Enhanced Thermal Stability of Conductive Mercury Telluride Colloidal Quantum Dot Thin Films Using Atomic Layer Deposition
Abstract
1. Introduction
2. Materials and Methods
2.1. Preparation of Trioctylphosphine Telluride (TOPTe)
2.2. Synthesis and Post-Synthesis Processing of p-Type HgTe Colloidal Quantum Dots
2.3. Synthesis and Post-Synthesis Processing of n-Type HgTe Colloidal Quantum Dots
2.4. Atomic Layer Deposition on Colloidal Quantum Dot Thin Films
2.5. Characterization of Optical Properties
2.6. Conductance and Field-Effect Transistor Measurements
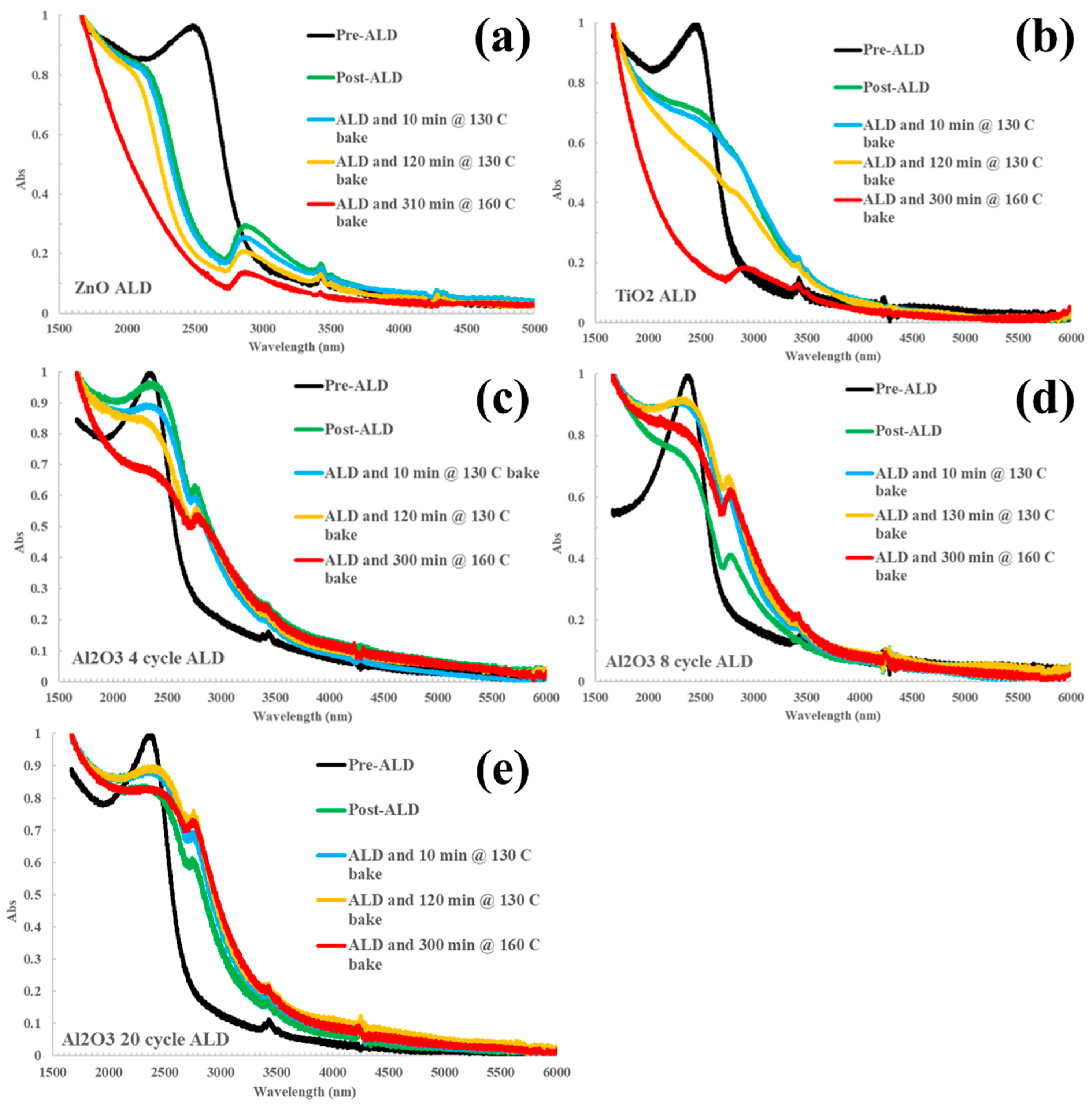
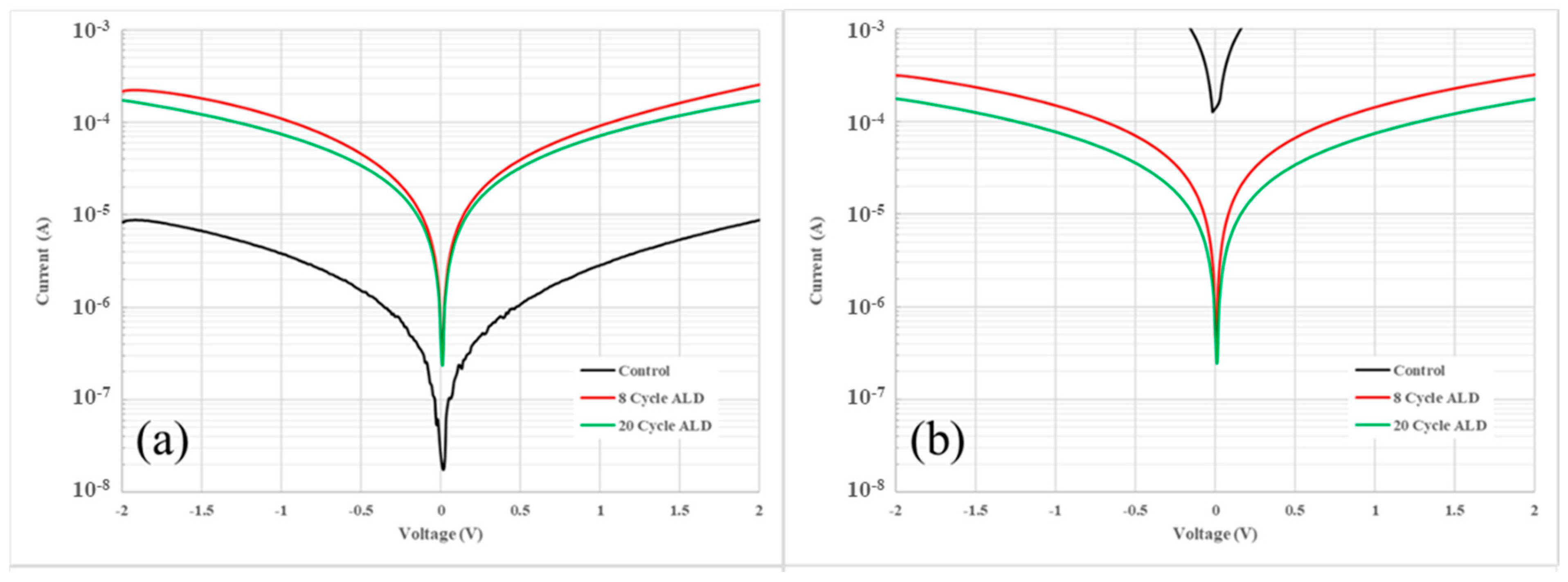
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Won, Y.-H.; Cho, O.; Kim, T.; Chung, D.-Y.; Kim, T.; Chung, H.; Jang, H.; Lee, J.; Kim, D.; Jang, E. Highly efficient and stable InP/ZnSe/ZnS quantum dot light-emitting diodes. Nature 2019, 575, 634–638. [Google Scholar] [CrossRef] [PubMed]
- Ling, X.; Yuan, J.; Ma, W. The Rise of Colloidal Lead Halide Perovskite Quantum Dot Solar Cells. Acc. Mater. Res. 2022, 3, 866–878. [Google Scholar] [CrossRef]
- Makarov, N.S.; Korus, D.; Freppon, D.; Ramasamy, K.; Houck, D.W.; Velarde, A.; Parameswar, A.; Bergren, M.R.; McDaniel, H. Minimizing Scaling Losses in High-Performance Quantum Dot Luminescent Solar Concentrators for Large-Area Solar Windows. ACS Appl. Mater. Interfaces 2022, 14, 29679–29689. [Google Scholar] [CrossRef] [PubMed]
- Steckel, J.S.; Josse, E.; Pattantyus-Abraham, A.G.; Bidaud, M.; Mortini, B.; Bilgen, H.; Arnaud, O.; Allegret-Maret, S.; Saguin, F.; Mazet, L.; et al. 1.62 μm Global Shutter Quantum Dot Image Sensor Optimized for Near and Shortwave Infrared. In Proceedings of the 2021 IEEE International Electron Devices Meeting (IEDM), San Francisco, CA, USA, 11–15 December 2021; pp. 23.4.1–23.4.4. [Google Scholar]
