Core–Shell Interface Engineering Strategies for Modulating Energy Transfer in Rare Earth-Doped Nanoparticles
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Composition of RENPs
2.3. Method 1: One-Pot Successive LBL Strategy
2.3.1. Syntheses of Ce-RENPs Shell Precursors
2.3.2. Synthesis of Ce-RENPs Core
2.3.3. Synthesis of Ce-RENPs-LBL Core@Shell 1 with Different Thicknesses of Shell 1
2.3.4. Synthesis of Ce-RENPs-LBL Core@Shell 1@Shell 2
2.4. Method 2: Modified SA Growth Strategy
2.4.1. Synthesis of Ce-RENPs Core
2.4.2. Syntheses of Ce-RENPs Shell 1 Precursor and Shell 2 Precursor
2.4.3. Synthesis of Ce-RENPs-SA Core@Shell 1 with Different Thicknesses of Shell 1
2.4.4. Synthesis of Ce-RENPs-SA Core@Shell 1@Shell 2
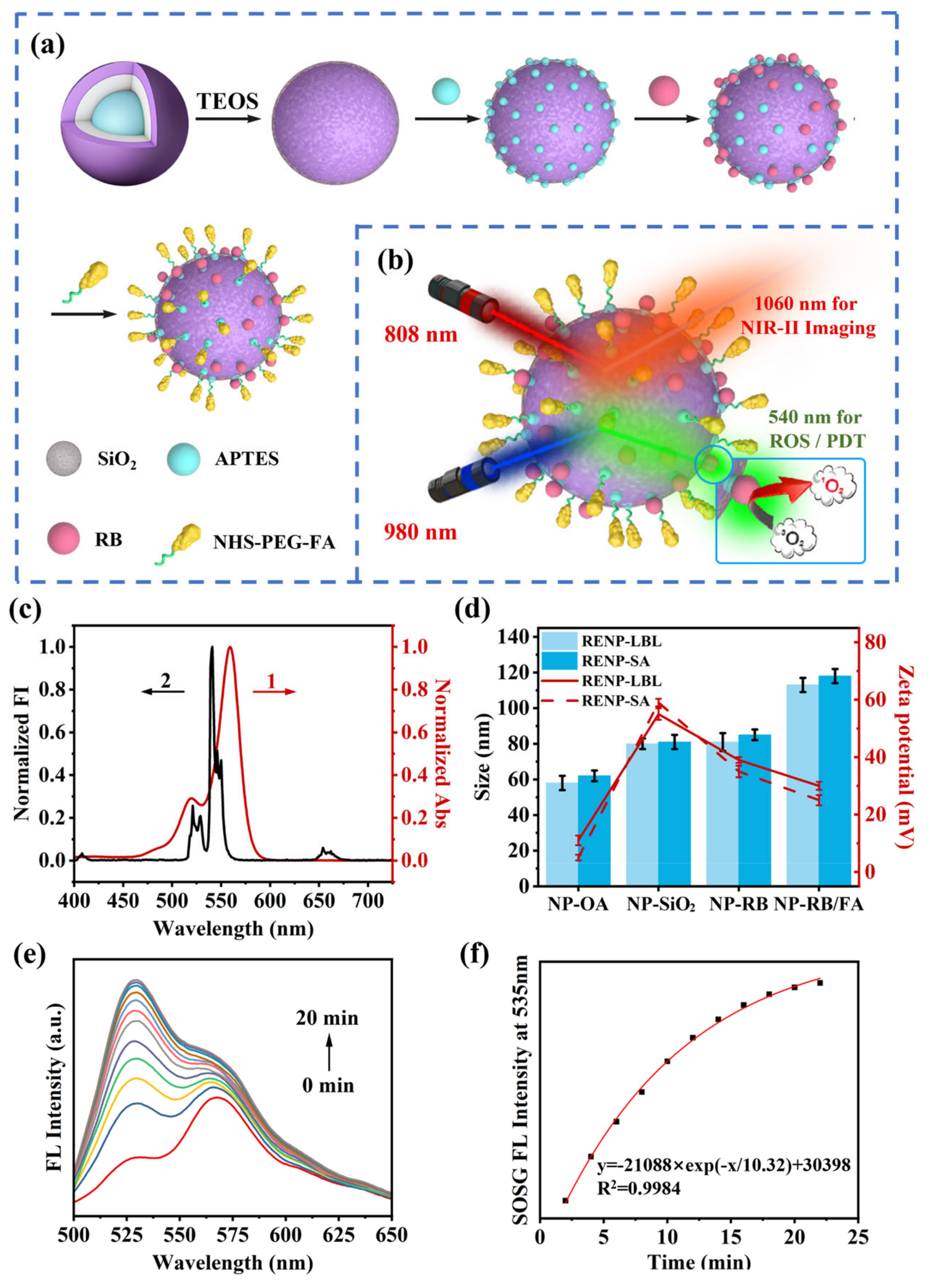
2.5. Synthesis of Ce-RENPs@SiO2-NH2
2.6. Synthesis of Ce-RENPs@SiO2-RB/FA
2.7. Cell Culture
2.8. ROS Detection
2.9. In Vivo Imaging
2.10. Characterization
3. Results and Discussion
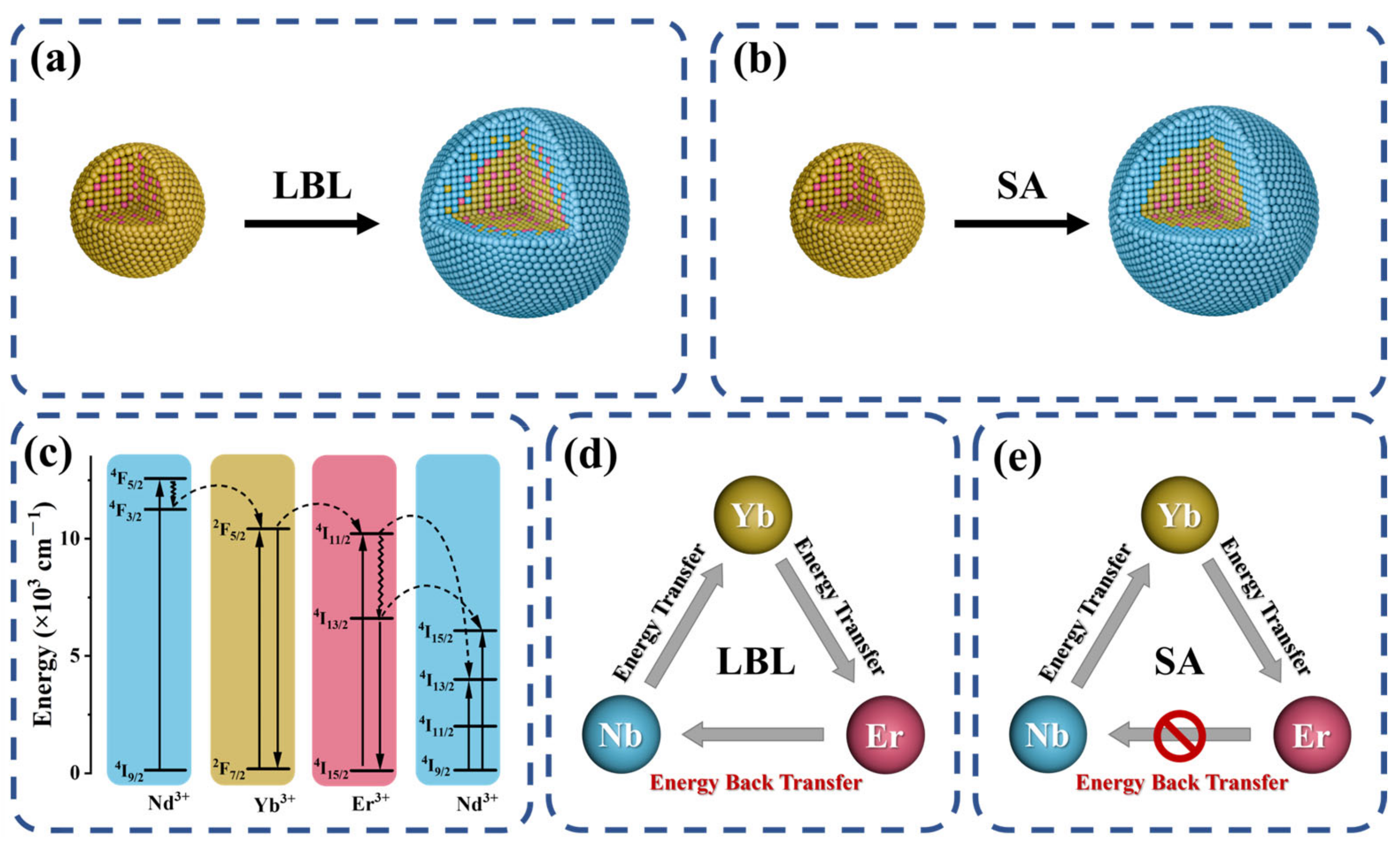
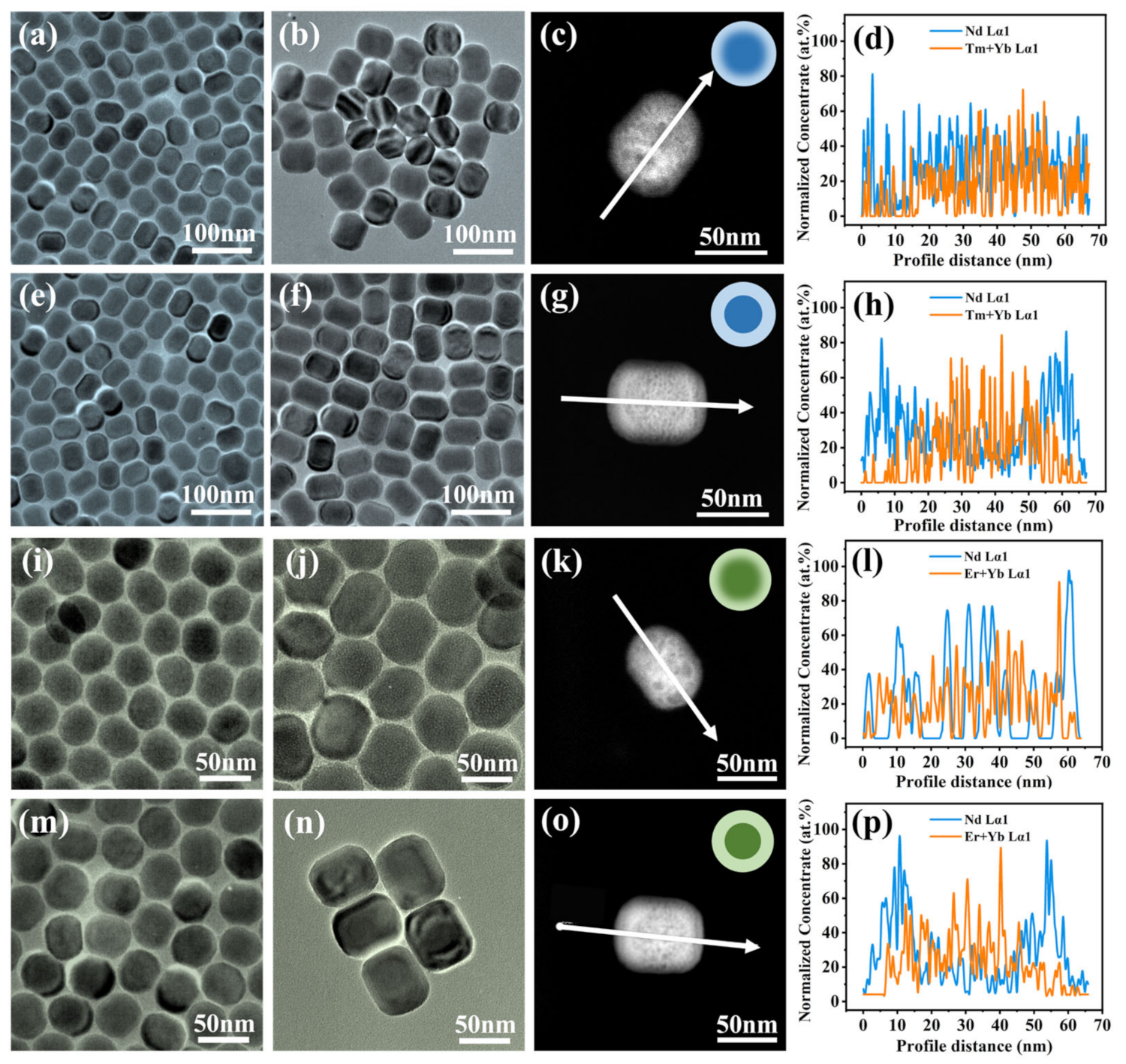
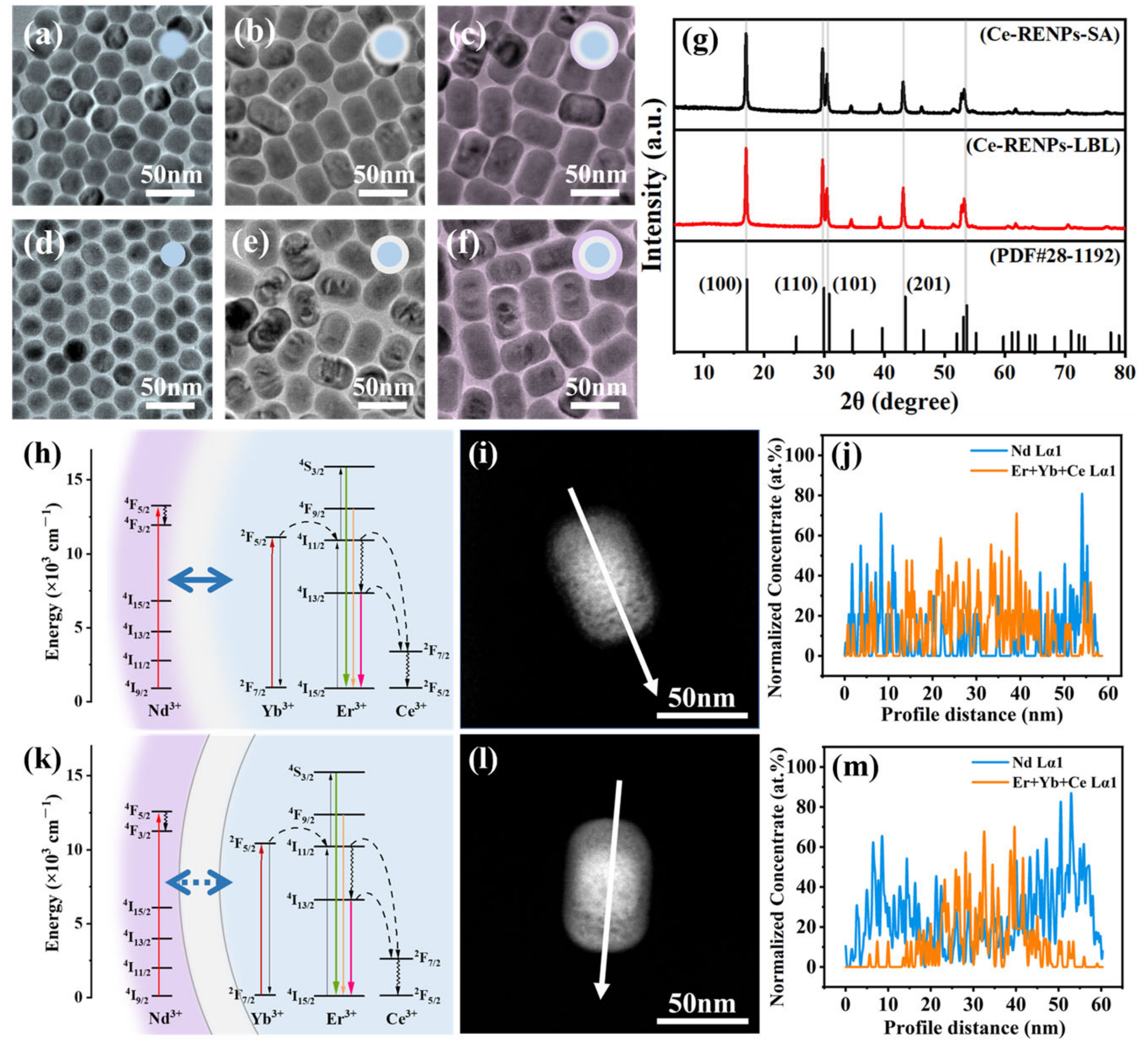
3.1. Synthesis Strategies and the Interface Characteristics
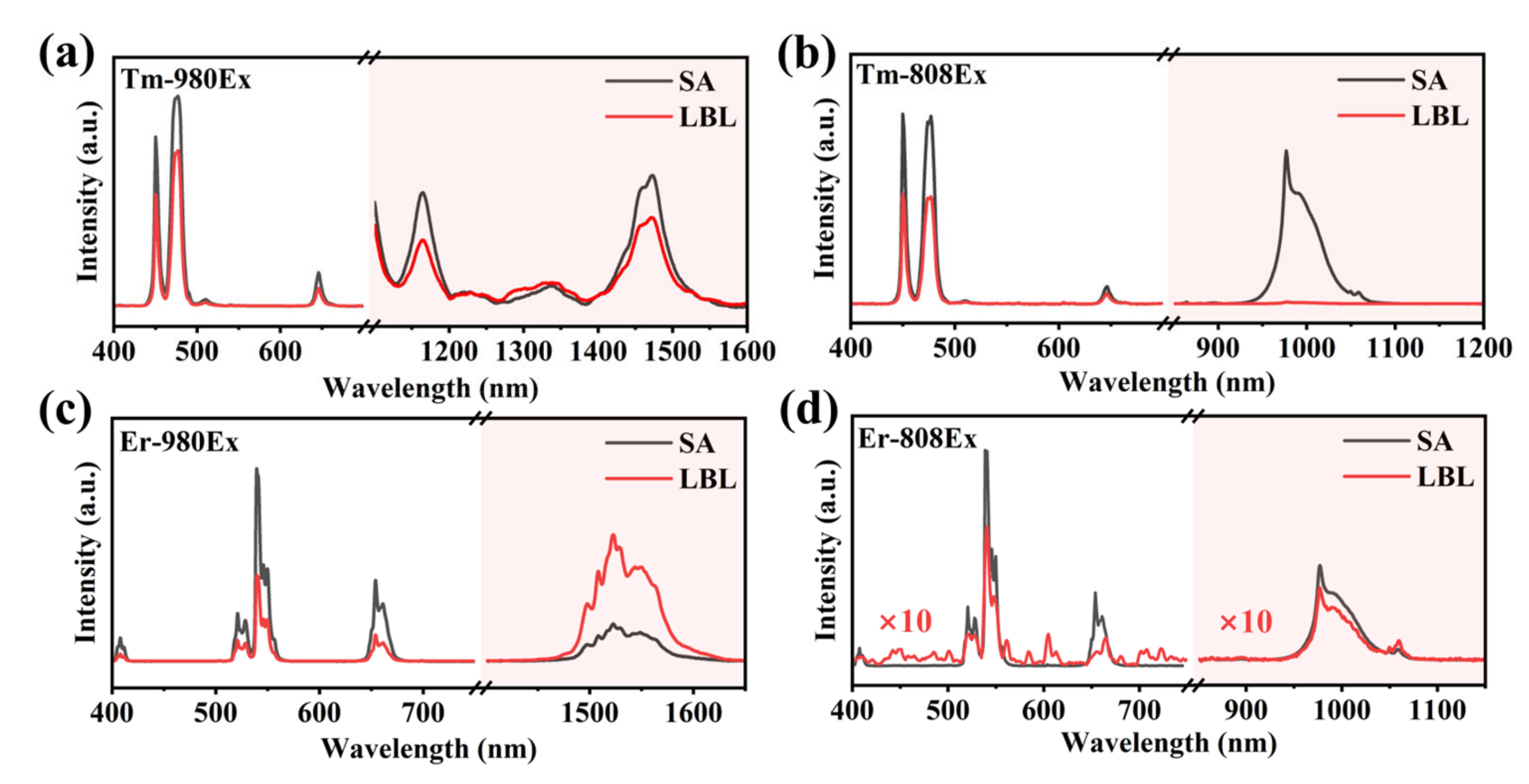
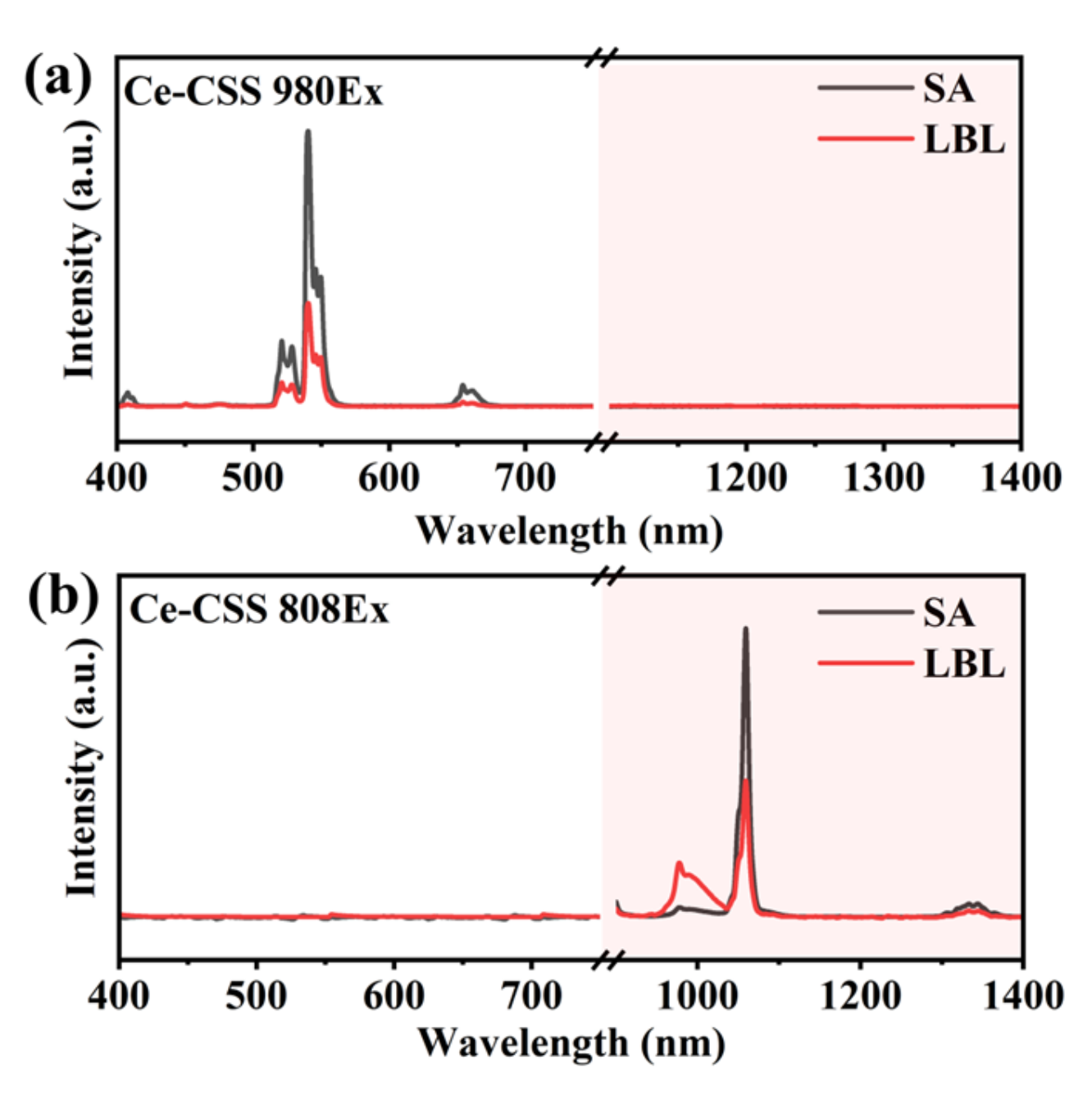
3.2. Interface Clarity and Energy Transfer
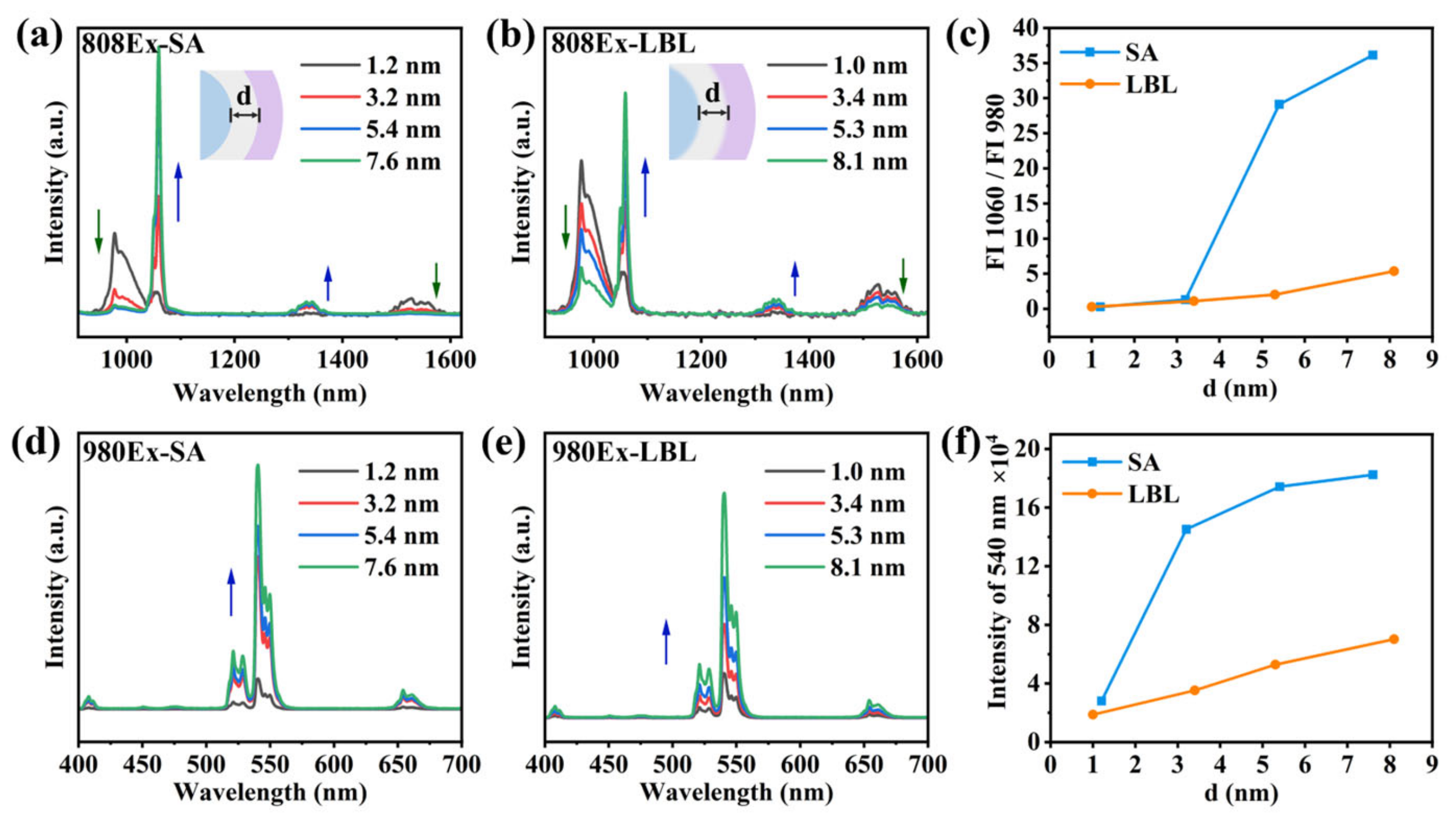
3.3. The Orthogonality of Luminescence and the Thickness of the Isolation Layer
3.4. ROS Generation and Detection of Ce-RENPs@SiO2-RB In Vitro and In Vivo
3.5. Enhanced NIR-II Imaging for SA Strategy
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| RENPs | Rare Earth Nanoparticles |
| LBL | Layer-by-Layer |
| SA | Seed-Assisted |
| NIR | Near Infrared |
| PDT | Photodynamic Therapy |
| ROS | Reactive Oxygen Species |
| TEM | Transmission Electron Microscopy |
| HAADF-STEM | High-angle annular dark-field scanning transmission electron microscopy |
