Research Progress on the Preparation of Manganese Dioxide Nanomaterials and Their Electrochemical Applications
Abstract
1. Introduction
2. Structure of MnO2 Nanoparticles
3. Synthesis of MnO2 Nanomaterials
3.1. Hydrothermal Method
| Structure of MnO2 | Targets | Synthesis Conditions | Results | Applications | Reference |
|---|---|---|---|---|---|
| δ-MnO2 | Pb (II) and U (VI) | - | The adsorption capacities were 41.32 and 492.61 mg g−1, respectively | Adsorbent | [29] |
| Pristine ε-MnO2 and ε-MnO2 of Mn-6-2-6 | Toluene | Manganese (II) nitrate hexahydrate, urea, glucose 180 °C | The conversion 41% and 85%, respectively | Catalysts | [30] |
| MnO2 | Tl (I) | KMnO4, MnSO4·H2O, 240 °C | Adsorption capacity was 450 mg g−1 | For removing thallium (Tl) from wastewater | [31] |
| MnO2 nanoparticles | MB (Methylene Blue) | KMnO4, CH3CH2OH, HCl | The adsorption capacities 22.2 mg g−1 after 60 min. | Removal of MB | [32] |
| α-MnO2, β-MnO2, and δ-MnO2 | MG (Methyl Glucoside) | - | The removal efficiency of MG 96.42%, 46.58%, 99.75%, respectively | For typical organic pollutant removal | [33] |
| MnO2 nanostructures | - | KMnO4, Mn (CH3COO)2 | The capacitance was 348.2 F g−1 and rate capability of 89% for 2000 cycles. | Electrode materials | [34] |
| δ-MnO2 | - | Mn-MOF, KMnO4, 120 °C | The capacitance was 416 F g−1 | Capacitors | [35] |

3.2. Sol–Gel Approach
| MnO2 Structure | Synthesis Conditions | Result | Applications | Reference |
|---|---|---|---|---|
| γ-MnO2 | MnAc2·4H2O, C6H8O7·H2O | Capacitance was 317 F g−1 | Supercapacitors | [42] |
| Mesoporous Silica/MnO2 composite (MS/MnO2) | Tetraethyl Orthosilicate, KMnO4 | Capacitance was 1158.50 F g−1 | Supercapacitors | [43] |
| Nanostructured MnO2 | - | The capacitance was 627.9 F g−1 | Supercapacitors | [44] |
| Nickel-doped layered MnO2 | KMnO4, Ni (NO3)2·6H2O | The capacitance was 140 mAh g−1 | Sodium-ion batteries | [45] |
| CF@CoFe2O4@MnO2 | FeCl3·6H2O, CoCl2·6H2O, CF (Carbon Fiber), KMnO4 | The microwave absorbing capacity can reach up −41 dB | Microwave absorbers | [46] |
| α-MnO2 and Cu-α-MnO2 | CuSO4·5H2O, KMnO4 | The maximum degradation of Methylene Blue (MB) by α-MnO2, 1% Cu-α-MnO2, 5% Cu-α-MnO2, and 10% Cu-αMnO2 were 97.9%, 98.3%, 98.7%, and 99.5%, respectively | Degradable MB | [47] |
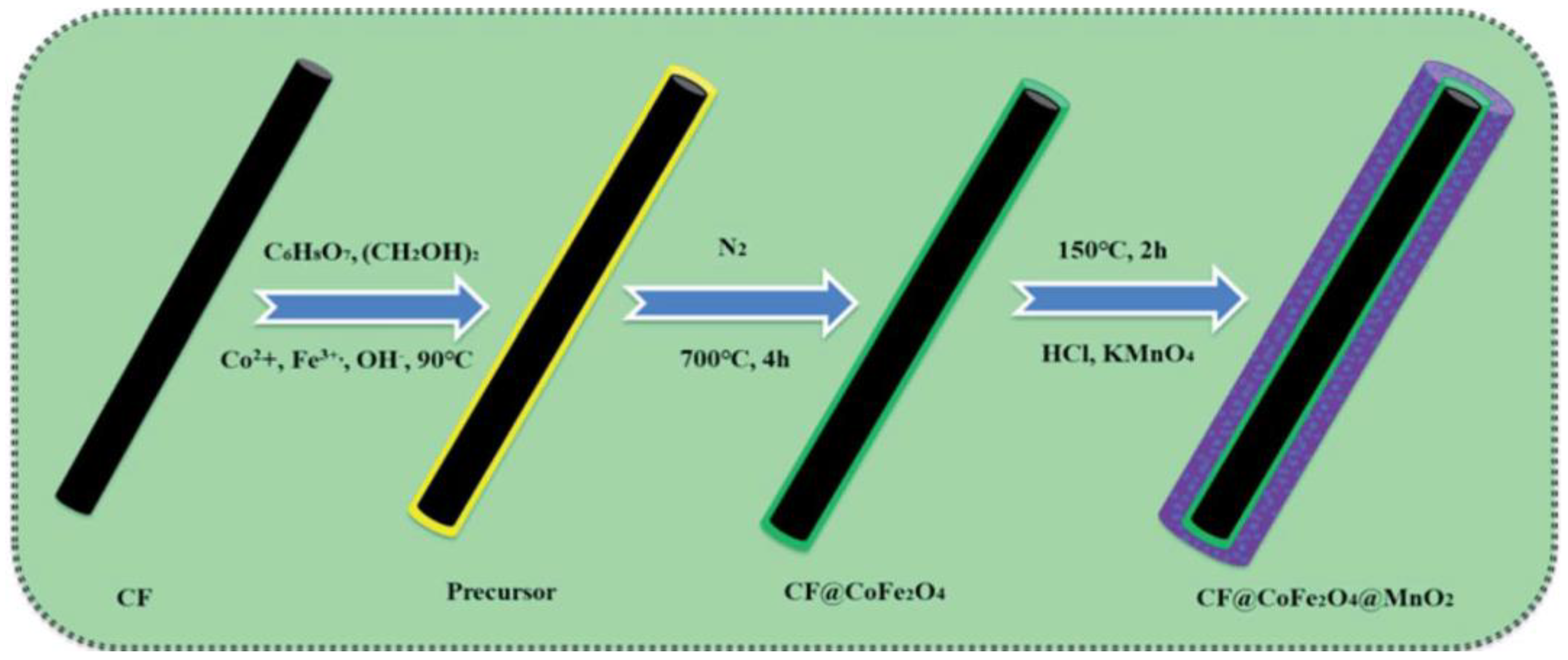
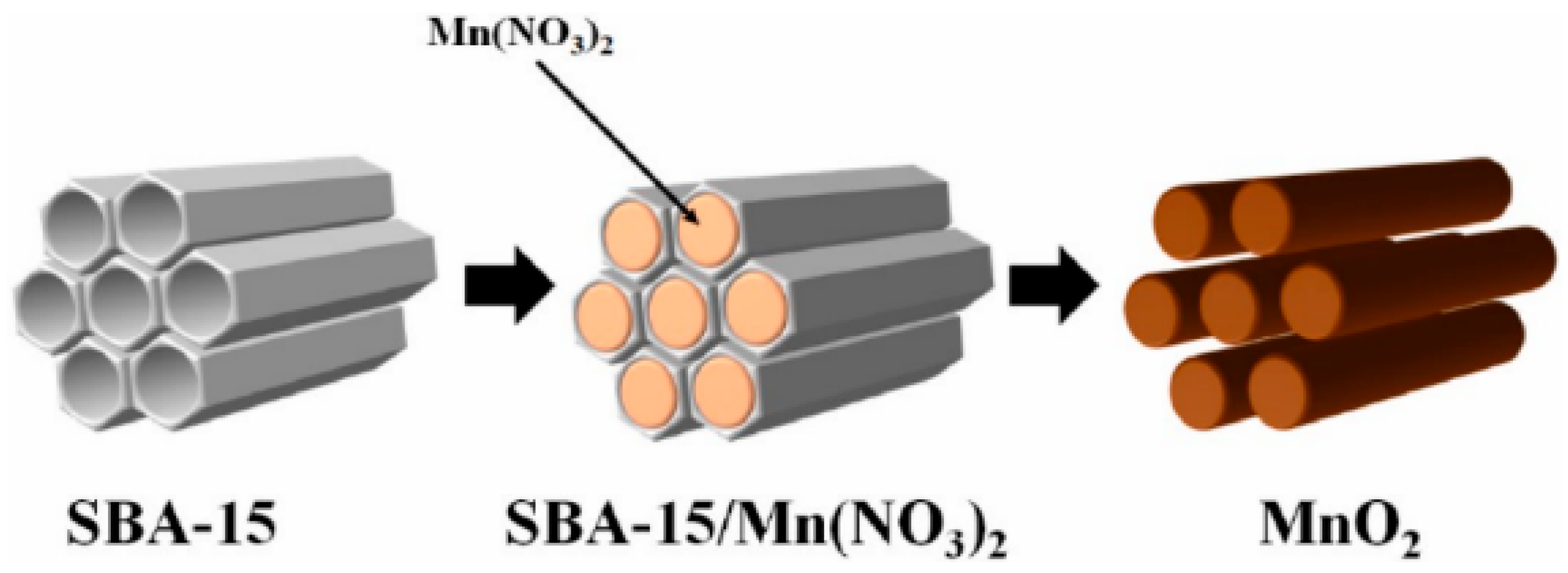
3.3. Template Method
3.3.1. Soft Template Approach
3.3.2. Hard Template Approach
3.4. Electrodeposition Method
3.5. Reflux Approach
3.6. Microemulsion Approach
3.7. Chemical Coprecipitation
3.8. Chemical Reduction Method
3.9. Green Synthesis Method
3.9.1. Plant Extraction Method
3.9.2. Environmentally Friendly Synthetic Methods Based on Microorganisms
4. Electrochemical Applications
4.1. Supercapacitors
4.2. Zn-MnO2 Batteries
4.3. MnO2/Carbon Nanomaterial Composites
5. Summary and Outlook
- i.
- Although there are several ways to prepare MnO2 nanoparticles, realizing large-scale, cost-effective and high-quality synthesis remains challenging. The high production cost makes the commercialization of manganese dioxide nanoparticles difficult, especially in cost-sensitive industries.
- ii.
- Although MnO2 nanoparticles have demonstrated excellent performance on the laboratory scale, a series of technical challenges need to be addressed in practical applications, for example, how to improve the stability and electrochemical properties of MnO2 nanoparticles. These issues need to be addressed by continuous research and technological innovations.
- iii.