- Peng, M.; Tao, Y.; Bao, D.; Wang, K.; Sun, X. Rational synthesis of core/shell heterostructured quantum dots for improved self-powered photodetection performance enabled by energy band engineering. Nano Energy 2024, 129 Pt A, 110033. [Google Scholar] [CrossRef]
- Zhang, H.; Prado, Y.; Alchaar, R.; Lehouelleur, H.; Cavallo, M.; Dang, T.H.; Khalili, A.; Bossavit, E.; Dabard, C.; Ledos, N.; et al. Infrared Imaging Using Thermally Stable HgTe/CdS Nanocrystals. Nano Lett. 2024, 24, 5039–5046. [Google Scholar] [CrossRef] [PubMed]
- Ackerman, M.M. Bringing Colloidal Quantum Dots to Detector Technologies. Inf. Disp. 2020, 36, 19–23. [Google Scholar] [CrossRef]
- Li, Z.; Tian, Y.; Teng, C.; Cao, H. Recent Advances in Barrier Layer of Cu Interconnects. Materials 2020, 13, 5049. [Google Scholar] [CrossRef] [PubMed]
- So, H.M.; Choi, H.; Shim, H.C.; Lee, S.M.; Jeong, S.; Chang, W.S. Atomic layer deposition effect on the electrical properties of Al2O3-passivated PbS quantum dot field-effect transistors. Appl. Phys. Lett. 2015, 106, 093507. [Google Scholar] [CrossRef]
- Liu, Y.; Tolentino, J.; Gibbs, M.; Ihly, R.; Perkins, C.L.; Liu, Y.; Crawford, N.; Hemminger, J.C.; Law, M. PbSe Quantum Dot Field-Effect Transistors with Air-Stable Electron Mobilities above 7 cm2V−1s−1. Nano Lett. 2013, 13, 1578–1587. [Google Scholar] [CrossRef] [PubMed]
- Crisp, R.W.; Hashemi, F.S.; Alkemade, J.; Kirkwood, N.; Grimaldi, G.; Kinge, S.; Siebbeles, L.D.; van Ommen, J.R.; Houtepen, A.J. Atomic Layer Deposition of ZnO on InP Quantum Dot Films for Charge Separation, Stabilization, and Solar Cell Formation. Adv. Mater. Interfaces 2020, 7, 1901600. [Google Scholar] [CrossRef]
