Green Phytic Acid-Assisted Synthesis of LiMn1-xFexPO4/C Cathodes for High-Performance Lithium-Ion Batteries
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials’ Synthesis
2.2. Material Characterization
2.3. Electrochemical Measurements
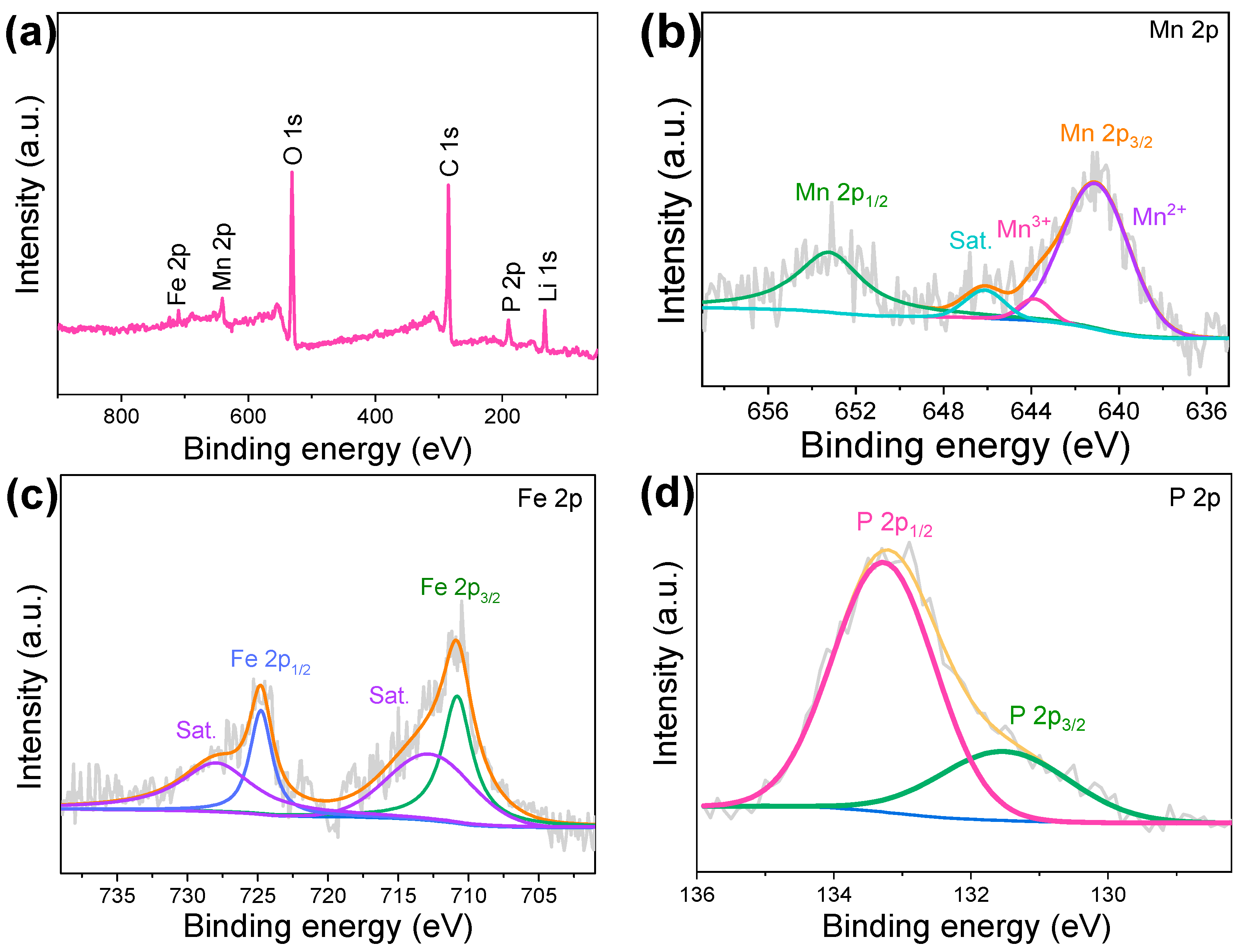
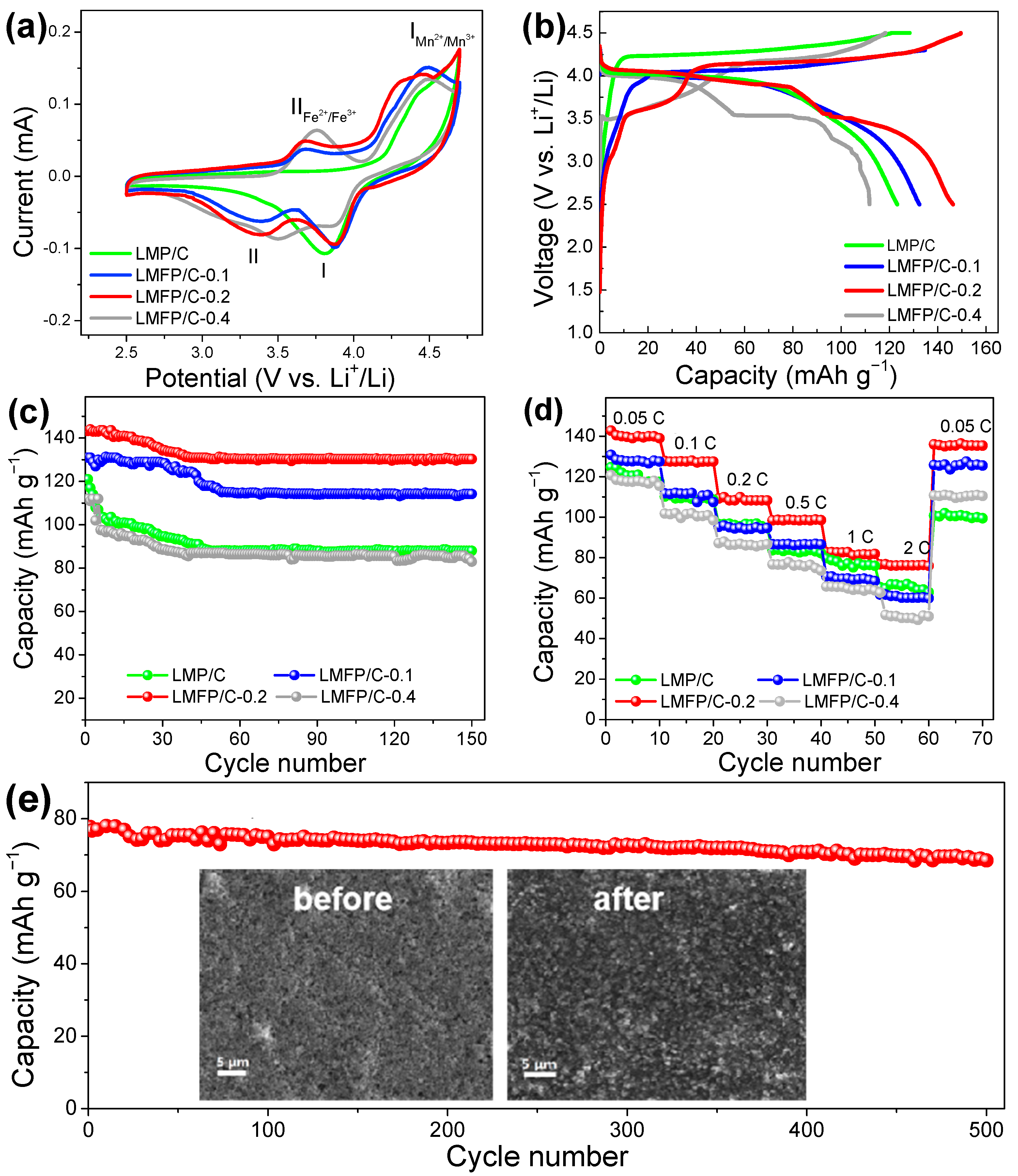
3. Results
4. Conclusions and Perspectives
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Gao, J.; Qin, Z.; Zhao, G.; Liu, Y.; Zhang, W.; Yao, H.; Zheng, Y.; Lin, Y.; Huang, Z.; Li, J. Modulating NCM622 electrode to efficiently boost the lithium storage and thermal safety of its full batteries. Energy Stor. Mater. 2024, 67, 103332. [Google Scholar] [CrossRef]
- Wang, W.; Li, W.; Jing, S.; Yang, H.; Wang, H.; Huang, L.; Mao, Y.; Pang, X.; Huang, Y.; Zhang, L. Quantitative lithium substitution of carboxyl hydrogens in polyacrylic acid binder enables robust SiO electrodes with durable lithium storage stability. J. Energy Chem. 2024, 97, 352–360. [Google Scholar] [CrossRef]
- Liu, J.; Zhang, Y.; Zhou, J.; Wang, Z.; Zhu, P.; Cao, Y.; Zheng, Y.; Zhou, X.; Yan, C.; Qian, T. Advances and prospects in improving the utilization efficiency of lithium for high energy density lithium batteries. Adv. Funct. Mater. 2023, 33, 2302055. [Google Scholar] [CrossRef]
- Shin, J.; Yang, J.; Sergey, C.; Song, M. Carbon nanofibers heavy laden with Li3V2(PO4)3 particles featuring superb kinetics for high-power lithium ion battery. Adv. Sci. 2017, 4, 1700128. [Google Scholar] [CrossRef]
- Yang, H.; Xiong, T.; Zhu, Z.; Xiao, R.; Yao, X.; Huang, Y.; Balogun, M. Deciphering the lithium storage chemistry in flexible carbon fiber-based self-supportive electrodes. Carbon Energy 2022, 4, 820–832. [Google Scholar] [CrossRef]
- Cao, M.-Y.; Liu, Z.-P.; Zhang, X.; Yang, L.; Xu, S.-W.; Weng, S.-T.; Zhang, S.-M.; Li, X.-Y.; Li, Y.-J.; Liu, T.-C.; et al. Feasibility of prelithiation in LiFePO4. Adv. Funct. Mater. 2022, 33, 2210032. [Google Scholar] [CrossRef]