- Despite the excellent MnO2 nanoparticle performances, the process of MnO2 preparation may generate some hazardous substances and wastes. This requires manufacturers to take environmental protection measures during the production process. In addition, the environmental impacts of nanomaterials need to be further studied and evaluated.
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Liu, J.; Meng, L.; Fei, Z.; Dyson, P.J.; Jing, X.; Liu, X. MnO2 nanosheets as an artificial enzyme to mimic oxidase for rapid and sensitive detection of glutathione. Biosens. Bioelectron. 2017, 90, 69–74. [Google Scholar] [CrossRef] [PubMed]
- Prasad, K.S.; Patra, A. Green synthesis of MnO2 nanorods using Phyllanthus amarus plant extract and their fluorescence studies. Green Process. Synth. 2017, 6, 549–554. [Google Scholar] [CrossRef]
- Jin, S.B.; Jeong, J.-M.; Son, S.G.; Park, S.H.; Lee, K.G.; Choi, B.G. Synthesis of two-dimensional holey MnO2/graphene oxide nanosheets with high catalytic performance for the glycolysis of poly(ethylene terephthalate). Mater. Today Commun. 2021, 26, 101857. [Google Scholar] [CrossRef]
- Gaire, M.; Liang, K.; Luo, S.; Subedi, B.; Adireddy, S.; Schroder, K.; Farnsworth, S.; Chrisey, D.B. Nanostructured manganese oxides electrode with ultra-long lifetime for electrochemical capacitors. RSC Adv. 2020, 10, 16817–16825. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Ji, Y. Nanostructured manganese dioxide for anticancer applications: Preparation, diagnosis, and therapy. Nanoscale 2020, 12, 17982–18003. [Google Scholar] [CrossRef] [PubMed]
- Yang, R.; Fan, Y.; Ye, R.; Tang, Y.; Cao, X.; Yin, Z.; Zeng, Z. MnO2-Based Materials for Environmental Applications. Adv. Mater. 2021, 33, 2004862. [Google Scholar] [CrossRef] [PubMed]
- Huang, S.-D.; Shang, C.; Zhang, X.-J.; Liu, Z.-P. Material discovery by combining stochastic surface walking global optimization with a neural network. Chem. Sci. 2017, 8, 6327–6337. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Chen, C.; Zhao, Y.; Jia, B. A Review on the Synthesis of Manganese Oxide Nanomaterials and Their Applications on Lithium-Ion Batteries. J. Nanomater. 2013, 2013, 736375. [Google Scholar] [CrossRef]
- Yadav, P.; Bhaduri, A.; Thakur, A. Manganese Oxide Nanoparticles: An Insight into Structure, Synthesis and Applications. ChemBioEng Rev. 2023, 10, 510–528. [Google Scholar] [CrossRef]
- Hayashi, E.; Yamaguchi, Y.; Kamata, K.; Tsunoda, N.; Kumagai, Y.; Oba, F.; Hara, M. Effect of MnO2 Crystal Structure on Aerobic Oxidation of 5-Hydroxymethylfurfural to 2,5-Furandicarboxylic Acid. J. Am. Chem. Soc. 2019, 141, 890–900. [Google Scholar] [CrossRef]
- Robinson, D.M.; Go, Y.B.; Mui, M.; Gardner, G.; Zhang, Z.; Mastrogiovanni, D.; Garfunkel, E.; Li, J.; Greenblatt, M.; Dismukes, G.C. Photochemical water oxidation by crystalline polymorphs of manganese oxides: Structural requirements for catalysis. J. Am. Chem. Soc. 2013, 135, 3494–3501. [Google Scholar] [CrossRef] [PubMed]
- Chabre, Y.; Pannetier, J. Structural and electrochemical properties of the proton/γ-MnO2 system. Prog. Solid State Chem. 1995, 23, 1–130. [Google Scholar] [CrossRef]
- Biswal, A.; Chandra Tripathy, B.; Sanjay, K.; Subbaiah, T.; Minakshi, M. Electrolytic manganese dioxide (EMD): A perspective on worldwide production, reserves and its role in electrochemistry. RSC Adv. 2015, 5, 58255–58283. [Google Scholar] [CrossRef]
- Salvador, G.M.S.; Silva, A.L.; Silva, L.P.C.; Passos, F.B.; Carvalho, N.M.F. Enhanced activity of Pd/α-MnO2 for electrocatalytic oxygen evolution reaction. Int. J. Hydrogen Energy 2021, 46, 26976–26988. [Google Scholar] [CrossRef]
- Gowrisankar, A.; Thangavelu, S. Effect of β-MnO2 on Controlled Polymorphism of VO2(x) (x = A, B, M Polymorphs) Microstructures Anchored on Two-Dimensional Reduced Graphene Oxide Nanosheets for Overall Water Splitting. J. Phys. Chem. C 2022, 126, 3419–3431. [Google Scholar] [CrossRef]
- Rojas, S. Durable MnO2 electrocatalysts by stronger Mn–O bonds. Nat. Catal. 2024, 7, 227–228. [Google Scholar] [CrossRef]
- Guo, C.; Liu, H.; Li, J.; Hou, Z.; Liang, J.; Zhou, J.; Zhu, Y.; Qian, Y. Ultrathin δ-MnO2 nanosheets as cathode for aqueous rechargeable zinc ion battery. Electrochim. Acta 2019, 304, 370–377. [Google Scholar] [CrossRef]
- Li, L.; Yang, Q.; Wang, D.; Peng, Y.; Yan, J.; Li, J.; Crittenden, J. Facile synthesis λ-MnO2 spinel for highly effective catalytic oxidation of benzene. Chem. Eng. J. 2021, 421, 127828. [Google Scholar] [CrossRef]
- Kim, C.-H.; Akase, Z.; Zhang, L.; Heuer, A.H.; Newman, A.E.; Hughes, P.J. The structure and ordering of ε-MnO2. J. Solid State Chem. 2006, 179, 753–774. [Google Scholar] [CrossRef]
- Zeng, L.; Zhang, G.; Huang, X.; Wang, H.; Zhou, T.; Xie, H. Tuning crystal structure of MnO2 during different hydrothermal synthesis temperature and its electrochemical performance as cathode material for zinc ion battery. Vacuum 2021, 192, 110398. [Google Scholar] [CrossRef]
- Hasegawa, Y.; Fukumoto, K.; Ishima, T.; Yamamoto, H.; Sano, M.; Miyake, T. Preparation of copper-containing mesoporous manganese oxides and their catalytic performance for CO oxidation. Appl. Catal. B Environ. 2009, 89, 420–424. [Google Scholar] [CrossRef]
- Peng, J.; Deng, F.; Shi, H.; Wang, Z.; Li, X.; Zou, J.; Luo, X. Target recognition and preferential degradation of toxic chemical groups by innovative group-imprinted photocatalyst with footprint cavity. Appl. Catal. B Environ. 2024, 340, 123179. [Google Scholar] [CrossRef]
- Mishra, R.K.; Prajapati, C.S.; Shahi, R.R.; Kushwaha, A.K.; Sahay, P.P. Influence of electrodeposition modes on the electrochemical performance of MnO2 films prepared using anionic MnO4− (Mn7+) precursor. Ceram. Int. 2018, 44, 5710–5718. [Google Scholar] [CrossRef]
- Cherian, E.; Rajan, A.; Gurunathan, D.B. Synthesis of manganese dioxide nanoparticles using co-precipitation method and its antimicrobial activity. Int. J. Mod. Sci. Technol. 2016, 01, 17–22. [Google Scholar]
- Burda, C.; Chen, X.; Narayanan, R.; El-Sayed, M.A. Chemistry and Properties of Nanocrystals of Different Shapes. Chem. Rev. 2005, 105, 1025–1102. [Google Scholar] [CrossRef]
- Chen, B.; Wu, B.; Yu, L.; Crocker, M.; Shi, C. Investigation into the Catalytic Roles of Various Oxygen Species over Different Crystal Phases of MnO2 for C6H6 and HCHO Oxidation. ACS Catal. 2020, 10, 6176–6187. [Google Scholar] [CrossRef]
- Yang, S.; Yang, H.; Yang, J.; Qi, H.; Kong, J.; Bo, Z.; Li, X.; Yan, J.; Cen, K.; Tu, X. Three-dimensional hollow urchin α-MnO2 for enhanced catalytic activity towards toluene decomposition in post-plasma catalysis. Chem. Eng. J. 2020, 402, 126154. [Google Scholar] [CrossRef]
- Aljafari, B.; Vijaya, S.; Takshi, A.; Anandan, S. Copper doped manganese dioxide as counter electrode for dye-sensitized solar cells. Arab. J. Chem. 2022, 15, 104068. [Google Scholar] [CrossRef]
- Yang, C.; Zhong, Y.; Li, L.; Ren, X.; Sun, Y.; Niu, D.; Liu, Y.; Yin, M.; Zhang, D. Lead and uranium sorption characteristics on hydrothermal synthesized delta manganese dioxide. J. Radioanal. Nucl. Chem. 2018, 317, 1399–1408. [Google Scholar] [CrossRef]
- Nguyen Dinh, M.T.; Nguyen, C.C.; Truong Vu, T.L.; Ho, V.T.; Truong, Q.D. Tailoring porous structure, reducibility and Mn4+ fraction of ε-MnO2 microcubes for the complete oxidation of toluene. Appl. Catal. A Gen. 2020, 595, 117473. [Google Scholar] [CrossRef]
- Li, K.; Li, H.; Xiao, T.; Long, J.; Zhang, G.; Li, Y.; Liu, X.; Liang, Z.; Zheng, F.; Zhang, P. Synthesis of manganese dioxide with different morphologies for thallium removal from wastewater. J. Environ. Manag. 2019, 251, 109563. [Google Scholar] [CrossRef] [PubMed]
