Finely Tunable Carbon Nanofiber Catalysts for the Efficient Production of HMF in Biphasic MIBK/H2O Systems
Abstract
1. Introduction
2. Experimental
2.1. Preparation of CNF-SO3HPTMS
2.2. Catalytic Tests
2.3. Recycling
2.4. Characterization Techniques
3. Results and Discussions
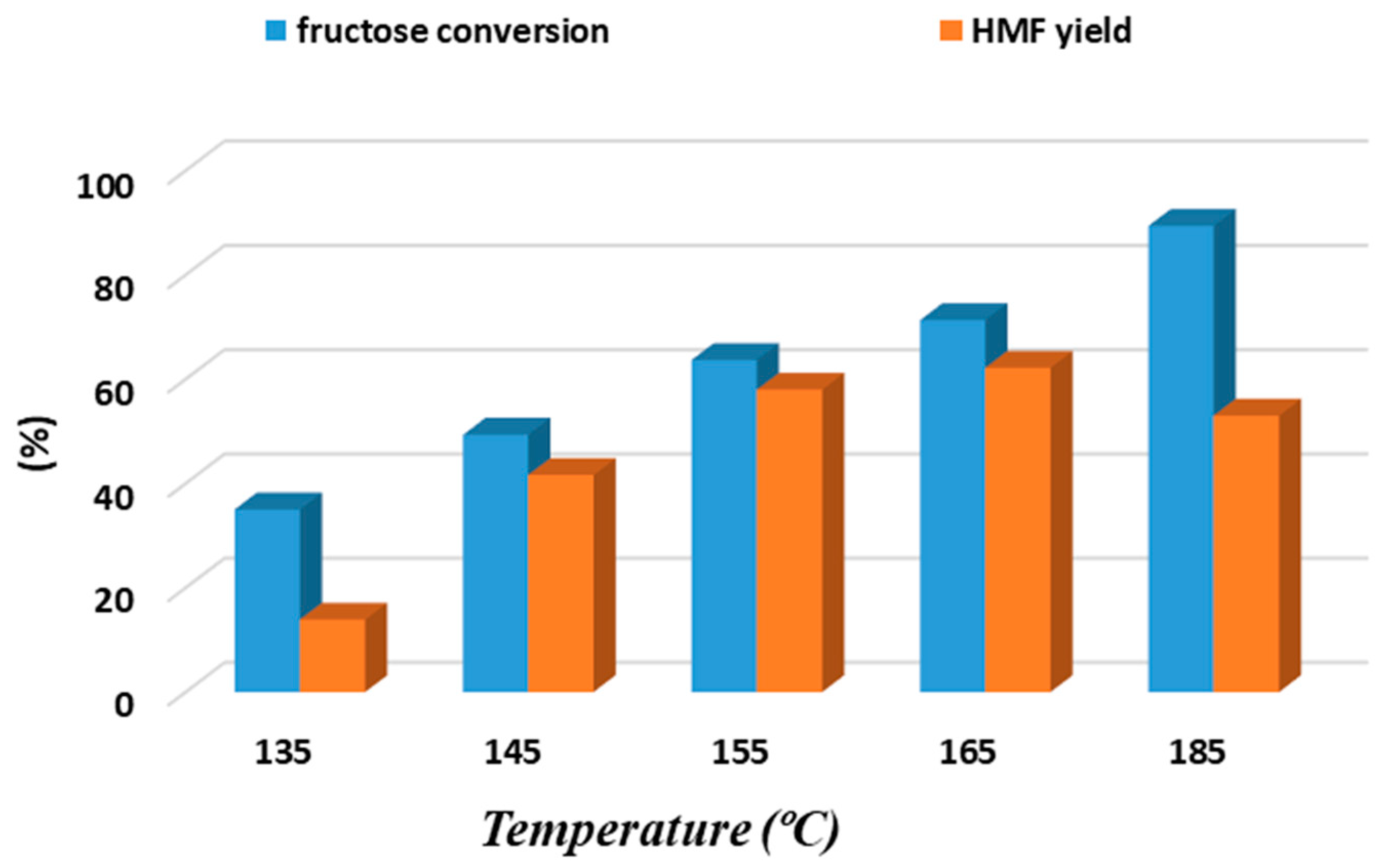
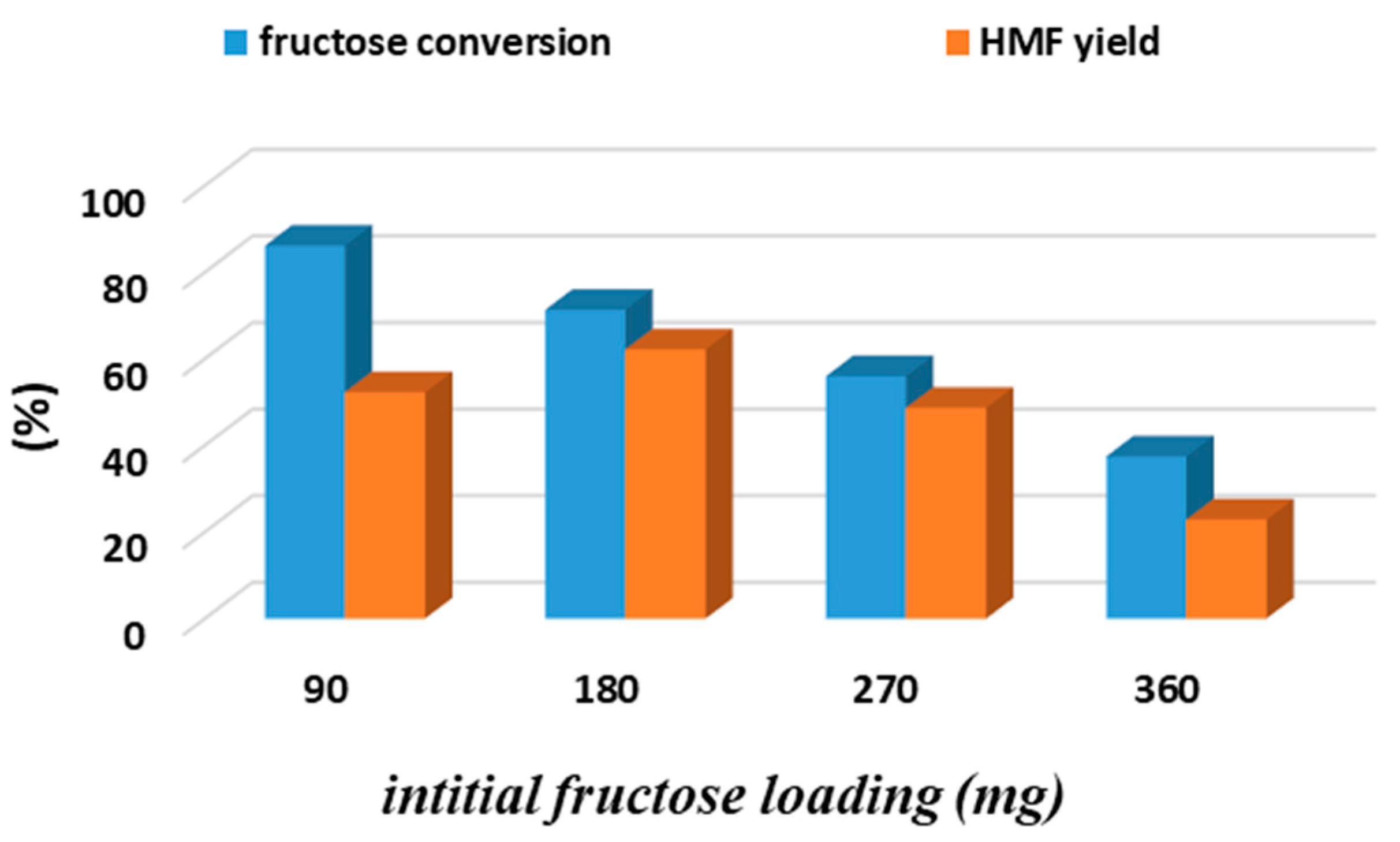
3.1. Biphasic Fructose Dehydration to HMF
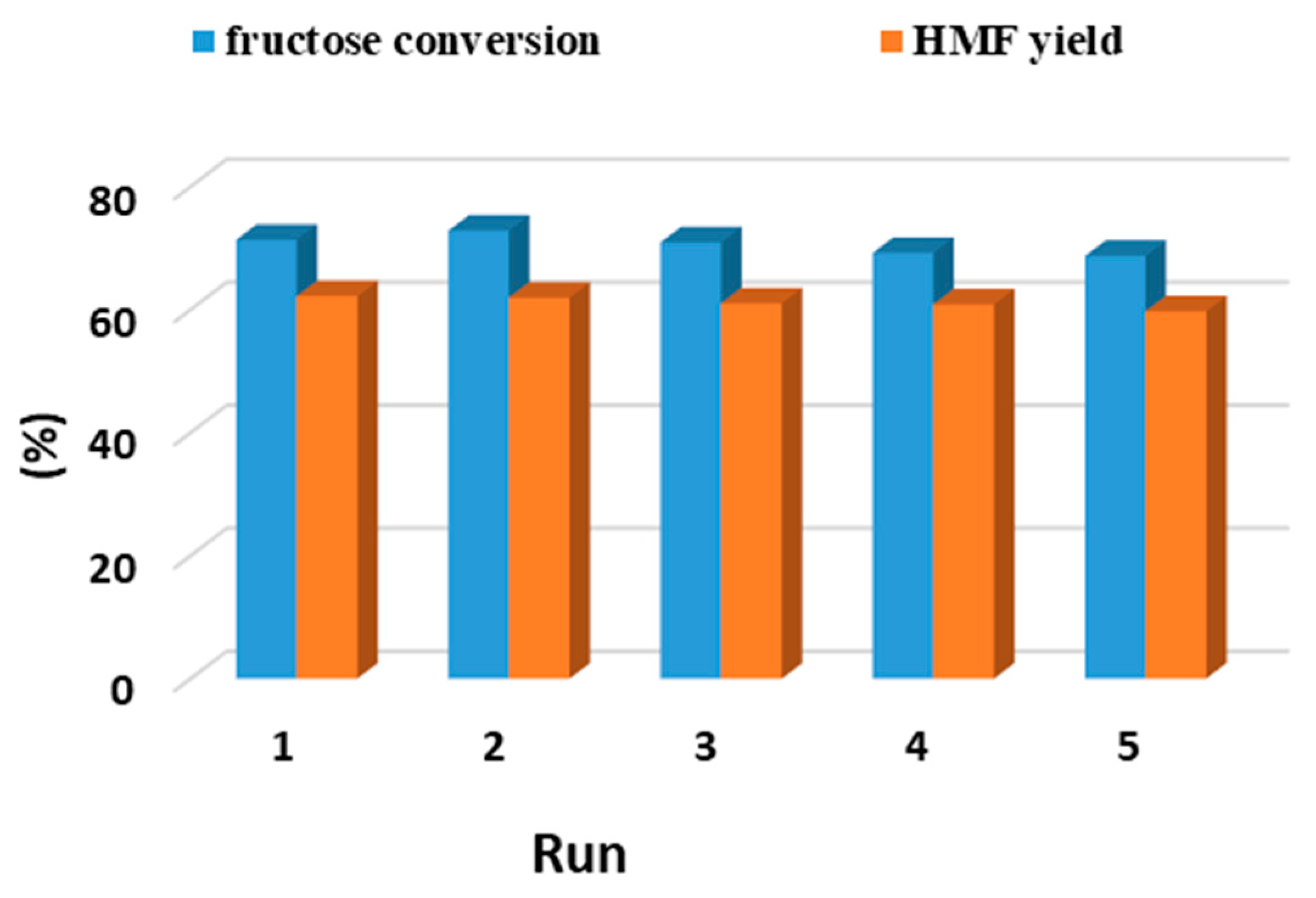
3.2. Catalyst Reuse
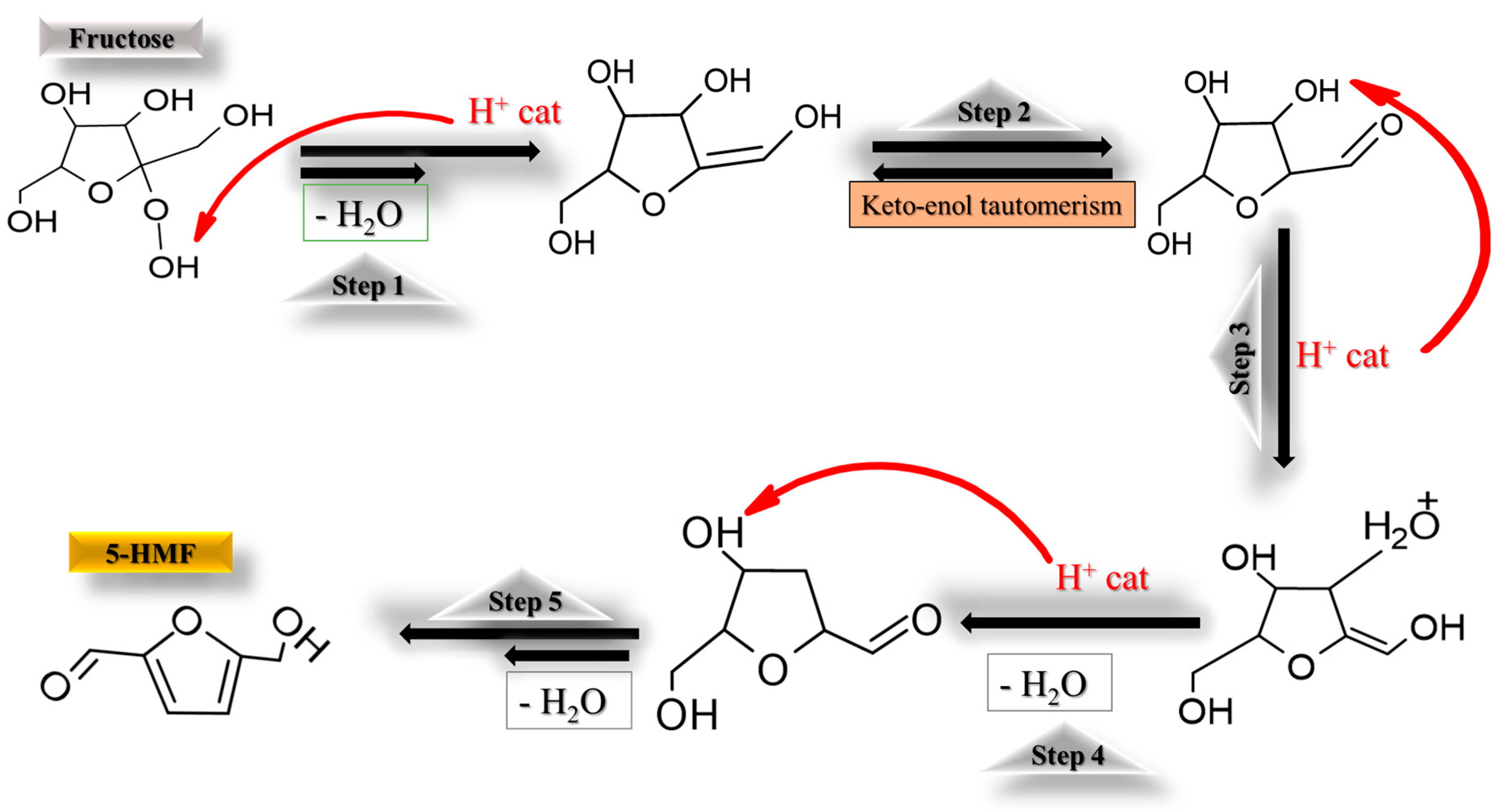
3.3. Catalyst Kinetics and Tentative Reaction Mechanism on Functionalized CNF
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Rout, P.K.; Nannaware, A.D.; Prakash, O.; Kalra, A.; Rajasekharan, R. Synthesis of hydroxymethylfurfural from cellulose using green processes: A promising biochemical and biofuel feedstock. Chem. Eng. Sci. 2016, 142, 318–346. [Google Scholar] [CrossRef]
- Kamm, B.; Schönicke, P.; Hille, C. Green biorefinery–Industrial implementation. Food Chem. 2016, 197, 1341–1345. [Google Scholar] [CrossRef] [PubMed]
- Morais, A.R.C.; Lopes, A.M.d.C.; Bogel-Łukasik, R. Carbon Dioxide in Biomass Processing: Contributions to the Green Biorefinery Concept. Chem. Rev. 2015, 115, 3–27. [Google Scholar] [CrossRef] [PubMed]
- Sarwono, A.; Man, Z.; Muhammad, N.; Khan, A.S.; Hamzah, W.S.W.; Rahim, A.H.A.; Ullah, Z.; Wilfred, C.D. A new approach of probe sonication assisted ionic liquid conversion of glucose, cellulose and biomass into 5-hydroxymethylfurfural. Ultrason. Sonochem. 2017, 37, 310–319. [Google Scholar] [CrossRef] [PubMed]
- Tong, X.; Ma, Y.; Li, Y. Biomass into chemicals: Conversion of sugars to furan derivatives by catalytic processes. Appl. Catal. A Gen. 2010, 385, 1–13. [Google Scholar] [CrossRef]
- James, O.O.; Maity, S.; Usman, L.A.; Ajanaku, K.O.; Ajani, O.O.; Siyanbola, T.O.; Sahu, S.; Chaubey, R. Towards the conversion of carbohydrate biomass feedstocks to biofuels via hydroxylmethylfurfural. Energy Environ. Sci. 2010, 3, 1833–1850. [Google Scholar] [CrossRef]
- Xue, Z.; Ma, M.-G.; Li, Z.; Mu, T. Advances in the conversion of glucose and cellulose to 5-hydroxymethylfurfural over heterogeneous catalysts. RSC Adv. 2016, 6, 98874–98892. [Google Scholar] [CrossRef]