| EDXS | Energy dispersive X-ray spectroscopy |
| XRD | X-ray diffraction |
| UCL | Upconversion luminescence |
| DSL | Down-shifting luminescence |
| Tm-RENPs | Tm3+-doped RENPs |
| Tm-RENPs-LBL | Tm-RENPs synthesized by LBL strategy |
| Tm-RENPs-SA | Tm-RENPs synthesized by SA strategy |
| Er-RENPs | Er3+-doped RENPs |
| Er-RENPs-LBL | Er-RENPs synthesized by LBL strategy |
| Er-RENPs-SA | Er-RENPs synthesized by SA strategy |
| Ce-RENPs | Ce3+-doped RENPs |
| Ce-RENPs-LBL | Ce-RENPs synthesized by LBL strategy |
| Ce-RENPs-SA | Ce-RENPs synthesized by SA strategy |
| NHS | N-hydroxy succinimide |
| EDC | 1-ethyl-(3-dimethyllaminopropyl) carbodiimide hydrochloride |
| HEPES | 2-[4-(2-hydroxyethyl)-1-piperazinyl] ethanesulfonic acid |
| DMF | N,N-dimethylformamide |
| APTES | 3-aminopropyl triethoxysilane |
| RB | Rose Bengal |
| FA | Folic acid |
| SOSG | Singlet oxygen sensor green reagent |
| CLSM | Confocal laser scanning microscope |
| LED | Light-emitting diode |
Appendix A
| Type of RENPs | Core Composition | Shell 1 Composition | Shell 2 Composition |
|---|---|---|---|
| Tm-RENPs | NaYF4: 0.5% Tm, 30% Yb | NaYF4: 10% Yb, 30% Nd | - |
| Er-RENPs | NaYF4: 2% Er, 20% Yb | NaYF4: 5% Nd | - |
| Ce-RENPs | NaYF4: 20% Ce, 2% Er, 20% Yb | NaYF4 | NaYF4: 5% Nd |
References
- Keshavarz, M.; Tan, B.; Venkatakrishnan, K. Multiplex Photoluminescent Silicon Nanoprobe for Diagnostic Bioimaging and Intracellular Analysis. Adv. Sci. 2018, 5, 1700548. [Google Scholar] [CrossRef] [PubMed]
- Zhu, X.; Su, Q.; Feng, W.; Li, F. Anti-Stokes shift luminescent materials for bio-applications. Chem. Soc. Rev. 2017, 46, 1025–1039. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Zhang, Y.; Takle, K.; Bilsel, O.; Li, Z.; Lee, H.; Zhang, Z.; Li, D.; Fan, W.; Duan, C.; et al. Dye-Sensitized Core/Active Shell Upconversion Nanoparticles for Optogenetics and Bioimaging Applications. ACS Nano 2016, 10, 1060–1066. [Google Scholar] [CrossRef] [PubMed]