- Abdullah, T.A.; Rasheed, R.T.; Juzsakova, T.; Al-Jammal, N.; Mallah, M.A.; Cuong, L.P.; Salman, A.D.; Domokos, E.; Ali, Z.; Cretescu, I. Preparation and characterization of MnO2-based nanoparticles at different annealing temperatures and their application in dye removal from water. Int. J. Environ. Sci. Technol. 2021, 18, 1499–1512. [Google Scholar] [CrossRef]
- Zhong, M.; Li, M.; Fan, Z.; Huang, W.; Hao, H.; Xia, Z.; Zhang, Q.; Peng, H.; Zhang, Y. Tuning the crystallinity of MnO2 oxidant to achieve highly efficient pollutant degradation. Chin. Chem. Lett. 2023, 34, 107189. [Google Scholar] [CrossRef]
- Kumar, Y.; Chopra, S.; Gupta, A.; Kumar, Y.; Uke, S.J.; Mardikar, S.P. Low temperature synthesis of MnO2 nanostructures for supercapacitor application. Mater. Sci. Energy Technol. 2020, 3, 566–574. [Google Scholar] [CrossRef]
- Yuan, Y.; Zhu, J.; Wang, Y.; Li, S.; Jin, P.; Chen, Y. Facile synthesis of manganese oxide nanostructures with different crystallographic phase and morphology for supercapacitors. J. Alloys Compd. 2020, 830, 154524. [Google Scholar] [CrossRef]
- Yadav, S.; Sharma, A. Importance and challenges of hydrothermal technique for synthesis of transition metal oxides and composites as supercapacitor electrode materials. J. Energy Storage 2021, 44, 103295. [Google Scholar] [CrossRef]
- Patra, T.; Mohanty, A.; Singh, L.; Muduli, S.; Parhi, P.K.; Sahoo, T.R. Effect of calcination temperature on morphology and phase transformation of MnO2 nanoparticles: A step towards green synthesis for reactive dye adsorption. Chemosphere 2022, 288, 132472. [Google Scholar] [CrossRef] [PubMed]
- Zayat, M.; Pardo, R.; Castellón, E.; Torres, L.; Almendro, D.; Parejo, P.G.; Álvarez, A.; Belenguer, T.; García-Revilla, S.; Balda, R.; et al. Optical and Electro-optical Materials Prepared by the Sol-Gel Method. Adv. Mater. 2011, 23, 5318–5323. [Google Scholar] [CrossRef] [PubMed]
- Reddy, R.N.; Reddy, R.G. Sol–gel MnO2 as an electrode material for electrochemical capacitors. J. Power Sources 2003, 124, 330–337. [Google Scholar] [CrossRef]
- Panimalar, S.; Logambal, S.; Thambidurai, R.; Inmozhi, C.; Uthrakumar, R.; Muthukumaran, A.; Rasheed, R.A.; Gatasheh, M.K.; Raja, A.; Kennedy, J.; et al. Effect of Ag doped MnO2 nanostructures suitable for wastewater treatment and other environmental pollutant applications. Environ. Res. 2022, 205, 112560. [Google Scholar] [CrossRef]
- Kusworo, T.D.; Kumoro, A.C.; Aryanti, N.; Kurniawan, T.A.; Dalanta, F.; Alias, N.H. Photocatalytic polysulfone membrane incorporated by ZnO-MnO2@SiO2 composite under UV light irradiation for the reliable treatment of natural rubber-laden wastewater. Chem. Eng. J. 2023, 451, 138593. [Google Scholar] [CrossRef]
- Wang, X.; Yuan, A.; Wang, Y. Supercapacitive behaviors and their temperature dependence of sol–gel synthesized nanostructured manganese dioxide in lithium hydroxide electrolyte. J. Power Sources 2007, 172, 1007–1011. [Google Scholar] [CrossRef]
- Pal, A.; Das, T.; Ghosh, S.; Nandi, M. Supercapacitor behaviour of manganese dioxide decorated mesoporous silica synthesized by a rapid sol–gel inverse micelle method. Dalton Trans. 2020, 49, 12716–12730. [Google Scholar] [CrossRef] [PubMed]
- Siddique, M.A.B.; Bithi, U.H.; Ahmed, A.N.; Gafur, M.A.; Reaz, A.H.; Roy, C.K.; Islam, M.M.; Firoz, S.H. Preparation of Manganese Oxide Nanoparticles with Enhanced Capacitive Properties Utilizing Gel Formation Method. ACS Omega 2022, 7, 48007–48017. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, V.H.; Huynh, L.T.N.; Nguyen, T.H.; Vu, T.P.; Le, M.L.P.; Grag, A.; Tran, V.M. Promising electrode material using Ni-doped layered manganese dioxide for sodium-ion batteries. J. Appl. Electrochem. 2018, 48, 793–800. [Google Scholar] [CrossRef]
- Feng, A.; Hou, T.; Jia, Z.; Wu, G. Synthesis of a hierarchical carbon fiber@cobalt ferrite@manganese dioxide composite and its application as a microwave absorber. RSC Adv. 2020, 10, 10510–10518. [Google Scholar] [CrossRef] [PubMed]
- Anggraini, R.; Siregar, S.S.; Awaluddin, A.; Linggawati, A. The Preliminary Studies on the Tremendous Degradation Rate of Methylene Blue with Cu-doped α-MnO2 Photocatalyst Under UV Light Irradation. J. Phys. Conf. Ser. 2021, 2049, 012060. [Google Scholar] [CrossRef]
- Fu, L.J.; Liu, H.; Li, C.; Wu, Y.P.; Rahm, E.; Holze, R.; Wu, H.Q. Electrode materials for lithium secondary batteries prepared by sol–gel methods. Prog. Mater. Sci. 2005, 50, 881–928. [Google Scholar] [CrossRef]
- Petkovich, N.D.; Stein, A. Controlling macro- and mesostructures with hierarchical porosity through combined hard and soft templating. Chem. Soc. Rev. 2013, 42, 3721–3739. [Google Scholar] [CrossRef]
- Zhang, L.; Jin, L.; Liu, B.; He, J. Templated Growth of Crystalline Mesoporous Materials: From Soft/Hard Templates to Colloidal Templates. Front. Chem. 2019, 7, 22. [Google Scholar] [CrossRef]
- Hou, B.; Liu, Y.; Li, Y.; Yuan, B.; Jia, M.; Jiang, F. Evolvement of soft templates in surfactant/cosurfactant system for shape control of ZnSe nanocrystals. Mater. Sci. Eng. B 2012, 177, 411–415. [Google Scholar] [CrossRef]
- Chen, J.; Meng, H.; Tian, Y.; Yang, R.; Du, D.; Li, Z.; Qu, L.; Lin, Y. Recent advances in functionalized MnO2 nanosheets for biosensing and biomedicine applications. Nanoscale Horiz. 2019, 4, 321–338. [Google Scholar] [CrossRef] [PubMed]
- Yuan, W.; Shen, P.K.; Jiang, S.P. Controllable synthesis of graphene supported MnO2 nanowires via self-assembly for enhanced water oxidation in both alkaline and neutral solutions. J. Mater. Chem. A 2014, 2, 123–129. [Google Scholar] [CrossRef]
- Tran, C.C.H.; Santos-Peña, J.; Damas, C. Theoretical and Practical Approach of Soft Template Synthesis for the Preparation of MnO2 Supercapacitor Electrode. J. Phys. Chem. C 2018, 122, 16–29. [Google Scholar] [CrossRef]
- Yang, J.; Li, K.; Li, C.; Gu, J. In Situ Coupling of Catalytic Centers into Artificial Substrate Mesochannels as Super-Active Metalloenzyme Mimics. Small 2021, 17, 2101455. [Google Scholar] [CrossRef] [PubMed]
- Bai, B.; Li, J.; Hao, J. 1D-MnO2, 2D-MnO2 and 3D-MnO2 for low-temperature oxidation of ethanol. Appl. Catal. B Environ. 2015, 164, 241–250. [Google Scholar] [CrossRef]
- Bai, B.; Qiao, Q.; Li, J.; Hao, J. Synthesis of three-dimensional ordered mesoporous MnO2 and its catalytic performance in formaldehyde oxidation. Chin. J. Catal. 2016, 37, 27–31. [Google Scholar] [CrossRef]
- Zhang, J.; Zhuang, T.; Liu, S.; Zhang, G.C.; Huo, K. Catalytic ozonation of phenol enhanced by mesoporous MnO2 prepared through nanocasting method with SBA-15 as template. J. Environ. Chem. Eng. 2020, 8, 103967. [Google Scholar] [CrossRef]
- Zhang, X.; Zhou, B.; Yin, S.; Wang, Y.; Zhang, X.; Meng, Q.; Meng, F.; Wei, C.; Wen, G. Mesoporous manganese dioxide prepared by nano-casting: An efficient catalyst for of methyl orange and oxalic acid degradation in aqueous solution. Vacuum 2022, 206, 111495. [Google Scholar] [CrossRef]
- Wang, C.; Liu, Z.; Wang, Q.; Guo, J.; Zhao, Q.; Lu, Y. MnO2@polypyrrole composite with hollow microsphere structure for electrode material of supercapacitors. J. Electroanal. Chem. 2021, 901, 115780. [Google Scholar] [CrossRef]
- Wu, J.; Ma, Q.; Lian, C.; Yuan, Y.; Long, D. Promoting polythionate intermediates formation by oxygen-deficient manganese oxide hollow nanospheres for high performance lithium-sulfur batteries. Chem. Eng. J. 2019, 370, 556–564. [Google Scholar] [CrossRef]