- Negahdar, L.; Delidovich, I.; Palkovits, R. Aqueous-phase hydrolysis of cellulose and hemicelluloses over molecular acidic catalysts: Insights into the kinetics and reaction mechanism. Appl. Catal. B Environ. 2016, 184, 285–298. [Google Scholar] [CrossRef]
- Liu, J.; Yang, M.; Gong, C.; Zhang, S.; Sheng, K.; Zhang, X. Insights into the glucose isomerization mechanism of Al-hydrochar catalyst probed by Al-oxide species transformation. J. Environ. Chem. Eng. 2021, 9, 106721. [Google Scholar] [CrossRef]
- Carraher, J.M.; Fleitman, C.N.; Tessonnier, J.-P. Kinetic and Mechanistic Study of Glucose Isomerization Using Homogeneous Organic Brønsted Base Catalysts in Water. ACS Catal. 2015, 5, 3162–3173. [Google Scholar] [CrossRef]
- Yu, I.K.; Tsang, D.C. Conversion of biomass to hydroxymethylfurfural: A review of catalytic systems and underlying mechanisms. Bioresour. Technol. 2017, 238, 716–732. [Google Scholar] [CrossRef] [PubMed]
- Zhu, L.; Fu, X.; Hu, Y.; Hu, C. Controlling the Reaction Networks for Efficient Conversion of Glucose into 5-Hydroxymethylfurfural. ChemSusChem 2020, 13, 4812–4832. [Google Scholar] [CrossRef] [PubMed]
- Hu, L.; Wu, Z.; Jiang, Y.; Wang, X.; He, A.; Song, J.; Xu, J.; Zhou, S.; Zhao, Y.; Xu, J. Recent advances in catalytic and autocatalytic production of biomass-derived 5-hydroxymethylfurfural. Renew. Sustain. Energy Rev. 2020, 134, 110317. [Google Scholar] [CrossRef]
- Agarwal, B.; Kailasam, K.; Sangwan, R.S.; Elumalai, S. Traversing the history of solid catalysts for heterogeneous synthesis of 5-hydroxymethylfurfural from carbohydrate sugars: A review. Renew. Sustain. Energy Rev. 2018, 82, 2408–2425. [Google Scholar] [CrossRef]
- Chen, S.S.; Maneerung, T.; Tsang, D.C.; Ok, Y.S.; Wang, C.-H. Valorization of biomass to hydroxymethylfurfural, levulinic acid, and fatty acid methyl ester by heterogeneous catalysts. Chem. Eng. J. 2017, 328, 246–273. [Google Scholar] [CrossRef]
- Tomer, R.; Biswas, P. Dehydration of glucose/fructose to 5-hydroxymethylfurfural (5-HMF) over an easily recyclable sulfated titania (SO42−/TiO2) catalyst. New J. Chem. 2020, 44, 20734–20750. [Google Scholar] [CrossRef]
- Qi, X.; Watanabe, M.; Aida, T.M.; Smith, J.R.L. Catalytic dehydration of fructose into 5-hydroxymethylfurfural by ion-exchange resin in mixed-aqueous system by microwave heating. Green Chem. 2008, 10, 799–805. [Google Scholar] [CrossRef]