- Mettenbrink, E.M.; Yang, W.; Wilhelm, S. Bioimaging with Upconversion Nanoparticles. Adv. Photonics Res. 2022, 3, 2200098. [Google Scholar] [CrossRef] [PubMed]
- Zhong, Y.; Ma, Z.; Zhu, S.; Yue, J.; Zhang, M.; Antaris, A.L.; Yuan, J.; Cui, R.; Wan, H.; Zhou, Y.; et al. Boosting the down-shifting luminescence of rareearth nanocrystals for biological imaging beyond 1500 nm. Nat. Commun. 2017, 8, 737. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Yang, H.; Liu, Y.; Zhou, Z.; Zhang, X.; Sang, M.; Xu, F.; Song, L.; Xia, T.; Zhang, Y.; et al. Awakening Allies for Breaking Microenvironment Barriers: NIR-II Guided Orthogonal Activation of Tumor-Infiltrating Mast Cells for Efficient Nano-Drug Delivery. Adv. Healthc. Mater. 2023, 12, 2300420. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Jin, D.; Stenzel, M.H. Polymer-Functionalized Upconversion Nanoparticles for Light/Imaging-Guided Drug Delivery. Biomacromolecules 2021, 22, 3168–3201. [Google Scholar] [CrossRef] [PubMed]
- Zheng, F.; Meng, T.; Jiang, D.; Sun, J.; Yao, H.; Zhu, J.J.; Min, Q. Nanomediator–Effector Cascade Systems for Amplified Protein Kinase Activity Imaging and Phosphorylation-Induced Drug Release In Vivo. Angew. Chem. Int. Ed. Engl. 2021, 60, 21565–21574. [Google Scholar] [CrossRef] [PubMed]
- Han, Y.; Noor, M.O.; Sedighi, A.; Uddayasankar, U.; Doughan, S.; Krull, U.J. Inorganic Nanoparticles as Donors in Resonance Energy Transfer for Solid-Phase Bioassays and Biosensors. Langmuir 2017, 33, 12839–12858. [Google Scholar] [CrossRef] [PubMed]
- Liang, Z.; Wang, X.; Zhu, W.; Zhang, P.; Yang, Y.; Sun, C.; Zhang, J.; Wang, X.; Xu, Z.; Zhao, Y.; et al. Upconversion Nanocrystals Mediated Lateral-Flow Nanoplatform for In Vitro Detection. ACS Appl. Mater. Interfaces 2017, 9, 3497–3504. [Google Scholar] [CrossRef] [PubMed]
