Reverse Electrodialysis with Continuous Random Variation in Nanochannel Shape: Salinity Gradient-Driven Power Generation
Abstract
1. Introduction
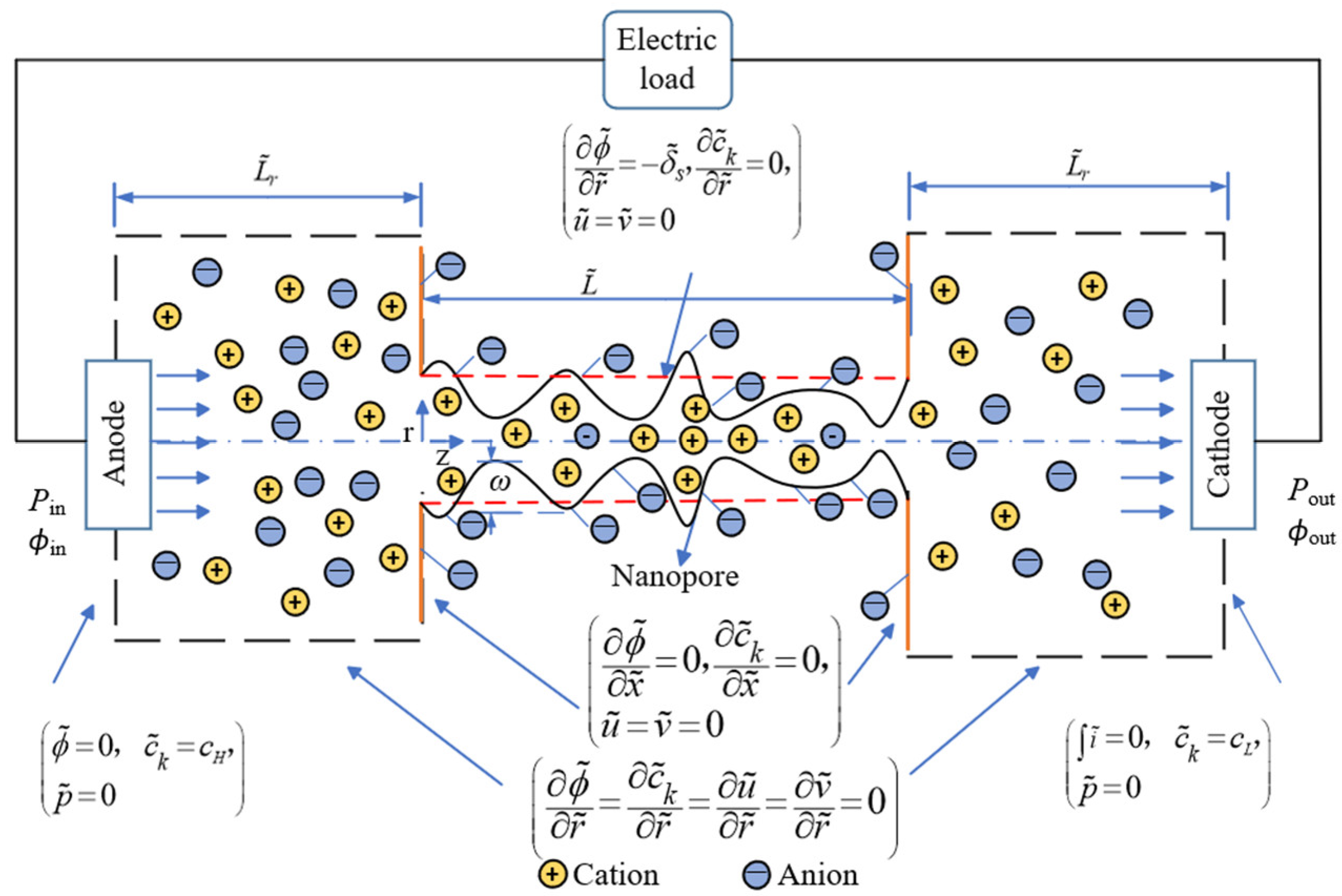
2. Mathematical Model
2.1. Numerical Model
2.2. Boundary Conditions
3. Results and Discussions
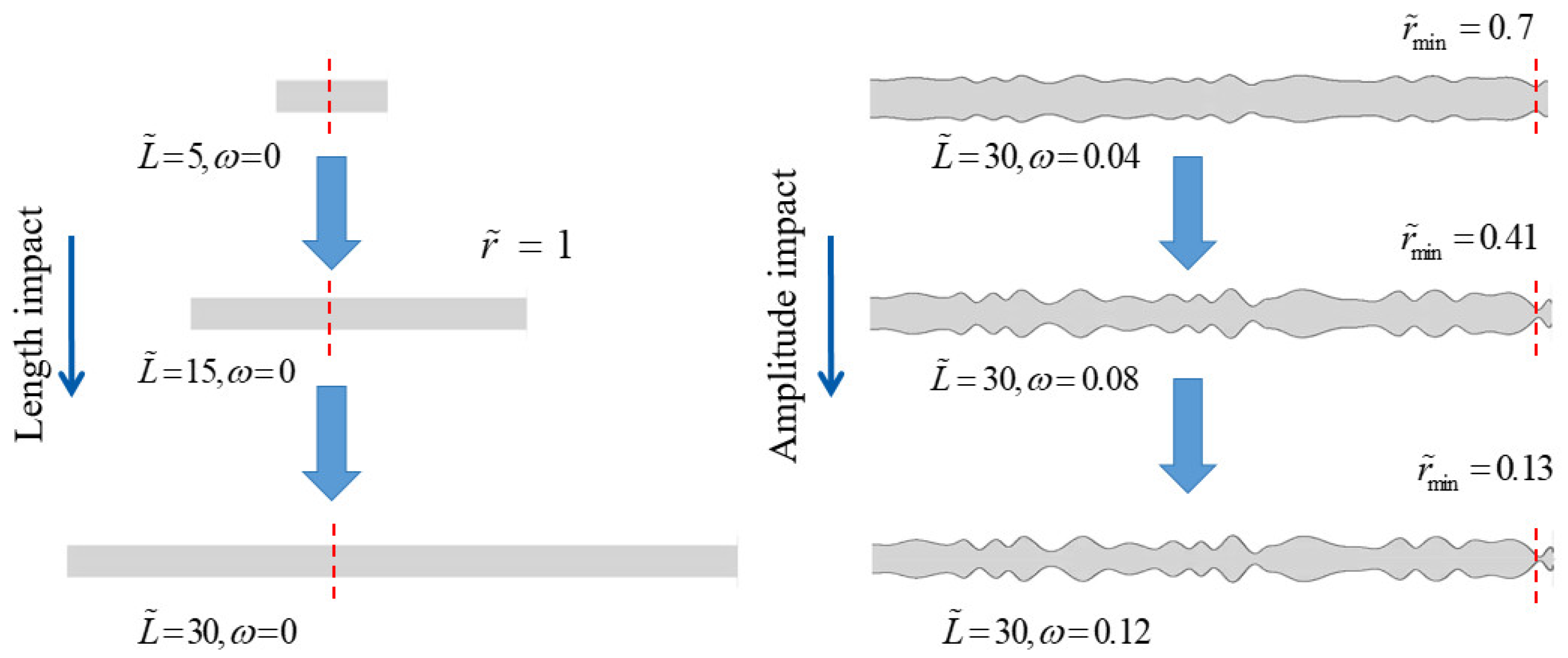
3.1. The Performance Impact of Nanochannel Length and Shape Amplitude
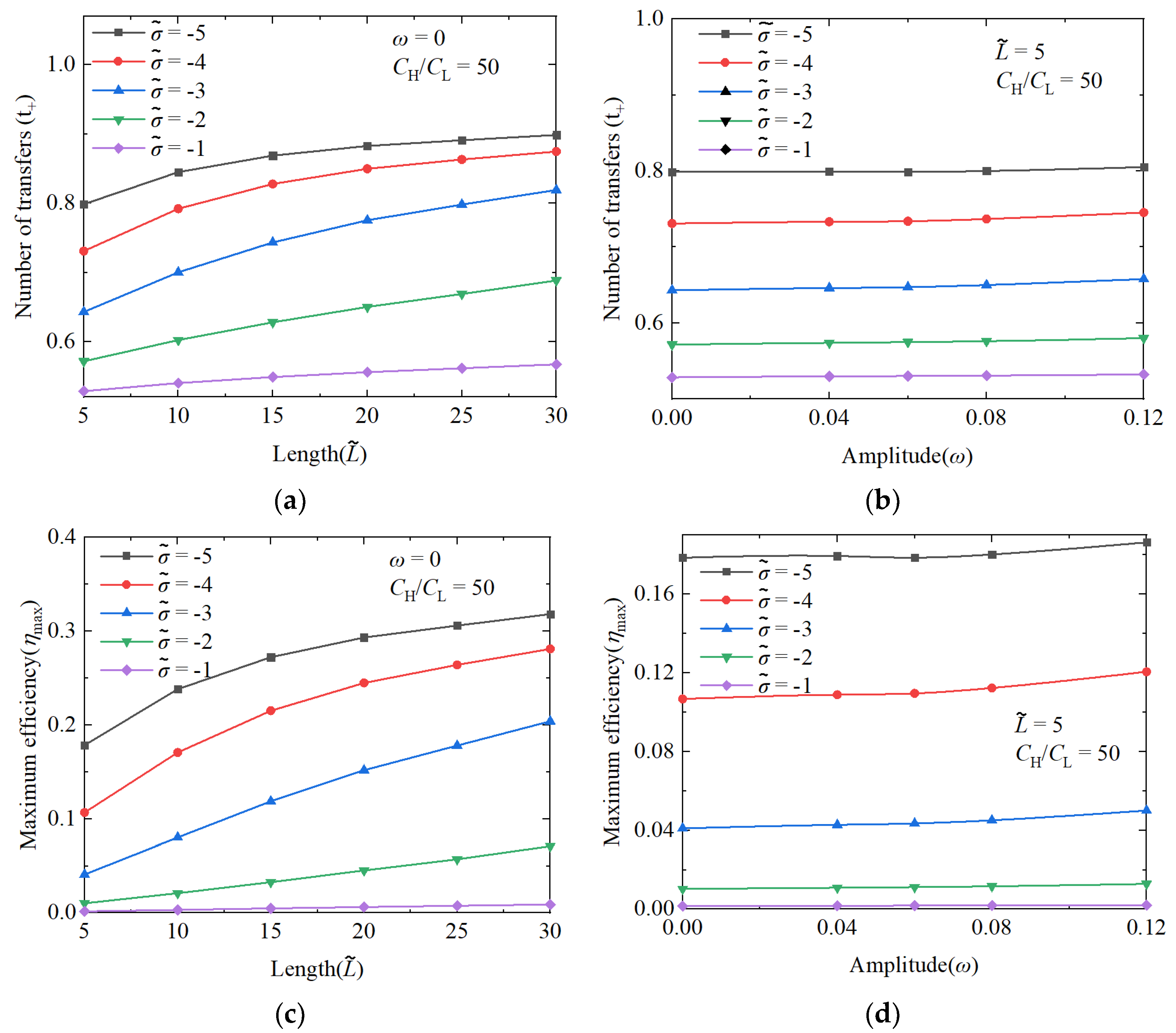
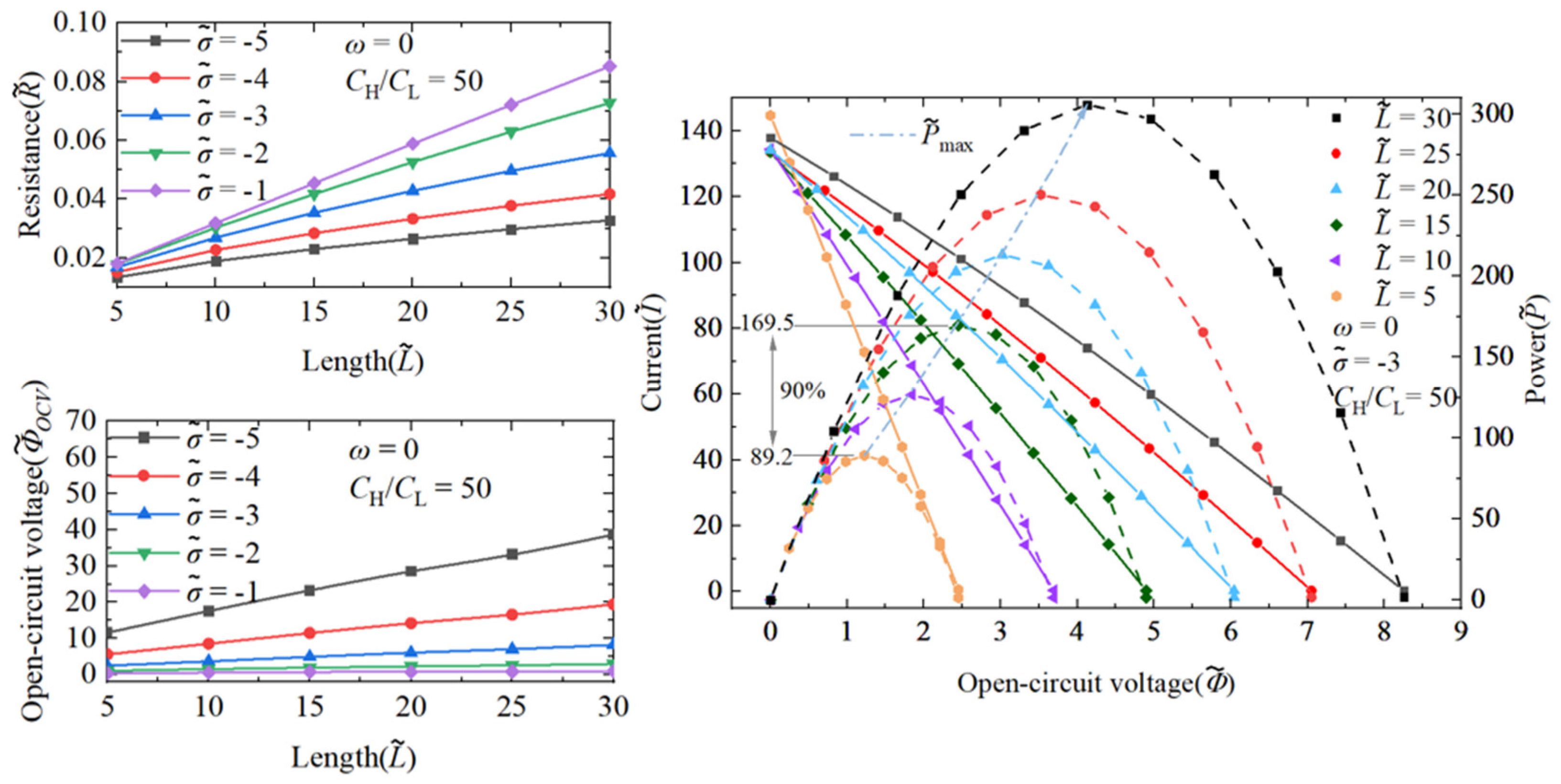
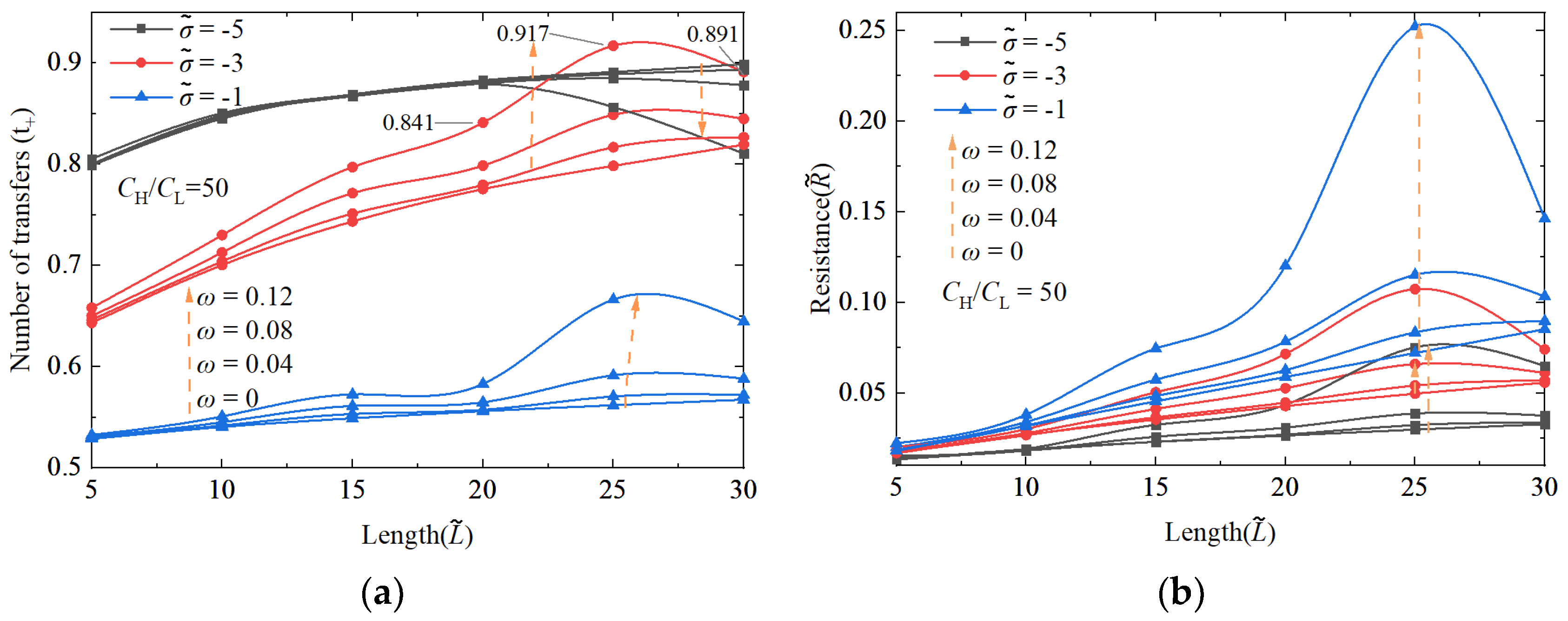
3.1.1. The Performance Impact of Nanochannel Length
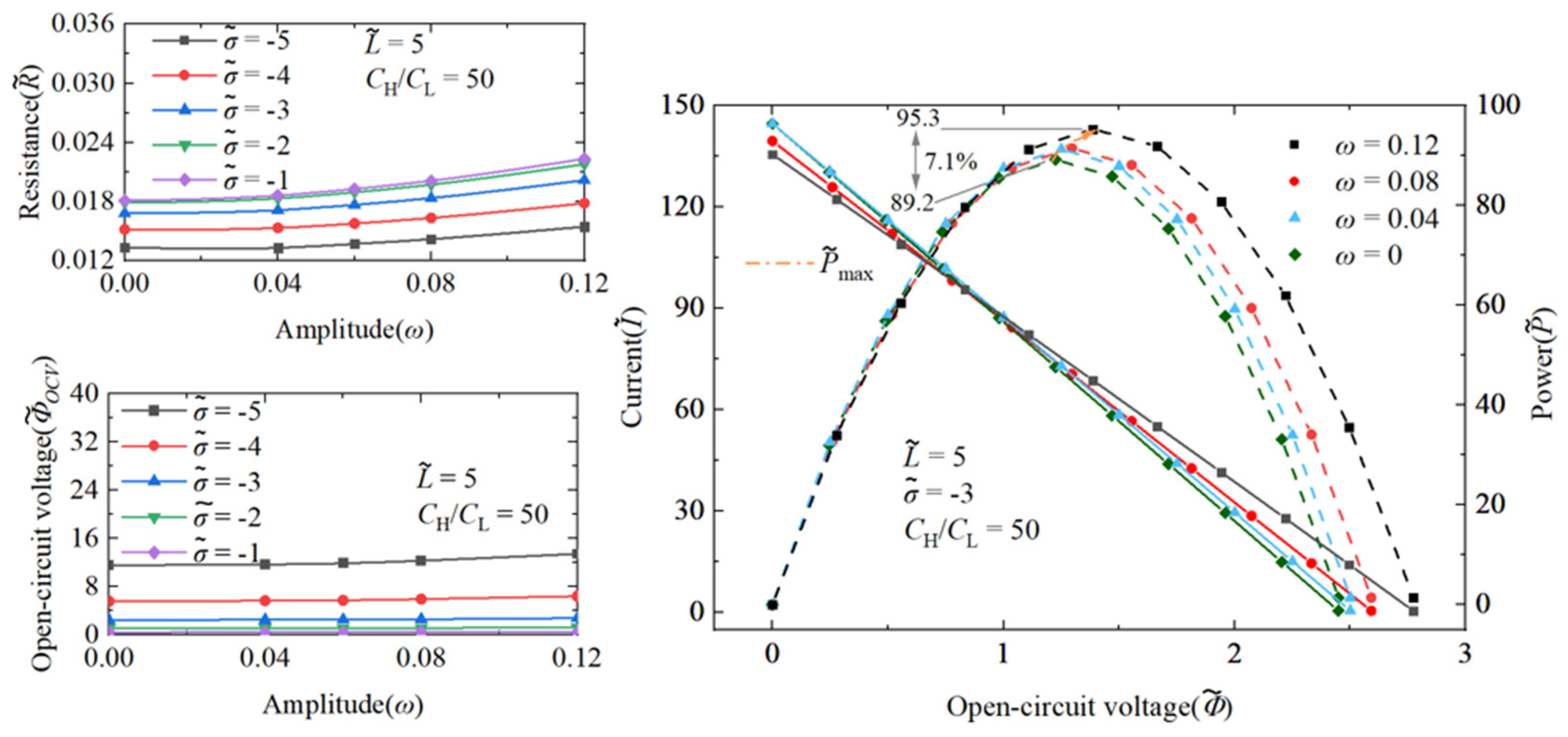
3.1.2. The Performance Impact of Nanochannel Shape Fluctuation Amplitude
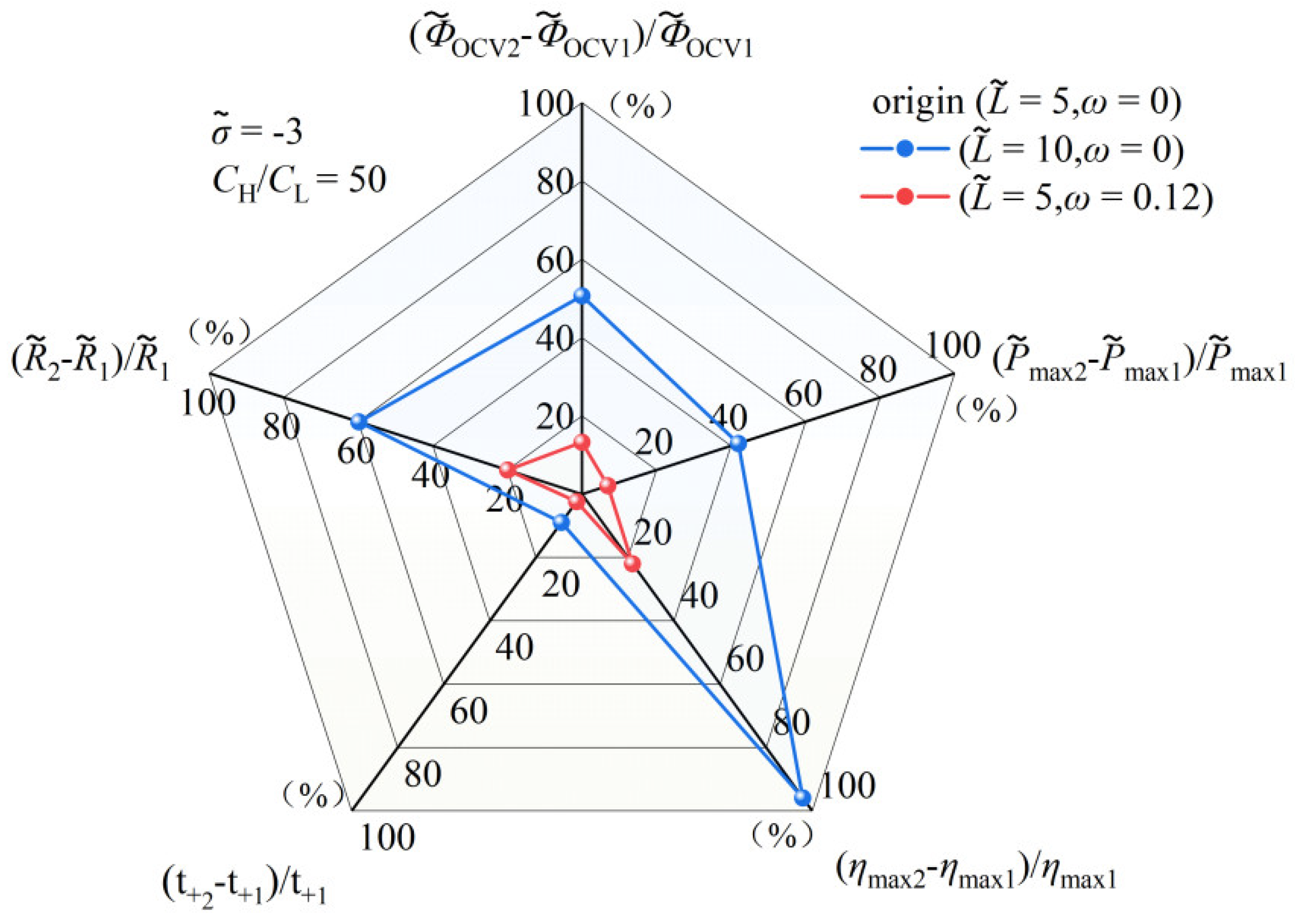
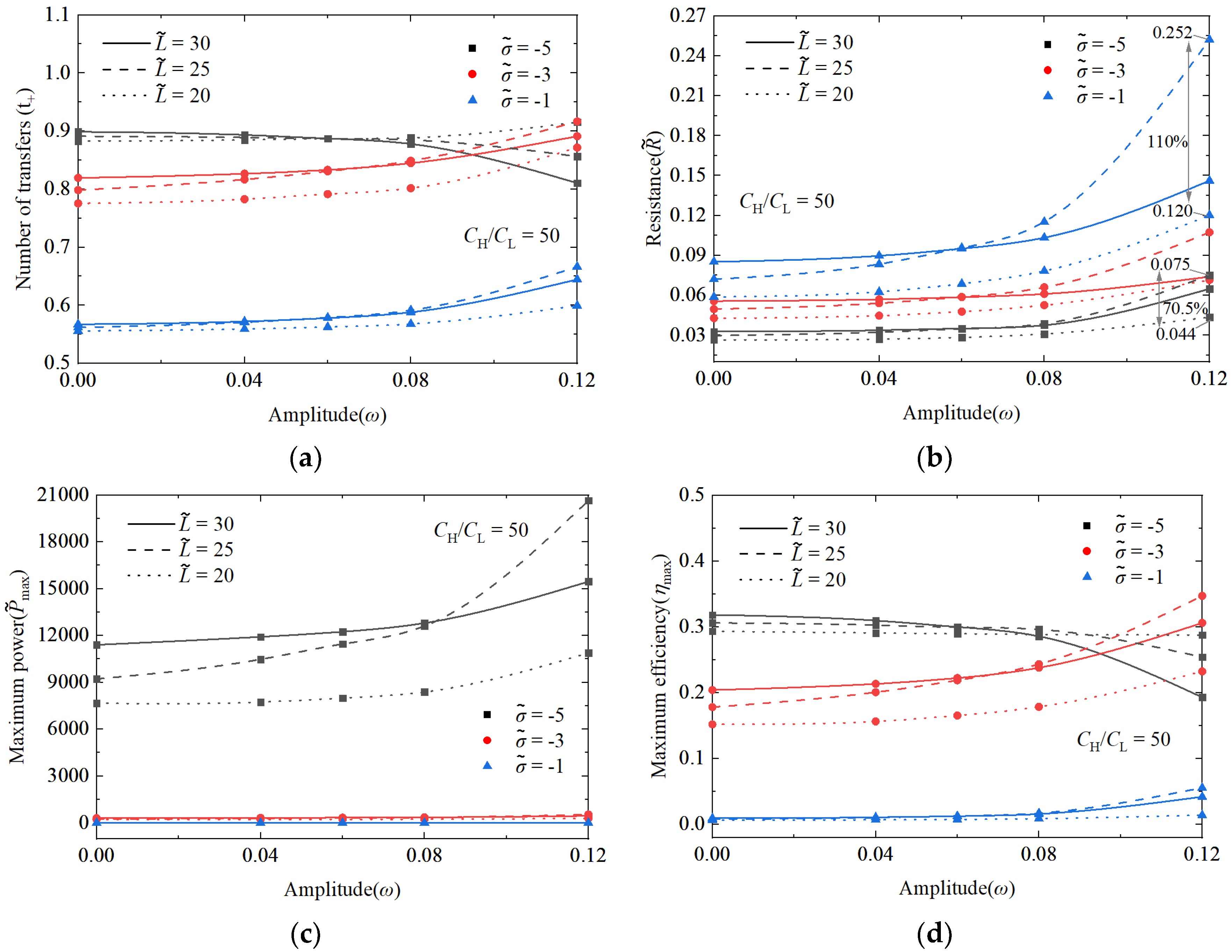
3.1.3. Comparison of Performance Effects of Nanochannel Length and Shape Fluctuation Amplitude
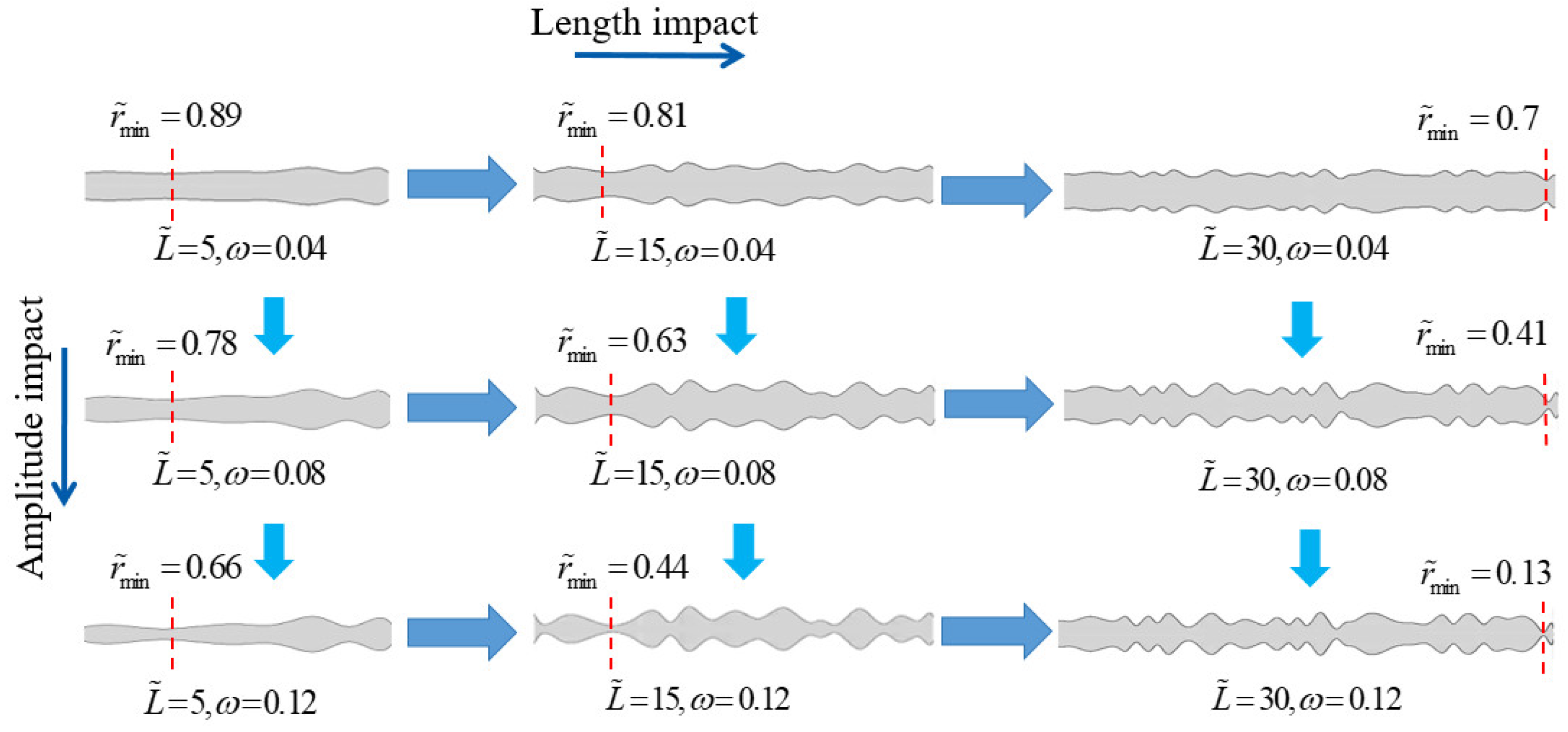
3.2. The Superimposed Effect of Nanochannel Length and Shape Amplitude
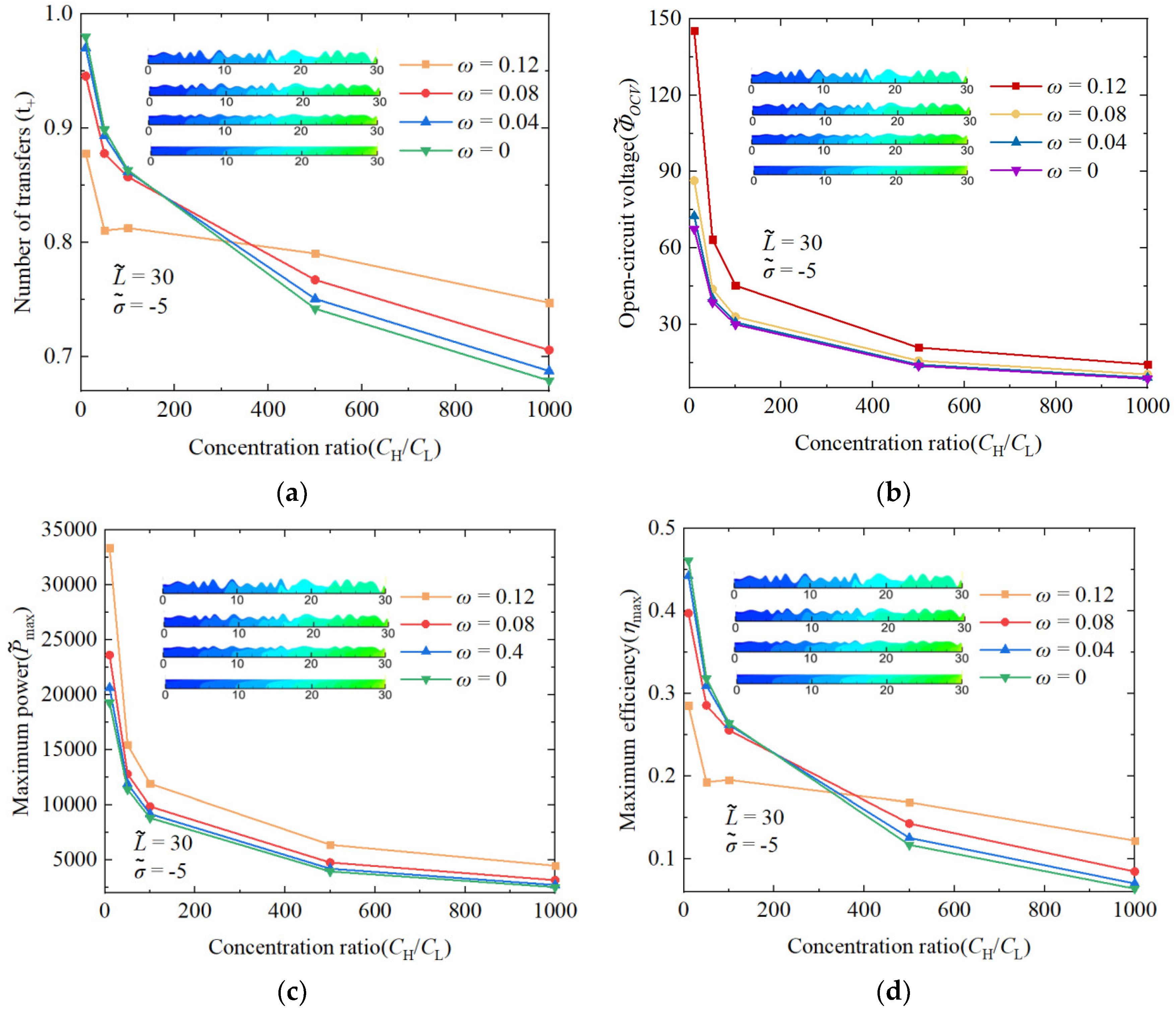
3.3. The Performance Impact of Randomness Nanochannels on Concentration Ratio
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Lindley, D. The energy should always work twice. Nature 2009, 458, 138–141. [Google Scholar] [CrossRef] [PubMed]
- Pacala, S.; Socolow, R. Stabilization Wedges: Solving the Climate Problem for the Next 50 Years with Current Technologies. Science 2004, 305, 968–972. [Google Scholar] [CrossRef] [PubMed]
- Melikoglu, M. Geothermal energy in Turkey and around the World: A review of the literature and an analysis based on Turkey’s Vision 2023 energy targets. Renew. Sustain. Energy Rev. 2017, 76, 485–492. [Google Scholar] [CrossRef]
