Nano-Biochar Prepared from High-Pressure Homogenization Improves Thermal Conductivity of Ethylene Glycol-Based Coolant
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
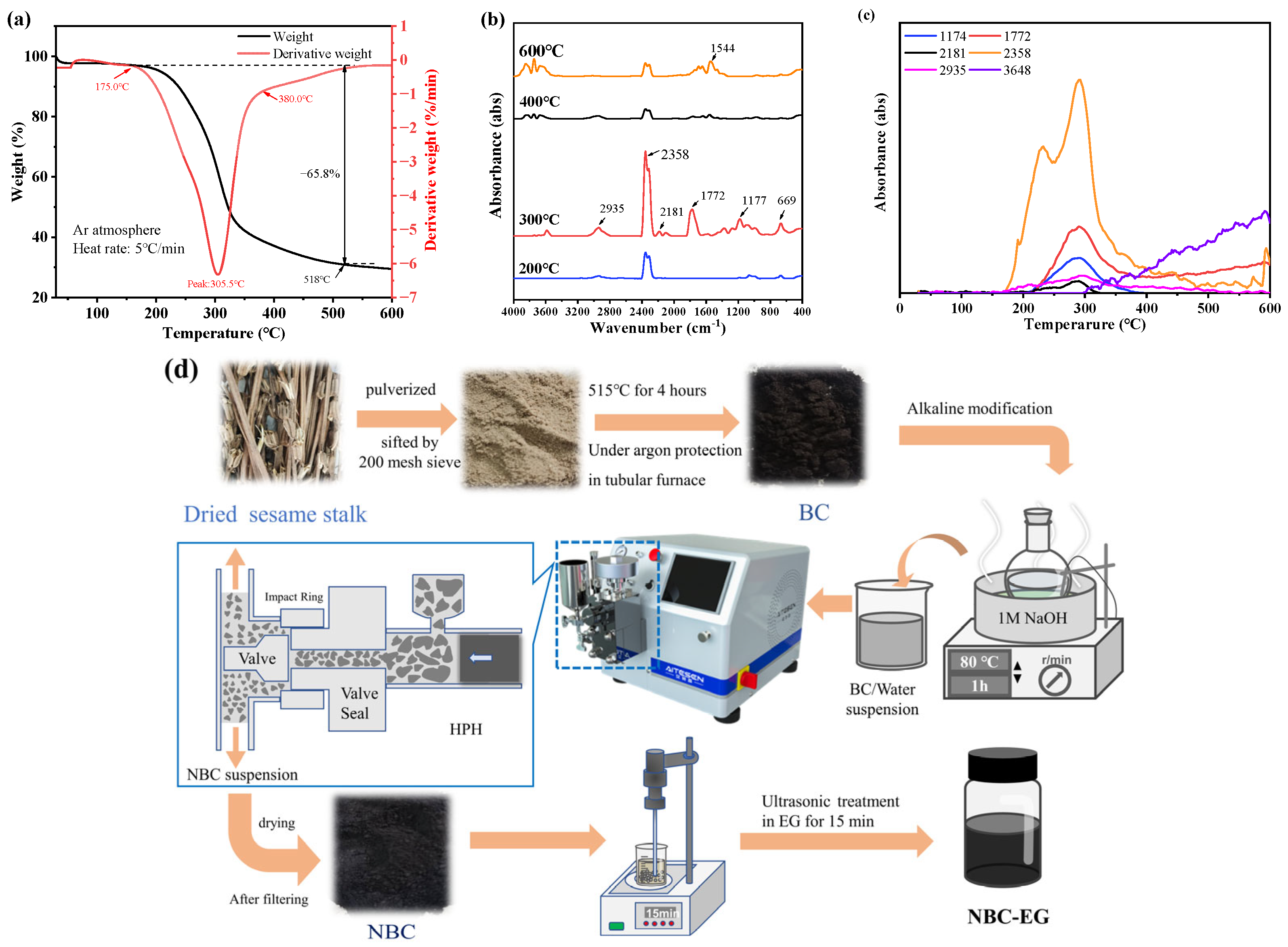
2.2. Preparation of Nano-Biochar
2.3. Characterization Technology
3. Results and Discussion
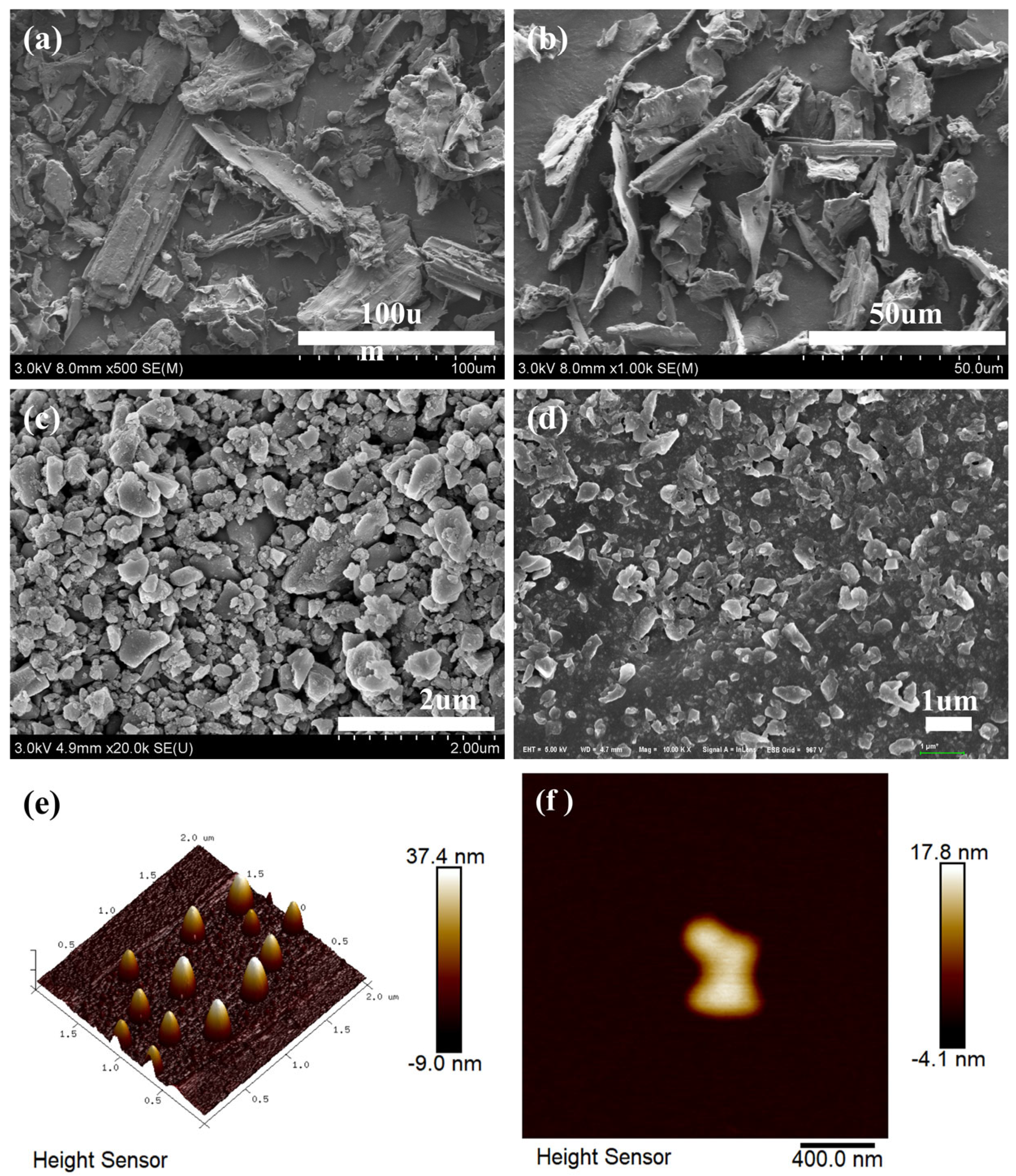
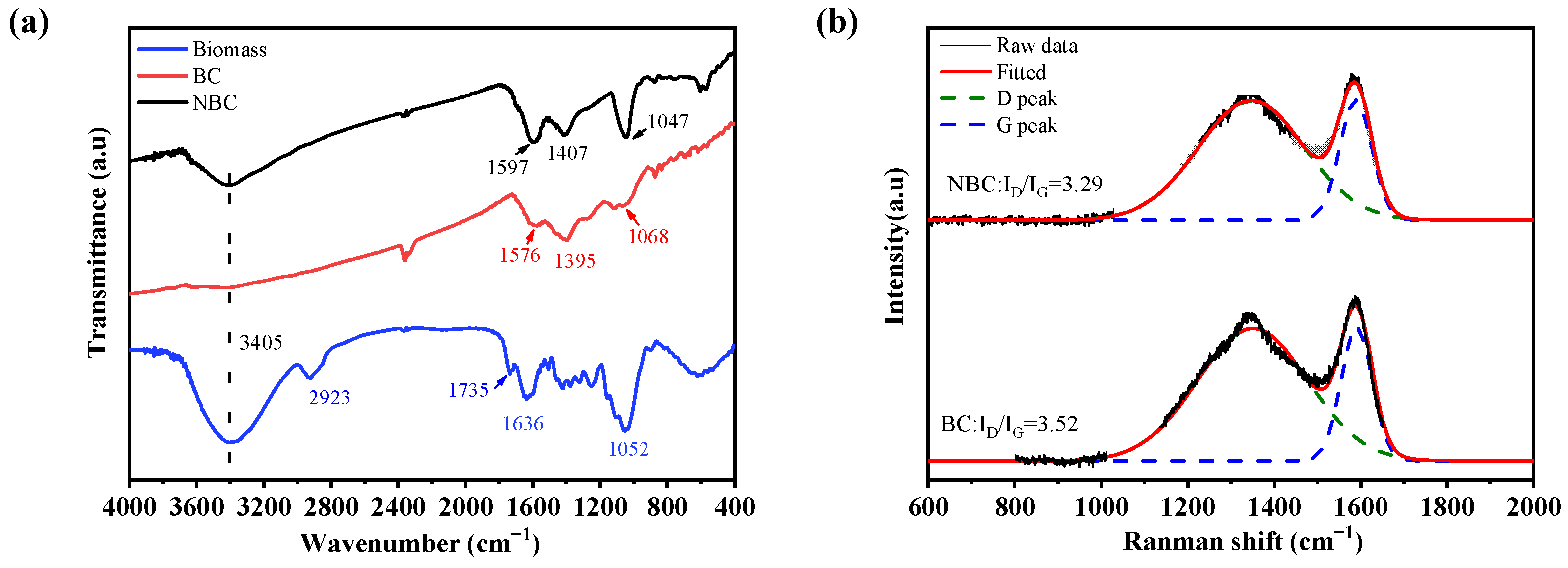
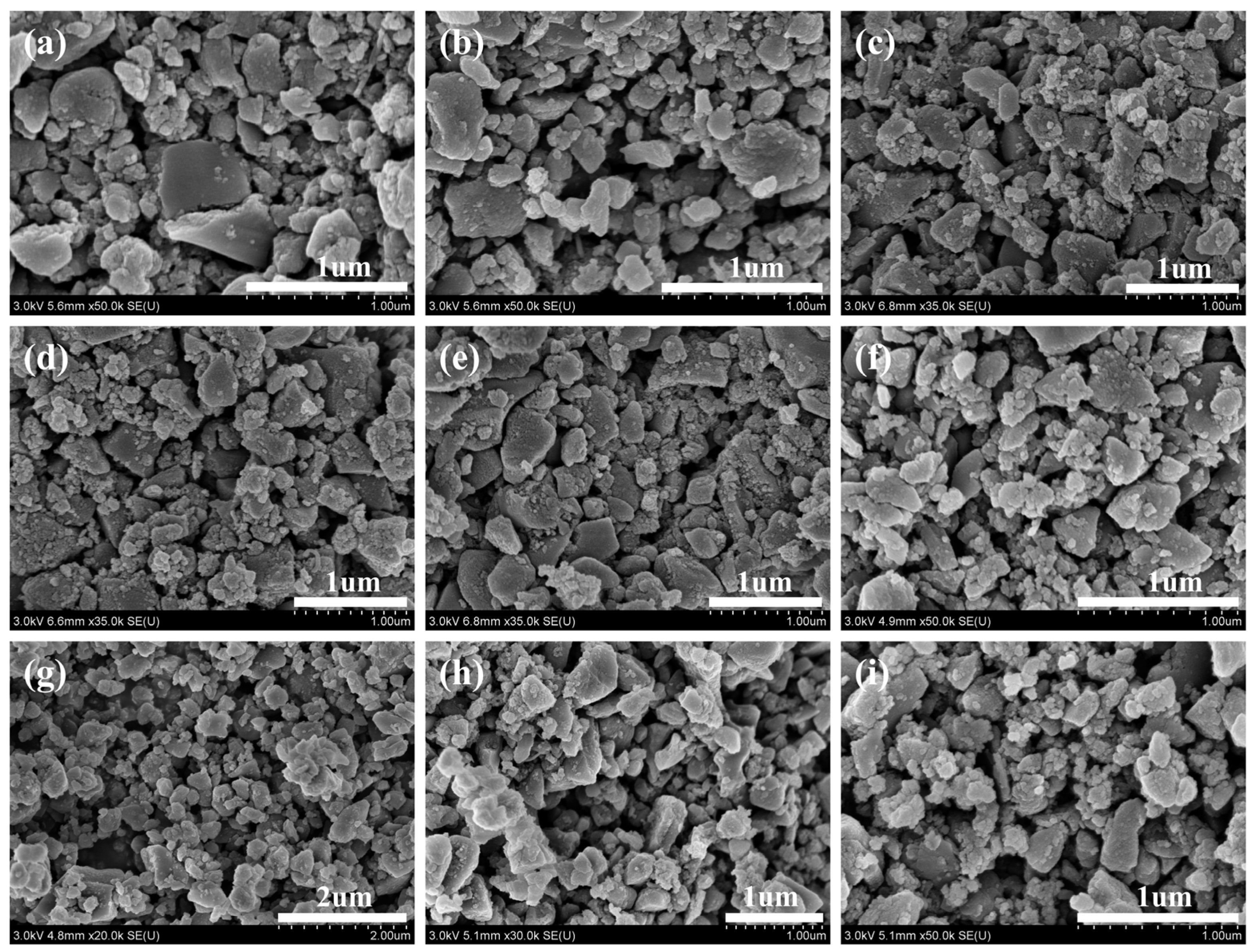
3.1. Characterization of Biochar
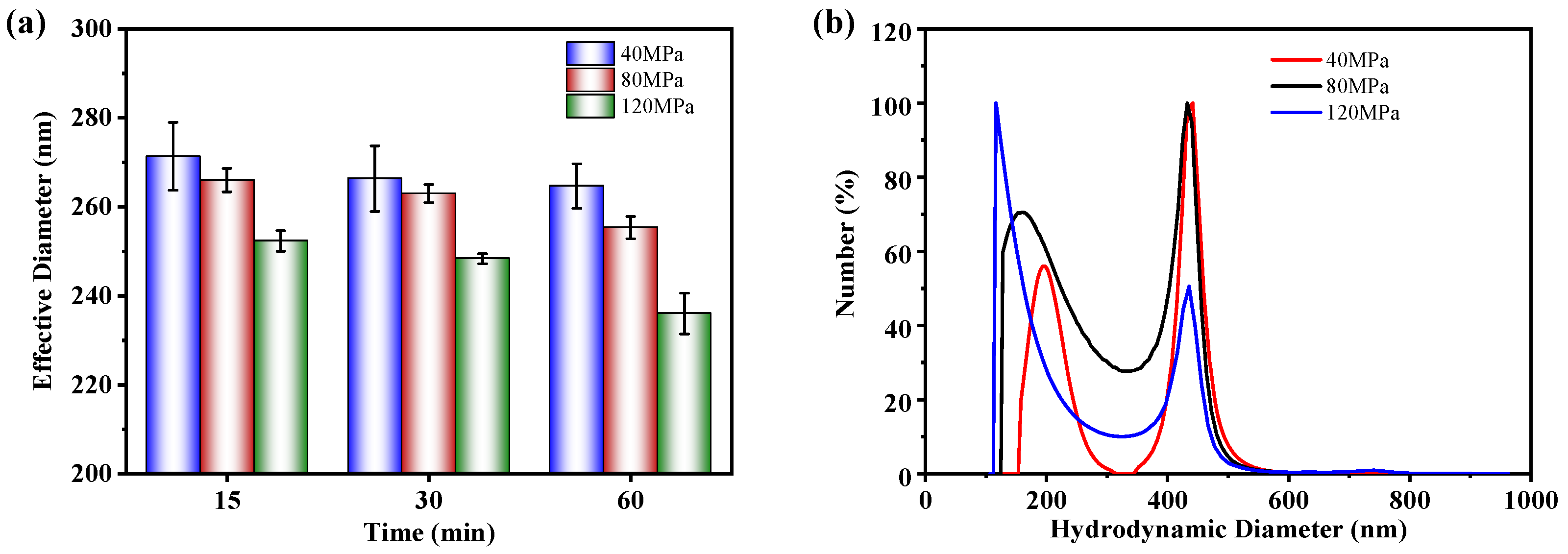
3.2. Dispersion Stability of NBC Coolant
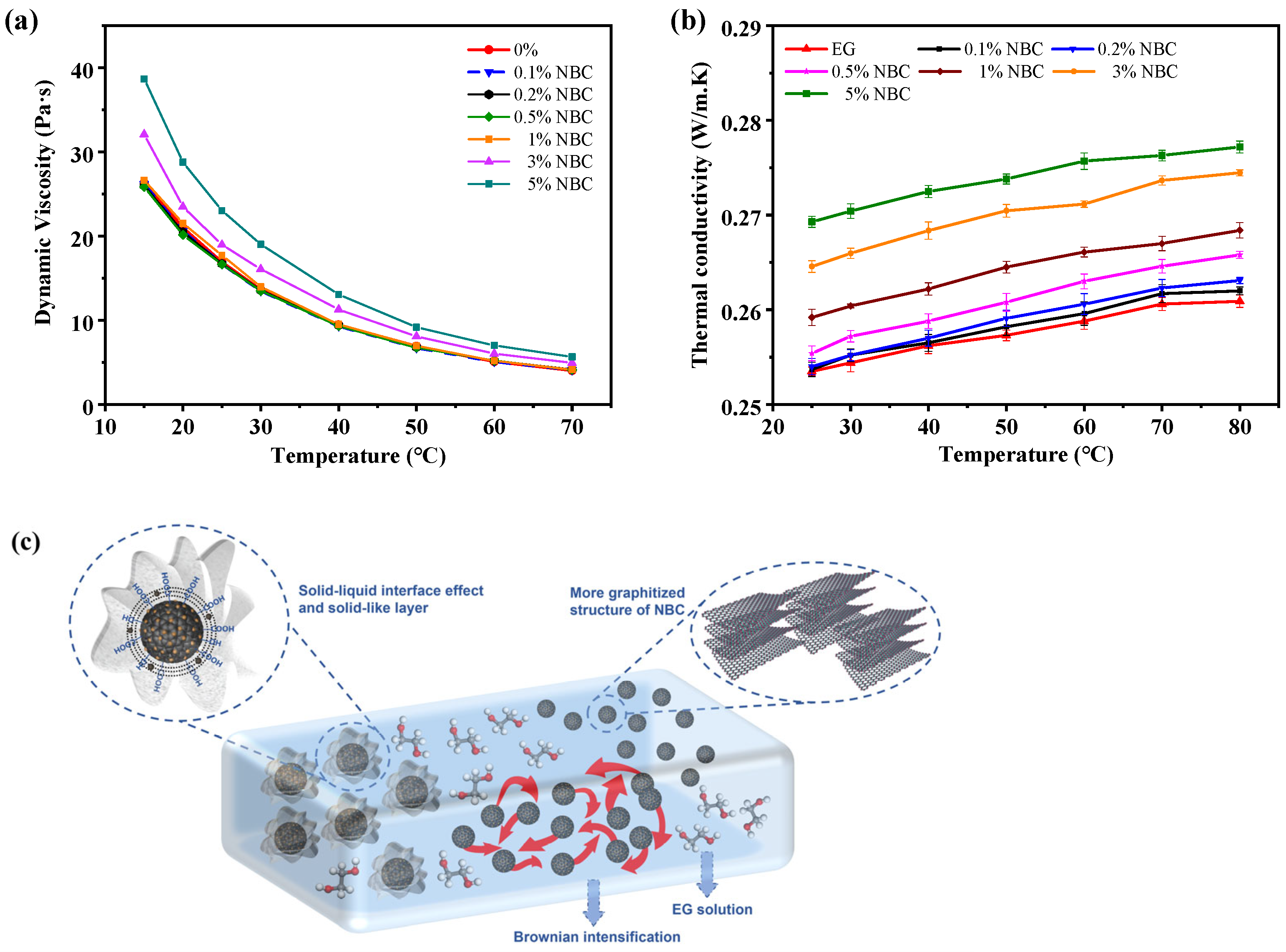
3.3. Viscosity Analysis of NBC Coolant
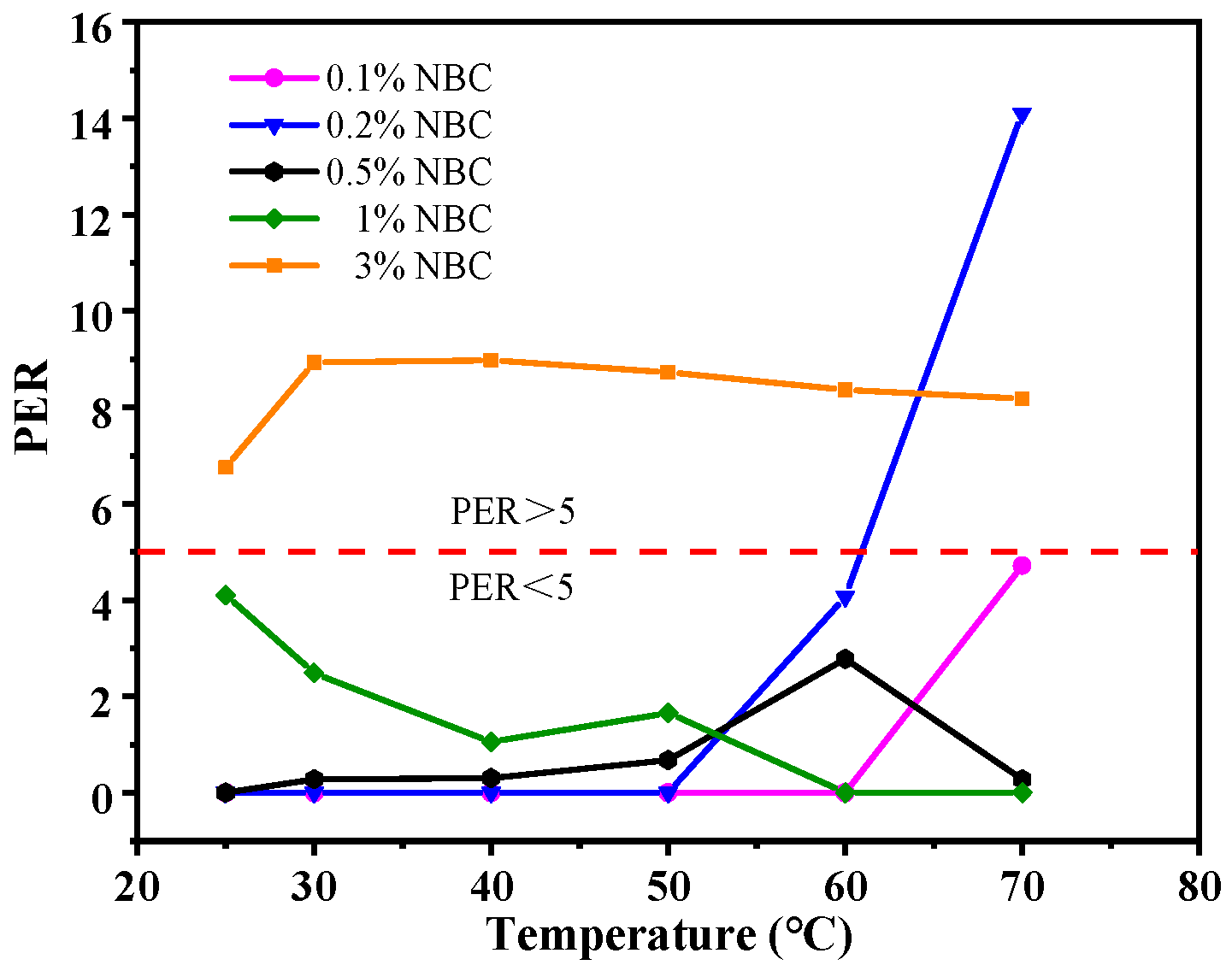
3.4. Thermal Conductivity of Coolants
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bao, Z.; Bing, N.; Zhu, X.; Xie, H.; Yu, W. Ti3C2Tx MXene contained nanofluids with high thermal conductivity, super colloidal stability and low viscosity. Chem. Eng. J. 2021, 406, 126390. [Google Scholar] [CrossRef]
- Kumar, R.; Mitra, A.; Srinivas, T. Role of nano-additives in the thermal management of lithium-ion batteries: A review. J. Energy Storage 2022, 48, 104059. [Google Scholar] [CrossRef]
- Kai, Y.; Ali, K.; Ahmad, S.; Ahmad, S.; Jamshed, W.; Raizah, Z.; Din, S.M.E. A case study of different magnetic strength fields and thermal energy effects in vortex generation of Ag-TiO2 hybrid nanofluid flow. Case Stud. Therm. Eng. 2023, 47, 103115. [Google Scholar] [CrossRef]
- Jilte, R.; Afzal, A.; Ağbulut, Ü.; Shaik, S.; Khan, S.A.; Linul, E.; Asif, M. Battery thermal management of a novel helical channeled cylindrical Li-ion battery with nanofluid and hybrid nanoparticle-enhanced phase change material. Int. J. Heat Mass Transf. 2023, 216, 124547. [Google Scholar] [CrossRef]
- Habib, D.; Abdal, S.; Ali, R.; Baleanu, D.; Siddique, I. On bioconvection and mass transpiration of micropolar nanofluid dynamics due to an extending surface in existence of thermal radiations. Case Stud. Therm. Eng. 2021, 27, 101239. [Google Scholar] [CrossRef]