- Peng, J.; Xu, W.; Teoh, C.L.; Han, S.; Kim, B.; Samanta, A.; Er, J.C.; Wang, L.; Yuan, L.; Liu, X.; et al. High-Efficiency In Vitro and In Vivo Detection of Zn2+ by Dye-Assembled Upconversion Nanoparticles. J. Am. Chem. Soc. 2015, 137, 2336–2342. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Wu, J.; Wei, W.; Zhang, Y.; Chen, Q. Upconversion Nanoparticles Based Sensing: From Design to Point-of-Care Testing. Small 2024, 20, 2311729. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Liu, T.; Tu, L.; Zuo, J.; Li, J.; Feng, Y.; Yao, C.J. Enhancing NIR-II Upconversion Monochromatic Emission for Temperature Sensing. Small 2024, 20, 2308748. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; An, Z.; Zhou, B. Optical multiplexing of upconversion in nanoparticles towards emerging applications. Chem. Eng. J. 2023, 452, 139649. [Google Scholar] [CrossRef]
- Lu, Y.; Zhao, J.; Zhang, R.; Liu, Y.; Liu, D.; Goldys, E.M.; Yang, X.; Xi, P.; Sunna, A.; Lu, J.; et al. Tunable lifetime multiplexing using luminescent nanocrystals. Nat. Photonics 2013, 8, 32–36. [Google Scholar] [CrossRef]
- Zhang, Q.; Gao, Y.; Cheng, L.; Li, Y.; Xu, S.; Chen, B. The Combination of Upconversion Nanoparticles and Perovskite Quantum Dots with Temperature-Dependent Emission Colors for Dual-Mode Anti-Counterfeiting Applications. Nanomaterials 2023, 13, 3102. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Chen, W.; Xie, X.; Zhang, Y.; Chao, Z.; Ma, H.; Liu, Y.; Ju, H. Energy Pumping by Surface Collectors on Upconversion Nanoparticles for Extended Transfer and Efficient Self-Evaluable Photodynamic Therapy. CCS Chem. 2022, 4, 1251–1262. [Google Scholar] [CrossRef]