- de Andres, A.; MacGillivray, A.; Roberts, O.; Guanche, R.; Jeffrey, H. Beyond LCOE: A study of ocean energy technology development and deployment attractiveness. Sustain. Energy Technol. Assess. 2017, 19, 1–16. [Google Scholar] [CrossRef]
- Brauns, E. Towards a worldwide sustainable and simultaneous large-scale production of renewable energy and potable water through salinity gradient power by combining reversed electrodialysis and solar power? Desalination 2008, 219, 312–323. [Google Scholar] [CrossRef]
- Logan, B.E.; Elimelech, M. Membrane-based processes for sustainable power generation using water. Nature 2012, 488, 313–319. [Google Scholar] [CrossRef]
- Rezazadeh, A.; Javanmard, P.; Rezazadeh, S.; Hosseini, M. Salinity Gradient Power: An overview of applications and experiences. In Proceedings of the 12th international Energy Conference (IEC 2018), Tehran, Iran, 19–20 June 2018. [Google Scholar]
- Zoungrana, A.; Cakmakci, M. From non-renewable energy to renewable by harvesting salinity gradient power by reverse electrodialysis: A review. Int. J. Energy Res. 2021, 45, 3495–3522. [Google Scholar] [CrossRef]
- Cao, L.; Guo, W.; Ma, W.; Wang, L.; Xia, F.; Wang, S.; Wang, Y.; Jiang, L.; Zhu, D. Towards understanding the nanofluidic reverse electrodialysis system: Well matched charge selectivity and ionic composition. Energy Environ. Sci. 2011, 4, 2259–2266. [Google Scholar] [CrossRef]