- Mukherjee, A.; Dumont, M.-J.; Raghavan, V. Review: Sustainable production of hydroxymethylfurfural and levulinic acid: Challenges and opportunities. Biomass-Bioenergy 2015, 72, 143–183. [Google Scholar] [CrossRef]
- Tao, F.; Song, H.; Chou, L. Dehydration of fructose into 5-hydroxymethylfurfural in acidic ionic liquids. RSC Adv. 2011, 1, 672–676. [Google Scholar] [CrossRef]
- Upare, P.P.; Hwang, D.W.; Hwang, Y.K.; Lee, U.-H.; Hong, D.-Y.; Chang, J.-S. An integrated process for the production of 2,5-dimethylfuran from fructose. Green Chem. 2015, 17, 3310–3313. [Google Scholar] [CrossRef]
- Chen, P.; Yamaguchi, A.; Hiyoshi, N.; Mimura, N. Efficient continuous dehydration of fructose to 5-hydroxymethylfurfural in ternary solvent system. Fuel 2023, 334, 126632. [Google Scholar] [CrossRef]
- Wei, Z.; Yao, E.; Cheng, Y.; Hu, J.; Liu, Y. Insight into the dehydration of high-concentration fructose to 5-hydroxymethylfurfural in oxygen-containing polar aprotic solvents. New J. Chem. 2022, 46, 10470–10476. [Google Scholar] [CrossRef]
- Zhu, L.-W.; Wang, J.-G.; Zhao, P.-P.; Song, F.; Sun, X.-Y.; Wang, L.-H.; Cui, H.-Y.; Yi, W.-M. Preparation of the Nb-P/SBA-15 catalyst and its performance in the dehydration of fructose to 5-hydroxymethylfurfural. J. Fuel Chem. Technol. 2017, 45, 651–659. [Google Scholar] [CrossRef]
- Morales-Leal, F.J.; de la Rosa, J.R.; Lucio-Ortiz, C.J.; De Haro-Del Rio, D.A.; Maldonado, C.S.; Wi, S.; Casabianca, L.B.; Garcia, C.D. Dehydration of fructose over thiol– and sulfonic– modified alumina in a continuous reactor for 5–HMF production: Study of catalyst stability by NMR. Appl. Catal. B Environ. 2019, 244, 250–261. [Google Scholar] [CrossRef] [PubMed]
- Maldonado, C.S.; De la Rosa, J.R.; Lucio-Ortiz, C.J.; Valente, J.S.; Castaldi, M.J. Synthesis and characterization of functionalized alumina catalysts with thiol and sulfonic groups and their performance in producing 5-hydroxymethylfurfural from fructose. Fuel 2017, 198, 134–144. [Google Scholar] [CrossRef]
- Yang, Z.; Qi, W.; Huang, R.; Fang, J.; Su, R.; He, Z. Functionalized silica nanoparticles for conversion of fructose to 5-hydroxymethylfurfural. Chem. Eng. J. 2016, 296, 209–216. [Google Scholar] [CrossRef]
