Optimization of Cyanide-Free Composite Electrodeposition Based on π-π Interactions Preparation of Silver-Graphene Composite Coatings for Electrical Contact Materials
Abstract
:1. Introduction
2. Materials and Methods
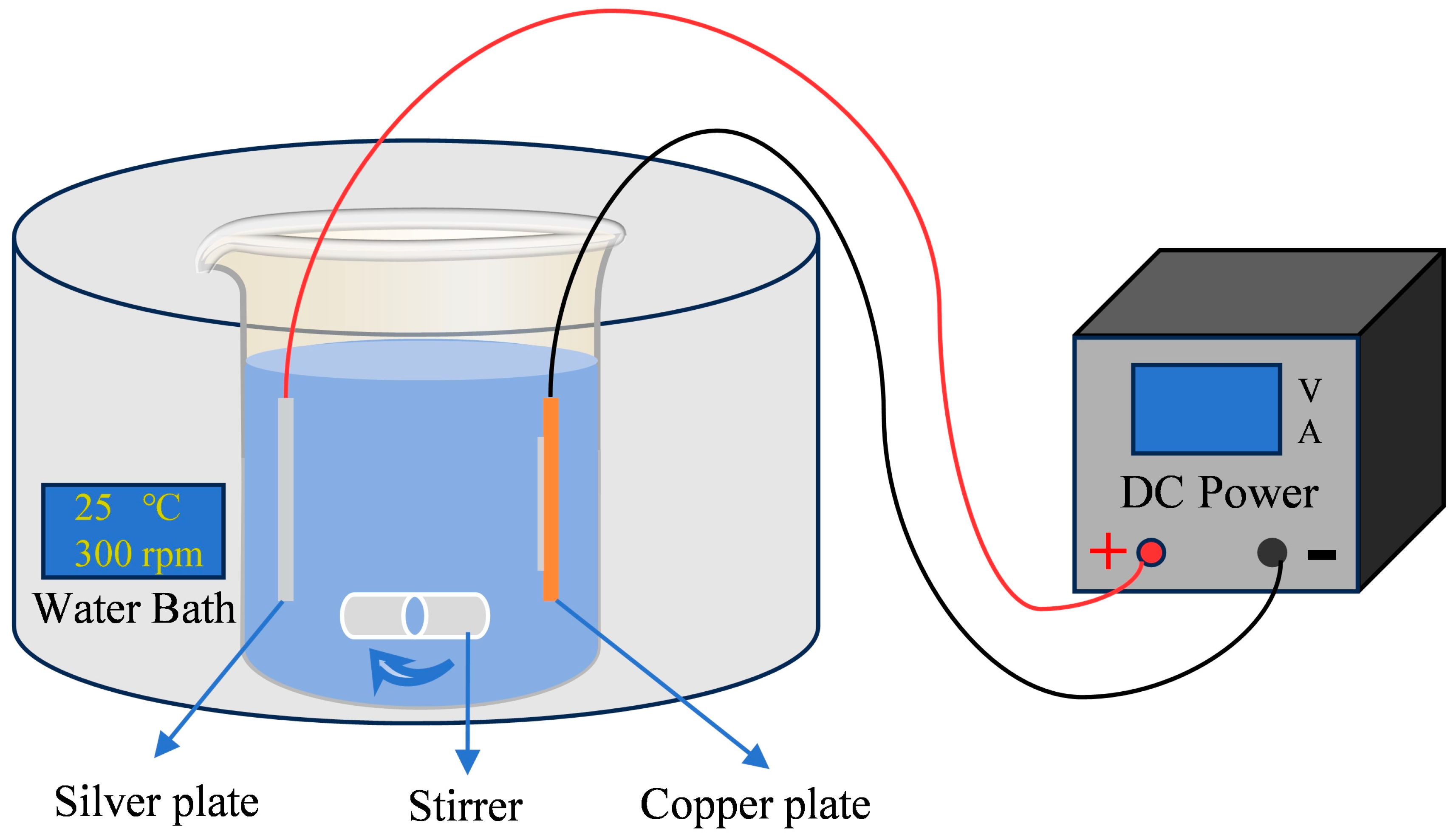
2.1. Preparation of Coating
2.2. Preparation of Electrical Contacts
2.3. Characterization and Test Methods
2.3.1. Characterization Methods
2.3.2. Mechanical Property Test Methods
2.3.3. Tribological Performance Test Methods
2.3.4. Combined Performance Test Methods
3. Results and Discussion
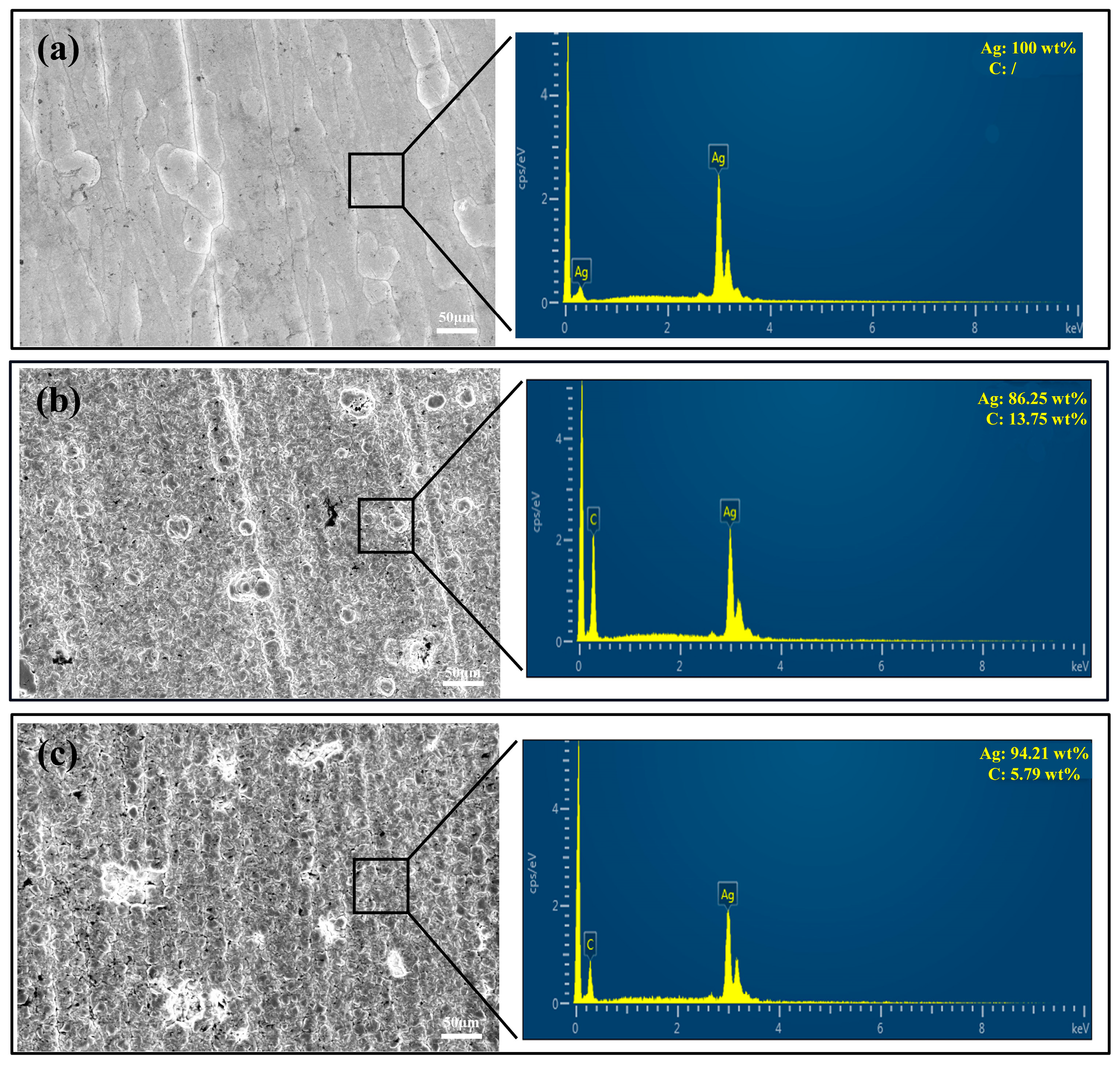
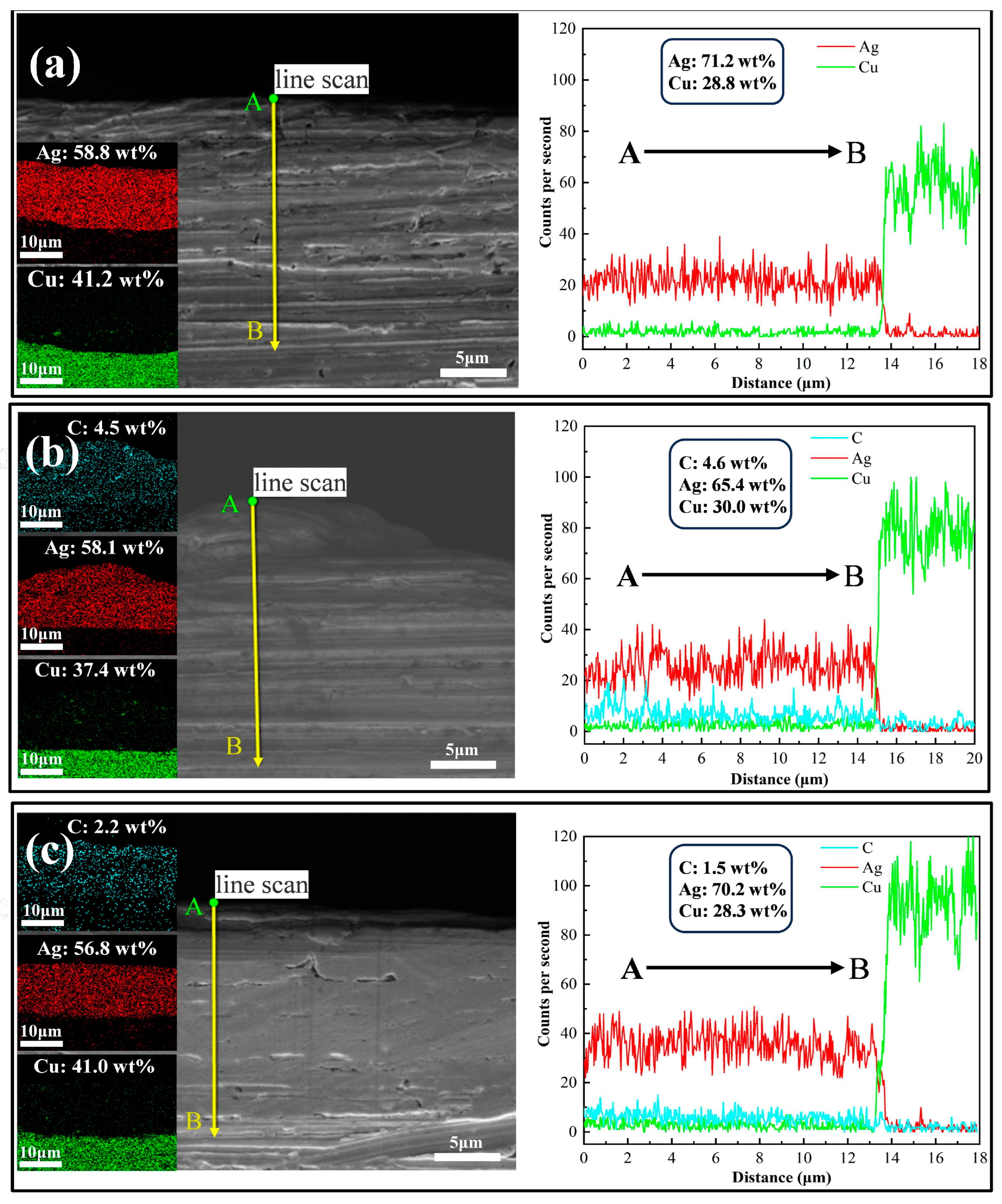
3.1. Coating Morphology
3.2. Dispersion of Graphene on Coating
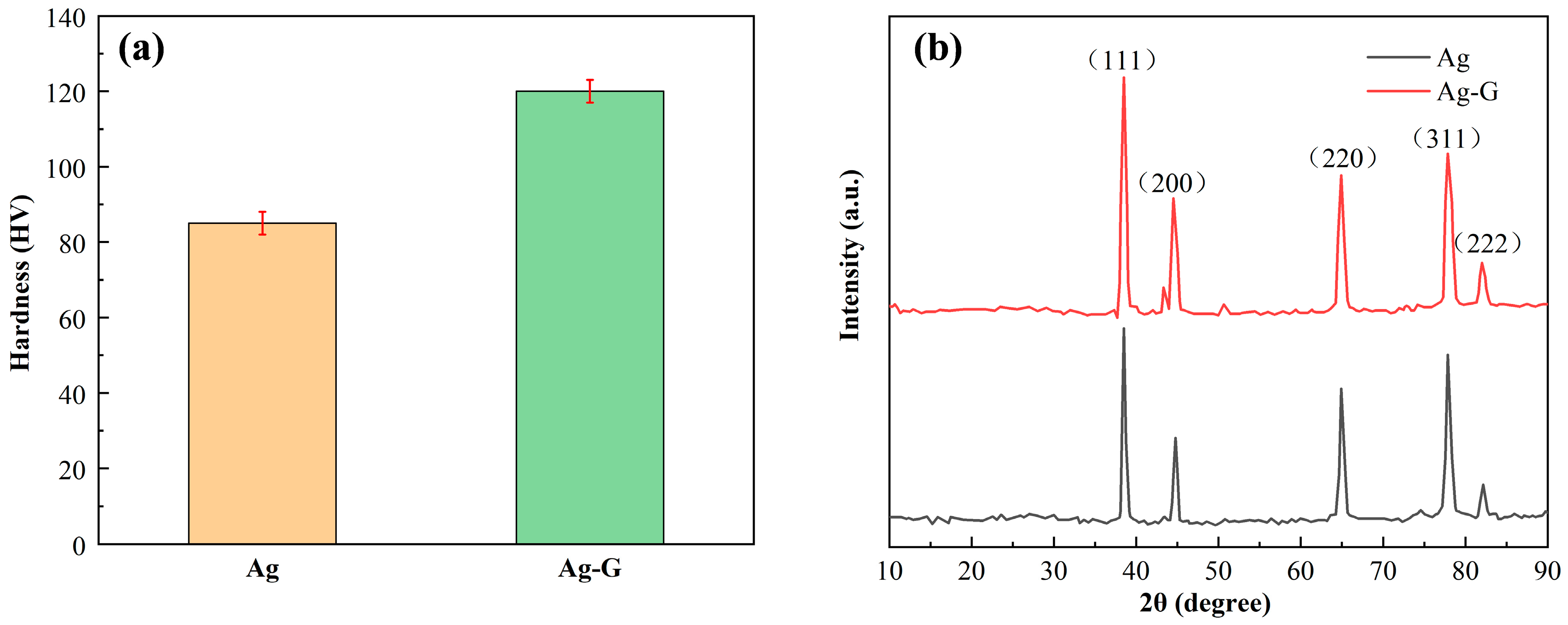
3.3. Mechanical Properties of Coating
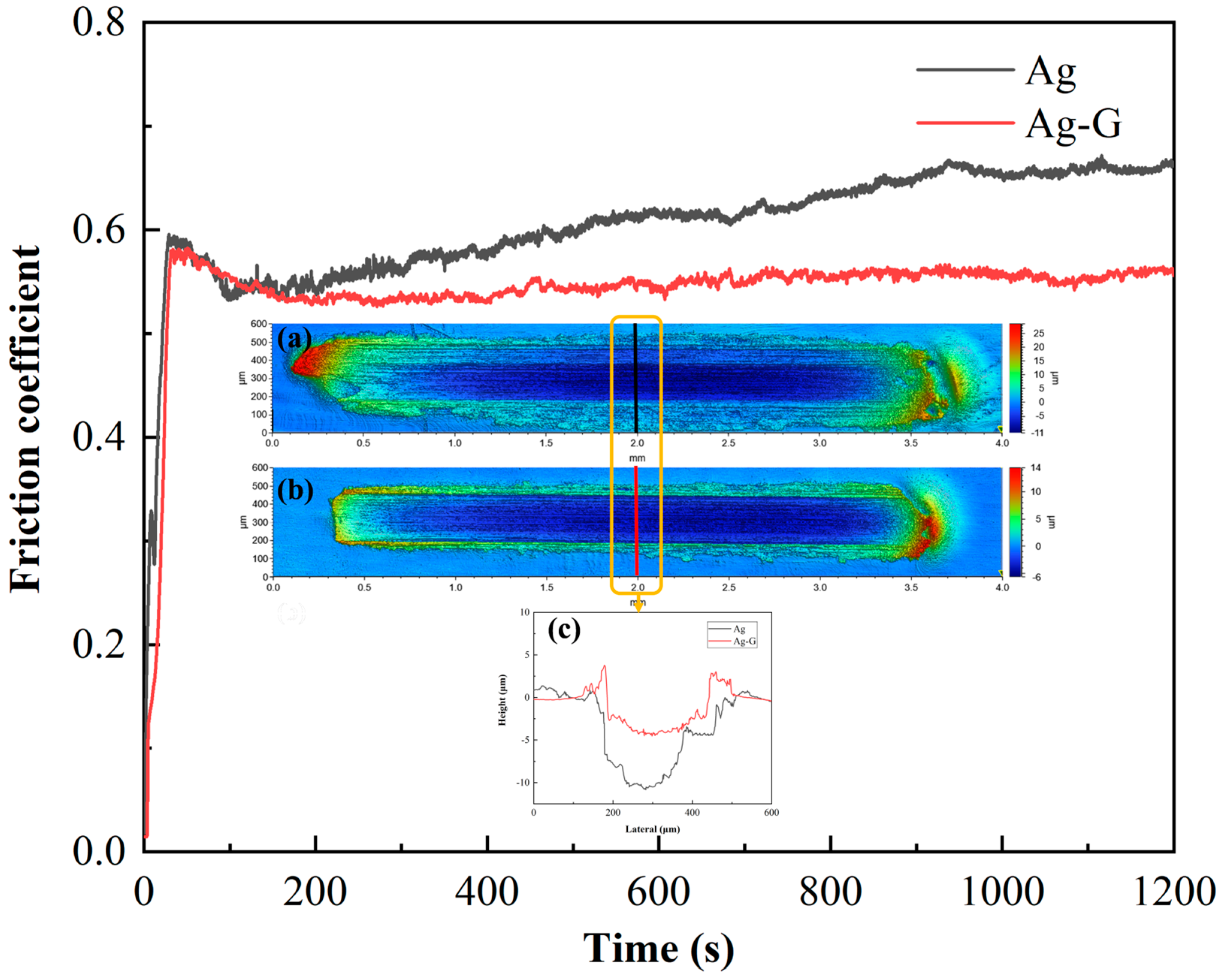
3.4. Tribological Properties of Coating
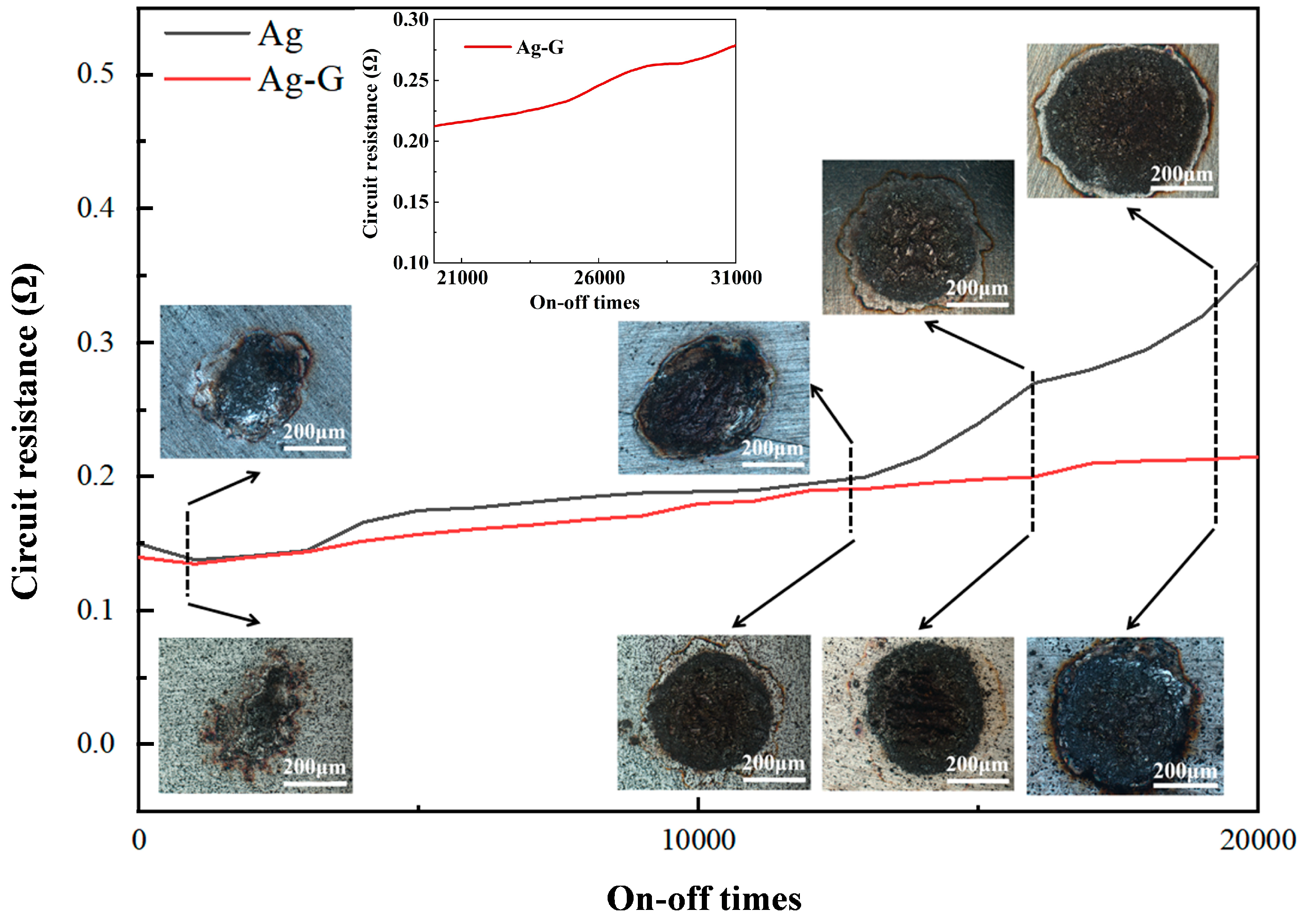
3.5. Overall Properties of the Coating
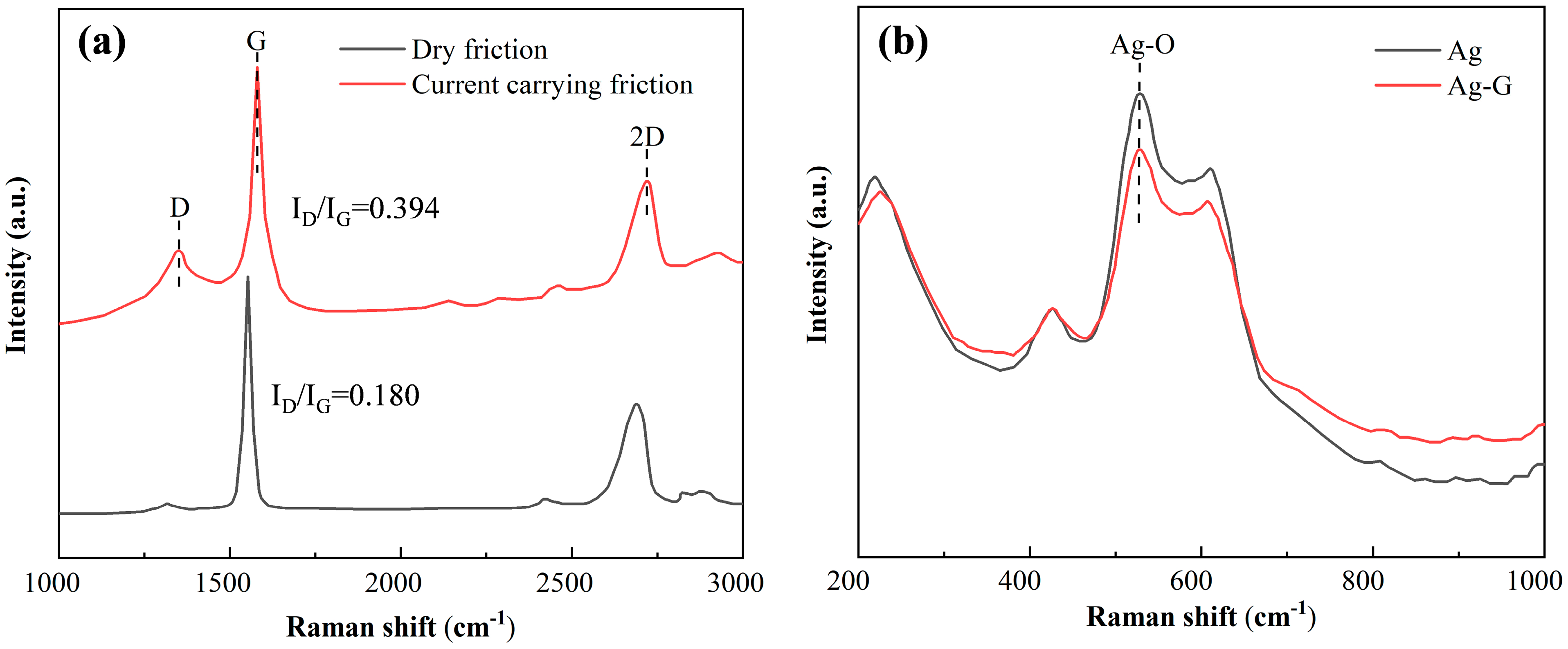
3.6. Composite Electrodeposition Mechanism
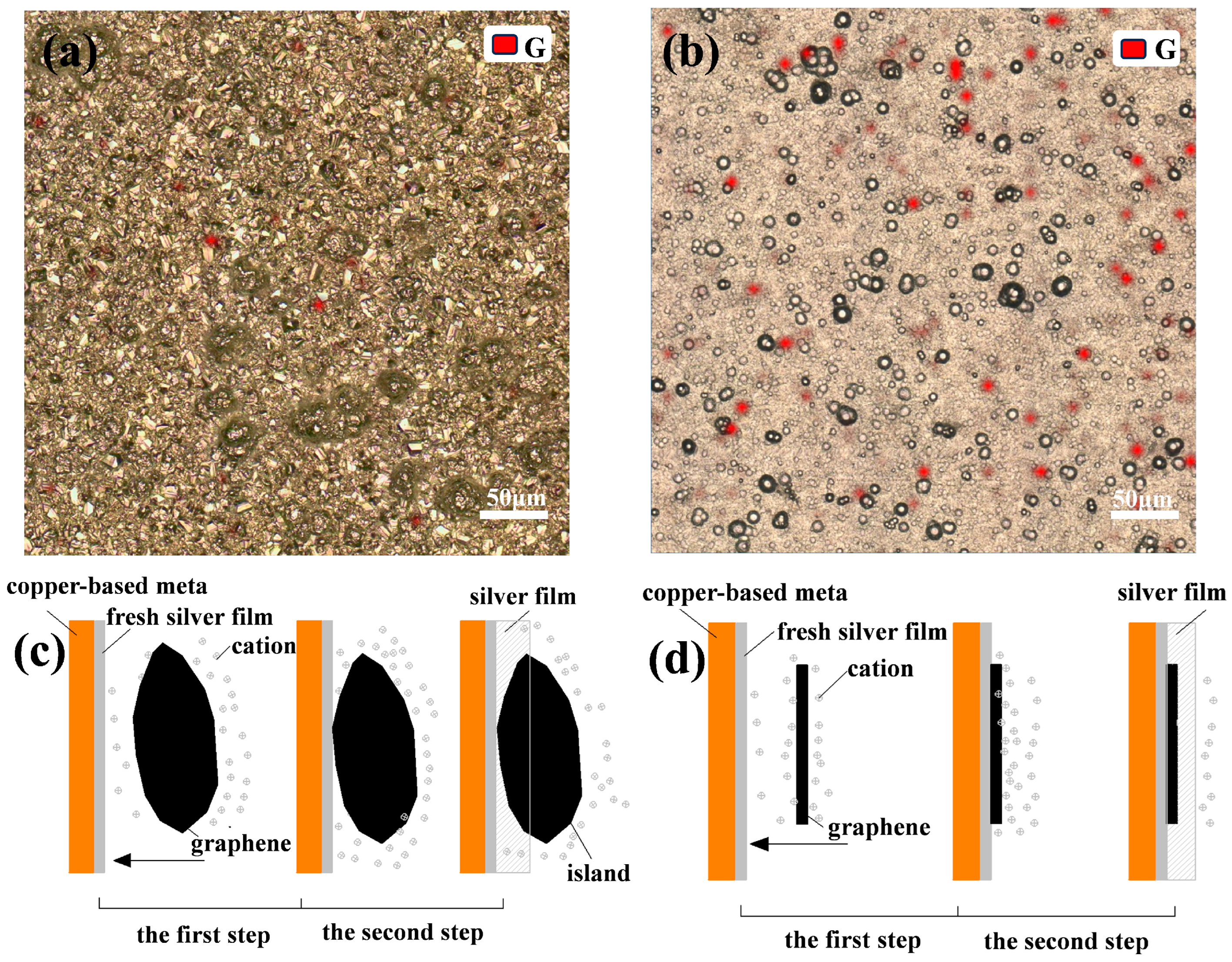
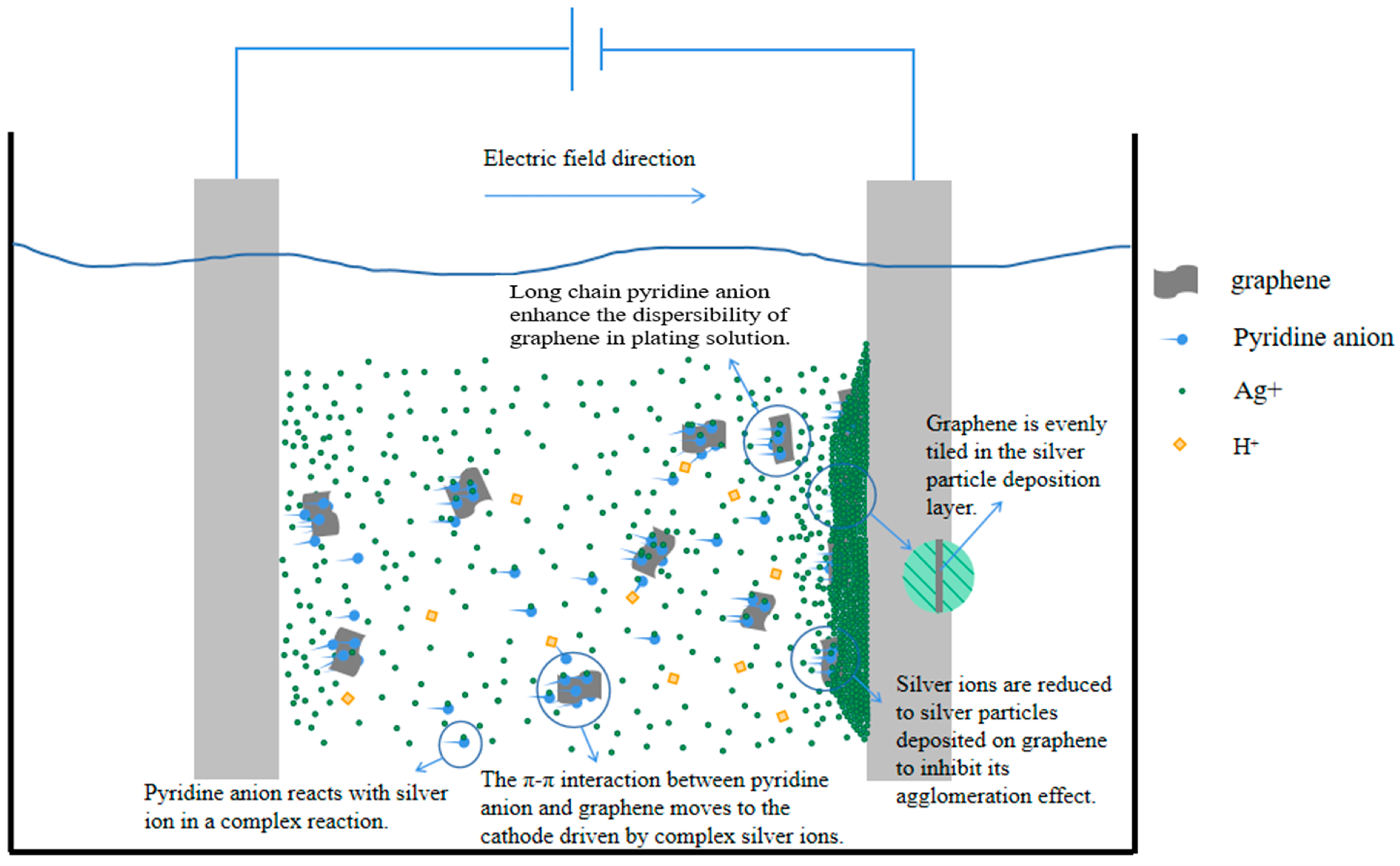
 . According to the relevant literature, there is a π-π interaction force between graphene with a six-membered ring structure and compounds with a conjugated structure [28], and the pyridine ring of nicotinic acid, the plating solution complex mentioned above, has a conjugated structure. Therefore, the complex is adsorbed on the graphene surface through the π-π interaction between the pyridine ring structure and graphene. On the one hand, the long chain structure of the pyridine anion inhibits the agglomeration of graphene, and on the other hand, under the action of an external electric field, the complex and the graphene as a whole move together toward the cathode and are deposited. As shown in Figure 11, these two reasons ensure the uniform distribution of graphene in the Ag-G composite coating.