- Sofiah, A.G.N.; Pasupuleti, J.; Samykano, M.; Rajamony, R.K.; Pandey, A.K.; Sulaiman, N.F.; Ramli, Z.A.C.; Tiong, S.K.; Koh, S.P. Potential coolants for fuel cell application: Multi-objective optimization of thermophysical properties and PPF calculation of hybrid palm oil nanofluids. Case Stud. Therm. Eng. 2024, 53, 103931. [Google Scholar] [CrossRef]
- Lei, S.; Xin, S.; Liu, S. Separate and integrated thermal management solutions for electric vehicles: A review. J. Power Sources 2022, 550, 232133. [Google Scholar] [CrossRef]
- Dhairiyasamy, R.; Saleh, B.; Govindasamy, M.; Aly, A.A.; Afzal, A.; Abdelrhman, Y. Effect of particle size on thermophysical and heat transfer properties of Ag nanofluid in a radiator—An experimental investigation. Inorg. Nano-Met. Chem. 2021, 53, 78–92. [Google Scholar] [CrossRef]
- Riahi, A.; Khamlich, S.; Balghouthi, M.; Khamliche, T.; Doyle, T.B.; Dimassi, W.; Guizani, A.; Maaza, M. Study of thermal conductivity of synthesized Al2O3-water nanofluid by pulsed laser ablation in liquid. J. Mol. Liq. 2020, 304, 112694. [Google Scholar] [CrossRef]
- Zhang, X.-C.; Xia, X.-L.; Li, D.-H.; Sun, C. Theoretical Modeling and Inverse Analysis of Thermal Conductivity of Skeletons in SiO2 Nano-Insulation Materials. Nanomaterials 2019, 9, 934. [Google Scholar] [CrossRef] [PubMed]
- Sobczak, J.; Vallejo, J.P.; Traciak, J.; Hamze, S.; Fal, J.; Estellé, P.; Lugo, L.; Żyła, G. Thermophysical profile of ethylene glycol based nanofluids containing two types of carbon black nanoparticles with different specific surface areas. J. Mol. Liq. 2021, 326, 115255. [Google Scholar] [CrossRef]
- Ali, N. Graphene-Based Nanofluids: Production Parameter Effects on Thermophysical Properties and Dispersion Stability. Nanomaterials 2022, 12, 357. [Google Scholar] [CrossRef] [PubMed]
- Marouzi, S.; Sabouri, Z.; Darroudi, M. Greener synthesis and medical applications of metal oxide nanoparticles. Ceram. Int. 2021, 47, 19632–19650. [Google Scholar] [CrossRef]