- Ding, L.; Xiao, D.; Lu, Z.; Deng, J.; Wei, Y.; Caro, J.; Wang, H. Oppositely Charged Ti3 C2 Tx MXene Membranes with 2D Nanofluidic Channels for Osmotic Energy Harvesting. Angew. Chem. Int. Ed. Engl. 2020, 59, 8720–8726. [Google Scholar] [CrossRef]
- Li, C.; Liu, Z.; Zhang, H.; Qiao, N.; Zhang, Z.; Zhou, J.; Tian, Z.Q. Surface charge and thermal dependence of energy conversion in nanochannels. Int. Commun. Heat Mass Transf. 2022, 135, 106121. [Google Scholar] [CrossRef]
- Xiao, T.; Lu, B.; Liu, Z.; Zhang, Q.; Zhai, J.; Diao, X. Action-potential-inspired osmotic power generation nanochannels. J. Membr. Sci. 2022, 642, 119999. [Google Scholar] [CrossRef]
- Qiao, N.; Zhang, Z.; Liu, Z.; Lu, W.; Li, C.Z. Ion current rectification in asymmetric nanochannels: Effects of nanochannel shape and surface charge. Int. J. Heat Mass Transf. 2023, 208, 124038. [Google Scholar] [CrossRef]
- He, Y.; Huang, Z.; Chen, B.; Tsutsui, M.; Miao, X.S.; Taniguchi, M. Electrokinetic Analysis of Energy Harvest from Natural Salt Gradients in Nanochannels. Sci. Rep. 2017, 7, 13156. [Google Scholar] [CrossRef]
- Huang, W.-C.; Hsu, J.-P. Ultrashort nanopores of large radius can generate anomalously high salinity gradient power. Electrochim. Acta 2020, 353, 136613. [Google Scholar] [CrossRef]
- Chein, R.; Liu, B. Energy conversion from electrolyte concentration gradient using charged nano-pores. Int. J. Green Energy 2016, 13, 1400–1411. [Google Scholar] [CrossRef]
- Guo, W.; Cao, L.; Xia, J.; Nie, F.-Q.; Ma, W.; Xue, J.; Song, Y.; Zhu, D.; Wang, Y.; Jiang, L. Energy Harvesting with Single-Ion-Selective Nanopores: A Concentration-Gradient-Driven Nanofluidic Power Source. Adv. Funct. Mater. 2010, 20, 1339–1344. [Google Scholar] [CrossRef]
