Investigation of the Crystallographic Evolution Sequence of Nano-Precipitation via HRTEM in Cu-Bearing Ultra-Low Carbon Steel
Abstract
1. Introduction
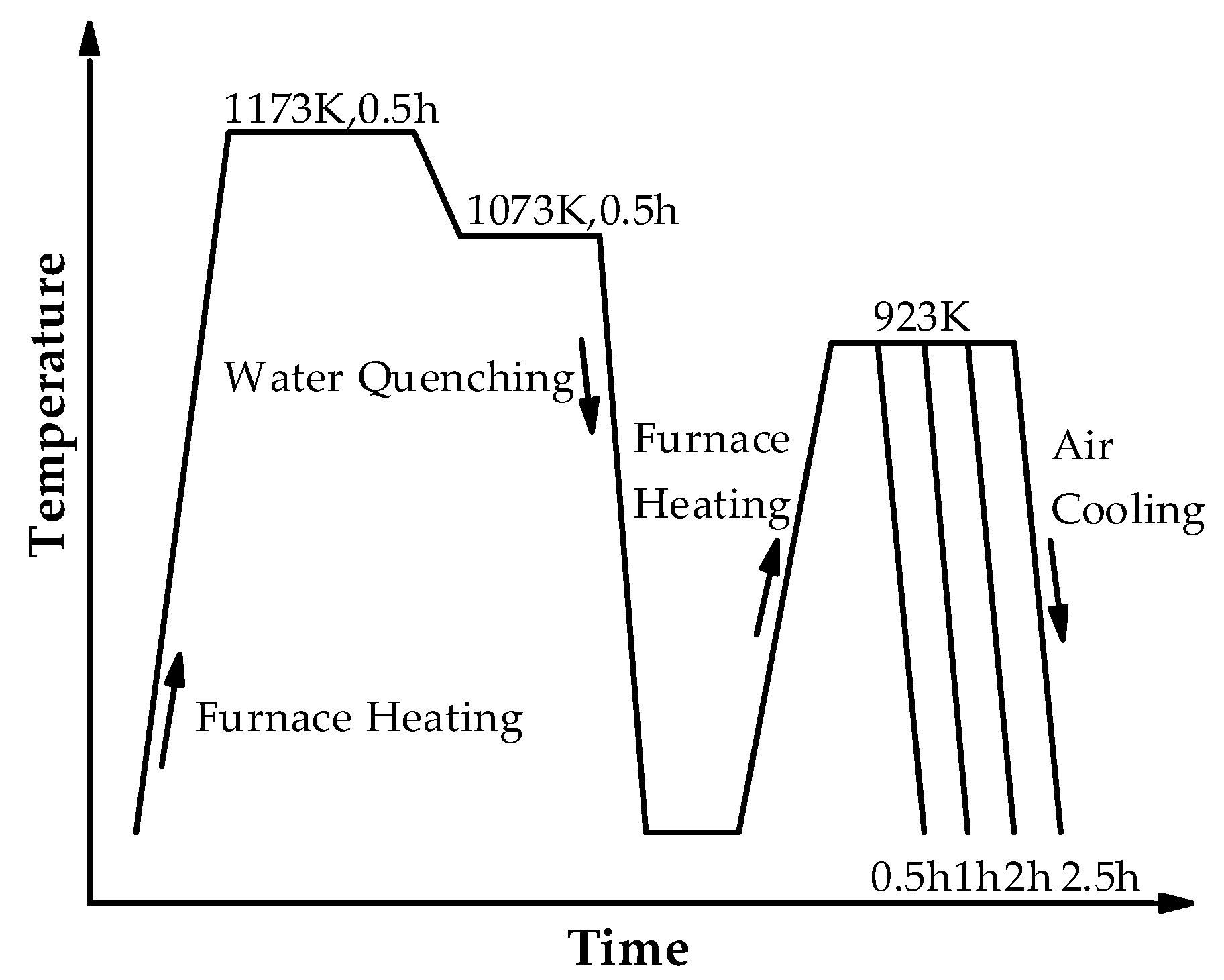
2. Materials and Methods
3. Results and Discussion
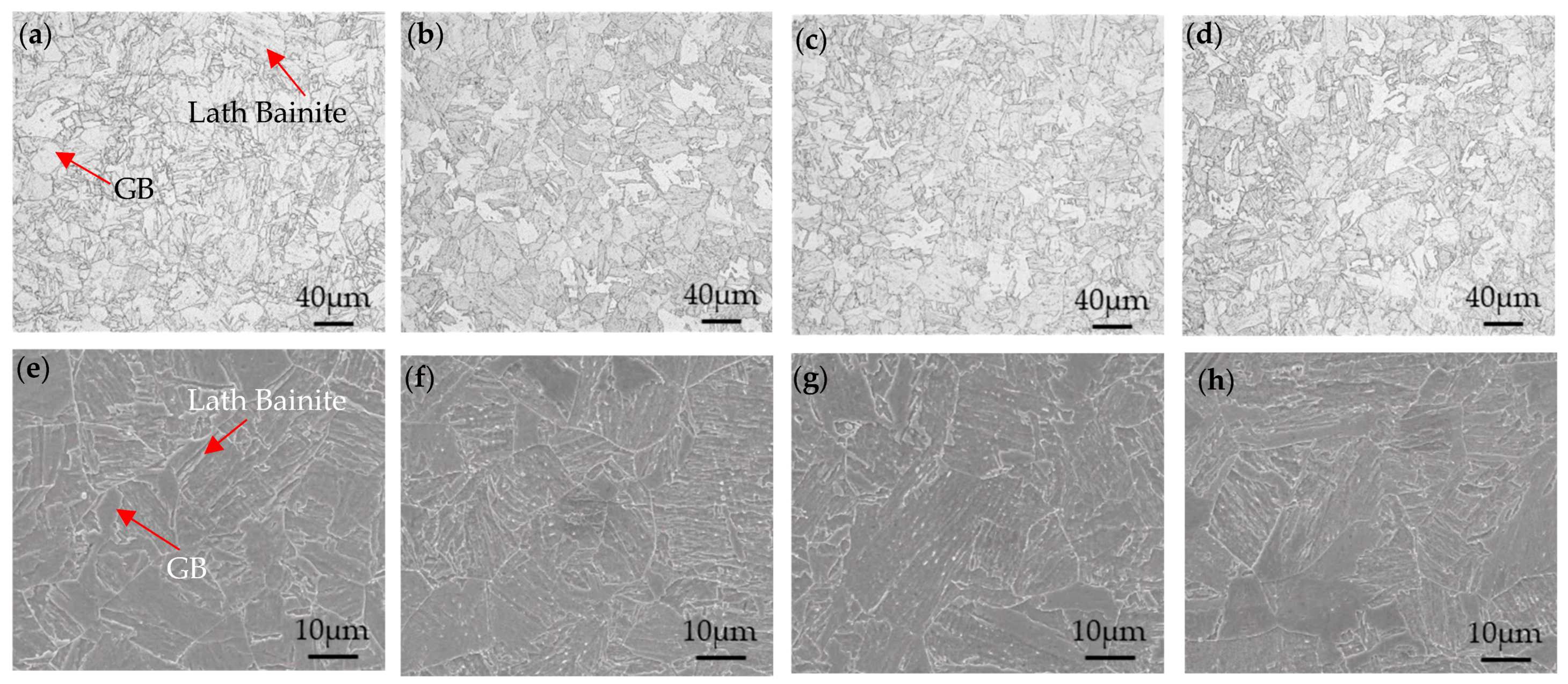