- Qiu, H.; Tan, M.; Ohulchanskyy, T.Y.; Lovell, J.F.; Chen, G. Recent Progress in Upconversion Photodynamic Therapy. Nanomaterials 2018, 8, 344. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Shao, Q.; Dong, Y.; Jiang, J. Phase-, shape- and size-controlled synthesis of NaYF4:Yb3+,Er3+ nanoparticles using rare-earth acetate precursors. J. Rare Earth 2014, 32, 1032–1036. [Google Scholar] [CrossRef]
- Dong, H.; Sun, L.-D.; Feng, W.; Gu, Y.; Li, F.; Yan, C.-H. Versatile Spectral and Lifetime Multiplexing Nanoplatform with Excitation Orthogonalized Upconversion Luminescence. ACS Nano 2017, 11, 3289–3297. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Z.; He, L.; Yang, Z.; Qiu, H.; Chen, X.; Yu, X.; Li, W. Ultra-wideband-responsive photon conversion through co-sensitization in lanthanide nanocrystals. Nat. Commun. 2023, 14, 827. [Google Scholar] [CrossRef] [PubMed]
- Zuo, J.; Tu, L.; Li, Q.; Feng, Y.; Que, I.; Zhang, Y.; Liu, X.; Xue, B.; Cruz, L.J.; Chang, Y.; et al. Near Infrared Light Sensitive Ultraviolet-Blue Nanophotoswitch for Imaging-Guided “Off-On” Therapy. ACS Nano 2018, 12, 3217–3225. [Google Scholar] [CrossRef] [PubMed]
- Yan, C.; Zhao, H.; Perepichka, D.F.; Rosei, F. Lanthanide Ion Doped Upconverting Nanoparticles: Synthesis, Structure and Properties. Small 2016, 12, 3888–3907. [Google Scholar] [CrossRef] [PubMed]
- Hudry, D.; Howard, I.A.; Popescu, R.; Gerthsen, D.; Richards, B.S. Structure–Property Relationships in Lanthanide-Doped Upconverting Nanocrystals: Recent Advances in Understanding Core–Shell Structures. Adv. Mater. 2019, 31, 1900623. [Google Scholar] [CrossRef] [PubMed]
- Huang, F.; Bagheri, N.; Wang, L.; Ågren, H.; Zhang, J.; Pu, R.; Zhan, Q.; Jing, Y.; Xu, W.; Widengren, J.; et al. Suppression of Cation Intermixing Highly Boosts the Performance of Core–Shell Lanthanide Upconversion Nanoparticles. J. Am. Chem. Soc. 2023, 145, 17621–17631. [Google Scholar] [CrossRef] [PubMed]