. According to the relevant literature, there is a π-π interaction force between graphene with a six-membered ring structure and compounds with a conjugated structure [28], and the pyridine ring of nicotinic acid, the plating solution complex mentioned above, has a conjugated structure. Therefore, the complex is adsorbed on the graphene surface through the π-π interaction between the pyridine ring structure and graphene. On the one hand, the long chain structure of the pyridine anion inhibits the agglomeration of graphene, and on the other hand, under the action of an external electric field, the complex and the graphene as a whole move together toward the cathode and are deposited. As shown in Figure 11, these two reasons ensure the uniform distribution of graphene in the Ag-G composite coating.4. Conclusions
5. Patents
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Rodrigues, R.; Du, Y.; Antoniazzi, A.; Cairoli, P. A Review of Solid-State Circuit Breakers. IEEE Trans. Power Electron. 2021, 36, 364–377. [Google Scholar] [CrossRef]
- Liu, Z.; Wang, L. Electrical Life Assessment of the Low-Voltage Circuit Breaker (LVCB) Considering Arc Voltage. Energies 2022, 15, 3070. [Google Scholar] [CrossRef]
- Yuan, X.; Fu, F.; He, R. Graphene-Enhanced Silver Composites for Electrical Contacts: A Review. J. Mater. Sci. 2024, 59, 3762–3779. [Google Scholar] [CrossRef]
- Zhao, D.S.; Zhang, S.M.; Duan, L.H.; Wang, Y.T.; Jiang, D.S.; Liu, W.B.; Zhang, B.S.; Yang, H. Effects of Ag on Electrical Properties of Ag/Ni/p-GaN Ohmic Contact. Chin. Phys. Lett. 2007, 24, 1741–1744. [Google Scholar] [CrossRef]
- Pompanon, F.; Fouvry, S.; Alquier, O. Influence of Humidity on the Endurance of Silver-Plated Electrical Contacts Subjected to Fretting Wear. Surf. Coat. Technol. 2018, 354, 246–256. [Google Scholar] [CrossRef]