- Kim, D.-K.; Duan, C.; Chen, Y.-F.; Majumdar, A. Power generation from concentration gradient by reverse electrodialysis in ion-selective nanochannels. Microfluid. Nanofluidics 2010, 9, 1215–1224. [Google Scholar] [CrossRef]
- Kwon, K.; Lee, S.J.; Li, L.; Han, C.; Kim, D. Energy harvesting system using reverse electrodialysis with nanoporous polycarbonate track-etch membranes. Int. J. Energy Res. 2014, 38, 530–537. [Google Scholar] [CrossRef]
- Hwang, J.; Kataoka, S.; Endo, A.; Daiguji, H. Enhanced energy harvesting by concentration gradient-driven ion transport in SBA-15 mesoporous silica thin films. Lab Chip 2016, 16, 3824–3832. [Google Scholar] [CrossRef]
- He, W.; Wang, Y.; Shaheed, M.H. Modelling of osmotic energy from natural salt gradients due to pressure retarded osmosis: Effects of detrimental factors and flow schemes. J. Membr. Sci. 2014, 471, 247–257. [Google Scholar] [CrossRef]
- Kim, J.; Kim, S.J.; Kim, D.-K. Energy harvesting from salinity gradient by reverse electrodialysis with anodic alumina nanopores. Energy 2013, 51, 413–421. [Google Scholar] [CrossRef]
- Gao, J.; Guo, W.; Feng, D.; Wang, H.; Zhao, D.; Jiang, L. High-performance ionic diode membrane for salinity gradient power generation. J. Am. Chem. Soc. 2014, 136, 12265–12272. [Google Scholar] [CrossRef]
- Zhang, Z.; Xie, G.; Xiao, K.; Kong, X.Y.; Li, P.; Tian, Y.; Wen, L.; Jiang, L. Asymmetric Multifunctional Heterogeneous Membranes for pH- and Temperature-Cooperative Smart Ion Transport Modulation. Adv. Mater. 2016, 28, 9613–9619. [Google Scholar] [CrossRef]