- Zhang, H.-J.; Cheng, Y.; Yuan, H.; Wang, Y.; Ma, Z.-H. An enhanced nonpolarity effect of silica-supported perfluoroalkyl sulfonylimide on catalytic fructose dehydration. Catal. Sci. Technol. 2017, 7, 4691–4699. [Google Scholar] [CrossRef]
- Wang, L.; Zhang, L.; Li, H.; Ma, Y.; Zhang, R. High selective production of 5-hydroxymethylfurfural from fructose by sulfonic acid functionalized SBA-15 catalyst. Compos. Part B Eng. 2019, 156, 88–94. [Google Scholar] [CrossRef]
- Bounoukta, C.E.; Megías-Sayago, C.; Ammari, F.; Ivanova, S.; Monzon, A.; Centeno, M.A.; Odriozola, J.A. Dehydration of glucose to 5-Hydroxymethlyfurfural on bifunctional carbon catalysts. Appl. Catal. B Environ. 2021, 286, 119938. [Google Scholar] [CrossRef]
- Bounoukta, C.E.; Megías-Sayago, C.; Ivanova, S.; Penkova, A.; Ammari, F.; Centeno, M.A.; Odriozola, J.A. Effect of the sulphonating agent on the catalytic behavior of activated carbons in the dehydration reaction of fructose in DMSO. Appl. Catal. A Gen. 2021, 617, 118108. [Google Scholar] [CrossRef]
- Martin, G.D.; Bounoukta, C.E.; Ammari, F.; Domínguez, M.I.; Monzón, A.; Ivanova, S.; Centeno, M. Fructose dehydration reaction over functionalized nanographitic catalysts in MIBK/H2O biphasic system. Catal. Today 2021, 366, 68–76. [Google Scholar] [CrossRef]
- Konwar, L.J.; Mäki-Arvela, P.; Mikkola, J.-P. SO3H-Containing Functional Carbon Materials: Synthesis, Structure, and Acid Catalysis. Chem. Rev. 2019, 119, 11576–11630. [Google Scholar] [CrossRef] [PubMed]
- Zhong, R.; Sels, B.F. Sulfonated mesoporous carbon and silica-carbon nanocomposites for biomass conversion. Appl. Catal. B Environ. 2018, 236, 518–545. [Google Scholar] [CrossRef]
- Araya-Lopez, C.; Conejeros, J.; Valdebenito, C.; Cabezas, R.; Merlet, G.; Marco, J.F.; Abarca, G.; Salazar, R.; Romero, J. Triazolium-based Ionic Liquids Supported on Alumina as Catalysts to Produce 5-HMF from Fructose. ChemCatChem 2022, 14, e2022000. [Google Scholar] [CrossRef]
- Zunita, M.; Yuan, D.M.; Laksono, A.S. Glucose conversion into hydroxymethylfurfural via ionic liquid-based processes. Chem. Eng. J. Adv. 2022, 11, 100307. [Google Scholar] [CrossRef]
- Shi, X.-L.; Zhang, M.; Li, Y.; Zhang, W. Polypropylene fiber supported ionic liquids for the conversion of fructose to 5-hydroxymethylfurfural under mild conditions. Green Chem. 2013, 15, 3438–3445. [Google Scholar] [CrossRef]
