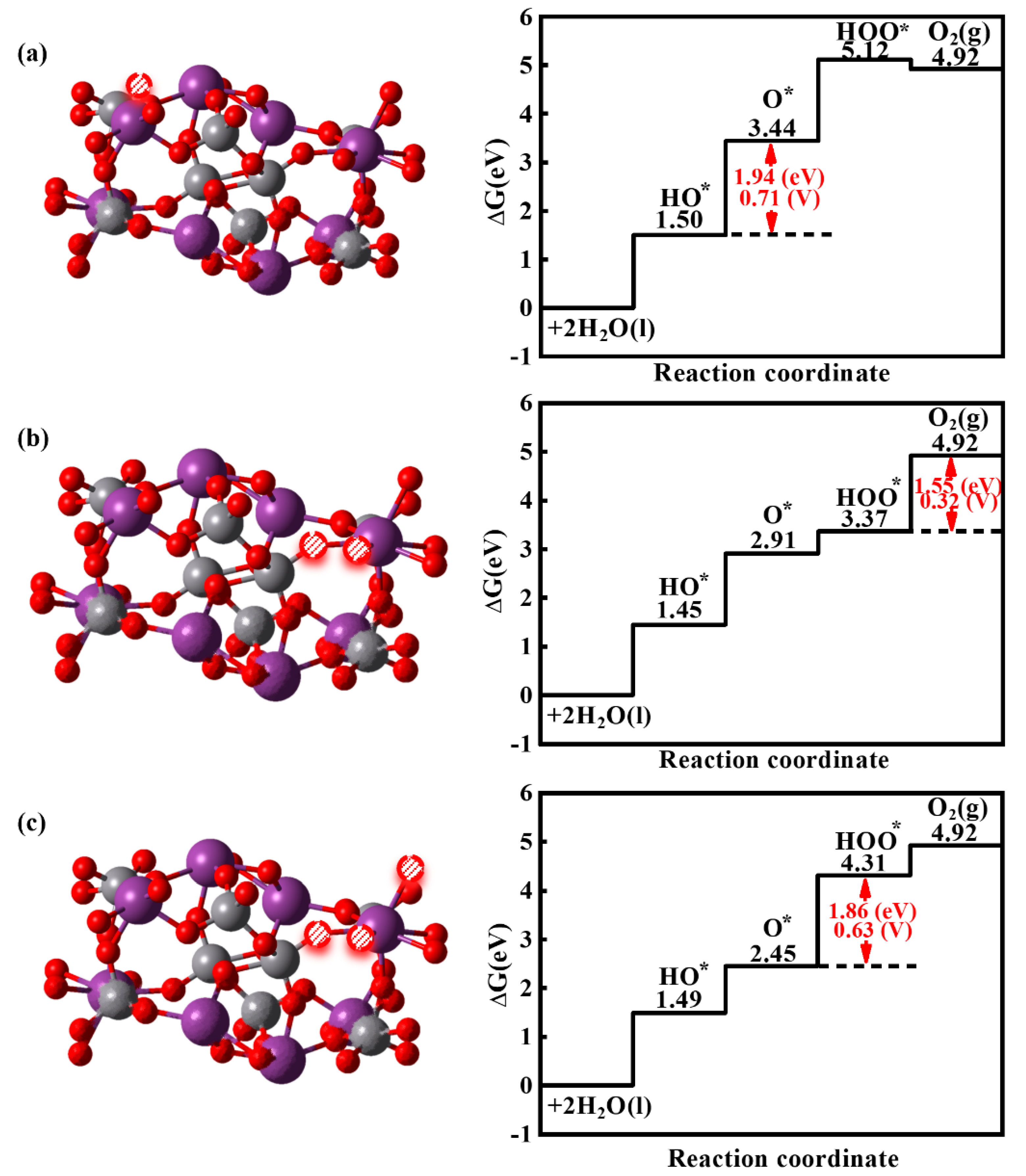
3.3. The Effect of the Ovac on the OER of the BiVO4 (110) Facet
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Cao, M.; Wang, X.; Cao, W.; Fang, X.; Wen, B.; Yuan, J. Thermally Driven Transport and Relaxation Switching Self-Powered Electromagnetic Energy Conversion. Small 2018, 14, 1800987. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Chang, X.; Lin, X.; Zhao, Z.J.; Gong, J. Theoretical insights into single-atom catalysts. Chem. Soc. Rev. 2020, 49, 8156–8178. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Xia, Y.; Li, H.; Wang, X.; Yu, Y.; Jiao, X.; Chen, D. Highly active deficient ternary sulfide photoanode for photoelectrochemical water splitting. Nat. Commun. 2020, 11, 3078. [Google Scholar] [CrossRef] [PubMed]
- Zhang, E.; Liu, Z.; Kato, M. Durable and efficient photoelectrochemical water splitting using TiO2 and 3C–SiC single crystals in a tandem structure. Sol. Energy Mater. Sol. Cells 2021, 230, 111260. [Google Scholar] [CrossRef]
- Low, J.; Yu, J.; Jaroniec, M.; Wageh, S.; Al-Ghamdi, A.A. Heterojunction Photocatalysts. Adv. Mater. 2017, 29, 1601694. [Google Scholar] [CrossRef] [PubMed]
- Han, S.; Noh, S.; Yu, Y.T.; Lee, C.R.; Lee, S.K.; Kim, J.S. Highly Efficient Photoelectrochemical Water Splitting Using GaN-Nanowire Photoanode with Tungsten Sulfides. ACS Appl. Mater. Interfaces 2020, 12, 58028–58037. [Google Scholar] [CrossRef] [PubMed]
- Cui, X.; Ren, P.; Ma, C.; Zhao, J.; Chen, R.; Chen, S.; Rajan, N.P.; Li, H.; Yu, L.; Tian, Z.; et al. Robust Interface Ru Centers for High-Performance Acidic Oxygen Evolution. Adv. Mater. 2020, 32, 1908126. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Xue, Z.; Liu, Q.; Jia, Y.; Li, Y.; Liu, K.; Lin, Y.; Liu, M.; Li, G.; Su, C.Y. Modulating electronic structure of metal-organic frameworks by introducing atomically dispersed Ru for efficient hydrogen evolution. Nat. Commun. 2021, 12, 1369. [Google Scholar] [CrossRef] [PubMed]
- Hu, E.; Feng, Y.; Nai, J.; Zhao, D.; Hu, Y.; Lou, X.W. Construction of hierarchical Ni–Co–P hollow nanobricks with oriented nanosheets for efficient overall water splitting. Energy Environ. Sci. 2018, 11, 872–880. [Google Scholar] [CrossRef]
- Xu, Z.; Ying, Y.; Zhang, G.; Li, K.; Liu, Y.; Fu, N.; Guo, X.; Yu, F.; Huang, H. Engineering nife layered double hydroxide by valence control and intermediate stabilization toward the oxygen evolution reaction. J. Mater. Chem. A 2020, 8, 26130–26138. [Google Scholar] [CrossRef]
- Kalanur, S.S.; Singh, R.; Seo, H. Enhanced solar water splitting of an ideally doped and work function tuned {002} oriented one-dimensional WO3 with nanoscale surface charge mapping insights. Appl. Catal. B Environ. 2021, 295, 12029. [Google Scholar] [CrossRef]