- Selvakumar, K.; Kumar, S.M.S.; Thangamuthu, R.; Rajput, P.; Bhattacharyya, D.; Jha, S.N. 2D and 3D Silica-Template-Derived MnO2 Electrocatalysts towards Enhanced Oxygen Evolution and Oxygen Reduction Activity. ChemElectroChem 2018, 5, 3980–3990. [Google Scholar] [CrossRef]
- Gu, W.; Li, C.; Qiu, J.; Yao, J. Facile fabrication of flower-like MnO2 hollow microspheres as high-performance catalysts for toluene oxidation. J. Hazard. Mater. 2021, 408, 124458. [Google Scholar] [CrossRef]
- Wang, H.-q.; Zheng, M.-b.; Chen, J.-h.; Ji, G.-b.; Cao, J.-m. Synthesis of MnO2 Microfiber with Secondary Nanostructure by Cotton Template. J. Nanotechnol. 2010, 2010, 479172. [Google Scholar] [CrossRef]
- Hsueh, H.-Y.; Yao, C.-T.; Ho, R.-M. Well-ordered nanohybrids and nanoporous materials from gyroid block copolymer templates. Chem. Soc. Rev. 2015, 44, 1974–2018. [Google Scholar] [CrossRef] [PubMed]
- Eric, M.G.; Vanessa, F.C.L.; Tulio, M. Metallic and Oxide Electrodeposition. In Modern Surface Engineering Treatments; Mahmood, A., Ed.; IntechOpen: Rijeka, Croatia, 2013; p. Ch. 5. [Google Scholar]
- Shirole, D.; Volpatti, G.; Guerini, A.; Zampini, D.; Cusatis, G.; Rotta Loria, A.F. Effects of electrodeposition in concrete mediated by electric currents of variable polarity. Cem. Concr. Res. 2023, 172, 107254. [Google Scholar] [CrossRef]
- Dai, X.; Zhang, M.; Li, J.; Yang, D. Effects of electrodeposition time on a manganese dioxide supercapacitor. RSC Adv. 2020, 10, 15860–15869. [Google Scholar] [CrossRef] [PubMed]
- Surendranath, Y.; Dincǎ, M.; Nocera, D.G. Electrolyte-Dependent Electrosynthesis and Activity of Cobalt-Based Water Oxidation Catalysts. J. Am. Chem. Soc. 2009, 131, 2615–2620. [Google Scholar] [CrossRef] [PubMed]
- Ye, Z.; Li, T.; Ma, G.; Peng, X.; Zhao, J. Morphology controlled MnO2 electrodeposited on carbon fiber paper for high-performance supercapacitors. J. Power Sources 2017, 351, 51–57. [Google Scholar] [CrossRef]
- Ren, H.; Zhang, L.; Zhang, J.; Miao, T.; Yuan, R.; Chen, W.; Wang, Z.; Yang, J.; Zhao, B. Na+ pre-intercalated Na0.11MnO2 on three-dimensional graphene as cathode for aqueous zinc ion hybrid supercapacitor with high energy density. Carbon 2022, 198, 46–56. [Google Scholar] [CrossRef]
- Shi, Y.; Zhang, M.; Zhao, J.; Zhang, L.; Cui, X.M.; Zhu, X.; Jin, D.; Gong, J.; Yang, D.; Li, J. Preparation of Manganese Dioxide Supercapacitors by Secondary Construction of Three-Dimensional Substrates and Ion Embedding. Electron. Mater. Lett. 2022, 18, 475–488. [Google Scholar] [CrossRef]
- Zhao, W.K.; Ma, Z.Q.; Zheng, J.Y.; Han, C.B.; Zhou, K.L.; Hao, M.Y.; Fang, D.C.; Xia, Y.; Yan, H. Dual-functional MnS2/MnO2 heterostructure catalyst for efficient acidic hydrogen evolution reaction and assisted degradation of organic wastewater. J. Energy Chem. 2023, 87, 215–224. [Google Scholar] [CrossRef]
- Zhou, H.; Zhi, X.; Zhai, H.-J. Promoted supercapacitive performances of electrochemically synthesized poly(3,4-ethylenedioxythiophene) incorporated with manganese dioxide. J. Mater. Sci. Mater. Electron. 2018, 29, 3935–3942. [Google Scholar] [CrossRef]
- Forghani, M.; McCarthy, J.; Cameron, A.P.; Davey, S.B.; Donne, S.W. Semiconductor Properties of Electrodeposited Manganese Dioxide for Electrochemical Capacitors: Mott-Schottky Analysis. J. Electrochem. Soc. 2021, 168, 020508. [Google Scholar] [CrossRef]
- Hu, X.; Cheng, F.; Han, X.; Zhang, T.; Chen, J. Oxygen Bubble-Templated Hierarchical Porous ε-MnO2 as a Superior Catalyst for Rechargeable Li–O2 Batteries. Small 2015, 11, 809–813. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Yang, L.; Song, Z.; Xu, C. Microstructures and capacitance performance of MnO2 films fabricated by ultrasonic-assisted electrodeposition. Appl. Surf. Sci. 2019, 478, 94–102. [Google Scholar] [CrossRef]
- Zhao, P.; Wang, N.; Yao, M.; Ren, H.; Hu, W. Hydrothermal electrodeposition incorporated with CVD-polymerisation to tune PPy@MnO2 interlinked core-shell nanowires on carbon fabric for flexible solid-state asymmetric supercapacitors. Chem. Eng. J. 2020, 380, 122488. [Google Scholar] [CrossRef]
- Meng, X.; Liu, T.; Qin, M.; Liu, Z.; Wang, W. Carbon-Free, Binder-Free MnO2@Mn Catalyst for Oxygen Reduction Reaction. ACS Appl. Mater. Interfaces 2023, 15, 20110–20119. [Google Scholar] [CrossRef]
- Wang, P.; Lin, Y.; Wan, L.; Wang, B. Construction of a Janus MnO2-NiFe Electrode via Selective Electrodeposition Strategy as a High-Performance Bifunctional Electrocatalyst for Rechargeable Zinc–Air Batteries. ACS Appl. Mater. Interfaces 2019, 11, 37701–37707. [Google Scholar] [CrossRef]
- Jeong, J.-H.; Park, J.W.; Lee, D.W.; Baughman, R.H.; Kim, S.J. Electrodeposition of α-MnO2/γ-MnO2 on Carbon Nanotube for Yarn Supercapacitor. Sci. Rep. 2019, 9, 11271. [Google Scholar] [CrossRef]
- Li, X.-b.; Xu, G.-r. Hydrothermal vs electrodeposition: How does deposition method affect the electrochemical capacitor performance of manganese dioxide? Ceram. Int. 2017, 43, 8963–8969. [Google Scholar] [CrossRef]
- Ali, H.; Mahto, B.; Barhoi, A.; Hussain, S. Visible light-driven photocatalytic thiol–ene/yne reactions using anisotropic 1D Bi2S3 nanorods: A green synthetic approach. Nanoscale 2023, 15, 14551–14563. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Sun, X.; Zhang, H.; Zhang, D.; Ma, Y. Microwave-assisted reflux rapid synthesis of MnO2 nanostructures and their application in supercapacitors. Electrochim. Acta 2013, 87, 637–644. [Google Scholar] [CrossRef]
- Said, M.I. Akhtenskite-nsutite phases: Polymorphic transformation, thermal behavior and magnetic properties. J. Alloys Compd. 2020, 819, 152976. [Google Scholar] [CrossRef]
- May, Y.A.; Wei, S.; Yu, W.Z.; Wang, W.W.; Jia, C.J. Highly Efficient CuO/α-MnO2 Catalyst for Low-Temperature CO Oxidation. Langmuir ACS J. Surf. Colloids 2020, 36, 11196–11206. [Google Scholar] [CrossRef] [PubMed]
- Kijima, N.; Sakata, Y.; Takahashi, Y.; Akimoto, J.; Kumagai, T.; Igarashi, K.; Shimizu, T. Synthesis and lithium ion insertion/extraction properties of hollandite-type MnO2 prepared by acid digestion of Mn2O3. Solid State Ion. 2009, 180, 616–620. [Google Scholar] [CrossRef]
- Antonio, C. Synthesis of NPs by Microemulsion Method. In Microemulsion; Juan, C.M., Ed.; IntechOpen: Rijeka, Croatia, 2018; p. Ch. 5. [Google Scholar]
- Tavakoli, A.; Sohrabi, M.; Kargari, A. A review of methods for synthesis of nanostructured metals with emphasis on iron compounds. Chem. Pap. 2007, 61, 151–170. [Google Scholar] [CrossRef]
- Xu, C.; Li, B.; Du, H.; Kang, F.; Zeng, Y. Electrochemical properties of nanosized hydrous manganese dioxide synthesized by a self-reacting microemulsion method. J. Power Sources 2008, 180, 664–670. [Google Scholar] [CrossRef]
- Zefirov, V.V.; Elmanovich, I.V.; Levin, E.E.; Abramchuk, S.S.; Kharitonova, E.P.; Khokhlov, A.A.; Kondratenko, M.S.; Gallyamov, M.O. Synthesis of manganese oxide electrocatalysts in supercritical carbon dioxide. J. Mater. Sci. 2018, 53, 9449–9462. [Google Scholar] [CrossRef]
- Tatarchuk, T.; Bououdina, M.; Judith Vijaya, J.; John Kennedy, L. Spinel Ferrite Nanoparticles: Synthesis, Crystal Structure, Properties, and Perspective Applications. In Nanophysics, Nanomaterials, Interface Studies, and Applications; Springer: Berlin/Heidelberg, Germany, 2017; pp. 305–325. [Google Scholar]
- Liu, Z.; Ji, H.; Wang, S.; Zhao, W.; Huang, Y.; Feng, H.; Wei, J.; Li, M. Enhanced Electrical and Mechanical Properties of a Printed Bimodal Silver Nanoparticle Ink for Flexible Electronics. Phys. Status Solidi A 2018, 215, 1800007. [Google Scholar] [CrossRef]
- Sivakumar, S.; Nelson Prabu, L. Synthesis and Characterization of α-MnO2 nanoparticles for Supercapacitor application. Mater. Today Proc. 2021, 47, 52–55. [Google Scholar] [CrossRef]