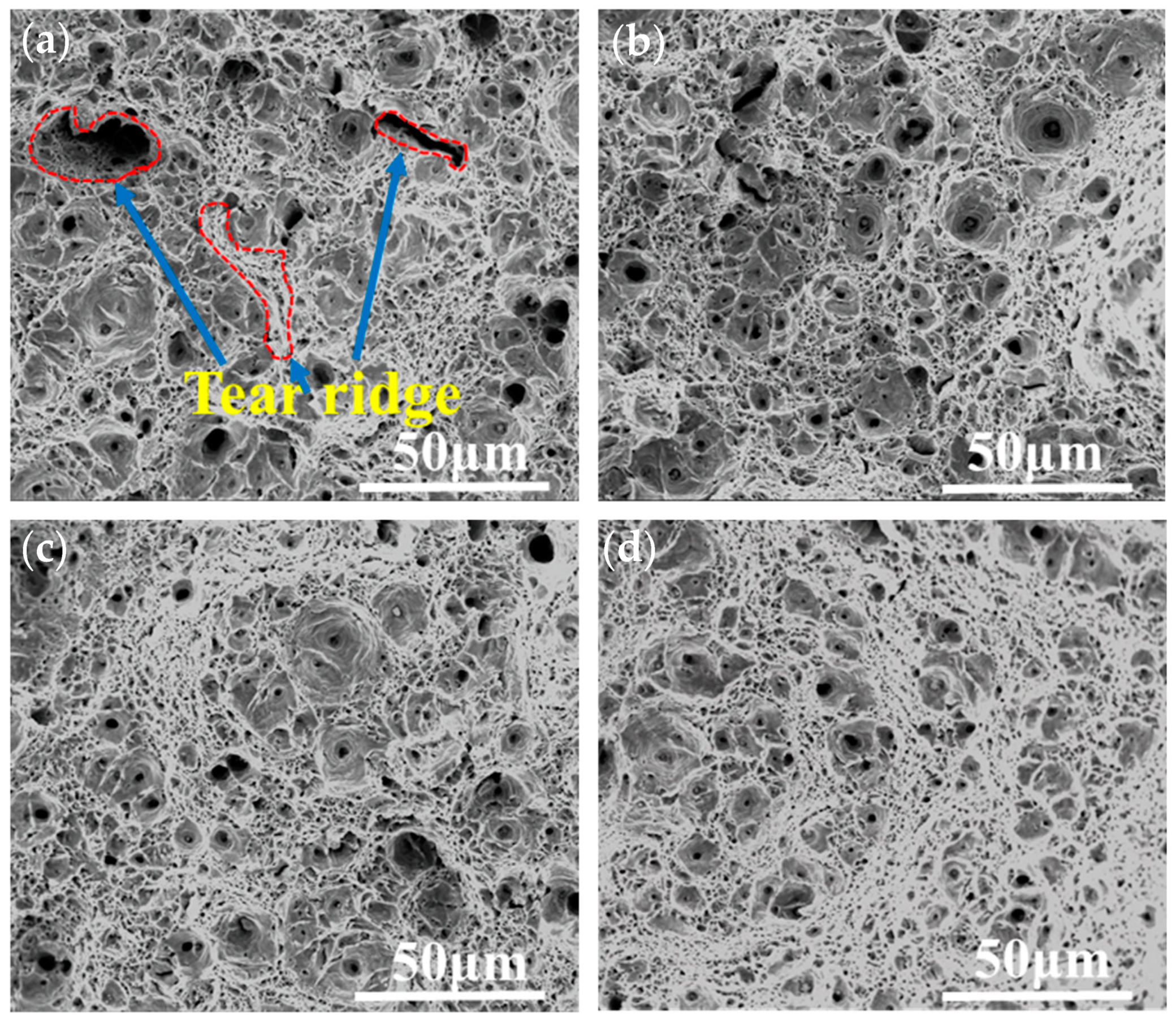
3.1. Microstructure Characterization
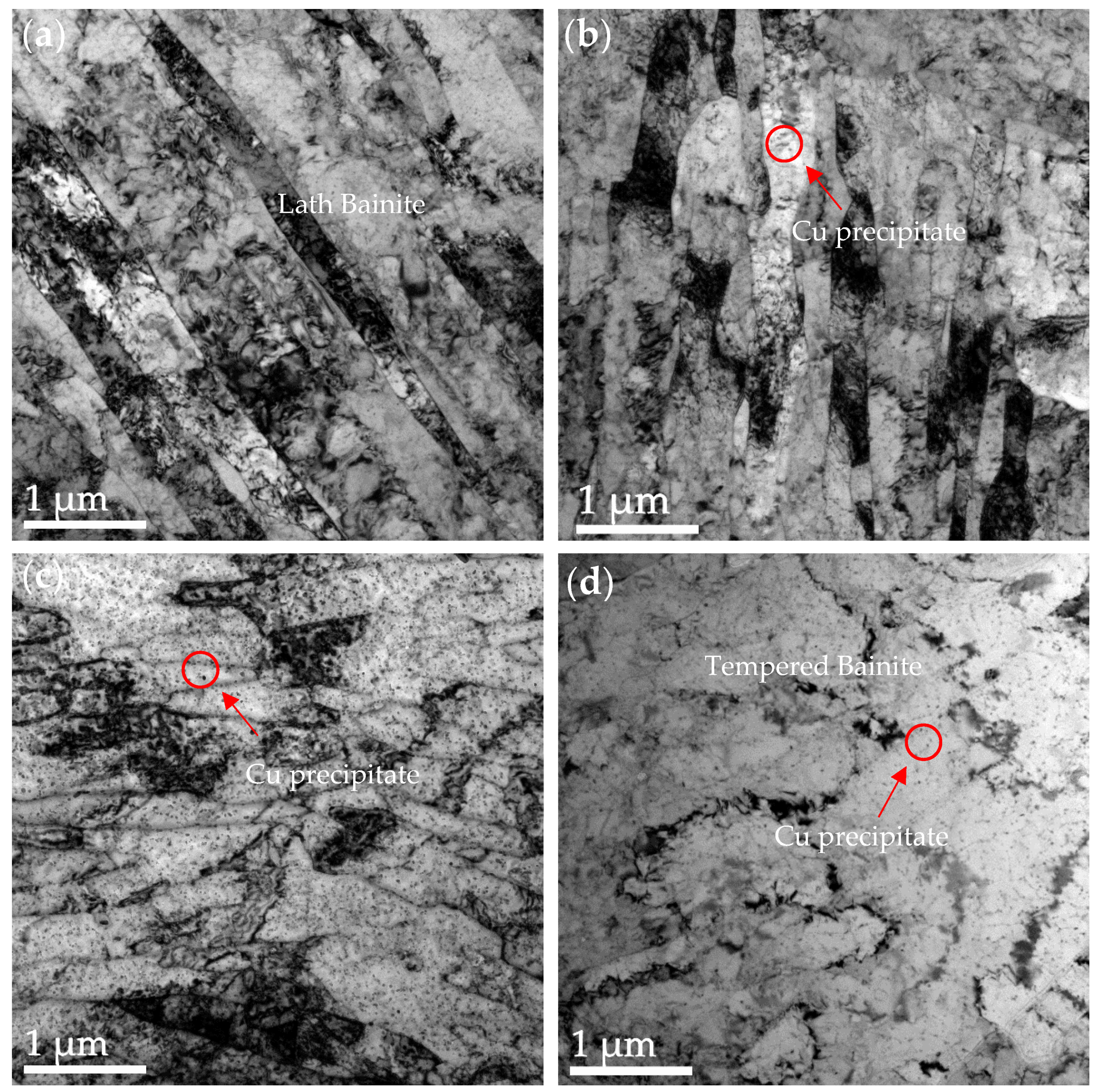
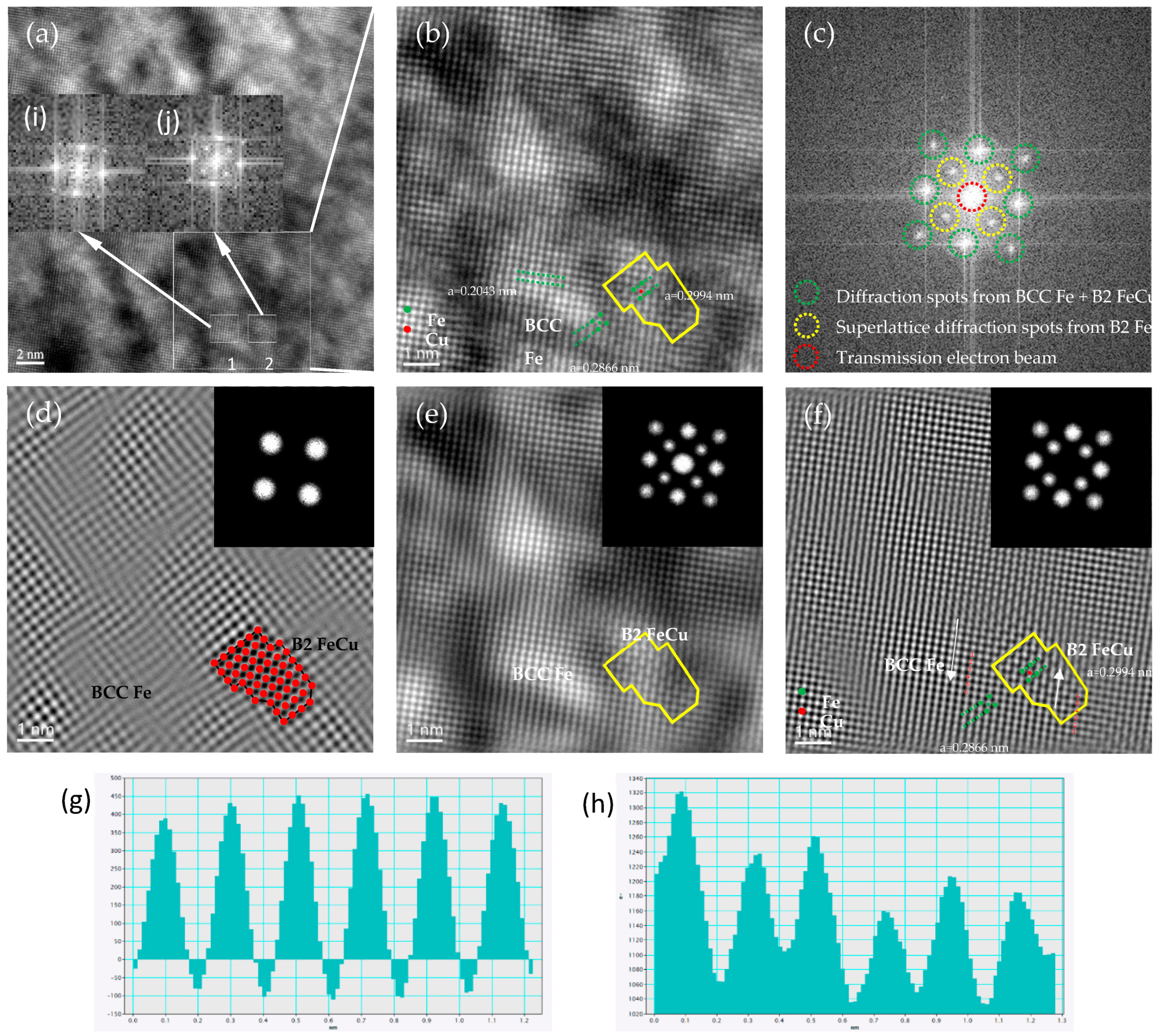
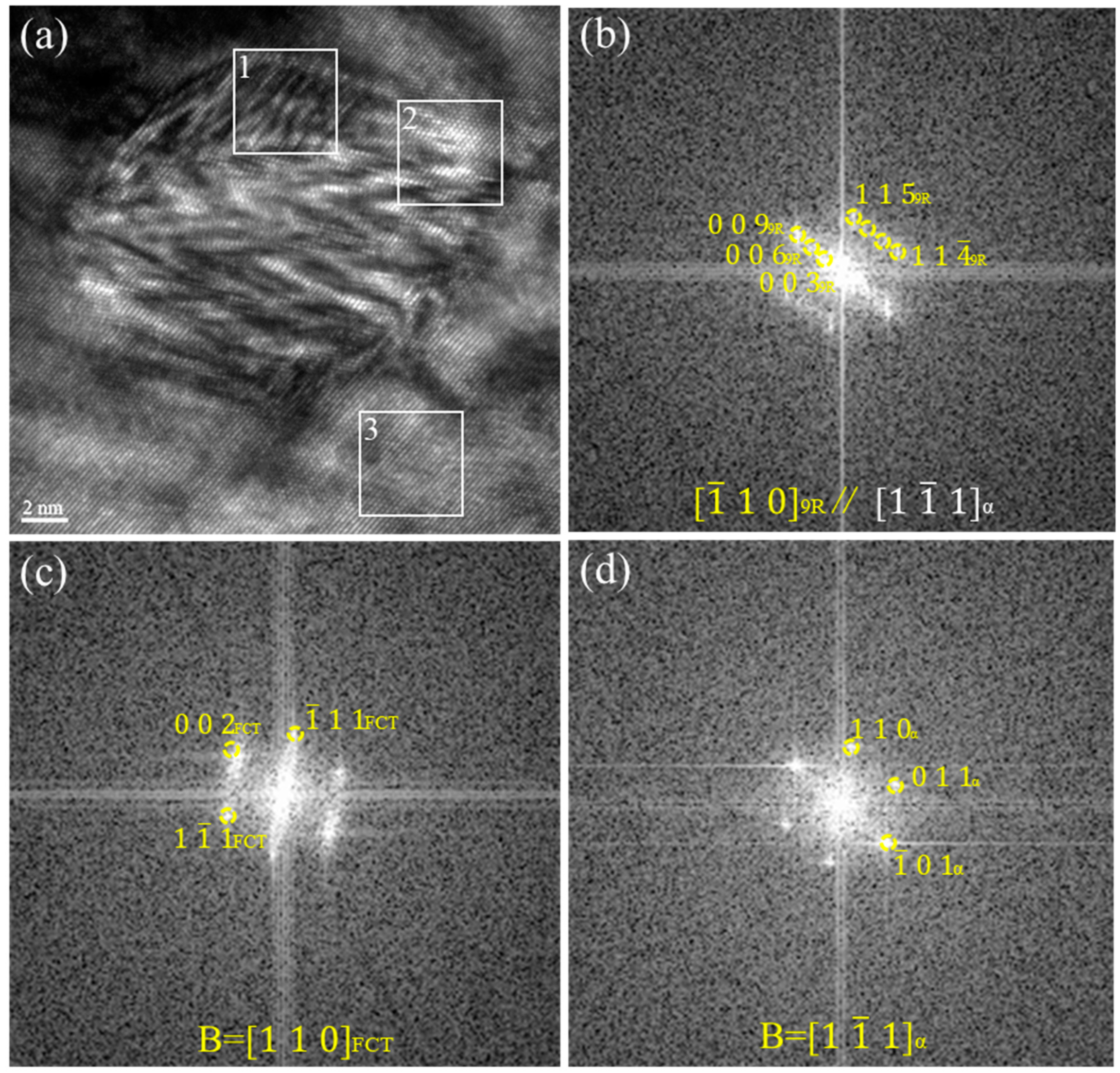
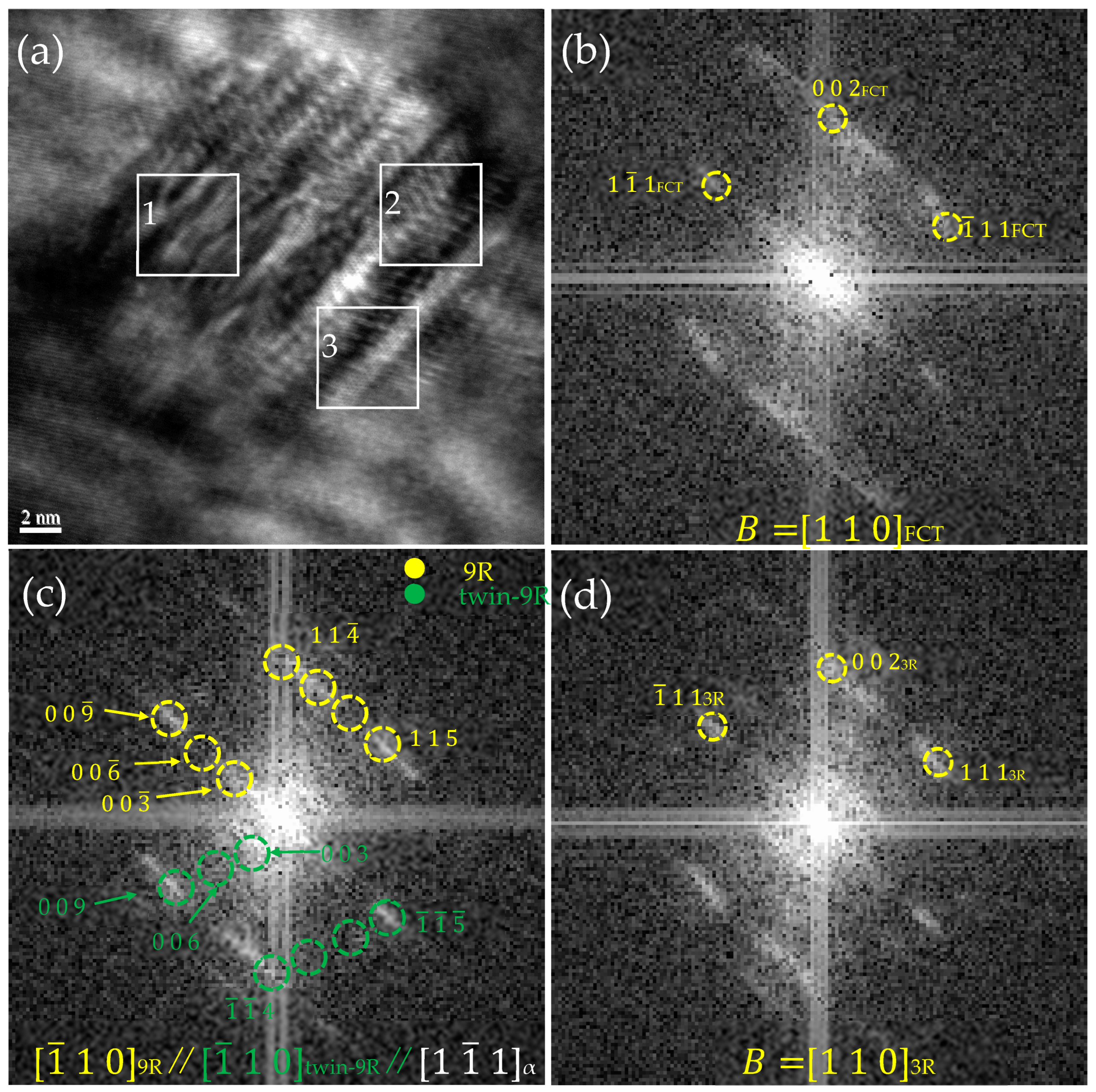
3.2. HRTEM Observations
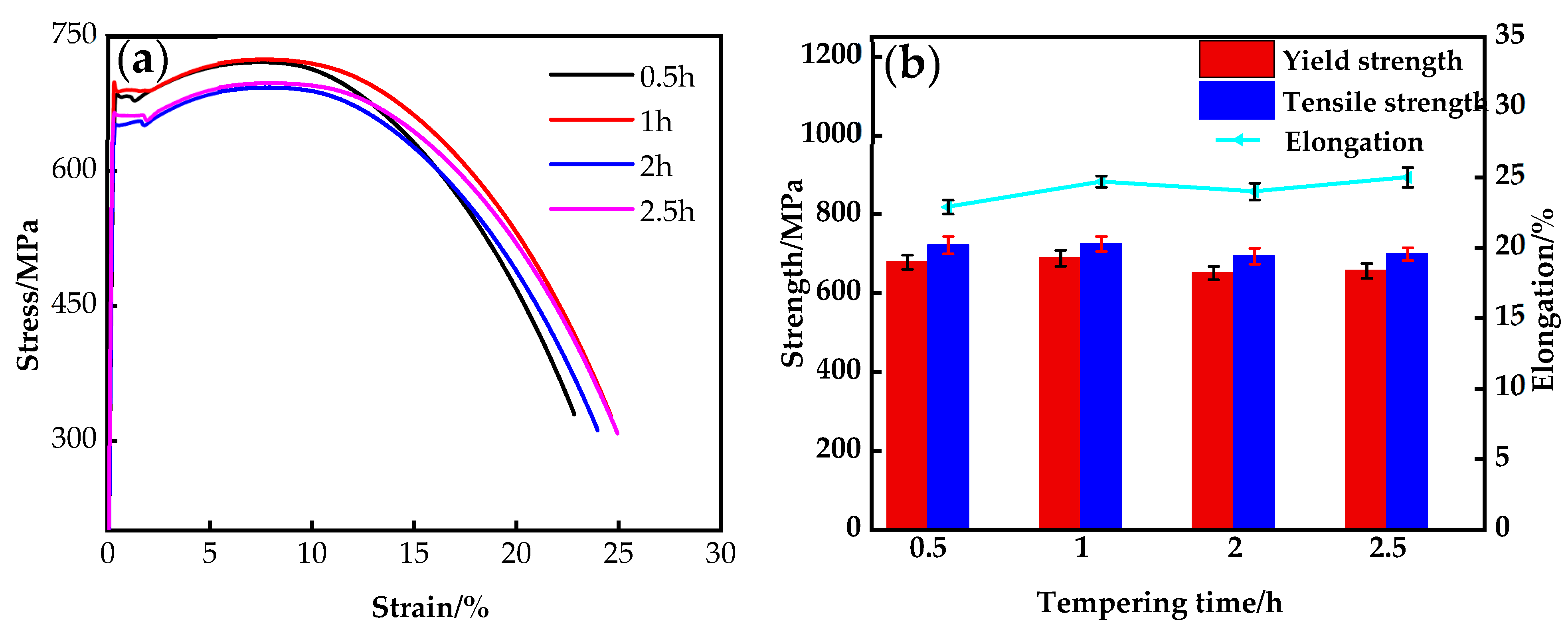
3.3. Mechanical Properties
3.4. The Crystallographic Evolution Sequence
3.5. Influence of Processing on Precipitation
4. Conclusions
- (1)
- There were two crystallographic evolution paths for the morphology and crystal structure changes during the precipitation process: B2→multi twin 9R→detwined 9R→FCT→FCC and B2→multi-twin 9R→detwinned 9R→3R→FCT→FCC. Through the simultaneous existence of 9R, 3R, and FCT structures in the same precipitated particles, it was proven that the evolution sequence of precipitates in Cu-bearing ultra-low carbon steel could have a stable 3R structure.
- (2)
- In addition, the morphology of the precipitated particles during the growth process changed from spherical to ellipsoidal and then to rod-shaped. By observing the nano-precipitates during different heat treatment processes, the structures of B2, 9R, 3R, and FCT were accurately determined.