- Park, Y.W.; Joo, H.G.; Lee, K.Y. Effect of Intermittent Fretting on Corrosion Behavior in Electrical Contact. Wear 2010, 268, 353–360. [Google Scholar] [CrossRef]
- Li, H.; Wang, X.; Liang, Y.; Fei, Y.; Zhang, H. Effect of Electrical Contact Mode on the Arc-Erosion Behavior of Titanium Diboride-Nickel Co-Reinforced and Nickel-Enhanced Silver-Based Electrical Contact Materials. J. Electron. Mater. 2022, 51, 1137–1147. [Google Scholar] [CrossRef]
- Satpathy, B.; Jena, S.; Das, S.; Das, K. A Comprehensive Review of Various Non-Cyanide Electroplating Baths for the Production of Silver and Gold Coatings. Int. Mater. Rev. 2023, 68, 825–861. [Google Scholar] [CrossRef]
- He, D.H.; Manory, R. A Novel Electrical Contact Material with Improved Self-Lubrication for Railway Current Collectors. Wear 2001, 249, 626–636. [Google Scholar] [CrossRef]
- Huang, S.; Feng, Y.; Ding, K.; Qian, G.; Liu, H.; Wang, Y. Friction and Wear Properties of Cu-Based Self-Lubricating Composites in Air and Vacuum Conditions. Acta Metall. Sin. (Engl. Lett.) 2012, 25, 391. [Google Scholar] [CrossRef]
- Liu, Y.; Senturk, B.S.; Mantese, J.V.; Aindow, M.; Alpay, S.P. Electrical and Tribological Properties of a Ni–18%Ru Alloy for Contact Applications. J. Mater. Sci. 2011, 46, 6563–6570. [Google Scholar] [CrossRef]
- Wang, J.; Feng, Y.; Li, S.; Lin, S. Influence of Graphite Content on Sliding Wear Characteristics of CNTs-Ag-G Electrical Contact Materials. Trans. Nonferrous Met. Soc. China 2009, 19, 113–118. [Google Scholar] [CrossRef]
- Gómez-Navarro, C.; Weitz, R.T.; Bittner, A.M.; Scolari, M.; Mews, A.; Burghard, M.; Kern, K. Electronic Transport Properties of Individual Chemically Reduced Graphene Oxide Sheets. Nano Lett. 2007, 7, 3499–3503. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Tan, Y.-W.; Stormer, H.L.; Kim, P. Experimental Observation of the Quantum Hall Effect and Berry’s Phase in Graphene. Nature 2005, 438, 201–204. [Google Scholar] [CrossRef]