- Yadav, P.; Bhaduri, A. Chemically synthesized manganese oxide nanorods for effectual organic dye removal and energy storage application. Mater. Chem. Phys. 2023, 299, 127495. [Google Scholar] [CrossRef]
- Yang, P.; Wang, J.; Wang, H.; Wang, S.; Yang, C.; He, Y. Physicochemical properties of different crystal forms of manganese dioxide prepared by a liquid phase method and their quantitative evaluation in capacitor and battery materials. Nanoscale Adv. 2023, 5, 3396–3413. [Google Scholar] [CrossRef]
- Quintero-Quiroz, C.; Acevedo, N.; Zapata-Giraldo, J.; Botero, L.E.; Quintero, J.; Zárate-Triviño, D.; Saldarriaga, J.; Pérez, V.Z. Optimization of silver nanoparticle synthesis by chemical reduction and evaluation of its antimicrobial and toxic activity. Biomater. Res. 2019, 23, 27. [Google Scholar] [CrossRef] [PubMed]
- Ferrando, R.; Jellinek, J.; Johnston, R.L. Nanoalloys: From Theory to Applications of Alloy Clusters and Nanoparticles. Chem. Rev. 2008, 108, 845–910. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Rong, G.; Xie, Y.; Huang, L.; Feng, C. Low-Temperature Synthesis of α-MnO2 Hollow Urchins and Their Application in Rechargeable Li+ Batteries. Inorg. Chem. 2006, 45, 6404–6410. [Google Scholar] [CrossRef] [PubMed]
- Khan, I.; Sadiq, M.; Khan, I.; Saeed, K. Manganese dioxide nanoparticles/activated carbon composite as efficient UV and visible-light photocatalyst. Environ. Sci. Pollut. Res. 2019, 26, 5140–5154. [Google Scholar] [CrossRef] [PubMed]
- Cremonezzi, J.M.d.O.; Tiba, D.Y.; Domingues, S.H. Fast synthesis of δ-MnO2 for a high-performance supercapacitor electrode. SN Appl. Sci. 2020, 2, 1689. [Google Scholar] [CrossRef]
- Narayanan, K.B.; Sakthivel, N. Green synthesis of biogenic metal nanoparticles by terrestrial and aquatic phototrophic and heterotrophic eukaryotes and biocompatible agents. Adv. Colloid Interface Sci. 2011, 169, 59–79. [Google Scholar] [CrossRef]
- Ahmed, S.; Annu; Chaudhry, S.A.; Ikram, S. A review on biogenic synthesis of ZnO nanoparticles using plant extracts and microbes: A prospect towards green chemistry. J. Photochem. Photobiol. B Biol. 2017, 166, 272–284. [Google Scholar] [CrossRef]
- Joshi, N.C.; Siddqui, F.; Salman, M.; Singh, A. Antibacterial Activity, Characterizations, and Biological Synthesis of Manganese Oxide Nanoparticles using the Extract of Aloe vera. Asian Pac. J. Health Sci. 2020, 7, 27–29. [Google Scholar] [CrossRef]
- Souri, M.; Shakeri, A. Optimization of Total Phenol and Tannin Content and Biological Activity of Dittrichia graveolens (L.) GREUTER. Curr. Bioact. Compd. 2020, 16, 124–132. [Google Scholar] [CrossRef]
- Hashem, A.M.; Abuzeid, H.; Kaus, M.; Indris, S.; Ehrenberg, H.; Mauger, A.; Julien, C.M. Green synthesis of nanosized manganese dioxide as positive electrode for lithium-ion batteries using lemon juice and citrus peel. Electrochim. Acta 2018, 262, 74–81. [Google Scholar] [CrossRef]
- Shehroz, H.; Ali, S.; Bibi, G.; Khan, T.; Jamil, S.; Khan, S.R.; Hashaam, M.; Naz, S. Comparative investigation of the catalytic application of α/β/γ-MnO2 nanoparticles synthesized by green and chemical approaches. Environ. Technol. 2024, 45, 1081–1091. [Google Scholar] [CrossRef] [PubMed]
- Ramesh, P.; Rajendran, A. Green synthesis of manganese dioxide nanoparticles photocatalytic and antimicrobial investigations. Int. J. Environ. Anal. Chem. 2023, 1–13. [Google Scholar] [CrossRef]
- Rahmat, M.; Kiran, S.; Gulzar, T.; Yusuf, M.; Nawaz, R.; Khalid, J.; Fatima, N.; Ullah, A.; Azam, M. Plant-assisted synthesis and characterization of MnO2 nanoparticles for removal of crystal violet dye: An environmental remedial approach. Environ. Sci. Pollut. Res. 2023, 30, 57587–57598. [Google Scholar] [CrossRef] [PubMed]
- Majani, S.S.; Sathyan, S.; Manoj, M.V.; Vinod, N.; Pradeep, S.; Shivamallu, C.; Venkatachalaiah, K.N.; Kollur, S.P. Eco-friendly synthesis of MnO2 nanoparticles using Saraca asoca leaf extract and evaluation of in vitro anticancer activity. Curr. Res. Green Sustain. Chem. 2023, 6, 100367. [Google Scholar] [CrossRef]
- Hoseinpour, V.; Souri, M.; Ghaemi, N. Green synthesis, characterisation, and photocatalytic activity of manganese dioxide nanoparticles. Micro Nano Lett. 2018, 13, 1560–1563. [Google Scholar] [CrossRef]
- Samsoon, S.; Azam, M.; Khan, A.; Ashraf, M.; Bhatti, H.N.; Alshawwa, S.Z.; Iqbal, M. Green-synthesized MnO2 nanofertilizer impact on growth, photosynthetic pigment, and non-enzymatic antioxidant of Vigna unguiculata cultivar. Biomass Convers. Biorefinery 2022, 1–10. [Google Scholar] [CrossRef]
- Lu, H.; Zhang, X.; Khan, S.A.; Li, W.; Wan, L. Biogenic Synthesis of MnO2 Nanoparticles with Leaf Extract of Viola betonicifolia for Enhanced Antioxidant, Antimicrobial, Cytotoxic, and Biocompatible Applications. Front. Microbiol. 2021, 12, 761084. [Google Scholar] [CrossRef]
- Srivastava, V.; Choubey, A.K. Study of adsorption of anionic dyes over biofabricated crystalline α-MnO2 nanoparticles. Environ. Sci. Pollut. Res. 2021, 28, 15504–15518. [Google Scholar] [CrossRef]
- Bhattacharjee, S.; Bardhan, M.; Ghosh, S.; Banerjee, A.; Pal, K.; Guha, A.; Mondal, D.; Basu, R.; Das, S.; Sinha, S.K. An in-vivo interpretation for validating the ameliorative efficacy of green synthesized MnO2 nano-conjugate using Carica Papaya (Papaya) leaf extract against acute hepatic damage. J. Drug Deliv. Sci. Technol. 2021, 66, 102774. [Google Scholar] [CrossRef]
- Ogunyemi, S.O.; Zhang, F.; Abdallah, Y.; Zhang, M.; Wang, Y.; Sun, G.; Qiu, W.; Li, B. Biosynthesis and characterization of magnesium oxide and manganese dioxide nanoparticles using Matricaria chamomilla L. extract and its inhibitory effect on Acidovorax oryzae strain RS-2. Artif. Cells Nanomed. Biotechnol. 2019, 47, 2230–2239. [Google Scholar] [CrossRef]
- Elsherif, S.A.; Abuzeid, H.M.; Hashem, A.M.; Abdel Ghany, N.A. Green synthesis of MnO2 via plant extracts and its composite with exfoliated graphene for high-performance asymmetric supercapacitors. J. Energy Storage 2023, 74, 109341. [Google Scholar] [CrossRef]
- Ghosh, A.; Hegde, R.V.; Limaye, A.S.; Thrilokraj, R.; Patil, S.A.; Dateer, R.B. Biogenic synthesis of δ-MnO2 nanoparticles: A sustainable approach for C-alkylation and quinoline synthesis via acceptorless dehydrogenation and borrowing hydrogen reactions. Appl. Organomet. Chem. 2023, 37, e7119. [Google Scholar] [CrossRef]
- Affrald, J. A comprehensive review of manganese dioxide nanoparticles and strategy to overcome toxicity. Nanomedicine 2022, 10, 1–15. [Google Scholar] [CrossRef]
- Beveridge, T.J.; Hughes, M.N.; Lee, H.; Leung, K.T.; Poole, R.K.; Savvaidis, I.; Silver, S.; Trevors, J.T. Metal-Microbe Interactions: Contemporary Approaches. Adv. Microb. Physiol. 1996, 38, 177–243. [Google Scholar]
- Bharde, A.; Rautaray, D.; Bansal, V.; Ahmad, A.; Sarkar, I.; Yusuf, S.M.; Sanyal, M.; Sastry, M. Extracellular Biosynthesis of Magnetite using Fungi. Small 2006, 2, 135–141. [Google Scholar] [CrossRef] [PubMed]
- Sinha, A.; Singh, V.N.; Mehta, B.R.; Khare, S.K. Synthesis and characterization of monodispersed orthorhombic manganese oxide nanoparticles produced by Bacillus sp. cells simultaneous to its bioremediation. J. Hazard. Mater. 2011, 192, 620–627. [Google Scholar] [CrossRef]
- Borah, D.; Rout, J.; Gogoi, D.; Nath Ghosh, N.; Bhattacharjee, C.R. Composition controllable green synthesis of manganese dioxide nanoparticles using an edible freshwater red alga and its photocatalytic activity towards water soluble toxic dyes. Inorg. Chem. Commun. 2022, 138, 109312. [Google Scholar] [CrossRef]