- Bohre, A.; Modak, A.; Chourasia, V.; Jadhao, P.R.; Sharma, K.; Pant, K.K. Recent advances in supported ionic liquid catalysts for sustainable biomass valorisation to high-value chemicals and fuels. Chem. Eng. J. 2022, 450, 138032. [Google Scholar] [CrossRef]
- Li, L.; Liu, S.; Liu, J. Surface modification of coconut shell based activated carbon for the improvement of hydrophobic VOC removal. J. Hazard. Mater. 2011, 192, 683–690. [Google Scholar] [CrossRef] [PubMed]
- Losch, P.; Pascual, A.M.; Boltz, M.; Ivanova, S.; Louis, B.; Montilla, F.; Odriozola, J.A. Ionic liquid immobilization on carbon nanofibers and zeolites: Catalyst design for the liquid-phase toluene chlorination. C. R. Chim. 2015, 18, 324–329. [Google Scholar] [CrossRef]
- Gao, Y.; Alecu, I.; Hashemi, H.; Glarborg, P.; Marshall, P. Reactions of hydrazine with the amidogen radical and atomic hydrogen. Proc. Combust. Inst. 2023, 39, 571–579. [Google Scholar] [CrossRef]
- Cobos, C.J.; Glarborg, P.; Marshall, P.; Troe, J. Re-evaluation of rate constants for the reaction N2H4 (+M) ⇄ NH2 + NH2 (+M). Combust. Flame 2023, 257, 112374. [Google Scholar] [CrossRef]
- Marshall, P.; Glarborg, P. Probing High-Temperature Amine Chemistry: Is the Reaction NH3 + NH2 ⇄ N2H3 + H2 Important? J. Phys. Chem. A 2023, 127, 2601–2607. [Google Scholar] [CrossRef] [PubMed]
- Dharaskar, S.A.; Wasewar, K.L.; Varma, M.N.; Shende, D.Z.; Yoo, C.K. Deep Removal of Sulfur from Model Liquid Fuels using 1-Butyl-3-Methylimidazolium Chloride. Procedia Eng. 2013, 51, 416–422. [Google Scholar] [CrossRef]
- Gui, M.M.; Yap, Y.X.; Chai, S.-P.; Mohamed, A.R. Multi-walled carbon nanotubes modified with (3-aminopropyl)triethoxysilane for effective carbon dioxide adsorption. Int. J. Greenh. Gas Control. 2013, 14, 65–73. [Google Scholar] [CrossRef]
- Arrigo, R.; Hävecker, M.; Wrabetz, S.; Blume, R.; Lerch, M.; McGregor, J.; Parrott, E.P.J.; Zeitler, J.A.; Gladden, L.F.; Knop-Gericke, A.; et al. Tuning the Acid/Base Properties of Nanocarbons by Functionalization via Amination. J. Am. Chem. Soc. 2010, 132, 9616–9630. [Google Scholar] [CrossRef] [PubMed]
- Shafeeyan, M.S.; Daud, W.M.A.W.; Houshmand, A.; Shamiri, A. A review on surface modification of activated carbon for carbon dioxide adsorption. J. Anal. Appl. Pyrolysis 2010, 89, 143–151. [Google Scholar] [CrossRef]