- Balandin, A.A.; Ghosh, S.; Bao, W.; Calizo, I.; Teweldebrhan, D.; Miao, F.; Lau, C.N. Superior Thermal Conductivity of Single-Layer Graphene. Nano Lett. 2008, 8, 902–907. [Google Scholar] [CrossRef] [PubMed]
- Park, S.; Lee, K.-S.; Bozoklu, G.; Cai, W.; Nguyen, S.T.; Ruoff, R.S. Graphene Oxide Papers Modified by Divalent Ions—Enhancing Mechanical Properties via Chemical Cross-Linking. ACS Nano 2008, 2, 572–578. [Google Scholar] [CrossRef]
- Soldano, C.; Mahmood, A.; Dujardin, E. Production, Properties and Potential of Graphene. Carbon 2010, 48, 2127–2150. [Google Scholar] [CrossRef]
- Nieto, A.; Bisht, A.; Lahiri, D.; Zhang, C.; Agarwal, A. Graphene Reinforced Metal and Ceramic Matrix Composites: A Review. Int. Mater. Rev. 2017, 62, 241–302. [Google Scholar] [CrossRef]
- Lv, W.Y.; Zheng, K.Q.; Zhang, Z.G. Performance Studies of Ag, Ag-graphite, and Ag-graphene Coatings on Cu Substrate for High-voltage Isolation Switch. Mater. Corros. 2018, 69, 1847–1853. [Google Scholar] [CrossRef]
- Gao, J.; Zhou, M.; Cheng, G.; Tang, M.; Sun, L.; Chen, Y.; Luo, C. Multilayer Coatings of Periodically Co-Deposited Graphene and Ag Substrate: Improving the Electrified Friction Interface by Modifying the Strength-Ductility Combination. Surf. Coat. Technol. 2024, 482, 130667. [Google Scholar] [CrossRef]
- Ranjan, R.; Bajpai, V. Graphene-Based Metal Matrix Nanocomposites: Recent Development and Challenges. J. Compos. Mater. 2021, 55, 2369–2413. [Google Scholar] [CrossRef]
- Khobragade, N.; Sikdar, K.; Kumar, B.; Bera, S.; Roy, D. Mechanical and Electrical Properties of Copper-Graphene Nanocomposite Fabricated by High Pressure Torsion. J. Alloys Compd. 2019, 776, 123–132. [Google Scholar] [CrossRef]
- Shang, W.; Li, J.; Rabiei Baboukani, A.; Wen, Y.; Kong, D.; Peng, N.; Jiang, J. Study on the Relationship between Graphene Dispersion and Corrosion Resistance of Graphene Composite Film. Appl. Surf. Sci. 2020, 511, 145518. [Google Scholar] [CrossRef]