- Ackerman, M.M.; Chen, M.; Guyot-Sionnest, P. HgTe colloidal quantum dot photodiodes for extended short-wave infrared detection. Appl. Phys. Lett. 2020, 116, 083502. [Google Scholar] [CrossRef]
- Yang, J.; Hu, H.; Lv, Y.; Yuan, M.; Wang, B.; He, Z.; Chen, S.; Wang, Y.; Hu, Z.; Yu, M.; et al. Ligand-Engineered HgTe Colloidal Quantum Dot Solids for Infrared Photodetectors. Nano Lett. 2022, 22, 3465–3472. [Google Scholar] [CrossRef] [PubMed]
- Ballinger, T.H.; Yates, J.T., Jr. IR Spectroscopic Detection of Lewis Acid Sites on Al2O3 Using Adsorbed CO Correlation with Al-OH Group Removal. Langmuir 1991, 7, 3041–3045. [Google Scholar] [CrossRef]
- Noei, H.; Qiu, H.; Wang, Y.; Löffler, E.; Wöll, C.; Muhler, M. The identification of hydroxyl groups on ZnO nanoparticles by infrared spectroscopy. Phys. Chem. Chem. Phys. 2008, 10, 7092–7097. [Google Scholar] [CrossRef] [PubMed]
- Primet, M.; Pichat, P.; Mathieu, M.V. Infrared study of the surface of titanium dioxides. I. Hydroxyl Groups. J. Phys. Chem. 1971, 75, 1216–1220. [Google Scholar] [CrossRef]
- Liu, Y.; Gibbs, M.; Perkins, C.L.; Tolentino, J.; Zarghami, M.H.; Bustamante, J.J., Jr.; Law, M. Robust, Functional Nanocrystal Solids by Infilling with Atomic Layer Deposition. Nano Lett. 2011, 11, 5349–5355. [Google Scholar] [CrossRef] [PubMed]
- Jeong, K.S.; Deng, Z.; Keuleyan, S.; Liu, H.; Guyot-Sionnest, P. Air-Stable n-Doped Colloidal HgS Quantum Dots. J. Phys. Chem. Lett. 2014, 5, 1139–1143. [Google Scholar] [CrossRef] [PubMed]


| ALD Material | Al2O3 | ZnO | TiO2 | |
|---|---|---|---|---|
| Metal precursor | TMA | DEZ | TDMAT | |
| Reactant | Water | Water | Water | |