- (3)
- By analyzing the microstructure and mechanical properties of the steel at different heat treatment processes, it was found that nano-precipitation could effectively improve the yield strength of Cu-bearing ultra-low carbon steel. Statistical analysis of the size and distribution characteristics of precipitation particles revealed that the yield strength of the steel reached its maximum when the precipitation size was mainly 9R structure between 12 and 26 nm.
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zhang, Z.W.; Liu, C.T.; Wen, Y.R.; Hirata, A.; Guo, S.; Chen, G.; Chen, M.W.; Chin, B.A. Influence of aging and thermomechanical treatments on the mechanical properties of a nanocluster-strengthened ferritic steel. Metall. Mater. Trans. A 2012, 43, 351–358. [Google Scholar] [CrossRef]
- Nizinkovskyi, R.; Halle, T.; Krüger, M. Investigation of the equilibrium morphology of fcc ϵ-Cu in Fe -Cu alloys using a non-local Allen–Cahn model. Mater. Today Commun. 2024, 38, 107635. [Google Scholar] [CrossRef]
- Misra, R.D.K.; Jia, Z.; O’Malley, R.; Jansto, S.J. Precipitation behavior during thin slab thermomechanical processing and isothermal aging of copper-bearing niobium-microalloyed highstrength structural steels: The effect on mechanical properties. Mater. Sci. Eng. A 2011, 528, 8772–8780. [Google Scholar] [CrossRef]
- Momeni, A.; Arabi, H.; Rezaei, A.; Badri, H.; Abbasi, S.M. Hot deformation behavior of austenite in HSLA-100 microalloyed steel. Mater. Sci. Eng. A 2011, 528, 2158–2163. [Google Scholar] [CrossRef]
- Jung, J.G.; Jung, M.; Lee, S.M.; Shin, E.; Shin, H.C.; Lee, Y.K. Cu precipitation kinetics during martensite tempering in a medium C steel. J. Alloys. Compd. 2013, 553, 299–307. [Google Scholar] [CrossRef]
- Yayla, P.; Kaluc, E.; Ural, K. Effects of welding processes on the mechanical properties of HY 80steel weldments. Mater. Des. 2007, 28, 1898–1906. [Google Scholar] [CrossRef]
- Osamura, K.; Okuda, H.; Takashima, M.; Asano, K.; Furusaka, M. Small-Angle Neutron Scattering Study of Phase Decomposition in Fe–Cu Binary Alloy. Mater. Trans. 1993, 34, 305. [Google Scholar] [CrossRef]
- Chen, C.; Thompson, A.W.; Bernstein, I.M. The correlation of microstructure and stress corrosion fracture of HY-130 steel weldments. Metall. Trans. A 1980, 11, 1723–1730. [Google Scholar] [CrossRef]
- Mulholland, M.D.; Seidman, D.N. Nanoscale co-precipitation and mechanical properties of ahigh-strength low-carbon steel. Acta Mater. 2011, 59, 1881–1897. [Google Scholar] [CrossRef]
- Xu, C.; Dai, W.J.; Chen, Y.; Qi, Z.X.; Zheng, G.; Cao, Y.D.; Zhang, J.P.; Bu, C.C.; Chen, G. Control of dislocation density maximizing precipitation strengthening effect. J. Mater. Sci. Technol. 2022, 127, 133–143. [Google Scholar] [CrossRef]
- Li, Y.Z.; Shen, Y.F.; Zhao, S.X.; Zhang, W.N.; Xue, W.Y. Strengthening a Medium-Carbon Low-Alloy Steel by Nanosized Grains: The Role of Asymmetrical Rolling. Nanomaterials 2023, 13, 956. [Google Scholar] [CrossRef] [PubMed]
- Yin, G.Q.; Yang, C.F.; Lu, Y.N. HREM Observation of Age-Precipitated Particles in Practical Cu-bearing Ultra-Low Carbon Steels. Mater. Sci. Technol. 2010, 26, 433–438. [Google Scholar] [CrossRef]
- Yoo, J.Y.; Choo, W.Y.; Park, T.W.; Kim, Y.W. Microstructures and age hardening characteristics of direct quenched Cu bearing HSLA steel. ISIJ Int. 1995, 35, 1034–1040. [Google Scholar] [CrossRef]
- Piyada, S.; Margarita, B.; Atipong, B.; Wutthigrai, S. Local Structure Investigation of Cu Precipitates in Modified18CrNiMo7-6 Steels by Synchrotron X-ray Absorption Spectroscopy. ISIJ Int. 2022, 62, 977–983. [Google Scholar] [CrossRef]
- Lee, T.H.; Kim, Y.O.; Kim, S.J. Crystallographic model for bcc-to-9R martensitic transformation of Cu precipitates in ferritic steel. Philos. Mag. 2007, 87, 209–224. [Google Scholar] [CrossRef]