- Ren, F.; Yin, L.; Wang, S.; Volinsky, A.A.; Tian, B. Cyanide-Free Silver Electroplating Process in Thiosulfate Bath and Microstructure Analysis of Ag Coatings. Trans. Nonferrous Met. Soc. China 2013, 23, 3822–3828. [Google Scholar] [CrossRef]
- Satpathy, B.; Jena, S.; Das, S.; Das, K. A Comparative Study of Electrodeposition Routes for Obtaining Silver Coatings from a Novel and Environment-Friendly Thiosulphate-Based Cyanide-Free Electroplating Bath. Surf. Coat. Technol. 2021, 424, 127680. [Google Scholar] [CrossRef]
- Jayakrishnan, S.; Natarajan, S.R.; Vasu, K.I. Alkaline Noncyanide Bath for Electrodeposition of Silver. Met. Finish. 1996, 94, 12–15. [Google Scholar] [CrossRef]
- Liu, A.; Ren, X.; Zhang, J.; Li, D.; An, M. Complexing Agent Study for Environmentally Friendly Silver Electrodeposition(II): Electrochemical Behavior of Silver Complex. RSC Adv. 2016, 6, 7348–7355. [Google Scholar] [CrossRef]
- Xu, Y.; Bai, H.; Lu, G.; Li, C.; Shi, G. Flexible Graphene Films via the Filtration of Water-Soluble Noncovalent Functionalized Graphene Sheets. J. Am. Chem. Soc. 2008, 130, 5856–5857. [Google Scholar] [CrossRef] [PubMed]
- Ferrari, A.C.; Meyer, J.C.; Scardaci, V.; Casiraghi, C.; Lazzeri, M.; Mauri, F.; Piscanec, S.; Jiang, D.; Novoselov, K.S.; Roth, S.; et al. Raman Spectrum of Graphene and Graphene Layers. Phys. Rev. Lett. 2006, 97, 187401. [Google Scholar] [CrossRef]
- Zhang, Z.; Chen, D.L. Contribution of Orowan Strengthening Effect in Particulate-Reinforced Metal Matrix Nanocomposites. Mater. Sci. Eng. A 2008, 483–484, 148–152. [Google Scholar] [CrossRef]
- He, Y.; Sun, W.T.; Wang, S.C.; Reed, P.A.S.; Walsh, F.C. An Electrodeposited Ni-P-WS2 Coating with Combined Super-Hydrophobicity and Self-Lubricating Properties. Electrochim. Acta 2017, 245, 872–882. [Google Scholar] [CrossRef]