- Steinbach, D.; Kruse, A.; Sauer, J. Pretreatment technologies of lignocellulosic biomass in water in view of furfural and 5-hydroxymethylfurfural production—A review. Biomass-Convers. Biorefinery 2017, 7, 247–274. [Google Scholar] [CrossRef]
- Binder, J.B.; Raines, R.T. Simple Chemical Transformation of Lignocellulosic Biomass into Furans for Fuels and Chemicals. J. Am. Chem. Soc. 2009, 131, 1979–1985. [Google Scholar] [CrossRef] [PubMed]
- Vasudevan, V.; Mushrif, S.H. Insights into the solvation of glucose in water, dimethyl sulfoxide (DMSO), tetrahydrofuran (THF) and N,N-dimethylformamide (DMF) and its possible implications on the conversion of glucose to platform chemicals. RSC Adv. 2015, 5, 20756–20763. [Google Scholar] [CrossRef]
- Swift, T.D.; Bagia, C.; Choudhary, V.; Peklaris, G.; Nikolakis, V.; Vlachos, D.G. Kinetics of Homogeneous Brønsted Acid Catalyzed Fructose Dehydration and 5-Hydroxymethyl Furfural Rehydration: A Combined Experimental and Computational Study. ACS Catal. 2014, 4, 259–267. [Google Scholar] [CrossRef]
- Patil, S.K.R.; Lund, C.R.F. Formation and Growth of Humins via Aldol Addition and Condensation during Acid-Catalyzed Conversion of 5-Hydroxymethylfurfural. Energy Fuels 2011, 25, 4745–4755. [Google Scholar] [CrossRef]
- Weingarten, R.; Cho, J.; Xing, R.; Conner, W.C.; Huber, G.W. Kinetics and Reaction Engineering of Levulinic Acid Production from Aqueous Glucose Solutions. ChemSusChem 2012, 5, 1280–1290. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez Quiroz, N.; Chen, T.-H.; Chen, T.-Y.; Caratzoulas, S.; Vlachos, D.G. Unexpected Kinetic Solvent Effects Enhance Activity and Selectivity in Biphasic Systems. ACS Catal. 2023, 13, 7942–7954. [Google Scholar] [CrossRef]
- Carniti, P.; Gervasini, A.; Biella, S.; Auroux, A. Niobic acid and niobium phosphate as highly acidic viable catalysts in aqueous medium: Fructose dehydration reaction. Catal. Today 2006, 118, 373–378. [Google Scholar] [CrossRef]
- Villanueva, N.I.; Marzialetti, T.G. Mechanism and kinetic parameters of glucose and fructose dehydration to 5-hydroxymethylfurfural over solid phosphate catalysts in water. Catal. Today 2018, 302, 100–107. [Google Scholar] [CrossRef]