- Han, G.; Xie, Z.J.; Li, Z.Y.; Lei, B.; Shang, C.J.; Misra, R.D.K. Evolution of crystal structure of Cu precipitates in a low carbon steel. Mater. Des. 2017, 135, 92–101. [Google Scholar] [CrossRef]
- Wen, Y.R.; Li, Y.P.; Hirata, A.; Zhang, Y.; Fujita, T.; Liu, C.T.; Chiba, A.; Chen, M.W. Synergistic alloying effect on microstructural evolution and mechanical properties of Cu precipitation-strengthened ferritic alloys. Acta Mater. 2013, 61, 7726–7740. [Google Scholar] [CrossRef]
- Kapoor, M.; Isheim, D.; Vaynman, S.; Fine, M.E.; Chung, Y.W. Effects of increased alloying element content on NiAl-type precipitate formation, loading rate sensitivity and ductility of Cu- and NiAl-precipitation-strengthened ferritic steels. Acta Mater. 2016, 104, 166–171. [Google Scholar] [CrossRef]
- Zhang, Z.W.; Liu, C.T.; Wang, X.L.; Miller, M.K.; Ma, D.; Chen, G.; Williams, J.R.; Chin, B.A. Effects of proton irradiation on nanocluster precipitation in ferritic steel containing fcc alloying additions. Acta Mater. 2012, 60, 3034–3046. [Google Scholar] [CrossRef]
- Hwang, G.C.; Lee, S.; Yoo, J.Y.; Choo, W.Y. Effect of direct quenching on microstructure and mechanical properties of copper-bearing high-strength alloy steels. Mater. Sci. Eng. A 1998, 252, 256–268. [Google Scholar] [CrossRef]
- Ray, P.K.; Ganguly, R.I.; Panda, A.K. Optimization of mechanical properties of an HSLA-100 steel through control of heat treatment variables. Mater. Sci. Eng. A 2003, 346, 122–131. [Google Scholar] [CrossRef]
- Ghosh, A.; Mishra, B.; Das, S.; Chatterjee, S. An ultra low carbon Cu bearing steel: Influence of thermomechanical processing and aging heat treatment on structure and properties. Mater. Sci. Eng. A 2004, 374, 43–55. [Google Scholar] [CrossRef]
- Han, G.; Xie, Z.J.; Lei, B.; Liu, W.Q.; Zhu, H.H.; Yan, Y.; Misra, R.D.K.; Shang, C.J. Simultaneous enhancement of strength and plasticity by nano B2 clusters and nano-γ phase in a low carbon low alloy steel. Mater. Sci. Eng. A 2018, 730, 119. [Google Scholar] [CrossRef]
- Yu, Y.; Hu, B.; Gao, M.; Xie, Z.; Rong, X.; Han, G.; Guo, H.; Shang, C. Determining role of heterogeneous microstructure in lowering yield ratio and enhancing impact toughness in high-strength low-alloy steel. Int. J. Miner. Metall. Mater. 2021, 28, 816–825. [Google Scholar] [CrossRef]
- Othen, P.J.; Jenkins, M.L.; Smith, G.D.W.; Phythian, W.J. Transmission electron microscope investigations of the structure of copper precipitates in thermally-aged Fe—Cu and Fe—Cu—Ni. Philos. Mag. Lett. 1991, 64, 383–391. [Google Scholar] [CrossRef]
- Wang, W.; Zhou, B.; Xu, G.; Chu, D.; Peng, J. High-resolution electron microscopy characterization of 2H and 9R variant in the ferritic steels containing copper. Mater. Charact. 2011, 62, 438. [Google Scholar] [CrossRef]
- Othen, P.J.; Jenkins, M.L.; Smith, G.D.W. High-resolution electron microscopy studies of the structure of Cu precipitates in α-Fe. Philos. Mag. A 1994, 70, 1. [Google Scholar] [CrossRef]
- Hardouin Duparc, H.A.; Doole, R.C.; Jenkins, M.L.; Barbu, A. A high-resolution electron microscopy study of copper precipitation in Fe-1.5 wt% Cu under electron irradiation. Philos. Mag. Lett. 1995, 71, 325. [Google Scholar] [CrossRef]
- He, S.M.; Van Dijk, N.H.; Paladugu, M.; Schut, H.; Kohlbrecher, J.; Tichelaar, F.D.; van der Zwaag, S. In situ determination of aging precipitation in deformed Fe-Cu and Fe-Cu-B-N alloys by time-resolved small-angle neutron scattering. Phys. Rev. B. 2010, 82, 174111. [Google Scholar] [CrossRef]
- Sun, M.; Zhang, W.; Liu, Z.; Wang, G. Direct observations on the crystal structure evolution of nano Cu-precipitates in an extremely low carbon steel. Mater. Lett. 2017, 15, 49–52. [Google Scholar] [CrossRef]
- Habibi, H.R. Atomic structure of the Cu precipitates in two stages hardening in maraging steel. Mater. Lett. 2005, 59, 1824–1827. [Google Scholar] [CrossRef]