- Ali, M.A.; Fayaz; Viegas, R.F.; Kumar, M.B.S.; Kannapiran, R.K.; Feroskhan, M. Enhancement of heat transfer in paraffin wax PCM using nano graphene composite for industrial helmets. J. Energy Storage 2019, 26, 100982. [Google Scholar] [CrossRef]
- Yoo, M.J.; Park, H.B. Effect of hydrogen peroxide on properties of graphene oxide in Hummers method. Carbon 2019, 141, 515–522. [Google Scholar] [CrossRef]
- Sikora, P.; Woliński, P.; Chougan, M.; Madraszewski, S.; Węgrzyński, W.; Papis, B.K.; Federowicz, K.; Ghaffar, S.H.; Stephan, D. A systematic experimental study on biochar-cementitious composites: Towards carbon sequestration. Ind. Crops Prod. 2022, 184, 115103. [Google Scholar] [CrossRef]
- Kang, X.; Geng, N.; Li, Y.; Li, X.; Yu, J.; Gao, S.; Wang, H.; Pan, H.; Yang, Q.; Zhuge, Y.; et al. Treatment of cadmium and zinc-contaminated water systems using modified biochar: Contaminant uptake, adsorption ability, and mechanism. Bioresour. Technol. 2022, 363, 127817. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Yin, W.; Zhao, T.-L.; Liu, M.; Yao, Q.-Z.; Zhou, G.-T. Efficient Removal of Congo Red, Methylene Blue and Pb(II) by Hydrochar-MgAlLDH Nanocomposite: Synthesis, Performance and Mechanism. Nanomaterials 2023, 13, 1145. [Google Scholar] [CrossRef] [PubMed]
- Twagirayezu, G.; Cheng, H.; Wu, Y.; Lu, H.; Huang, S.; Fang, X.; Irumva, O. Insights into the influences of biochar on the fate and transport of pesticides in the soil environment: A critical review. Biochar 2024, 6, 9. [Google Scholar] [CrossRef]
- Salimi, P.; Tieuli, S.; Taghavi, S.; Venezia, E.; Fugattini, S.; Lauciello, S.; Prato, M.; Marras, S.; Li, T.; Signoretto, M.; et al. Sustainable lithium-ion batteries based on metal-free tannery waste biochar. Green Chem. 2022, 24, 4119–4129. [Google Scholar] [CrossRef]