- Alvares, J.; Gaonkar, S.; Naik, C.; Asogekar, P.; Furtado, I. Characterization of Mn3O4-MnO2 nanocomposites biosynthesized by cell lysate of Haloferax alexandrinus GUSF-1. J. Basic Microbiol. 2023, 63, 996–1006. [Google Scholar] [CrossRef]
- Iravani, S.; Varma, R.S. Bacteria in Heavy Metal Remediation and Nanoparticle Biosynthesis. ACS Sustain. Chem. Eng. 2020, 8, 5395–5409. [Google Scholar] [CrossRef]
- Gahlawat, G.; Choudhury, A.R. A review on the biosynthesis of metal and metal salt nanoparticles by microbes. RSC Adv. 2019, 9, 12944–12967. [Google Scholar] [CrossRef] [PubMed]
- Zhang, K.; Han, X.; Hu, Z.; Zhang, X.; Tao, Z.; Chen, J. Nanostructured Mn-based oxides for electrochemical energy storage and conversion. Chem. Soc. Rev. 2015, 44, 699–728. [Google Scholar] [CrossRef] [PubMed]
- Liao, X.; Pan, C.; Pan, Y.; Yin, C. Synthesis of three-dimensional β-MnO2/PPy composite for high-performance cathode in zinc-ion batteries. J. Alloys Compd. 2021, 888, 161619. [Google Scholar] [CrossRef]
- Wu, R.; Kwan, K.W.; Wang, Y.; Ngan, A.H.W. Air-Working Electrochemical Actuator and Ionic Sensor Based on Manganese Dioxide/Gelatin-Glycerol Composites. Adv. Mater. Technol. 2023, 8, 2202062. [Google Scholar] [CrossRef]
- Wu, Y.; Xu, Z.; Ren, R.; Lv, N.; Yang, J.; Zhang, J.; Ren, H.; Dong, S.; Dong, X. Flexible Ammonium-Ion Pouch Cells Based on a Tunneled Manganese Dioxide Cathode. ACS Appl. Mater. Interfaces 2023, 15, 12434–12442. [Google Scholar] [CrossRef]
- Benedet, M.; Gallo, A.; Maccato, C.; Rizzi, G.A.; Barreca, D.; Lebedev, O.I.; Modin, E.; McGlynn, R.; Mariotti, D.; Gasparotto, A. Controllable Anchoring of Graphitic Carbon Nitride on MnO2 Nanoarchitectures for Oxygen Evolution Electrocatalysis. ACS Appl. Mater. Interfaces 2023, 15, 47368–47380. [Google Scholar] [CrossRef] [PubMed]
- Miller, J.R.; Simon, P. Electrochemical Capacitors for Energy Management. Science 2008, 321, 651–652. [Google Scholar] [CrossRef]
- Zhang, Y.-Z.; Wang, Y.; Cheng, T.; Yao, L.-Q.; Li, X.; Lai, W.-Y.; Huang, W. Printed supercapacitors: Materials, printing and applications. Chem. Soc. Rev. 2019, 48, 3229–3264. [Google Scholar] [CrossRef]
- Krishnamoorthy, K.; Pazhamalai, P.; Mariappan, V.K.; Manoharan, S.; Kesavan, D.; Kim, S.-J. Two-Dimensional Siloxene–Graphene Heterostructure-Based High-Performance Supercapacitor for Capturing Regenerative Braking Energy in Electric Vehicles. Adv. Funct. Mater. 2021, 31, 2008422. [Google Scholar] [CrossRef]
- Jiao, X.; Wang, J.; Yuan, Z.; Zhang, C. Smart current collector for high-energy-density and high-contrast electrochromic supercapacitors toward intelligent and wearable power application. Energy Storage Mater. 2023, 54, 254–265. [Google Scholar] [CrossRef]
- Jin, W.-Y.; Ovhal, M.M.; Lee, H.B.; Tyagi, B.; Kang, J.-W. Scalable, All-Printed Photocapacitor Fibers and Modules based on Metal-Embedded Flexible Transparent Conductive Electrodes for Self-Charging Wearable Applications. Adv. Energy Mater. 2021, 11, 2003509. [Google Scholar] [CrossRef]
- Fic, K.; Platek, A.; Piwek, J.; Frackowiak, E. Sustainable materials for electrochemical capacitors. Mater. Today 2018, 21, 437–454. [Google Scholar] [CrossRef]
- Ideta, K.; Kim, D.-W.; Kim, T.; Nakabayashi, K.; Miyawaki, J.; Park, J.-I.; Yoon, S.-H. Effect of pore size in activated carbon on the response characteristic of electric double layer capacitor. J. Ind. Eng. Chem. 2021, 102, 321–326. [Google Scholar] [CrossRef]
- Teng, W.; Zhou, Q.; Wang, X.; Che, H.; Hu, P.; Li, H.; Wang, J. Hierarchically interconnected conducting polymer hybrid fiber with high specific capacitance for flexible fiber-shaped supercapacitor. Chem. Eng. J. 2020, 390, 124569. [Google Scholar] [CrossRef]
- Wang, H.; Diao, Y.; Lu, Y.; Yang, H.; Zhou, Q.; Chrulski, K.; D’Arcy, J.M. Energy storing bricks for stationary PEDOT supercapacitors. Nat. Commun. 2020, 11, 3882. [Google Scholar] [CrossRef] [PubMed]
- Chang, X.; El-Kady, M.F.; Huang, A.; Lin, C.-W.; Aguilar, S.; Anderson, M.; Zhu, J.Z.J.; Kaner, R.B. 3D Graphene Network with Covalently Grafted Aniline Tetramer for Ultralong-Life Supercapacitors. Adv. Funct. Mater. 2021, 31, 2102397. [Google Scholar] [CrossRef]
- Zhang, L.; Shi, D.; Liu, T.; Jaroniec, M.; Yu, J. Nickel-based materials for supercapacitors. Mater. Today 2019, 25, 35–65. [Google Scholar] [CrossRef]
- Zhang, A.; Zhao, R.; Hu, L.; Yang, R.; Yao, S.; Wang, S.; Yang, Z.; Yan, Y.-M. Adjusting the Coordination Environment of Mn Enhances Supercapacitor Performance of MnO2. Adv. Energy Mater. 2021, 11, 2101412. [Google Scholar] [CrossRef]
- Brousse, K.; Pinaud, S.; Nguyen, S.; Fazzini, P.-F.; Makarem, R.; Josse, C.; Thimont, Y.; Chaudret, B.; Taberna, P.-L.; Respaud, M.; et al. Facile and Scalable Preparation of Ruthenium Oxide-Based Flexible Micro-Supercapacitors. Adv. Energy Mater. 2020, 10, 1903136. [Google Scholar] [CrossRef]
- Wang, L.; Xie, X.; Dinh, K.N.; Yan, Q.; Ma, J. Synthesis, characterizations, and utilization of oxygen-deficient metal oxides for lithium/sodium-ion batteries and supercapacitors. Coord. Chem. Rev. 2019, 397, 138–167. [Google Scholar] [CrossRef]
- Devi, N.; Goswami, M.; Saraf, M.; Singh, B.; Mobin, S.M.; Singh, R.K.; Srivastava, A.K.; Kumar, S. Physicochemical and electrochemical behaviours of manganese oxide electrodes for supercapacitor application. J. Energy Storage 2020, 28, 101228. [Google Scholar] [CrossRef]
- Yao, B.; Chandrasekaran, S.; Zhang, J.; Xiao, W.; Qian, F.; Zhu, C.; Duoss, E.B.; Spadaccini, C.M.; Worsley, M.A.; Li, Y. Efficient 3D Printed Pseudocapacitive Electrodes with Ultrahigh MnO2 Loading. Joule 2019, 3, 459–470. [Google Scholar] [CrossRef]
- Bagal, I.V.; Chodankar, N.R.; Waseem, A.; Ali Johar, M.; Patil, S.J.; Abdullah, A.; Afifi Hassan, M.; Han, Y.-K.; Ryu, S.-W. CF4 plasma-treated porous silicon nanowire arrays laminated with MnO2 nanoflakes for asymmetric pseudocapacitors. Chem. Eng. J. 2021, 419, 129515. [Google Scholar] [CrossRef]
- Tynan, B.; Zhou, Y.; Brown, S.A.; Dai, L.; Rider, A.N.; Wang, C.H. Structural supercapacitor electrodes for energy storage by electroless deposition of MnO2 on carbon nanotube mats. Compos. Sci. Technol. 2023, 238, 110016. [Google Scholar] [CrossRef]
- Mladenova, B.; Pashova, K.; Hinkov, I.; Dimitrova, M.; Stoyanova, A. Green synthesis of MnO2 using Calendula officinalis and Tilia cordata extracts for application in supercapacitors. Monatshefte Für Chem.-Chem. Mon. 2024, 155, 341–348. [Google Scholar] [CrossRef]
- Li, P.; Wu, J.; Tang, L.; Liu, H.; Xu, Y.; Zhang, D. Interconnected δ–MnO2 nanosheets anchored on porous carbon derived from reed residue waste as high-performance electrode for supercapacitor. Ionics 2023, 29, 3629–3639. [Google Scholar] [CrossRef]
- Mladenova, B.; Dimitrova, M.; Stoyanova, A. MnO2/AgNPs Composite as Flexible Electrode Material for Solid-State Hybrid Supercapacitor. Batteries 2024, 10, 122. [Google Scholar] [CrossRef]
- Sayah, A.; Boumaza, N.; Habelhames, F.; Bahloul, A.; Bencherif, H.; Tounsi, A.; Lamiri, L.; Nessark, B. Electrodeposition mode effects on the electrochemical performance of MnO2–NiO eco-friendly material for supercapacitor electrode application. J. Mater. Sci. Mater. Electron. 2024, 35, 62. [Google Scholar] [CrossRef]
- Khalid, M.U.; Zulfiqar, S.; Khan, M.N.; Shakir, I.; Warsi, M.F.; Cochran, E.W. Electrochemical performance enhancement of MnO2 nanowires through silver incorporation for next-generation supercapacitors. Mater. Adv. 2024, 5, 6170–6184. [Google Scholar] [CrossRef]