- Zhang, Z.; Rahman, M.M.; Bajer, B.; Scharnagl, N.; Abetz, V. Highly selective isoporous block copolymer membranes with tunable polyelectrolyte brushes in soft nanochannels. J. Membr. Sci. 2022, 646, 120266. [Google Scholar] [CrossRef]
- Cao, L.; Xiao, F.; Feng, Y.; Zhu, W.; Geng, W.; Yang, J.; Zhang, X.; Li, N.; Guo, W.; Jiang, L. Anomalous Channel-Length Dependence in Nanofluidic Osmotic Energy Conversion. Adv. Funct. Mater. 2017, 27, 1604302. [Google Scholar] [CrossRef]
- Tseng, S.; Li, Y.-M.; Lin, C.-Y.; Hsu, J.-P. Salinity gradient power: Influences of temperature and nanopore size. Nanoscale 2016, 8, 2350–2357. [Google Scholar] [CrossRef]
- Qi, H.; Zhang, Z.; Li, Z.; Nan, H.; Bi, K.; Chen, Y. Synergic Effects of the Nanopore Size and Surface Charge on the Ion Selectivity of Graphene Membranes. J. Phys. Chem. C 2020, 125, 507–514. [Google Scholar] [CrossRef]
- Yi, G.; Du, L.; Wei, G.; Zhang, H.; Yu, H.; Quan, X.; Chen, S. Selective molecular separation with conductive MXene/CNT nanofiltration membranes under electrochemical assistance. J. Membr. Sci. 2022, 658, 120719. [Google Scholar] [CrossRef]
- Feng, Y.; Zhu, W.; Guo, W.; Jiang, L. Bioinspired Energy Conversion in Nanofluidics: A Paradigm of Material Evolution. Adv. Mater. 2017, 29, 1702773. [Google Scholar] [CrossRef]
- Yeh, H.-C.; Chang, C.-C.; Yang, R.-J. Reverse electrodialysis in conical-shaped nanopores: Salinity gradient-driven power generation. RSC Adv. 2014, 4, 2705–2714. [Google Scholar] [CrossRef]