- Agarwal, R.; Pawar, N.; Supriya; Rawat, P.; Rai, D.; Kumar, R.; Naik B, S. Thermo-mechanical behavior of cementitious material with partial replacement of Class-II biochar with Accelerated Carbonation Curing (ACC). Ind. Crops Prod. 2023, 204, 117335. [Google Scholar] [CrossRef]
- Bordoloi, U.; Das, D.; Kashyap, D.; Patwa, D.; Bora, P.; Muigai, H.H.; Kalita, P. Synthesis and comparative analysis of biochar based form-stable phase change materials for thermal management of buildings. J. Energy Storage 2022, 55, 105801. [Google Scholar] [CrossRef]
- Lv, L.; Huang, S.; Zhou, C.; Ma, W. Biochar activated by potassium carbonate to load organic phase change material: Better performance and environmental friendliness. Ind. Crops Prod. 2023, 204, 117184. [Google Scholar] [CrossRef]
- Xiong, T.; Ok, Y.S.; Dissanayake, P.D.; Tsang, D.C.W.; Kim, S.; Kua, H.W.; Shah, K.W. Preparation and thermal conductivity enhancement of a paraffin wax-based composite phase change material doped with garlic stem biochar microparticles. Sci. Total Environ. 2022, 827, 154341. [Google Scholar] [CrossRef] [PubMed]
- Anupama; Khare, P. A comprehensive evaluation of inherent properties and applications of nano-biochar prepared from different methods and feedstocks. J. Clean. Prod. 2021, 320, 128759. [Google Scholar] [CrossRef]
- Wang, Y.; Hou, X.; Zhang, L.; Ali, M.K.A.; Jiang, H.; Ma, Y. Development of nano biochar as a lubricating oil additive for tribological applications. J. Clean. Prod. 2023, 421, 138519. [Google Scholar] [CrossRef]
- Salaberria, A.M.; Fernandes, S.C.M.; Diaz, R.H.; Labidi, J. Processing of α-chitin nanofibers by dynamic high pressure homogenization: Characterization and antifungal activity against A. niger. Carbohydr. Polym. 2015, 116, 286–291. [Google Scholar] [CrossRef] [PubMed]
- Wei, D.; Liu, Q.; Liu, Z.; Liu, J.; Zheng, X.; Pei, Y.; Tang, K. Modified nano microfibrillated cellulose/carboxymethyl chitosan composite hydrogel with giant network structure and quick gelation formability. Int. J. Biol. Macromol. 2019, 135, 561–568. [Google Scholar] [CrossRef] [PubMed]