- Xiang, L.; Shen, Q.; Zhang, Y.; Bai, W.; Nie, C. One-Step Electrodeposited Ni-Graphene Composite Coating with Excellent Tribological Properties. Surf. Coat. Technol. 2019, 373, 38–46. [Google Scholar] [CrossRef]
- De, M.; Gupta, S.P.S. Lattice Imperfection Studies in Polycrystalline Materials by X-Ray Diffraction Line-Profile Analysis. Pramana-J. Phys. 1984, 23, 721–744. [Google Scholar] [CrossRef]
- Salim, E.T.; Ismail, R.A.; Halbos, H.T. Growth of Nb2O5 Film Using Hydrothermal Method: Effect of Nb Concentration on Physical Properties. Mater. Res. Express 2019, 6, 116429. [Google Scholar] [CrossRef]
- Zhang, J.; Chen, Z.; Wu, H.; Zhao, J.; Jiang, Z. Effect of Graphene on the Tribolayer of Aluminum Matrix Composite during Dry Sliding Wear. Surf. Coat. Technol. 2019, 358, 907–912. [Google Scholar] [CrossRef]
- Grandin, M.; Wiklund, U. Friction, Wear and Tribofilm Formation on Electrical Contact Materials in Reciprocating Sliding against Silver-Graphite. Wear 2013, 302, 1481–1491. [Google Scholar] [CrossRef]
- Toselli, M.; Saccani, A.; Pilati, F. Thermo-Oxidative Resistance of Crosslinked Polyethylene (XLPE) Coated by Hybrid Coatings Containing Graphene Oxide. Surf. Coat. Technol. 2014, 258, 503–508. [Google Scholar] [CrossRef]
- Wu, J.-B.; Lin, M.-L.; Cong, X.; Liu, H.-N.; Tan, P.-H. Raman Spectroscopy of Graphene-Based Materials and Its Applications in Related Devices. Chem. Soc. Rev. 2018, 47, 1822–1873. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Cai, W.; An, J.; Kim, S.; Nah, J.; Yang, D.; Piner, R.; Velamakanni, A.; Jung, I.; Tutuc, E.; et al. Large-Area Synthesis of High-Quality and Uniform Graphene Films on Copper Foils. Science 2009, 324, 1312–1314. [Google Scholar] [CrossRef]
- Qiang, H.; Sun, Q.; Zheng, X.; Qi, M.; Chen, M.; Luo, N.; Xu, C.; Ye, X. Laser Direct Fabrication Graphene on Silver-Based Contact as High-End Electrical Product. J. Mater. Sci. 2023, 58, 8178–8188. [Google Scholar] [CrossRef]