- Hsu, J.-P.; Lin, S.-C.; Lin, C.-Y.; Tseng, S. Power generation by a pH-regulated conical nanopore through reverse electrodialysis. J. Power Sources 2017, 366, 169–177. [Google Scholar] [CrossRef]
- Hsu, J.-P.; Su, T.-C.; Lin, C.-Y.; Tseng, S. Power generation from a pH-regulated nanochannel through reverse electrodialysis: Effects of nanochannel shape and non-uniform H+ distribution. Electrochim. Acta 2019, 294, 84–92. [Google Scholar] [CrossRef]
- Zhang, Z.; Yang, S.; Zhang, P.; Zhang, J.; Chen, G.; Feng, X. Mechanically strong MXene/Kevlar nanofiber composite membranes as high-performance nanofluidic osmotic power generators. Nat. Commun. 2019, 10, 2920. [Google Scholar] [CrossRef]
- Macha, M.; Marion, S.; Nandigana, V.V.R.; Radenovic, A. 2D materials as an emerging platform for nanopore-based power generation. Nat. Rev. Mater. 2019, 4, 588–605. [Google Scholar] [CrossRef]
- Shao, J.J.; Raidongia, K.; Koltonow, A.R.; Huang, J. Self-assembled two-dimensional nanofluidic proton channels with high thermal stability. Nat. Commun. 2015, 6, 7602. [Google Scholar] [CrossRef]
- Gao, J.; Feng, Y.; Guo, W.; Jiang, L. Nanofluidics in two-dimensional layered materials: Inspirations from nature. Chem. Soc. Rev. 2017, 46, 5400–5424. [Google Scholar] [CrossRef]
- Zhang, X.; Wen, Q.; Wang, L.; Ding, L.; Yang, J.; Ji, D.; Zhang, Y.; Jiang, L.; Guo, W. Asymmetric Electrokinetic Proton Transport through 2D Nanofluidic Heterojunctions. ACS Nano 2019, 13, 4238–4245. [Google Scholar] [CrossRef]
- Ji, J.; Kang, Q.; Zhou, Y.; Feng, Y.; Chen, X.; Yuan, J.; Guo, W.; Wei, Y.; Jiang, L. Osmotic Power Generation with Positively and Negatively Charged 2D Nanofluidic Membrane Pairs. Adv. Funct. Mater. 2017, 27, 1603623. [Google Scholar] [CrossRef]