- Melchior, S.; Codrich, M.; Gorassini, A.; Mehn, D.; Ponti, J.; Verardo, G.; Tell, G.; Calzolai, L.; Calligaris, S. Design and advanced characterization of quercetin-loaded nano-liposomes prepared by high-pressure homogenization. Food Chem. 2023, 428, 136680. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.; Teng, A.; Zhao, K.; Zhang, K.; Zhao, H.; Duan, S.; Li, S.; Guo, Y.; Wang, W. A top-down approach to improve collagen film’s performance: The comparisons of macro, micro and nano sized fibers. Food Chem. 2020, 309, 125624. [Google Scholar] [CrossRef] [PubMed]
- Emran, M.; El-Gamal, E.H.; Mokhiamar, O.; Elsamni, O.; Rashad, M. A novel solar disk chamber reactor for agricultural waste recycling and biochar production. Clean Technol. Environ. Policy 2024, 26, 467–479. [Google Scholar] [CrossRef]
- Bi, H.; Ni, Z.; Tian, J.; Wang, C.; Jiang, C.; Zhou, W.; Bao, L.; Sun, H.; Lin, Q. The effect of biomass addition on pyrolysis characteristics and gas emission of coal gangue by multi-component reaction model and TG-FTIR-MS. Sci. Total Environ. 2021, 798, 149290. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Chen, X.; Han, W.; Han, Y.; Guo, M.; Peng, Z.; Fan, Z.; Shi, Y.; Wan, S. Tetracycline Removal by Hercynite-Biochar from the Co-Pyrolysis of Red Mud-Steel Slag-Sludge. Nanomaterials 2022, 12, 2595. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Wang, F.; Miao, Y.; Mai, Y.; Li, H.; Chen, X.; Chen, J. A lignin-biochar with high oxygen-containing groups for adsorbing lead ion prepared by simultaneous oxidization and carbonization. Bioresour. Technol. 2020, 307, 123165. [Google Scholar] [CrossRef] [PubMed]
- Luo, M.; Lin, H.; Li, B.; Dong, Y.; He, Y.; Wang, L. A novel modification of lignin on corncob_based biochar to enhance removal of cadmium from water. Bioresour. Technol. 2018, 259, 312–318. [Google Scholar] [CrossRef] [PubMed]
- Jiao, Y.; Yi, Y.; Fang, Z.; Tsang, P.E. Selective removal of oxytetracycline by molecularly imprinted magnetic biochar. Bioresour. Technol. 2024, 395, 130394. [Google Scholar] [CrossRef] [PubMed]