- Xie, Z.; Chen, D.; Zhai, J.; Huang, Y.; Ji, H. Charge separation via synergy of homojunction and electrocatalyst in BiVO4 for photoelectrochemical water splitting. Appl. Catal. B Environ. 2023, 334, 122865. [Google Scholar] [CrossRef]
- Montoya, J.H.; Seitz, L.C.; Chakthranont, P.; Vojvodic, A.; Jaramillo, T.F.; Norskov, J.K. Materials for solar fuels and chemicals. Nat. Mater. 2016, 16, 70–81. [Google Scholar] [CrossRef] [PubMed]
- Kalanur, S.S.; Lee, Y.J.; Seo, H. A versatile synthesis strategy and band insights of monoclinic clinobisvanite BIVO4 thin films for enhanced photoelectrochemical water splitting activity. Appl. Surf. Sci. 2021, 562, 150078. [Google Scholar] [CrossRef]
- Hu, P.; Jia, Z.; Che, H.; Zhou, W.; Liu, N.; Li, F.; Wang, J. Engineering hybrid CoMoS4/Ni3S2 nanostructures as efficient bifunctional electrocatalyst for overall water splitting. J. Power Sources 2019, 416, 95–103. [Google Scholar] [CrossRef]
- Da Silva, G.C.; Mayrhofer, K.J.J.; Ticianelli, E.A.; Cherevko, S. Dissolution Stability: The Major Challenge in the Regenerative Fuel Cells Bifunctional Catalysis. J. Electrochem. Soc. 2018, 165, F1376–F1384. [Google Scholar] [CrossRef]
- Zhai, P.; Zhang, Y.; Wu, Y.; Gao, J.; Zhang, B.; Cao, S.; Zhang, Y.; Li, Z.; Sun, L.; Hou, J. Engineering active sites on hierarchical transition bimetal oxides/sulfides heterostructure array enabling robust overall water splitting. Nat. Commun. 2020, 11, 5462. [Google Scholar] [CrossRef]
- Jung, H.-Y.; Park, S.; Popov, B.N. Electrochemical studies of an unsupported Ptir electrocatalyst as a bifunctional oxygen electrode in a unitized regenerative fuel cell. J. Power Sources 2009, 191, 357–361. [Google Scholar] [CrossRef]
- Suryanto, B.H.R.; Wang, Y.; Hocking, R.K.; Adamson, W.; Zhao, C. Overall electrochemical splitting of water at the heterogeneous interface of nickel and iron oxide. Nat. Commun. 2019, 10, 5599. [Google Scholar] [CrossRef]
- Hosseini, H.; Roushani, M. Rational design of hollow core-double shells hybrid nanoboxes and nanopipes composed of hierarchical Cu-Ni-Co selenides anchored on nitrogen-doped carbon skeletons as efficient and stable bifunctional electrocatalysts for overall water splitting. Chem. Eng. J. 2020, 402, 126174. [Google Scholar] [CrossRef]
- Petala, A.; Noe, A.; Frontistis, Z.; Drivas, C.; Kennou, S.; Mantzavinos, D.; Kondarides, D.I. Synthesis and characterization of CoOx/BiVO4 photocatalysts for the degradation of propyl paraben. J. Hazard. Mater. 2019, 372, 52–60. [Google Scholar] [CrossRef] [PubMed]
- Babu, P.; Mohanty, S.; Naik, B.; Parida, K. Serendipitous Assembly of Mixed Phase BiVO4 on B-Doped g-C3N4: An Appropriate p–n Heterojunction for Photocatalytic O2 evolution and Cr(VI) reduction. Inorg. Chem. 2019, 58, 12480–12491. [Google Scholar] [CrossRef] [PubMed]