- Xiang, F.; Liang, S.; Shi, T.; Zhang, W. The impact of the distribution of surface charge on the ionic current rectification of nanochannel diodes. Jpn. J. Appl. Phys. 2022, 61, 105001. [Google Scholar] [CrossRef]
- Bao, B.; Hao, J.; Bian, X.; Zhu, X.; Xiao, K.; Liao, J.; Zhou, J.; Zhou, Y.; Jiang, L. 3D Porous Hydrogel/Conducting Polymer Heterogeneous Membranes with Electro-/pH-Modulated Ionic Rectification. Adv. Mater. 2017, 29, 1702926. [Google Scholar] [CrossRef]
- Xiao, K.; Giusto, P.; Wen, L.; Jiang, L.; Antonietti, M. Nanofluidic Ion Transport and Energy Conversion through Ultrathin Free-Standing Polymeric Carbon Nitride Membranes. Angew. Chem. Int. Ed. Engl. 2018, 57, 10123–10126. [Google Scholar] [CrossRef] [PubMed]
- Jiao, Y.; Zhao, C.; Yang, C.; Kang, Y.; Gao, X.; Wang, H.; Song, L.; He, B. A numerical study on ion concentration polarization and electric circuit performance of an electrokinetic battery. Electrophoresis 2020, 41, 811–820. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Qu, Z.; Wang, Q.; Iqbal, M. Geometry design and mechanism analysis of artificial nanoroughness for enhanced osmotic energy conversion. Energy Convers. Manag. 2022, 27, 116373. [Google Scholar] [CrossRef]
- Mai, V.-P.; Yang, R.-J. Boosting power generation from salinity gradient on high-density nanoporous membrane using thermal effect. Appl. Energy 2020, 274, 115294. [Google Scholar] [CrossRef]


| Classification | Symbol | Dimensionless Group | Dimensionless Parameter |
|---|---|---|---|