- Cibati, A.; Foereid, B.; Bissessur, A.; Hapca, S. Assessment of Miscanthus × giganteus derived biochar as copper and zinc adsorbent: Study of the effect of pyrolysis temperature, pH and hydrogen peroxide modification. J. Clean. Prod. 2017, 162, 1285–1296. [Google Scholar] [CrossRef]
- Kang, X.; Zhang, Q.; Liu, X.; Song, J.; Guo, H.; Wang, L. The interface mechanism of sludge biochar activating persulfate to remove tetracycline: The role of the C-O-Fe bridge at the carbon surface. J. Clean. Prod. 2023, 384, 135514. [Google Scholar] [CrossRef]
- Zhang, X.; Chu, Y.; Zhang, H.; Hu, J.; Wu, F.; Wu, X.; Shen, G.; Yang, Y.; Wang, B.; Wang, X. A mechanistic study on removal efficiency of four antibiotics by animal and plant origin precursors-derived biochars. Sci. Total Environ. 2021, 772, 145468. [Google Scholar] [CrossRef] [PubMed]
- Egyir, M.; Luyima, D.; Park, S.-J.; Lee, K.S.; Oh, T.-K. Volatilisations of ammonia from the soils amended with modified and nitrogen-enriched biochars. Sci. Total Environ. 2022, 835, 155453. [Google Scholar] [CrossRef] [PubMed]
- El-Shafie, A.S.; Barah, F.G.; Abouseada, M.; El-Azazy, M. Performance of Pristine versus Magnetized Orange Peels Biochar Adapted to Adsorptive Removal of Daunorubicin: Eco-Structuring, Kinetics and Equilibrium Studies. Nanomaterials 2023, 13, 1444. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Wang, X.; Fang, D. A review on C1s XPS-spectra for some kinds of carbon materials. Fuller. Nanotub. Carbon Nanostructures 2020, 28, 1048–1058. [Google Scholar] [CrossRef]
- Xiong, S.; Gong, D.; Deng, Y.; Tang, R.; Li, L.; Zhou, Z.; Zheng, J.; Yang, L.; Su, L. Facile one-pot magnetic modification of Enteromorpha prolifera derived biochar: Increased pore accessibility and Fe-loading enhances the removal of butachlor. Bioresour. Technol. 2021, 337, 125407. [Google Scholar] [CrossRef] [PubMed]