- Lee, Y.-Y.; Wu, K.C.-W. Conversion and kinetics study of fructose-to-5-hydroxymethylfurfural (HMF) using sulfonic and ionic liquid groups bi-functionalized mesoporous silica nanoparticles as recyclable solid catalysts in DMSO systems. Phys. Chem. Chem. Phys. 2012, 14, 13914–13917. [Google Scholar] [CrossRef]
- Saghandali, F.; Kazemeini, M.; Sadjadi, S. Halloysite-supported silicotungstic acid as an efficient catalyst for dehydration of fructose to 5-hydroxymethylfurfural. J. Phys. Chem. Solids 2024, 184, 111697. [Google Scholar] [CrossRef]
- Chhabra, T.; Dhingra, S.; Nagaraja, C.; Krishnan, V. Influence of Lewis and Brønsted acidic sites on graphitic carbon nitride catalyst for aqueous phase conversion of biomass derived monosaccharides to 5-hydroxymethylfurfural. Carbon 2021, 183, 984–998. [Google Scholar] [CrossRef]
- Nunes, R.S.; Reis, G.M.; Vieira, L.M.; Mandelli, D.; Carvalho, W.A. Ultra-Fast Selective Fructose Dehydration Promoted by a Kraft Lignin Sulfonated Carbon Under Microwave Heating. Catal. Lett. 2021, 151, 398–408. [Google Scholar] [CrossRef]
- Hu, J.; Yu, M.; Li, Y.; Shen, X.; Cheng, S.; Xu, T.; Ge, C.; Yu, Y.; Ju, Z. Dehydration mechanism of fructose to 5-hydroxymethylfurfural catalyzed by functionalized ionic liquids: A density functional theory study. New J. Chem. 2023, 47, 11525–11532. [Google Scholar] [CrossRef]
- Hafizi, H.; Chermahini, A.N.; Saraji, M.; Mohammadnezhad, G. The catalytic conversion of fructose into 5-hydroxymethylfurfural over acid-functionalized KIT-6, an ordered mesoporous silica. Chem. Eng. J. 2016, 294, 380–388. [Google Scholar] [CrossRef]
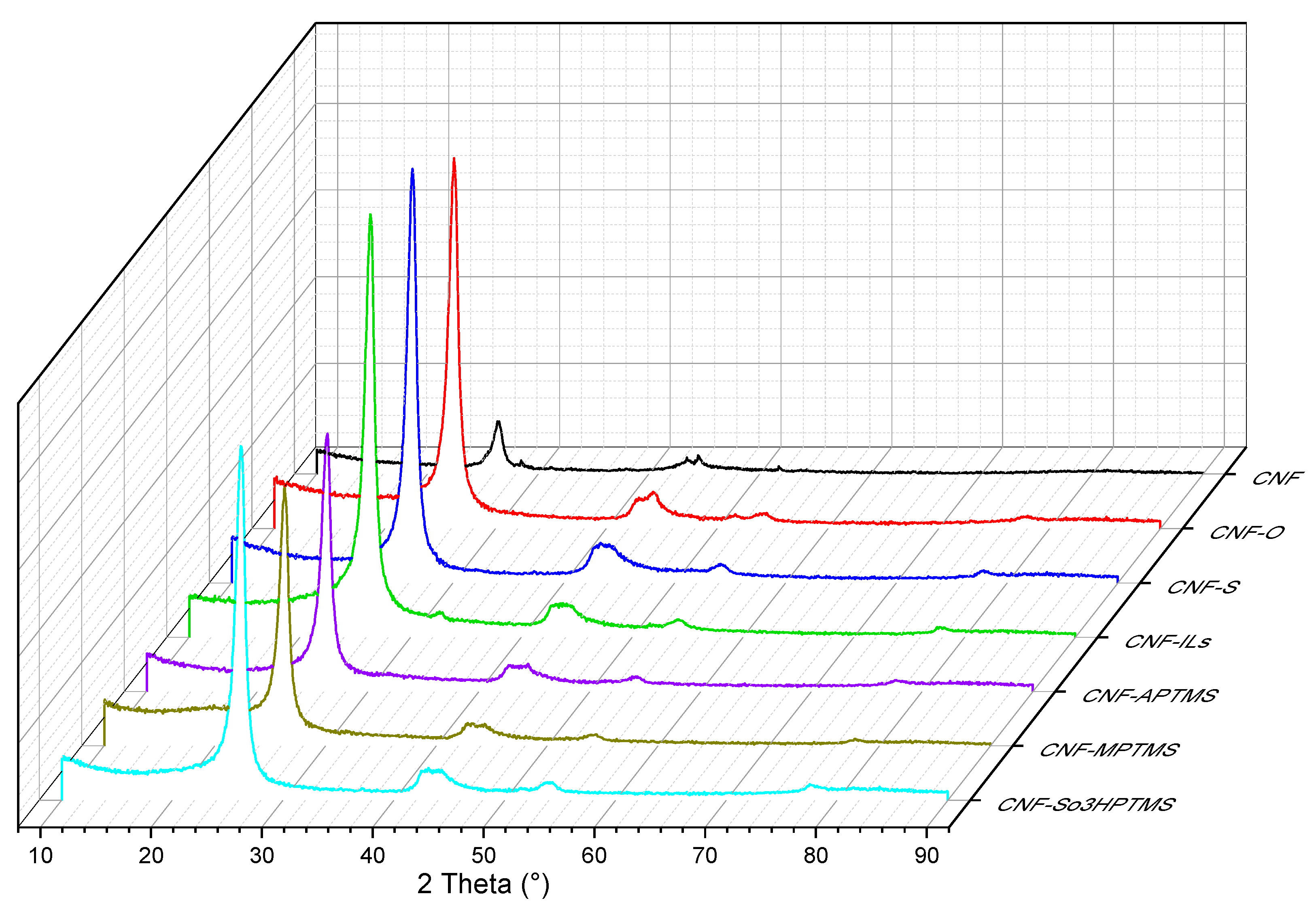

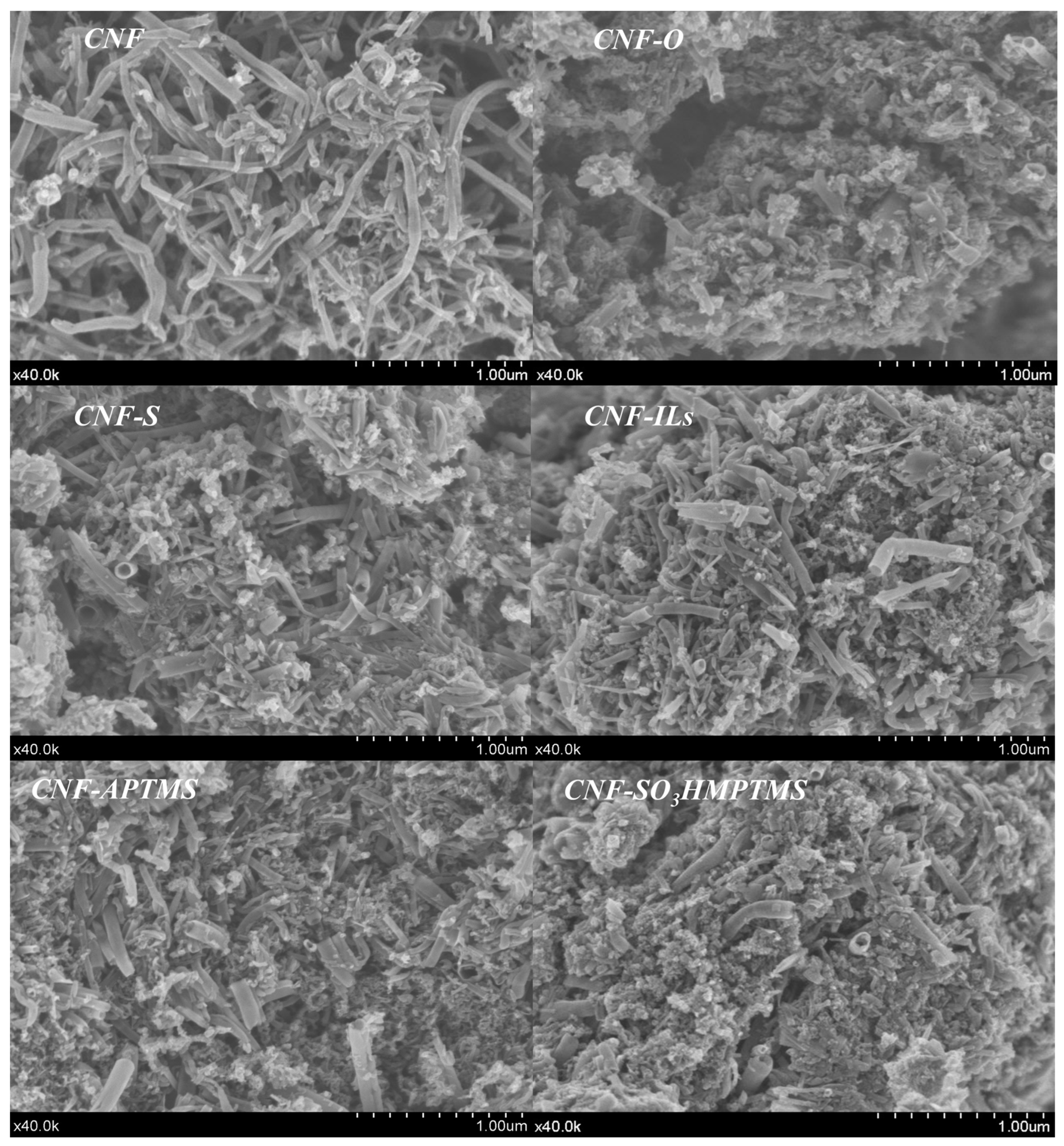
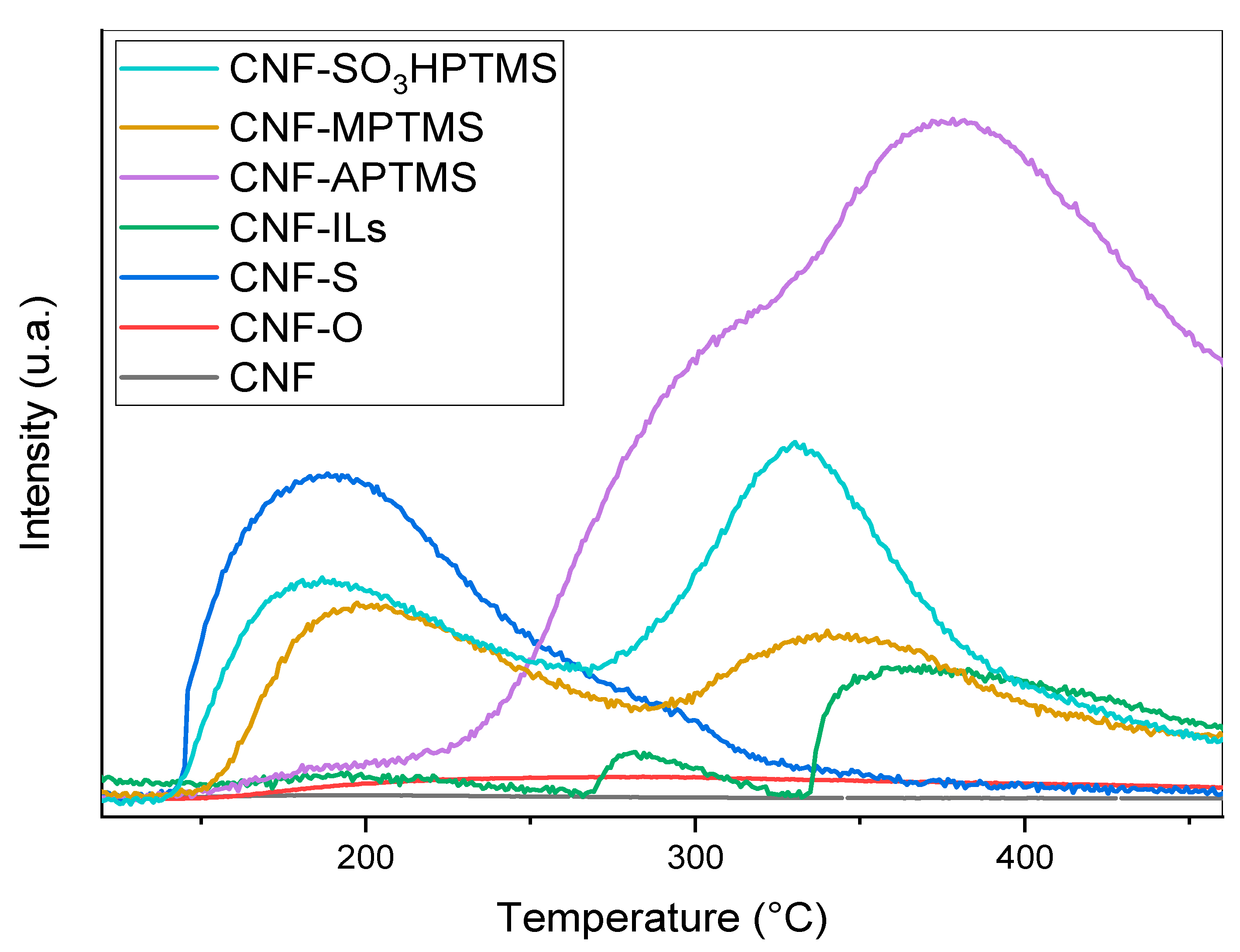
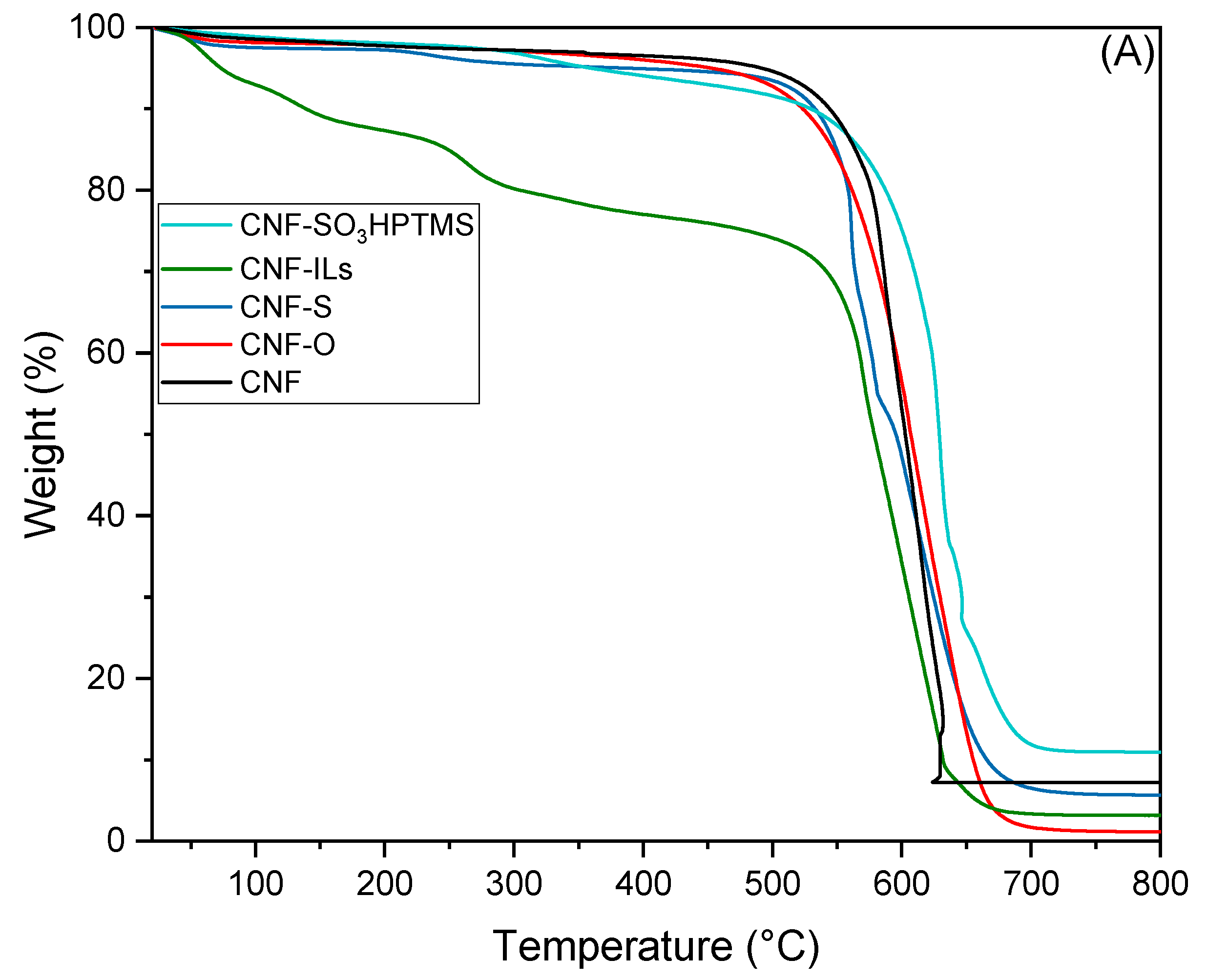
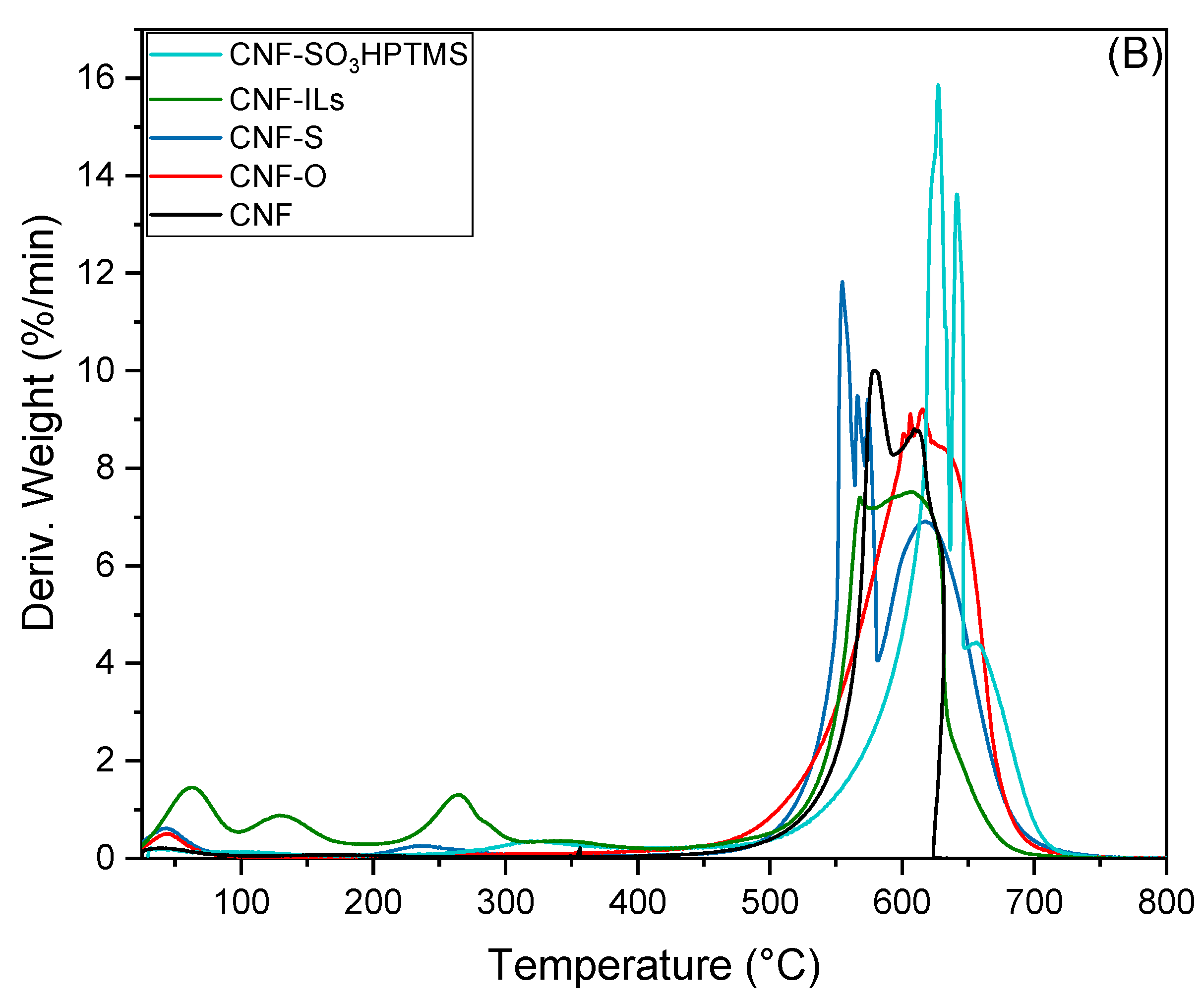
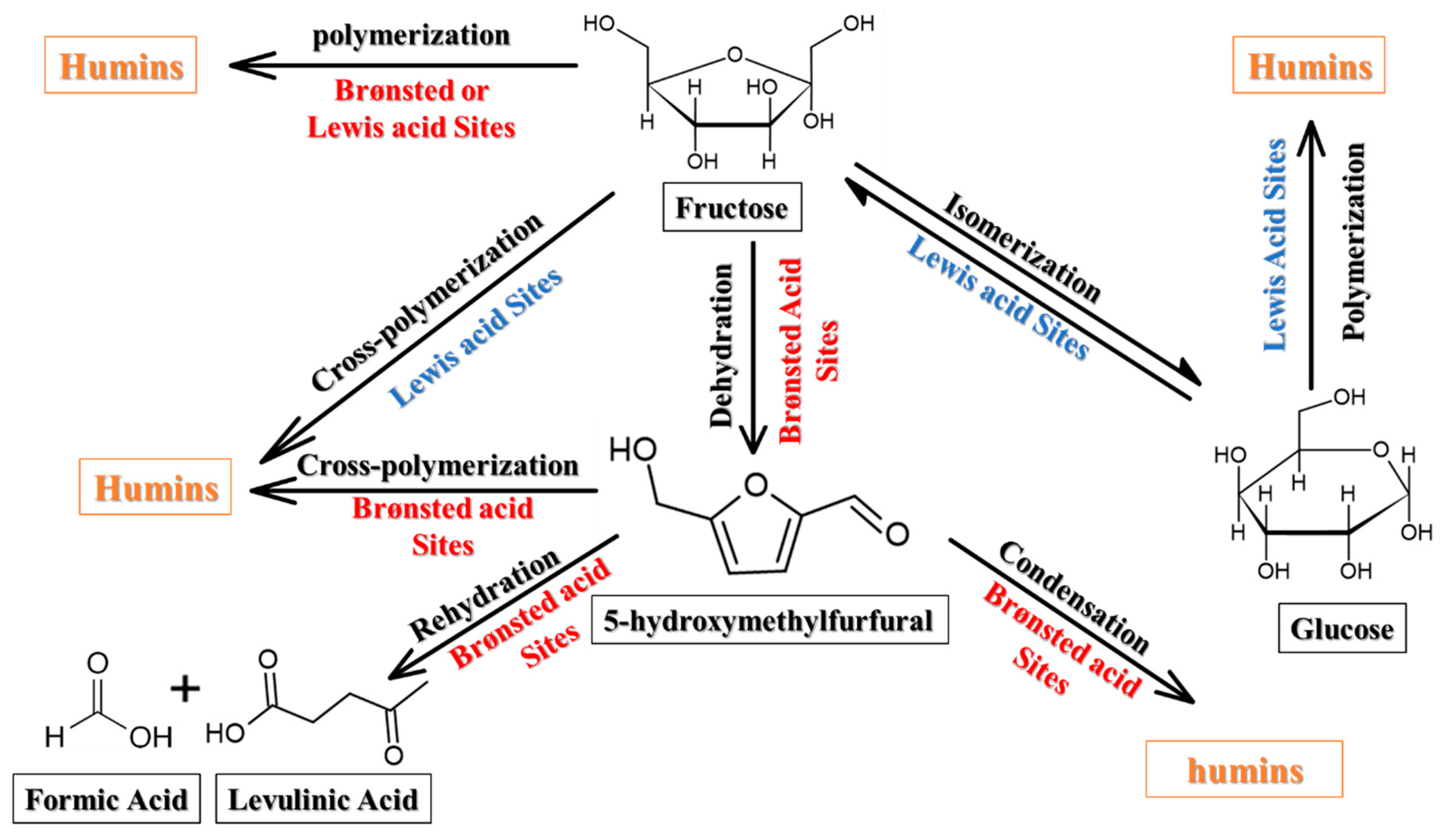

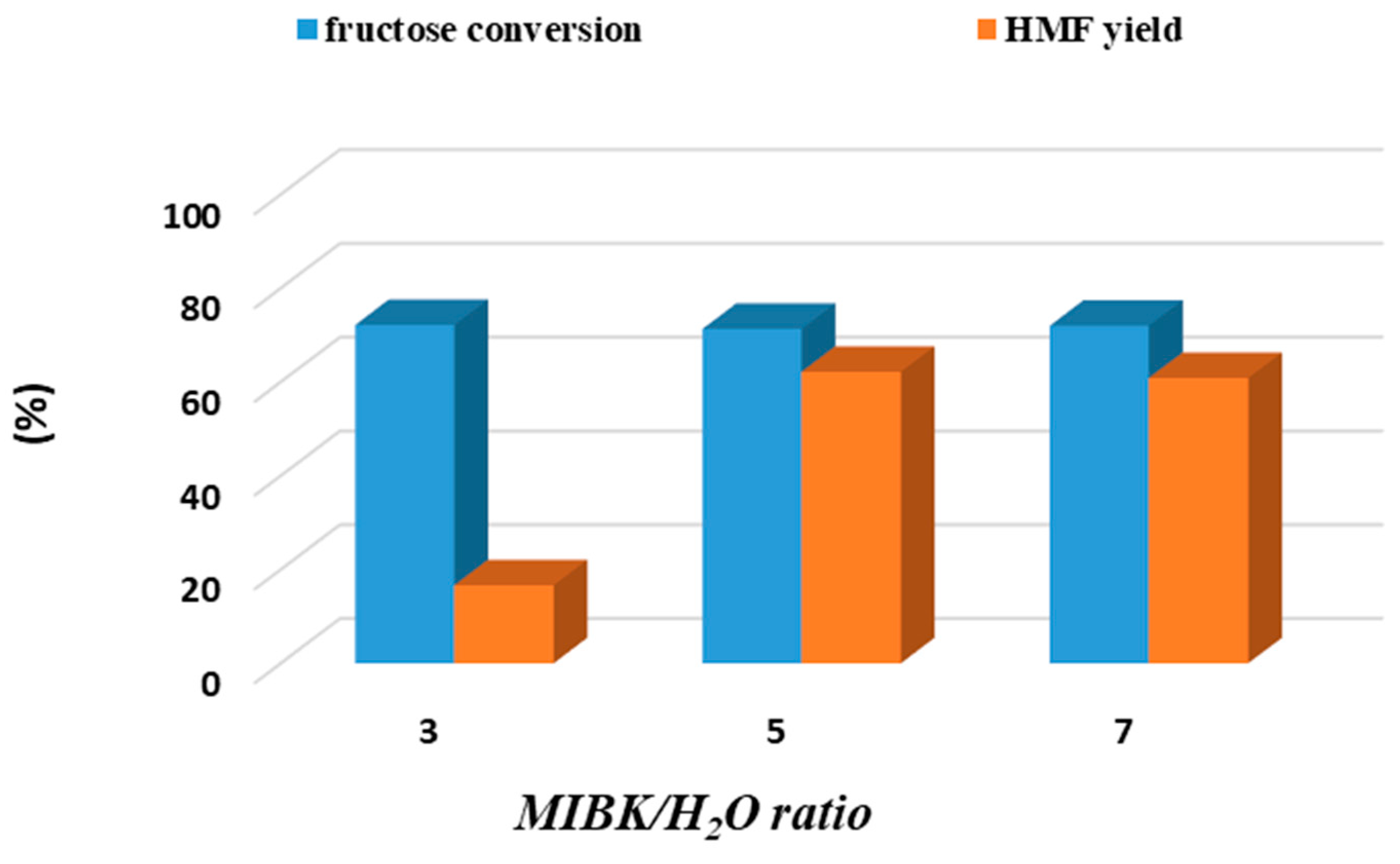
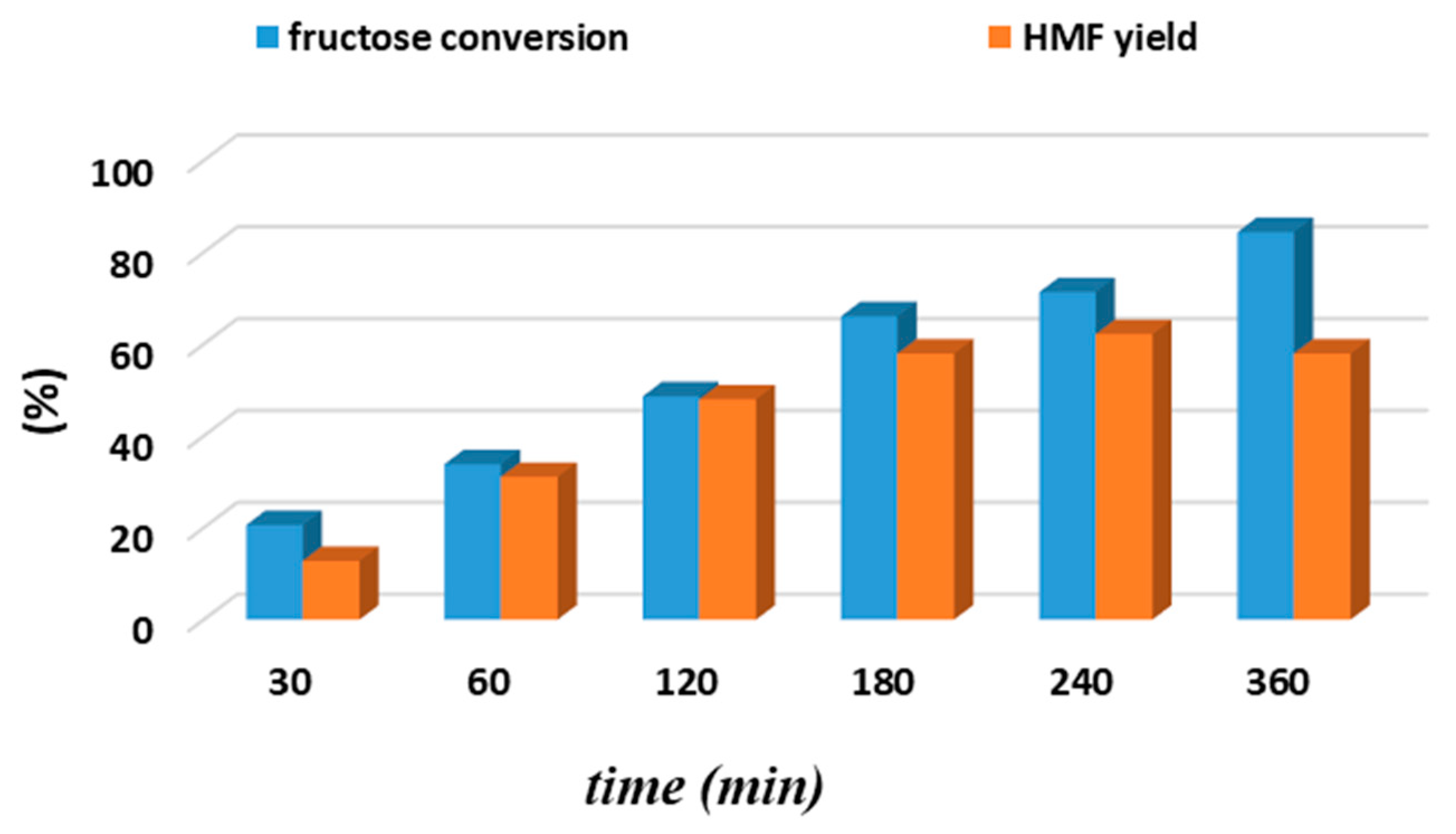
| Sample | BET Area (m2/g) | Pore Size (nm) | Pore Volume (cm3/g) | Surface Micro (m2/g) | Surface Meso (m2/g) |
|---|---|---|---|---|---|
| CNF | 160 | 15.5 | 0.621 | 9.2 | 161 |
| CNF-O | 178 | 15.4 | 1.188 | 9.6 | 215 |
| CNF-S | 178 | 13.0 | 0.780 | 18.7 | 180 |
| CNF-Ils | 152 | 12.0 | 0.606 | 7.7 | 158 |
| CNF-APTMS | 133 | 16.9 | 0.687 | 0 | 172 |