| Length | |||
| Concentration | |||
| Potential | |||
| Electric strength | |||
| Velocity | |||
| Pressure | |||
| Charge density | |||
| Electric current density | |||
| Electric conductivity | |||
| Electric resistance | |||
| Reynolds number | —— | ||
| Peclet number | —— |
| Given Parameters | |||||
|---|---|---|---|---|---|
| 5 | 0.12 | 50 | 1 | −5 | |
| Results | |||||
| Number of units | 9978 | 11,084 | 25,342 | 37,366 | 51,534 |
| 12.7 | 13.3 | 13.4 | 13.4 | 13.4 | |
| Computing time (s) | 24 | 524 | 1386 | 1875 | 2591 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, R.; Zhou, J.; Bu, T.; Li, H.; Jiao, Y. Reverse Electrodialysis with Continuous Random Variation in Nanochannel Shape: Salinity Gradient-Driven Power Generation. Nanomaterials 2024, 14, 1302. https://doi.org/10.3390/nano14151302
Zhao R, Zhou J, Bu T, Li H, Jiao Y. Reverse Electrodialysis with Continuous Random Variation in Nanochannel Shape: Salinity Gradient-Driven Power Generation. Nanomaterials. 2024; 14(15):1302. https://doi.org/10.3390/nano14151302
Chicago/Turabian StyleZhao, Runchen, Jinhui Zhou, Tianqi Bu, Hao Li, and Yanmei Jiao. 2024. "Reverse Electrodialysis with Continuous Random Variation in Nanochannel Shape: Salinity Gradient-Driven Power Generation" Nanomaterials 14, no. 15: 1302. https://doi.org/10.3390/nano14151302
APA StyleZhao, R., Zhou, J., Bu, T., Li, H., & Jiao, Y. (2024). Reverse Electrodialysis with Continuous Random Variation in Nanochannel Shape: Salinity Gradient-Driven Power Generation. Nanomaterials, 14(15), 1302. https://doi.org/10.3390/nano14151302