- Jin, S.; Ma, X.; Pan, J.; Zhu, C.; Saji, S.E.; Hu, J.; Xu, X.; Sun, L.; Yin, Z. Oxygen vacancies activating surface reactivity to favor charge separation and transfer in nanoporous BiVO4 photoanodes. Appl. Catal. B Environ. 2021, 281, 119477. [Google Scholar] [CrossRef]
- Gaikwad, M.; Ghorpade, U.; Suryawanshi, U.; Kumar, P.; Jang, S.; Jang, S.; Tran, L.; Lee, J.; Bae, H.; Shin, S.; et al. Rapid Synthesis of Ultrathin Ni:FeOOH with In Situ-Induced Oxygen Vacancies for Enhanced Water Oxidation Activity and Stability of BiVO4 Photoanodes. ACS Appl. Mater. Interfaces 2023, 15, 21123–21133. [Google Scholar] [CrossRef] [PubMed]
- Baral, B.; Parida, K. {040/110} Facet Isotype Heterojunctions with Monoclinic Scheelite BiVO4. Inorg. Chem. 2020, 59, 10328–10342. [Google Scholar] [CrossRef]
- Tan, H.L.; Tahini, H.A.; Wen, X.; Wong, R.J.; Tan, X.; Iwase, A.; Kudo, A.; Amal, R.; Smith, S.C.; Ng, Y.H. Interfacing BiVO4 with Reduced Graphene Oxide for Enhanced Photoactivity: A Tale of Facet Dependence of Electron Shuttling. Small 2016, 12, 5295–5302. [Google Scholar] [CrossRef] [PubMed]
- Tan, H.L.; Wen, X.; Amal, R.; Ng, Y.H. BiVO4 {010} and {110} Relative Exposure Extent: Governing Factor of Surface Charge Population and Photocatalytic Activity. J. Phys. Chem. Lett. 2016, 7, 1400–1405. [Google Scholar] [CrossRef] [PubMed]
- Murugan, C.; Pandikumar, A. Reinforcement of Visible-Light Harvesting and Charge-Transfer Dynamics of BiVO4 Photoanode via Formation of p−n Heterojunction with CuO for Efficient Photoelectrocatalytic Water Splitting. ACS Appl. Energy Mater. 2022, 5, 6618–6632. [Google Scholar] [CrossRef]
- Mary, A.S.; Murugan, C.; Murugan, P.; Pandikumar, A. Unravelling the Superior Photoelectrochemical Water Oxidation Performance of the Al-Incorporated CoOOH Cocatalyst-Loaded BiVO4 Photoanode. ACS Sustain. Chem. Eng. 2023, 11, 13656–13667. [Google Scholar] [CrossRef]
- Murugan, C.; Mary, A.S.; Pandikumar, A. Fabrication and Performance Validation of BiVO4 Photoanode-Based Prototype Photoelectrochemical Cells with Different Sizes and Reducing the Photocurrent Density Loss with Different Conductive Patterns. Ind. Eng. Chem. Res. 2024, 63, 4329–4337. [Google Scholar] [CrossRef]
- Mary, A.S.; Murugan, C.; Mahendiran, D.; Murugan, P.; Pandikumar, A. Investigation of Mn incorporation into NiOOH electrocatalyst loaded on BiVO4 photoanode for enhanced photoelectrochemical water splitting: Experimental and theoretical approach. Mater. Today Energy 2024, 41, 101541. [Google Scholar] [CrossRef]
- Murugan, C.; Mary, A.S.; Velmurugan, R.; Subramanian, B.; Murugan, P.; Pandikumar, A. Investigating the interfacial charge transfer between electrodeposited BiVO4 and pulsed laser-deposited Co3O4 p-n junction photoanode in photoelectrocatalytic water splitting. Chem. Eng. J. 2024, 483, 149104. [Google Scholar] [CrossRef]