| CNF-MPTMS | 101 | 17.3 | 0.555 | 0 | 128 |
| CNF-SO3HPTMS | 131 | 14.4 | 0.561 | 0 | 172 |
| Catalyst | La (Å) | Lc (Å) | I(D)/I(G) (a.u.) | pH | TPD-NH3 Area (×1010 a.u.) | Normalized TPD-NH3 Area/BET (×1012 (a.u.)·g−1·m2) |
|---|---|---|---|---|---|---|
| CNF | 87 | 90 | 0.88 | 7.03 | 0.07 | 0.04375 |
| CNF-O | 60 | 88 | 1.23 | 4.8 | 1.68 | 0.94382 |
| CNF-S | 79 | 88 | 1.05 | 3.75 | 1.92 | 1.07865 |
| CNF-Ils | 98 | 86 | 0.98 | 7.87 | 1.02 | 0.67105 |
| CNF-APTMS | 115 | 90 | 0.95 | 8.52 | 3.98 | 5.48872 |
| CNF-MPTMS | 103 | 84 | 1.21 | 5.05 | 5.34 | 3.94059 |
| CNF-SO3HPTMS | 63 | 88 | 1.25 | 4.84 | 7.3 | 4.07634 |
| Sample | C | O | Si | S | Cl | Ni | N * |
|---|---|---|---|---|---|---|---|
| CNF | 94.99 | 3.6 | 0.09 | 0.41 | 0.1 | 0.16 | |
| CNF-O | 87.45 | 11.78 | 0.06 | 0.09 | 0.1 | 0.36 | |
| CNF-S | 88.47 | 10.32 | 0.06 | 0.59 | 0.1 | ||
| CNF-ILs | 88.98 | 9.61 | 0.03 | 0.11 | 1.18 | 0.1 | 4.62 |
| CNF-APTMS | 87.24 | 11.50 | 1.04 | 0.09 | 0.1 | 1.40 | |
| CNF-MPTMS | 80.39 | 15.15 | 2.19 | 2.18 | 0.1 | ||
| CNF-SO3HPTMS | 80.33 | 15.97 | 1.59 | 1.99 | 0.12 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bounoukta, C.E.; Megías-Sayago, C.; Rendón, N.; Ammari, F.; Centeno, M.A.; Ivanova, S. Finely Tunable Carbon Nanofiber Catalysts for the Efficient Production of HMF in Biphasic MIBK/H2O Systems. Nanomaterials 2024, 14, 1293. https://doi.org/10.3390/nano14151293
Bounoukta CE, Megías-Sayago C, Rendón N, Ammari F, Centeno MA, Ivanova S. Finely Tunable Carbon Nanofiber Catalysts for the Efficient Production of HMF in Biphasic MIBK/H2O Systems. Nanomaterials. 2024; 14(15):1293. https://doi.org/10.3390/nano14151293
Chicago/Turabian StyleBounoukta, Charf Eddine, Cristina Megías-Sayago, Nuria Rendón, Fatima Ammari, Miguel Angel Centeno, and Svetlana Ivanova. 2024. "Finely Tunable Carbon Nanofiber Catalysts for the Efficient Production of HMF in Biphasic MIBK/H2O Systems" Nanomaterials 14, no. 15: 1293. https://doi.org/10.3390/nano14151293
APA StyleBounoukta, C. E., Megías-Sayago, C., Rendón, N., Ammari, F., Centeno, M. A., & Ivanova, S. (2024). Finely Tunable Carbon Nanofiber Catalysts for the Efficient Production of HMF in Biphasic MIBK/H2O Systems. Nanomaterials, 14(15), 1293. https://doi.org/10.3390/nano14151293