- Radunz, S.; Schavkan, A.; Wahl, S.; Würth, C.; Tschiche, H.R.; Krumrey, M.; Resch-Genger, U. Evolution of Size and Optical Properties of Upconverting Nanoparticles during High-Temperature Synthesis. J. Phys. Chem. C 2018, 122, 28958–28967. [Google Scholar] [CrossRef]
- Kulkarni, M.B.; Goel, S. Microfluidic devices for synthesizing nanomaterials—A review. Nano Express 2020, 1, 032004. [Google Scholar] [CrossRef]
- Li, X.; Shen, D.; Yang, J.; Yao, C.; Che, R.; Zhang, F.; Zhao, D. Successive Layer-by-Layer Strategy for Multi-Shell Epitaxial Growth: Shell Thickness and Doping Position Dependence in Upconverting Optical Properties. Chem. Mater. 2012, 25, 106–112. [Google Scholar] [CrossRef]
- Johnson, N.J.J.; Korinek, A.; Dong, C.; van Veggel, F.C.J.M. Self-Focusing by Ostwald Ripening: A Strategy for Layer-by-Layer Epitaxial Growth on Upconverting Nanocrystals. J. Am. Chem. Soc. 2012, 134, 11068–11071. [Google Scholar] [CrossRef] [PubMed]
- Guo, H.; Li, Z.; Qian, H.; Hu, Y.; Muhammad, I.N. Seed-mediated synthesis of NaYF4: Yb, Er/NaGdF4 nanocrystals with improved upconversion fluorescence and MR relaxivity. Nat. Nanotechnol. 2010, 21, 125602. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Peng, D.; Ju, Q.; Wang, F. Photon upconversion in core–shell nanoparticles. Chem. Soc. Rev. 2015, 44, 1318–1330. [Google Scholar] [CrossRef] [PubMed]
- Hudry, D.; De Backer, A.; Popescu, R.; Busko, D.; Howard, I.A.; Bals, S.; Zhang, Y.; Pedrazo-Tardajos, A.; Van Aert, S.; Gerthsen, D.; et al. Interface Pattern Engineering in Core-Shell Upconverting Nanocrystals: Shedding Light on Critical Parameters and Consequences for the Photoluminescence Properties. Small 2021, 17, e2104441. [Google Scholar] [CrossRef] [PubMed]
- Dühnen, S.; Haase, M. Study on the Intermixing of Core and Shell in NaEuF4/NaGdF4 Core/Shell Nanocrystals. Chem. Mater. 2015, 27, 8375–8386. [Google Scholar] [CrossRef]