- Hu, M.; Liu, X.; Zhou, C.; Wang, D.; Xiao, Q.; Guan, X.; Zhang, S.; Xu, Z. Comparative Study of the Current-Carrying Tribological Properties of Carbon Graphite Composites with Different Hardnesses. Int. J. Mech. Sci. 2023, 245, 108133. [Google Scholar] [CrossRef]


| Composition and Conditions | Ag Plating Solution (Nicotinic Acid) | Ag-G Composite Plating Solution (Nicotinic Acid) | Ag-G Composite Plating Solution (Sodium Thiosulfate) |
|---|---|---|---|
| AgNO3 (Silver nitrate) | 45 g/L | 45 g/L | 45 g/L |
| C6H5NO2 (Nicotinic acid) | 100 g/L | 100 g/L | / |
| CH3COONH4 (Ammonium acetate) | 77 g/L | 77 g/L | / |
| K2CO3 (Potassium carbonate anhydrous) | 70 g/L | 70 g/L | / |
| KOH (Potassium hydroxide) | 45 g/L | 45 g/L | / |
| NH3·H2O (Ammonia solution) | 32 mL/L | 32 mL/L | / |
| Na2S2O3 (Sodium thiosulfate) | / | / | 250 g/L |
| K2S2O5 (Potassium disulfite) | / | / | 45 g/L |
| CH5N3S (Thiosemicarbazide) | / | / | 0.6 g/L |
| (CH2CH2NH)n (Ethylene imine polymer) | / | / | 0.6 g/L |
| C12H25SO4Na (Sodium dodecyl sulfate) | / | 0.05 g/L | 0.05 g/L |
| C12H25SO3Na (Sodium laurylsulfonate) | / | 0.05 g/L | 0.05 g/L |
| HO(CH2CH2O)nH (Polyethylene glycol 400) | / | 0.05 g/L | 0.05 g/L |
| Graphene | / | 0.5 g/L | 0.5 g/L |
| Temperature | 25 ± 1 °C | 25 ± 1 °C | 25 ± 1 °C |
| pH | 9.5–10.0 | 9.5–10.0 | 6.0–7.0 |
| Current density | 0.24 A/dm2 | 0.24 A/dm2 | 0.24 A/dm2 |
| Electrolyte agitation | 300 rpm | 300 rpm | 300 rpm |
| Deposition thickness | 13–16 μm | 13–16 μm | 13–16 μm |
| Anode | Ag | Ag | Ag |
| Crystal Face | 2θ (°) | β (°) | D (nm) | δ × 10−3 (nm−2) | Coating |
|---|---|---|---|---|---|
| (111) | 38.50529 | 0.44091 | 18.87275 | 2.80756 | Ag |
| (200) | 44.72020 | 0.47910 | 17.73013 | 3.18109 | Ag |
| (220) | 64.85535 | 0.56278 | 16.53768 | 3.65637 | Ag |