- Li, Z.; Peng, Z.; Zhang, H.; Hu, T.; Hu, M.; Zhu, K.; Wang, X. [100]-Oriented LiFePO4 nanoflakes toward high rate Li-ion battery cathode. Nano Lett. 2016, 16, 795–799. [Google Scholar] [CrossRef]
- Tian, X.; Zhou, Y.; Tu, X.; Zhang, Z.; Du, G. Well-dispersed LiFePO4 nanoparticles anchored on a three-dimensional graphene aerogel as high-performance positive electrode materials for lithium-ion batteries. J. Power Sources 2017, 340, 40–50. [Google Scholar] [CrossRef]
- Su, J.; Wu, X.-L.; Yang, C.-P.; Lee, J.-S.; Kim, J.; Guo, Y.-G. Self-assembled LiFePO4/C nano/microspheres by using phytic acid as phosphorus source. J. Phys. Chem. C 2012, 116, 5019–5024. [Google Scholar] [CrossRef]
- Lung-Hao Hu, B.; Wu, F.Y.; Lin, C.T.; Khlobystov, A.N.; Li, L.J. Graphene-modified LiFePO(4) cathode for lithium ion battery beyond theoretical capacity. Nat. Commun. 2013, 4, 1687. [Google Scholar] [CrossRef] [PubMed]
- Huang, Q.; Yang, J.; Ng, C.B.; Jia, C.; Wang, Q. A redox flow lithium battery based on the redox targeting reactions between LiFePO4 and iodide. Energy Environ. Sci. 2016, 9, 917–921. [Google Scholar] [CrossRef]
- Herrera, J.O.; Camacho-Montes, H.; Fuentes, L.E.; Álvarez-Contreras, L. LiMnP4: Review on synthesis and electrochemical properties. J. Mater. Sci. Chem. Eng. 2015, 03, 54–64. [Google Scholar] [CrossRef][Green Version]
- Tu, W.-H.; Chen, Z.-W.; Cao, Y.-P.; Duan, J.-G.; He, J.J.; Li, R.-L.; Dong, P.; Wang, X.-S.; Zhang, Y.-J.; Wang, D. Hydrothermal synthesize of bi-functional interface structure modified LiMnPO4@LiFePO4/C nanorods as cathode materials for lithium-ion batteries. J. Power Sources 2024, 613, 234919. [Google Scholar] [CrossRef]