| Temperature | 80 °C | 80 °C | 80 °C | |
| First half cycle (metal precursor) | N2 flow | 5 sccm | 10 sccm | 10 sccm |
| Metal precursor pulse time | 150 ms | 80 ms | 700 ms | |
| Pulse delay | 10 s | 10 s | 10 s | |
| Number of pulses | 6 | 6 | 6 | |
| Exposure time | 600, 1200 s | 400 s | 120 s | |
| Purge time | 90 s | 90 s | 240, 480 s | |
| Second half cycle (water) | N2 flow | 5 sccm | 10 sccm | 5 sccm |
| Water pulse time | 80 ms | 80 ms | 80 ms | |
| Water pulse delay | 10 s | 10 s | 10 s | |
| Number of water pulses | 6 | 6 | 6 | |
| Exposure time | 400, 800 s | 400 s | 240 s | |
| Purge time | 120 s | 120 s | 240, 480 s | |
| Number of cycles | 4, 8, 20 | 8 | 20 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Malachosky, E.W.; Ackerman, M.M.; Stan, L. Enhanced Thermal Stability of Conductive Mercury Telluride Colloidal Quantum Dot Thin Films Using Atomic Layer Deposition. Nanomaterials 2024, 14, 1354. https://doi.org/10.3390/nano14161354
Malachosky EW, Ackerman MM, Stan L. Enhanced Thermal Stability of Conductive Mercury Telluride Colloidal Quantum Dot Thin Films Using Atomic Layer Deposition. Nanomaterials. 2024; 14(16):1354. https://doi.org/10.3390/nano14161354
Chicago/Turabian StyleMalachosky, Edward W., Matthew M. Ackerman, and Liliana Stan. 2024. "Enhanced Thermal Stability of Conductive Mercury Telluride Colloidal Quantum Dot Thin Films Using Atomic Layer Deposition" Nanomaterials 14, no. 16: 1354. https://doi.org/10.3390/nano14161354
APA StyleMalachosky, E. W., Ackerman, M. M., & Stan, L. (2024). Enhanced Thermal Stability of Conductive Mercury Telluride Colloidal Quantum Dot Thin Films Using Atomic Layer Deposition. Nanomaterials, 14(16), 1354. https://doi.org/10.3390/nano14161354