- Liu, L.; Li, X.; Fan, Y.; Wang, C.; El-Toni, A.M.; Alhoshan, M.S.; Zhao, D.; Zhang, F. Elemental Migration in Core/Shell Structured Lanthanide Doped Nanoparticles. Chem. Mater. 2019, 31, 5608–5615. [Google Scholar] [CrossRef]
- Chan, E.M.; Levy, E.S.; Cohen, B.E. Rationally Designed Energy Transfer in Upconverting Nanoparticles. Adv. Mater. 2015, 27, 5753–5761. [Google Scholar] [CrossRef] [PubMed]
- Lei, Z.; Ling, X.; Mei, Q.; Fu, S.; Zhang, J.; Zhang, Y. An Excitation Navigating Energy Migration of Lanthanide Ions in Upconversion Nanoparticles. Adv. Mater. 2020, 32, 1906225. [Google Scholar] [CrossRef] [PubMed]
- Hudry, D.; Busko, D.; Popescu, R.; Gerthsen, D.; Abeykoon, A.M.M.; Kübel, C.; Bergfeldt, T.; Richards, B.S. Direct Evidence of Significant Cation Intermixing in Upconverting Core@Shell Nanocrystals: Toward a New Crystallochemical Model. Chem. Mater. 2017, 29, 9238–9246. [Google Scholar] [CrossRef]
- Zhong, Y.; Tian, G.; Gu, Z.; Yang, Y.; Gu, L.; Zhao, Y.; Ma, Y.; Yao, J. Elimination of Photon Quenching by a Transition Layer to Fabricate a Quenching-Shield Sandwich Structure for 800 nm Excited Upconversion Luminescence of Nd3+-Sensitized Nanoparticles. Adv. Mater. 2013, 26, 2831–2837. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Zhou, M.; Zhou, M.-T.; Wei, H.-L.; Su, Y.; Su, Q. Simultaneous ultraviolet-C and near-infrared enhancement in heterogeneous lanthanide nanocrystals. Nanoscale 2022, 14, 4595–4603. [Google Scholar] [CrossRef]
- Zhou, B.; Yan, L.; Huang, J.; Liu, X.; Tao, L.; Zhang, Q. NIR II-responsive photon upconversion through energy migration in an ytterbium sublattice. Nat. Photonics 2020, 14, 760–766. [Google Scholar] [CrossRef]