| (311) | 77.75365 | 0.66403 | 15.19660 | 4.33018 | Ag |
| (111) | 38.38691 | 0.54018 | 15.39892 | 4.21715 | Ag-G |
| (200) | 44.55782 | 0.53871 | 15.75906 | 4.02660 | Ag-G |
| (220) | 64.80095 | 0.59909 | 15.53067 | 4.14590 | Ag-G |
| (311) | 77.71745 | 0.68990 | 14.62303 | 4.67654 | Ag-G |
| Coating Layer | Pure Ag Coating | Ag-G Composite Coating | ||
|---|---|---|---|---|
| Roughness degree | Cathode | Anode | Cathode | Anode |
| (Unit: μm) | 3.583 | 4.525 | 3.182 | 3.491 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sun, L.; Chen, X.; Zhou, M.; Gao, J.; Luo, C.; Li, X.; You, S.; Wang, M.; Cheng, G. Optimization of Cyanide-Free Composite Electrodeposition Based on π-π Interactions Preparation of Silver-Graphene Composite Coatings for Electrical Contact Materials. Nanomaterials 2024, 14, 1349. https://doi.org/10.3390/nano14161349
Sun L, Chen X, Zhou M, Gao J, Luo C, Li X, You S, Wang M, Cheng G. Optimization of Cyanide-Free Composite Electrodeposition Based on π-π Interactions Preparation of Silver-Graphene Composite Coatings for Electrical Contact Materials. Nanomaterials. 2024; 14(16):1349. https://doi.org/10.3390/nano14161349
Chicago/Turabian StyleSun, Luyi, Xin Chen, Ming Zhou, Jingwei Gao, Chaogui Luo, Xiao Li, Shengli You, Mingyue Wang, and Gangqiang Cheng. 2024. "Optimization of Cyanide-Free Composite Electrodeposition Based on π-π Interactions Preparation of Silver-Graphene Composite Coatings for Electrical Contact Materials" Nanomaterials 14, no. 16: 1349. https://doi.org/10.3390/nano14161349
APA StyleSun, L., Chen, X., Zhou, M., Gao, J., Luo, C., Li, X., You, S., Wang, M., & Cheng, G. (2024). Optimization of Cyanide-Free Composite Electrodeposition Based on π-π Interactions Preparation of Silver-Graphene Composite Coatings for Electrical Contact Materials. Nanomaterials, 14(16), 1349. https://doi.org/10.3390/nano14161349