- Wang, W.; Strohbeen, P.J.; Lee, D.; Zhou, C.; Kawasaki, J.K.; Choi, K.-S.; Liu, M.; Galli, G. The Role of Surface Oxygen Vacancies in BiVO4. Chem. Mater. 2020, 32, 2899–2909. [Google Scholar] [CrossRef]
- Li, H.; Yu, H.; Quan, X.; Chen, S.; Zhao, H. Improved Photocatalytic Performance of Heterojunction by Controlling the Contact Facet: High Electron Transfer Capacity between TiO2 and the {110} Facet of BiVO4 Caused by Suitable Energy Band Alignment. Adv. Funct. Mater. 2015, 25, 3074–3080. [Google Scholar] [CrossRef]
- Wang, Y.; Tan, G.; Li, B.; Dang, M.; Lv, L.; Wang, M.; Zhang, D.; Ren, H.; Xia, A. Enhanced NIR photocatalytic of Ag-RGO@{010}BiVO4/rgo@{110} BiVO4 photocatalysts induced by resonance effect of transverse electric of RGO and transverse magnetic of Ag. Appl. Surf. Sci. 2019, 489, 1–12. [Google Scholar] [CrossRef]
- Qiu, W.; Xiao, S.; Ke, J.; Wang, Z.; Tang, S.; Zhang, K.; Qian, W.; Huang, Y.; Huang, D.; Tong, Y.; et al. Freeing the Polarons to Facilitate Charge Transport in BiVO4 from Oxygen Vacancies with an Oxidative 2D Precursor. Angew. Chem. Int. Ed. 2019, 58, 19087–19095. [Google Scholar] [CrossRef] [PubMed]
- Pan, J.; Ma, X.; Zhang, W.; Hu, J. Enhancing the photocatalytic hydrogen production activity of BiVO4 [110] facets using oxygen vacancies. RSC Adv. 2021, 12, 540–545. [Google Scholar] [CrossRef]
- Blöchl, P.E. Projector augmented-wave method. Phys. Rev. B. 1994, 50, 17953–17979. [Google Scholar] [CrossRef]
- Perdew, J.P.; Burke, K.; Ernzerhof, M. Generalized Gradient Approximation Made Simple. Phys. Rev. Lett. 1996, 77, 3865–3868. [Google Scholar] [CrossRef]
- Kresse, G.; Hafner, J. Ab initio molecular-dynamics simulation of the liquid-metal-amorphous-semiconductor transition in germanium. Phys. Rev. B 1994, 49, 14251–14269. [Google Scholar] [CrossRef]
- Kresse, G.; Furthmüller, J. Efficiency of ab-initio total energy calculations for metals and semiconductors using a plane-wave basis set. Comput. Mater. Sci. 1996, 6, 15–50. [Google Scholar] [CrossRef]
- Thalluri, S.M.; Martinez Suarez, C.; Hussain, M.; Hernandez, S.; Virga, A.; Saracco, G.; Russo, N. Evaluation of the Parameters Affecting the Visible-Light-Induced Photocatalytic Activity of Monoclinic BiVO4 for Water Oxidation. Indust. Engineer. Chem. Res. 2013, 52, 17414–17418. [Google Scholar] [CrossRef]
- Anke, B.; Rohloff, M.; Willinger, M.G.; Hetaba, W.; Fischer, A.; Lerch, M. Improved photoelectrochemical performance of bismuth vanadate by partial O/F-substitution. Solid State Sci. 2017, 63, 1–8. [Google Scholar] [CrossRef]