- Chan, E.M.; Han, G.; Goldberg, J.D.; Gargas, D.J.; Ostrowski, A.D.; Schuck, P.J.; Cohen, B.E.; Milliron, D.J. Combinatorial Discovery of Lanthanide-Doped Nanocrystals with Spectrally Pure Upconverted Emission. Nano Lett. 2012, 12, 3839–3845. [Google Scholar] [CrossRef] [PubMed]
- Zhu, X.; Liu, X.; Zhang, H.; Zhao, M.; Pei, P.; Chen, Y.; Yang, Y.; Lu, L.; Yu, P.; Sun, C.; et al. High-Fidelity NIR-II Multiplexed Lifetime Bioimaging with Bright Double Interfaced Lanthanide Nanoparticles. Angew. Chem. Int. Ed. Engl. 2021, 60, 23545–23551. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.; Li, K.L.; Dai, M.; Fu, Z. Towards core–shell engineering for efficient luminescence and temperature sensing. J. Colloid Interface Sci. 2024, 673, 249–257. [Google Scholar] [CrossRef] [PubMed]
- Lin, H.; Shen, Y.; Chen, D.; Lin, L.; Wilson, B.C.; Li, B.; Xie, S. Feasibility Study on Quantitative Measurements of Singlet Oxygen Generation Using Singlet Oxygen Sensor Green. J. Fluoresc. 2012, 23, 41–47. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhou, Z.; Liu, Y.; Guo, L.; Wang, T.; Yan, X.; Wei, S.; Qiu, D.; Chen, D.; Zhang, X.; Ju, H. Core–Shell Interface Engineering Strategies for Modulating Energy Transfer in Rare Earth-Doped Nanoparticles. Nanomaterials 2024, 14, 1326. https://doi.org/10.3390/nano14161326
Zhou Z, Liu Y, Guo L, Wang T, Yan X, Wei S, Qiu D, Chen D, Zhang X, Ju H. Core–Shell Interface Engineering Strategies for Modulating Energy Transfer in Rare Earth-Doped Nanoparticles. Nanomaterials. 2024; 14(16):1326. https://doi.org/10.3390/nano14161326
Chicago/Turabian StyleZhou, Zhaoxi, Yuan Liu, Lichao Guo, Tian Wang, Xinrong Yan, Shijiong Wei, Dehui Qiu, Desheng Chen, Xiaobo Zhang, and Huangxian Ju. 2024. "Core–Shell Interface Engineering Strategies for Modulating Energy Transfer in Rare Earth-Doped Nanoparticles" Nanomaterials 14, no. 16: 1326. https://doi.org/10.3390/nano14161326