- Tan, X.; Shaaban, M.; Yang, J.; Cai, Y.; Wang, B.; Peng, Q.A. Efficient removal of hexavalent chromium from an aquatic system using nanoscale zero-valent iron supported by ramie biochar. Nanomaterials 2021, 11, 2698. [Google Scholar] [CrossRef] [PubMed]
- Tang, D.; Lu, L.; Luo, Z.; Yang, B.; Ke, J.; Lei, W.; Zhen, H.; Zhuang, Y.; Sun, J.; Chen, K.; et al. Heteroatom-Doped Hierarchically Porous Biochar for Supercapacitor Application and Phenol Pollutant Remediation. Nanomaterials 2022, 12, 2586. [Google Scholar] [CrossRef] [PubMed]
- Yao, C.; Li, F.; Chen, T.; Tang, Y. Green preparation of cellulose nanofibers via high-pressure homogenization and their film-forming properties. Ind. Crops Prod. 2023, 206, 117575. [Google Scholar] [CrossRef]
- Sofiah, A.G.N.; Pasupuleti, J.; Samykano, M.; Pandey, A.K.; Rajamony, R.K.; Sulaiman, N.F.; Che Ramli, Z.A. A class of promising fuel cell performance: International status on the application of nanofluids for thermal management systems. Mater. Today Sustain. 2024, 26, 100709. [Google Scholar] [CrossRef]
- Zhao, Y.; Min, X.; Huang, Z.; Liu, Y.; Wu, X.; Fang, M. Honeycomb-like structured biological porous carbon encapsulating PEG: A shape-stable phase change material with enhanced thermal conductivity for thermal energy storage. Energy Build. 2018, 158, 1049–1062. [Google Scholar] [CrossRef]
- Wang, J.; Prasher, R.; Song, D.; Phelan, P. Measurements of nanofluid viscosity and its implications for thermal applications. Appl. Phys. Lett. 2006, 89, 133108. [Google Scholar]
- Garg, J.; Poudel, B.; Chiesa, M.; Gordon, J.; Ma, J.; Wang, J.; Ren, Z.; Kang, Y.; Ohtani, H.; Nanda, J. Enhanced thermal conductivity and viscosity of copper nanoparticles in ethylene glycol nanofluid. J. Appl. Phys. 2008, 103, 74301. [Google Scholar] [CrossRef]
- Li, H.; Wang, L.; He, Y.; Hu, Y.; Zhu, J.; Jiang, B. Experimental investigation of thermal conductivity and viscosity of ethylene glycol based ZnO nanofluids. Appl. Therm. Eng. 2014, 88, 363–368. [Google Scholar] [CrossRef]