- Lardhi, S.; Cavallo, L.; Harb, M. Significant Impact of Exposed Facets on the BiVO4 Material Performance for Photocatalytic Water Splitting Reactions. J. Phys. Chem. Lett. 2020, 11, 5497–5503. [Google Scholar] [CrossRef]
- Pan, J.; Wang, R.; Xu, X.; Hu, J.; Ma, L. Transition metal doping activated basal-plane catalytic activity of two-dimensional 1T’-ReS2 for hydrogen evolution reaction: A first-principles calculation study. Nanoscale 2019, 11, 10402–10409. [Google Scholar] [CrossRef] [PubMed]
- Hinnemann, B.; Moses, P.G.; Bonde, J.; Jorgensen, K.P.; Nielsen, J.H.; Horch, S.; Chorkendorff, I.; Norskov, J.K. Biomimetic Hydrogen Evolution: MoS2 Nanoparticles as Catalyst for Hydrogen Evolution. J. Am. Chem. Soc. 2005, 127, 5308–5309. [Google Scholar] [CrossRef] [PubMed]
- Hu, J.; Zhao, X.; Chen, W.; Su, H.; Chen, Z. Theoretical Insight into the Mechanism of Photoelectrochemical Oxygen Evolution Reaction on BiVO4 Anode with Oxygen Vacancy. J. Phys. Chem. C 2017, 121, 18702–18709. [Google Scholar] [CrossRef]
- Zhang, X.; Yang, Z.; Lu, Z.; Wang, W. Bifunctional CoNx embedded graphene electrocatalysts for OER and ORR: A theoretical evaluation. Carbon 2018, 130, 112–119. [Google Scholar] [CrossRef]
- Nørskov, J.K.; Rossmeisl, J.; Logadottir, A.; Lindqvist, L.; Kitchin, J.R.; Bligaard, T.; Jónsson, H. Origin of the overpotential for oxygen reduction at a fuel-cell cathode. J. Phys. Chem. B 2004, 108, 17886–17892. [Google Scholar] [CrossRef]
- Liang, Q.; Brocks, G.; Bieberle-Hütter, A. Oxygen evolution reaction (OER) mechanism under alkaline and acidic conditions. J. Phys. Energy 2021, 3, 026001. [Google Scholar] [CrossRef]
- Zhou, X.; Dong, H.; Ren, A.M. Exploring the mechanism of water-splitting reaction in NiOx/β-Ga2O3 photocatalysts by first-principles calculations. Phys. Chem. Chem. Phys. 2016, 18, 11111–11119. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fu, H.; Qi, Q.; Li, Y.; Pan, J.; Zhong, C. Oxygen-Vacancy-Induced Enhancement of BiVO4 Bifunctional Photoelectrochemical Activity for Overall Water Splitting. Nanomaterials 2024, 14, 1270. https://doi.org/10.3390/nano14151270
Fu H, Qi Q, Li Y, Pan J, Zhong C. Oxygen-Vacancy-Induced Enhancement of BiVO4 Bifunctional Photoelectrochemical Activity for Overall Water Splitting. Nanomaterials. 2024; 14(15):1270. https://doi.org/10.3390/nano14151270
Chicago/Turabian StyleFu, Huailiang, Qingxiu Qi, Yushu Li, Jing Pan, and Chonggui Zhong. 2024. "Oxygen-Vacancy-Induced Enhancement of BiVO4 Bifunctional Photoelectrochemical Activity for Overall Water Splitting" Nanomaterials 14, no. 15: 1270. https://doi.org/10.3390/nano14151270
APA StyleFu, H., Qi, Q., Li, Y., Pan, J., & Zhong, C. (2024). Oxygen-Vacancy-Induced Enhancement of BiVO4 Bifunctional Photoelectrochemical Activity for Overall Water Splitting. Nanomaterials, 14(15), 1270. https://doi.org/10.3390/nano14151270