- Gupta, M.K.; Kumar, Y.; Shukla, V.K. Hydrothermal Synthesis of a Layered ZnO@MnO2 Nanocomposite for High-Performance Supercapacitor Electrodes. J. Electron. Mater. 2024, 53, 2050–2061. [Google Scholar] [CrossRef]
- Mofokeng, T.P.; Shabalala, S.; Haruna, A.B.; Mwonga, P.V.; Tetana, Z.N.; Ozoemena, K.I. Scalable synthesis of K+/Na+ pre-intercalated α-MnO2 via Taylor fluid flow-assisted hydrothermal reaction for high-performance asymmetric supercapacitors. J. Electroanal. Chem. 2023, 948, 117809. [Google Scholar] [CrossRef]
- Zhu, Y.; Xu, H.; Tang, J.; Jiang, X.; Bao, Y. Synthesis of γ-MnO2/PANI Composites for Supercapacitor Application in Acidic Electrolyte. J. Electrochem. Soc. 2021, 168, 030542. [Google Scholar] [CrossRef]
- Pundir, S.; Upadhyay, S.; Priya, R.; Kumar, N.; Chetana, S.; Hossain, I.; Joshi, N.C.; Pandey, O.P. Synthesis of 1D β-MnO2 for high-performance supercapacitor application. J. Solid State Electrochem. 2023, 27, 531–538. [Google Scholar] [CrossRef]
- Devi, R.; Kumar, V.; Kumar, S.; Bulla, M.; Sharma, S.; Sharma, A. Electrochemical Analysis of MnO2 (α, β, and γ)-Based Electrode for High-Performance Supercapacitor Application. Appl. Sci. 2023, 13, 7907. [Google Scholar] [CrossRef]
- Sinan-Tatli, N.; Unur-Yilmaz, E. PANI-grafted radially porous MnO2 for supercapacitor applications. J. Solid State Electrochem. 2024, 28, 2593–2603. [Google Scholar] [CrossRef]
- Jadhav, S.A.; Dhas, S.D.; Patil, K.T.; Moholkar, A.V.; Patil, P.S. Polyaniline (PANI)-manganese dioxide (MnO2) nanocomposites as efficient electrode materials for supercapacitors. Chem. Phys. Lett. 2021, 778, 138764. [Google Scholar] [CrossRef]
- Xie, C.; Li, T.; Deng, C.; Song, Y.; Zhang, H.; Li, X. A highly reversible neutral zinc/manganese battery for stationary energy storage. Energy Environ. Sci. 2020, 13, 135–143. [Google Scholar] [CrossRef]
- Chen, J.; Liang, J.; Zhou, Y.; Sha, Z.; Lim, S.; Huang, F.; Han, Z.; Brown, S.A.; Cao, L.; Wang, D.-W.; et al. A vertical graphene enhanced Zn–MnO2 flexible battery towards wearable electronic devices. J. Mater. Chem. A 2021, 9, 575–584. [Google Scholar] [CrossRef]
- Sambandam, B.; Mathew, V.; Kim, S.; Lee, S.; Kim, S.; Hwang, J.Y.; Fan, H.J.; Kim, J. An analysis of the electrochemical mechanism of manganese oxides in aqueous zinc batteries. Chem 2022, 8, 924–946. [Google Scholar] [CrossRef]
- Wu, D.; King, S.T.; Sadique, N.; Ma, L.; Ehrlich, S.N.; Ghose, S.; Bai, J.; Zhong, H.; Yan, S.; Bock, D.C.; et al. Operando investigation of aqueous zinc manganese oxide batteries: Multi-stage reaction mechanism revealed. J. Mater. Chem. A 2023, 11, 16279–16292. [Google Scholar] [CrossRef]
- Shen, X.; Wang, X.; Zhou, Y.; Shi, Y.; Zhao, L.; Jin, H.; Di, J.; Li, Q. Highly Reversible Aqueous Zn-MnO2 Battery by Supplementing Mn2+-Mediated MnO2 Deposition and Dissolution. Adv. Funct. Mater. 2021, 31, 2101579. [Google Scholar] [CrossRef]
- Liu, Z.; Yang, Y.; Lu, B.; Liang, S.; Fan, H.J.; Zhou, J. Insights into complexing effects in acetate-based Zn-MnO2 batteries and performance enhancement by all-round strategies. Energy Storage Mater. 2022, 52, 104–110. [Google Scholar] [CrossRef]
- Ma, K.; Yang, G.; Wang, C. Towards storable and durable Zn-MnO2 batteries with hydrous tetraglyme electrolyte. J. Energy Chem. 2023, 80, 432–441. [Google Scholar] [CrossRef]
- Xie, J.; Liu, G.; Sun, J.; Zheng, R.; Zhao, W.; Chu, T.; Lin, H.; Xu, Y.; Gao, S.; Sui, Z. α-MnO2/CNTs with cross-linked reticular structure: Towards ultralong life zinc-ion batteries. Diam. Relat. Mater. 2022, 125, 109024. [Google Scholar] [CrossRef]
- Cai, X.; Li, H.; Li, J.; Yan, H.; Liu, Y.; Yu, H.; Yan, L.; Zhang, L.; Shu, J. Hydrothermal synthesis of β-MnO2 nanorods for highly efficient zinc-ion storage. Ionics 2021, 27, 3943–3950. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, Y.; Gao, G.; Fan, Y.; Wang, R.; Feng, J.; Yang, L.; Meng, A.; Zhao, J.; Li, Z. Effectively Modulating Oxygen Vacancies in Flower-Like δ-MnO2 Nanostructures for Large Capacity and High-Rate Zinc-Ion Storage. Nano-Micro Lett. 2023, 15, 219. [Google Scholar] [CrossRef] [PubMed]
- Chao, D.; Ye, C.; Xie, F.; Zhou, W.; Zhang, Q.; Gu, Q.; Davey, K.; Gu, L.; Qiao, S.-Z. Atomic Engineering Catalyzed MnO2 Electrolysis Kinetics for a Hybrid Aqueous Battery with High Power and Energy Density. Adv. Mater. 2020, 32, 2001894. [Google Scholar] [CrossRef]
- Panda, M.R.; El Meragawi, S.; Mirshekarloo, M.S.; Chen, W.; Shaibani, M.; Majumder, M. Acidity-Aided Surface Modification Strategy to Enhance In Situ MnO2 Deposition for High Performance Zn-MnO2 Battery Prototypes. Small 2024, 2311933. [Google Scholar] [CrossRef]
- Lv, W.; Shen, Z.; Li, X.; Meng, J.; Yang, W.; Ding, F.; Ju, X.; Ye, F.; Li, Y.; Lyu, X.; et al. Discovering Cathodic Biocompatibility for Aqueous Zn–MnO2 Battery: An Integrating Biomass Carbon Strategy. Nano-Micro Lett. 2024, 16, 109. [Google Scholar] [CrossRef] [PubMed]
- Shi, Y.-Y.; Liao, S.-Y.; Wang, Q.-F.; Xu, X.-Y.; Wang, X.-Y.; Gu, X.-Y.; Hu, Y.-G.; Zhu, P.-L.; Sun, R.; Wan, Y.-J. Enhancing the Interaction of Carbon Nanotubes by Metal–Organic Decomposition with Improved Mechanical Strength and Ultra-Broadband EMI Shielding Performance. Nano-Micro Lett. 2024, 16, 134. [Google Scholar] [CrossRef] [PubMed]
- Ouda, E.; Yousf, N.; Magar, H.S.; Hassan, R.Y.A.; Duraia, E.-S.M. Electrochemical properties of MnO2-based carbon nanomaterials for energy storage and electrochemical sensing. J. Mater. Sci. Mater. Electron. 2023, 34, 731. [Google Scholar] [CrossRef]
- Tian, W.; Ren, P.; Hou, X.; Xue, R.; Chen, Z.; Guo, Z.; Jin, Y.; Ren, F. MnO2 porous carbon composite from cellulose enabling high gravimetric/volumetric performance for supercapacitor. Int. J. Biol. Macromol. 2024, 261, 129977. [Google Scholar] [CrossRef] [PubMed]
- Jereil, S.D.; Vijayalakshmi, K.; Monamary, A. Substantial effect of Pd incorporation in MnO2 synthesized by spray pyrolysis on MWCNTs/Ta electrode for better H2O2 sensitivity. Ceram. Int. 2019, 45, 3782–3790. [Google Scholar] [CrossRef]
- Xu, N.; Nie, Q.; Luo, L.; Yao, C.; Gong, Q.; Liu, Y.; Zhou, X.-D.; Qiao, J. Controllable Hortensia-like MnO2 Synergized with Carbon Nanotubes as an Efficient Electrocatalyst for Long-Term Metal–Air Batteries. ACS Appl. Mater. Interfaces 2019, 11, 578–587. [Google Scholar] [CrossRef]
- Li, Z.; Xiao, D.; Xu, C.; Li, Z.; Bi, S.; Xu, H.; Dou, H.; Zhang, X. MnO2/carbon nanotube free-standing electrode recycled from spent manganese-oxygen battery as high-performance supercapacitor material. J. Mater. Sci. 2022, 57, 8818–8827. [Google Scholar] [CrossRef]
- Rosaiah, P.; Divya, P.; Sambasivam, S.; Tighezza, A.M.; Kalaivani, V.; Muthukrishnaraj, A.; Ayyar, M.; Niyitanga, T.; Kim, H. Carbon based manganese oxide (MnO2, MnO2/MWCNT and MnO2/rGO) composite electrodes for high-stability Li-ion batteries. Carbon Lett. 2024, 34, 215–225. [Google Scholar] [CrossRef]
- Zhou, X.; Chen, S.; Zhang, Y.; Yu, B.; Chen, Y.; Liu, Y.; Li, S.; Liu, L.; Jin, H.; Deng, J.; et al. Three-Dimensional Conductive Interface and Tip Structure of MnO2 Electrode Facilitate Superior Zinc Ion Batteries. Small Struct 2024, 2400057. [Google Scholar] [CrossRef]

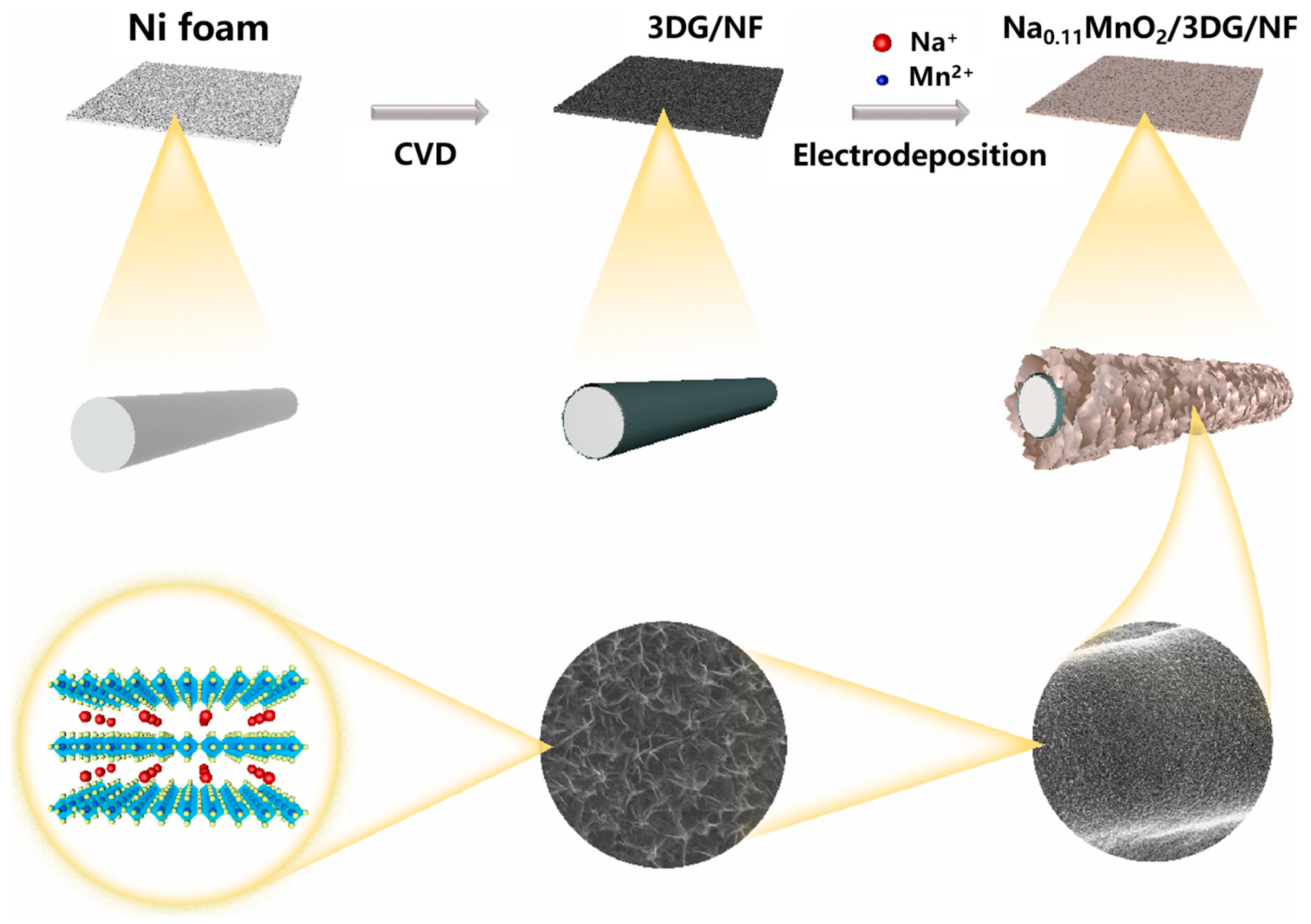


| Crystalline Morphology | Structure Type | Tunnels (n × m) | Dimension | Reference |
|---|---|---|---|---|
| α-MnO2 | Hollandite | (2 × 2) | 1D | [14] |
| β-MnO2 | Pyrolusite | (1 × 1) | 1D | [15] |
| γ-MnO2 | Nsutite | (1 × 1)/(1 × 2) | 1D | [16] |
| δ-MnO2 | Birnessite | (1 × ∞) | 2D | [17] |
| λ-MnO2 | Spinel | (1 × 1) | 3D | [18] |
| ε-MnO2 | - | (1 × 1)/(1 × 2) | 3D | [19] |
| Preparation Products | Formwork | Experimental Data | Applications | Reference |
|---|---|---|---|---|