- Bajguirani, H.R.H.; Jenkins, M.L. High-resolution electron microscopy analysis of the structure of copper precipitates in a martensitic stainless steel of type PH 15-5. Philos. Mag. Lett. 1996, 73, 155–162. [Google Scholar] [CrossRef]
- Monzen, R.; Jenkins, M.L.; Sutton, A.P. The bcc-to-9R martensitic transformation of Cu precipitates and the relaxation process of elastic strains in an Fe-Cu alloy. Philos. Mag. A. 2000, 80, 711–723. [Google Scholar] [CrossRef]
- Blackstock, J.J.; Ackland, G.J. Phase transitions of copper precipitates in Fe–Cu alloys. Philos. Mag. A 2001, 81, 2127–2148. [Google Scholar] [CrossRef]
- Monzen, R.; Iguchi, M.; Jenkins, M.L. Structural changes of 9R copper precipitates in an aged Fe-Cu alloy. Philos. Mag. Lett. 2000, 80, 137–148. [Google Scholar] [CrossRef]
- Heo, Y.U.; Kim, Y.K.; Kim, J.S.; Kim, J.K. Phase transformation of Cu precipitates from bcc to fcc in Fe–3Si–2Cu alloy. Acta Mater. 2013, 61, 519–528. [Google Scholar] [CrossRef]
- Shi, L.; Yan, Z.; Liu, Y. Improved toughness and ductility in ferrite/acicular ferrite dual-phase steel through intercritical heat treatment. Mater. Sci. Eng. A 2014, 590, 7–15. [Google Scholar] [CrossRef]
- Jiang, J.; Wu, H.; Liang, J. Microstructural characterization and impact toughness of a jackup rig rack steel treated by intercritical heat treatment. Mater. Sci. Eng. A 2013, 587, 359–364. [Google Scholar] [CrossRef]
- Liu, Y.; Shi, L.; Liu, C. Effect of step quenching on microstructures and mechanical properties of HSLA steel. Mater. Sci. Eng. A 2016, 675, 371–378. [Google Scholar] [CrossRef]
- Park, K.S.; Park, K.; Lee, D.L. Effect of heat treatment path on the cold formability of drawn dual-phase steels. Mater. Sci. Eng. A 2007, 449, 1135–1138. [Google Scholar] [CrossRef]
- Jefferson, Z.; Liu, A.; Walle, V.; Ghosh, G.; Asta, M. Structure, energeticsand mechanical stability of Fe-Cu bcc alloys from first-principles calculations. Phys. Rev. B 2005, 72, 144109. [Google Scholar] [CrossRef]
- Wang, J.; Zhou, L.; Kawazoe, Y. Ab initio studies on the structural and magnetic properties of FeCu superlattices. Phys. Rev. B 1999, 60, 3025. [Google Scholar] [CrossRef]
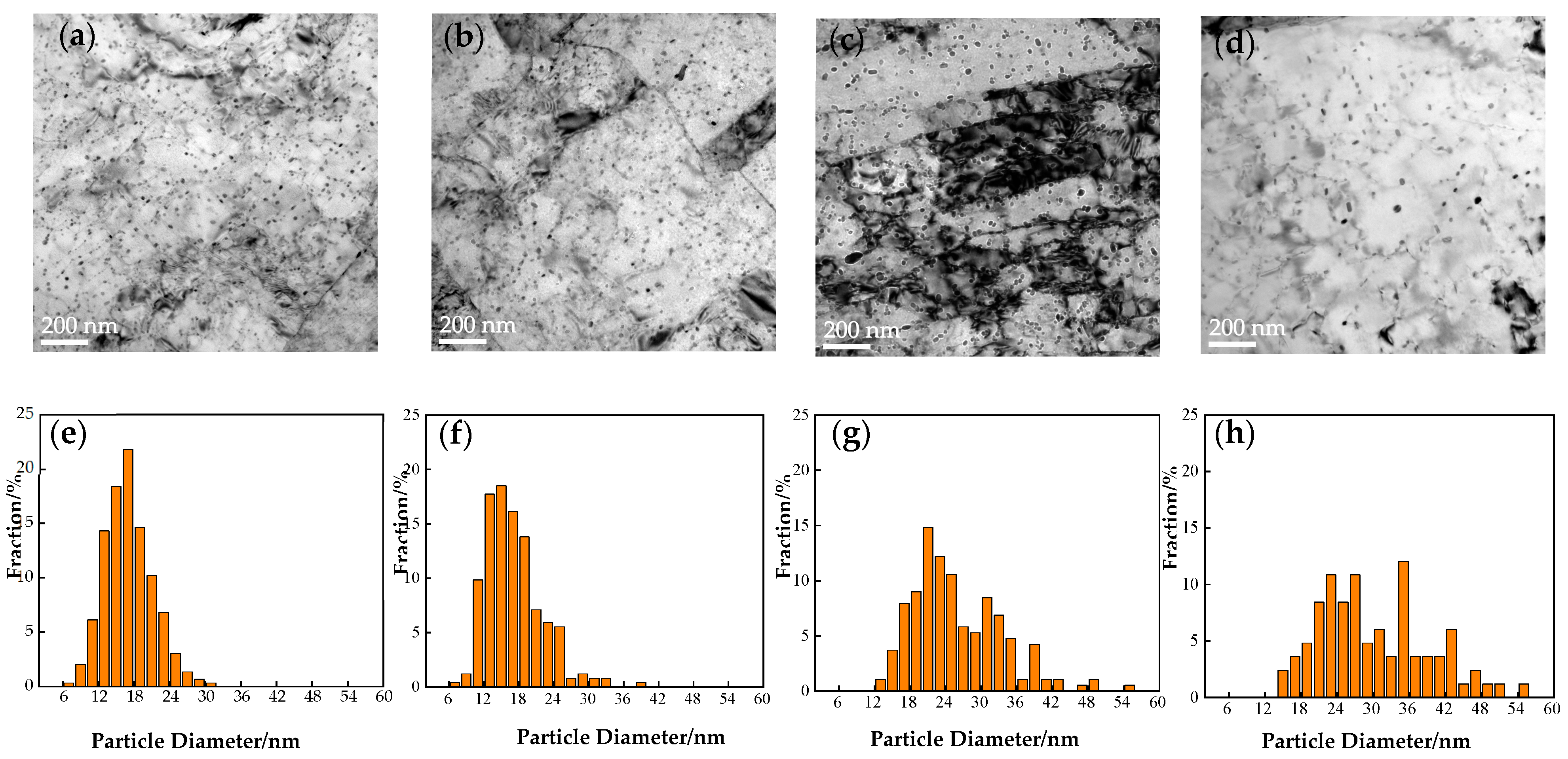


| Heat Treatment Time/h | Average Size/nm | Number Density/m−3 |
|---|---|---|
| 0.5 | 18.5 ± 3.18 | (2.08 ± 0.5) × 1021 |
| 1 | 18.6 ± 2.9 | (1.78 ± 0.43) × 1021 |
| 2 | 28.4 ± 4.8 | (1.59 ± 0.39) × 1021 |
| 2.5 | 29.2 ± 5.38 | (8.36 ± 2.04) × 1020 |
| Tempering Time/h | YS/MPa | UTS/MPa | Elongation/% |
|---|---|---|---|
| 0.5 h | 678 | 721 | 22.9 |
| 1 h | 688 | 724 | 24.7 |
| 2 h | 650 | 693 | 24 |
| 2.5 h | 656 | 698 | 25 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, W.; Gao, Z.; Wei, H.; Zhang, H.; Chen, Z.; Xue, W.; Shen, Y.; Liu, Z. Investigation of the Crystallographic Evolution Sequence of Nano-Precipitation via HRTEM in Cu-Bearing Ultra-Low Carbon Steel. Nanomaterials 2024, 14, 1335. https://doi.org/10.3390/nano14161335
Zhang W, Gao Z, Wei H, Zhang H, Chen Z, Xue W, Shen Y, Liu Z. Investigation of the Crystallographic Evolution Sequence of Nano-Precipitation via HRTEM in Cu-Bearing Ultra-Low Carbon Steel. Nanomaterials. 2024; 14(16):1335. https://doi.org/10.3390/nano14161335
Chicago/Turabian StyleZhang, Weina, Zhanjie Gao, Hao Wei, Huimin Zhang, Zejin Chen, Wenying Xue, Yongfeng Shen, and Zhenyu Liu. 2024. "Investigation of the Crystallographic Evolution Sequence of Nano-Precipitation via HRTEM in Cu-Bearing Ultra-Low Carbon Steel" Nanomaterials 14, no. 16: 1335. https://doi.org/10.3390/nano14161335
APA StyleZhang, W., Gao, Z., Wei, H., Zhang, H., Chen, Z., Xue, W., Shen, Y., & Liu, Z. (2024). Investigation of the Crystallographic Evolution Sequence of Nano-Precipitation via HRTEM in Cu-Bearing Ultra-Low Carbon Steel. Nanomaterials, 14(16), 1335. https://doi.org/10.3390/nano14161335