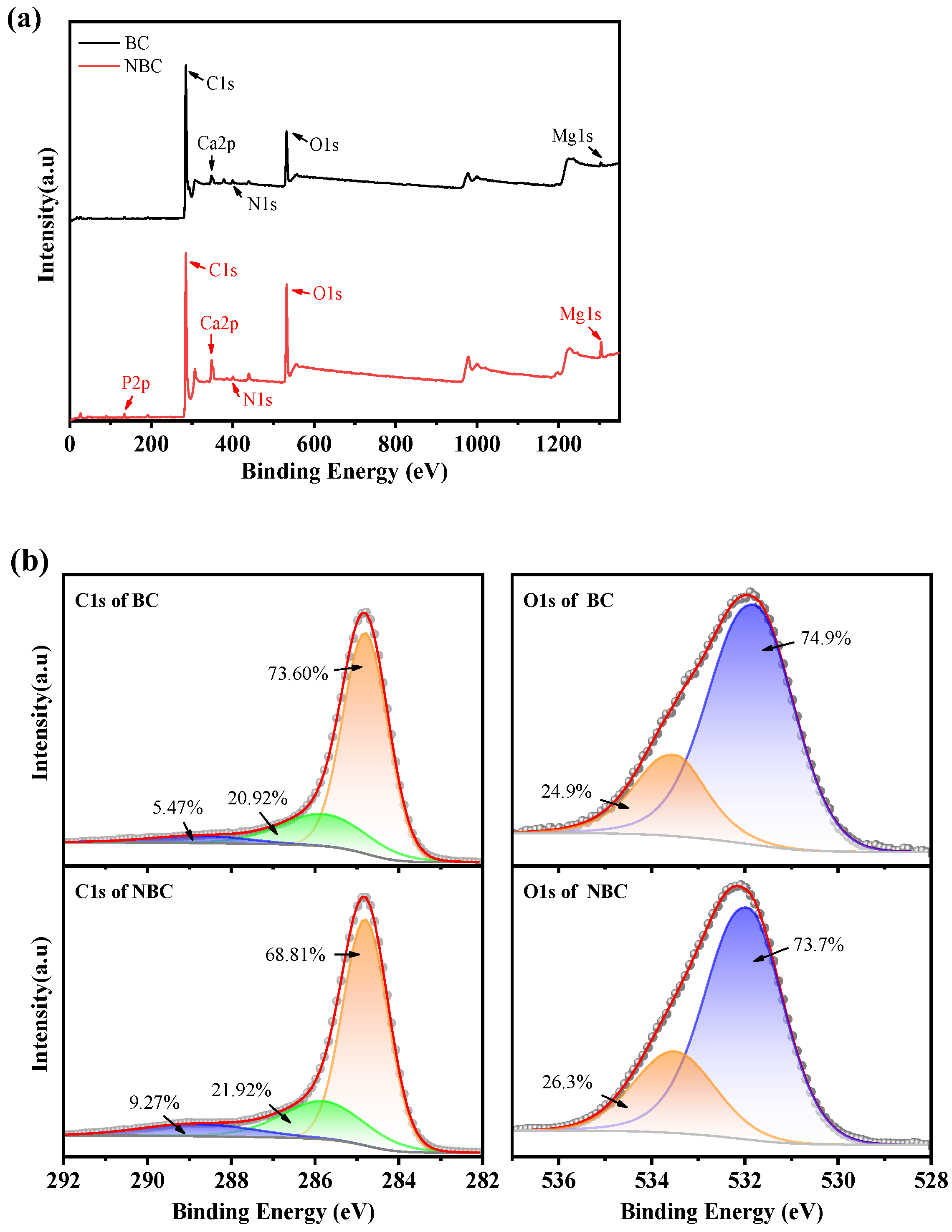

| Element/% | C | O | N | Mg | Ca | P |
|---|---|---|---|---|---|---|
| BC | 81.0 | 13.7 | 2.1 | 0.6 | 1.7 | 0.9 |
| NBC | 73.4 | 19.9 | 1.4 | 1.5 | 2.6 | 1.2 |
| Reference | Materials/Concentration | Base Fluid | Dispersity Stability | Viscosity | Enhancement of Thermal Conductivity |
|---|---|---|---|---|---|
| [11] | Carbon black (30 nm)/2 wt.% | EG | 4.8% | ||
| Carbon black (13 nm)/2 wt.% | EG | 1.21% | |||
| [9] | Nano Al2O3/0.7 vol% | Water | 4.2% | ||
| [1] | M-Ti3C2Tx MXene/5 vol% | EG | 400% | 53.1% | |
| S-Ti3C2Tx MXene/5 vol% | EG | 30 days | 76% | 64.9% | |
| [51] | Nano ZnO/3.5 wt.% | EG | 15% | 5.2% | |
| Nano ZnO/7 wt.% | EG | 50% | 9.13% | ||
| [23] | Bamboo biochar/(14.3 wt.%) | Stearic acid | 5.2% | ||
| Pine biochar/(14.3 wt.%) | Stearic acid | 2.1% | |||
| Walnut biochar/(14.3 wt.%) | Stearic acid | 8.6% | |||
| Corncob biochar/(14.3 wt.%) | Stearic acid | 1.0% | |||
| [22] | Biochar (40 wt.%) | PCM (OM35) | 22% | ||
| [24] | Garlic stem biochar/(5 wt.%) | Paraffin wax | 7.2% | ||
| This study | Nano-biochar/(3 wt.%) | EG | 28 days | 17% | 5.2% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Y.; Hou, X.; Yu, H.; Guan, W.; Ma, Y.; Ali, M.K.A. Nano-Biochar Prepared from High-Pressure Homogenization Improves Thermal Conductivity of Ethylene Glycol-Based Coolant. Nanomaterials 2024, 14, 1308. https://doi.org/10.3390/nano14151308
Wang Y, Hou X, Yu H, Guan W, Ma Y, Ali MKA. Nano-Biochar Prepared from High-Pressure Homogenization Improves Thermal Conductivity of Ethylene Glycol-Based Coolant. Nanomaterials. 2024; 14(15):1308. https://doi.org/10.3390/nano14151308
Chicago/Turabian StyleWang, Youheng, Xianjun Hou, Hong Yu, Weiwei Guan, Yuxin Ma, and Mohamed Kamal Ahmed Ali. 2024. "Nano-Biochar Prepared from High-Pressure Homogenization Improves Thermal Conductivity of Ethylene Glycol-Based Coolant" Nanomaterials 14, no. 15: 1308. https://doi.org/10.3390/nano14151308