| MnO2@polypyrrole | Polystyrene | The specific capacitance, energy density, and power density were 63 F g−1, 42 Wh kg−1 and 1100 W kg−1, separately. | Supercapacitors | [60] |
| S/MnO2-280H | S | The capacitances of 1053 and 551 mAh g−1 following 400 cycles | Cathodes with Li-S batteries | [61] |
| MnO2 (KIT-6) | KIT-6 | The bifunctional activity measurable value of 1.28 V | Electrocatalysts | [62] |
| Flower-like MnO2 | MnCO3 microspheres | 90% removal of 1000-ppm toluene | Catalyst | [63] |
| Synthetic Structure | Measurement Conditions | Performance | Applications | Reference |
|---|---|---|---|---|
| MnO2/poly (3,4-ethylenediox-ythiophene) (PEDOT) | 10 mV s−1 | Capacitance was 89.7 mF cm−2 | Supercapacitors | [74] |
| γ-MnO2 | 0.025 V s−1 | The capacitance was 43.1 F g−1 | Capacitor electrodes | [75] |
| ε-MnO2 | 100 mAh g−1 | The discharge capacity delivered by the cell was 5700 mAh g−1 | Li-O2 Catalysts | [76] |
| MnO2 nanostructures | 1 A g−1 | Capacitance and stability were 369 F g−1 and 97% following 1000 cycles | Supercapacitors | [77] |
| MnO2 nanowires | 1 mA cm−2 | The stability was 92.6% after 10,000 cycles | Supercapacitors | [78] |
| MnO2@Mn | 0.86 V | The catalyst showed good stability after a 30h timed current test with little or no decay | Catalysts | [79] |
| MnO2-NiFe/Ni | 50 mA cm−2 | The power density was 93.95 mW cm−2 | Oxygen electrocatalysts | [80] |
| α-MnO2/γ-MnO2 | 193 µW cm−2 | The energy density was 93.8 µWh cm−2 | Supercapacitors | [81] |
| Plant Organism | Nanoparticle Structures of MnO2 | Particle Size | Effect | Appliance | Reference |
|---|---|---|---|---|---|
| Flower extract | MnO2 nanorods | 100 nm | Decolorization of the target dye was 91.3%. TOC and COD were reduced by 90.6% and 92.1% separately. | Removal of crystalline violet dye | [109] |
| Saraca asoca leaves extract | MnO2 nanoparticles | 18 nm | The semi-inhibitory concentration values of 20 µg/mL for both MCF-7 and MDAMB-231 cells | Considerable cytotoxic effects on cancer cells | [110] |
| Yucca gloriosa leaf extract | MnO2 nanoparticle | 80 nm | The photocatalytic efficiency for 20 min was 33% | Photocatalytic activity and good degradation of organic dyes | [111] |
| Potato leaf extract | MnO2 nanoparticle | 26 nm | Significant increases of 67.1% in plant growth activity, 52.8% in photosynthetic pigments, and 56.25% in non-enzymatic antioxidant activity in soil, respectively | Multi-aspect enhancer | [112] |
| Extract of viola betonicifolia | Green synthesized MnO2 nanoparticles and Chemically Synthesized MnO2 Nanoparticles | 10.5 ± 0.85 nm | Cell survival (79.33 ± 0.75%), (73.54 ± 0.82%), respectively | Used to provide antimicrobial coatings | [113] |
| Extract of ficus retusa plant | α-MnO2 nanoparticles | 30~50 nm | The adsorption capacities for Mo and Mr dyes were 116.1 and 74.02 mg g−1, separately | Adsorbent | [114] |
| Papaya leaf extract | MnO2 nano-conjugate | 30~40 nm | The urea and cholesterol reduced to 94 ± 2.16 | For the treatment of hyperbilirubinaemia | [115] |
| Chamomile flower extract | MnO2 nanoparticles | 16.5 nm | The percentage of apoptotic cells in RS-2 ranged from 0.97% to 99.94% | Strong inhibitory effect on rice strain RS-2 | [116] |
| Plant extracts | α-MnO2 | 2.8~4.5 nm | The capacitance and stability were 500 F g−1 and 71%, separately, after 7000 cycles | Supercapacitors | [117] |
| Mango lead extract | δ-MnO2 nanoparticles | 1.5~2.5 nm | The efficiency with >96% removal of cationic pollutants | Cation adsorbent | [118] |
| Synthetic Structure | Measurement Conditions | Performance | Applications | Reference |
|---|---|---|---|---|
| β-MnO2/Polypyrrole | 0.2 A g−1 | Specific discharge capacity of 361.7 mAh g−1 | Zinc-ion batteries | [128] |
| Manganese dioxide/gelatin-glycerol | ±2 V | High bending actuation (20-mm deflection, >360° scan angle, and 2.5-mm radius of curvature) and different shape change | Air-working actuator | [129] |
| α-MnO2 | 0.1 A g −1 | Capacity was 190 mAh g−1 and the stability was after 50,000 cycles in (NH4)2SO4 | Ammonium-ion energy storage | [130] |
| MnO2/graphitic carbon nitride (g-CN) | 5 mV/s | The optimal composite system achieved a current density of 10 mA/cm2 with an overpotential of 430 mV and exhibited a Tafel slope of approximately 70 mV/dec | Electrocatalysts | [131] |
| Material | Preparation Method | Specific Capacitance | Cycling Life | Energy Density | Reference |
|---|---|---|---|---|---|
| α-MnO2 | Plant extraction method | 90 F g−1 at 1 A g−1 | 98% after 1000 cycles | 37 Wh kg−1 | [150] |
| δ-MnO2 | Chemical reduction method | 116.61 F g−1 at 1 A g−1 | 98.7% after 10,000 cycles | 22.7 Wh kg−1 | [151] |
| MnO2/Ag | Chemical reduction method | 115 F g−1 at 0.2 A g−1 | 75% after 1000 cycles | 45 Wh kg−1 | [152] |
| MnO2-NiO | Electrodeposition method | 375 F g−1 at 0.5 A g−1 | 56.81% after 1000 cycles | - | [153] |
| Ag0.05 MnO2 | Hydrothermal method | 1027 F g−1 at 1 A g−1 | 93.16% after 10,000 cycles | - | [154] |
| ZnO@MnO2 | Hydrothermal method | 839.9 F g−1 at 0.3 A g−1 | 92% after 10,000 cycles | 74.6 Wh kg−1 | [155] |
| α-MnO2 | Hydrothermal method | 47 F g−1 at 0.5 A g−1 | 94% after 5000 cycles | 21 Wh kg−1 | [156] |
| λ-MnO2/polyaniline | Hydrothermal method | 232.1 F g−1 at 0.2 A g−1 | 78.65% after 3000 cycles | 66.4 Wh kg−1 | [157] |
| β-MnO2 | Hydrothermal method | 212.85 F g−1 at 0.2 A g−1 | 97.5% after 5000 cycles | - | [158] |
| γ-MnO2 | Hydrothermal method | 103 F g−1 at 1 A g−1 | - | - | [159] |
| Polyaniline-MnO2 | Templates method | 765 F g−1 at 0.25 A g−1 | 80% after 14,000 cycles | - | [160] |
| Polyaniline-MnO2 | chemical co-precipitation method | 417 F g−1 at 5 mV s −1 | - | 7.2 Wh kg−1 | [161] |
| Cathode | Preparation Method | Electrolyte | Plateau (V) | Capacity (mAh g−1) | Cycling Life | Reference |
|---|---|---|---|---|---|---|
| α-MnO2 | Hydrothermal method | 2 M ZnSO4 + 0.1 M MnSO4 | 0.8–2.0 | 302 | 78.4% after 2000 cycles | [169] |
| β-MnO2 | Hydrothermal method | 2 M ZnSO4 + 0.1 M MnSO4 + 0.1 M Na2SO4 | 1.0–1.9 | 325 | 94% after 1000 cycles | [170] |
| δ-MnO2-x | Hydrothermal method | 2 M ZnSO4 + 0.1 M MnSO4 | 0.9–1.9 | 551.8 | 83% after 1500 cycles | [171] |
| ε-MnO2 | Hydrothermal method | 3 M MnSO4 + 0.3 M H2SO4 + 0.06 M NiSO4 | 1.16–3.4 | 270 | 99% after 450 cycles | [172] |
| β-MnO2 | Electrodeposition method | 1 M ZnSO4 + 1 M MnSO4 | 1.8–2.2 | - | ≈100% after 400 cycles | [173] |
| γ-MnO2 | Electrodeposition method | 0.5 M Mn (CH3COO)2 + 0.5 M Na2SO4 | - | 391.2 | 92.17% after 3000 cycles | [174] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xie, C.; Xu, Z.; Zheng, Y.; Wang, S.; Dai, M.; Xiao, C. Research Progress on the Preparation of Manganese Dioxide Nanomaterials and Their Electrochemical Applications. Nanomaterials 2024, 14, 1283. https://doi.org/10.3390/nano14151283
Xie C, Xu Z, Zheng Y, Wang S, Dai M, Xiao C. Research Progress on the Preparation of Manganese Dioxide Nanomaterials and Their Electrochemical Applications. Nanomaterials. 2024; 14(15):1283. https://doi.org/10.3390/nano14151283
Chicago/Turabian StyleXie, Chunsheng, Zesheng Xu, Yujian Zheng, Shuo Wang, Min Dai, and Chun Xiao. 2024. "Research Progress on the Preparation of Manganese Dioxide Nanomaterials and Their Electrochemical Applications" Nanomaterials 14, no. 15: 1283. https://doi.org/10.3390/nano14151283
APA StyleXie, C., Xu, Z., Zheng, Y., Wang, S., Dai, M., & Xiao, C. (2024). Research Progress on the Preparation of Manganese Dioxide Nanomaterials and Their Electrochemical Applications. Nanomaterials, 14(15), 1283. https://doi.org/10.3390/nano14151283