- Qukahou, S.; Elomrani, A.; Maymoun, M.; Sbiaai, K.; Hasnaoui, A. Feeling the strain: Enhancing the electrochemical performance of olivine LiMnPO4 as cathode materials for Li-ion batteries through strain effects. J. Energy Storage 2024, 75, 109663. [Google Scholar] [CrossRef]
- Zhang, B.-C.; Meng, W.; Gong, Y.-F.; Hu, G.-R.; Peng, Z.-D.; Du, K.; Makuza, B.; Wu, J.-H.; Xie, X.-M.; Cao, Y.-B. [001]-oriented LiMn0.6Fe0.4PO4/C nanorod microspheres contributing high-rate performance to olivine-structured cathode for lithium-ion battery. Mater. Today Energy 2022, 30, 101162. [Google Scholar] [CrossRef]
- Nie, P.; Shen, L.; Zhang, F.; Chen, L.; Deng, H.; Zhang, X. Flower-like LiMnPO4 hierarchical microstructures assembled from single-crystalline nanosheets for lithium-ion batteries. CrystEngComm 2012, 14, 4284. [Google Scholar] [CrossRef]
- Chen, D.; Wei, W.; Wang, R.; Lang, X.F.; Tian, Y.; Guo, L. Facile synthesis of 3D hierarchical foldaway-lantern-like LiMnPO4 by nanoplate self-assembly, and electrochemical performance for Li-ion batteries. Dalton Trans. 2012, 41, 8822–8828. [Google Scholar] [CrossRef]
- Li, H.; Zhou, H. Enhancing the performances of Li-ion batteries by carbon-coating: Present and future. Chem. Commun. 2012, 48, 1201–1217. [Google Scholar] [CrossRef]
- Jaafar, Z.; Jumah, T.; Mahmood, N. Enhanced electrical performance of LiMnPO4 by carbon coating for solid-state battery applications. J. Ceram. Int. 2024, 50, 22897–22904. [Google Scholar] [CrossRef]
- He, H.; Lu, X.; Jiang, W.; Zhao, H.; Zhang, Y.; Qiu, H.; He, W. Cetyltrimethylammonium bromide induced generation of highly (200) planes-exposed LiMnPO4 nanosheets coated by nitrogen-doped carbon for high-performance batteries. J. Energy Storage 2024, 93, 112378. [Google Scholar] [CrossRef]
- Xu, H.; Wu, C.; Wei, X.; Gao, S. Hierarchically porous carbon materials with controllable proportion of micropore area by dual-activator synthesis for high-performance supercapacitors. J. Mater. Chem. A 2018, 6, 15340–15347. [Google Scholar] [CrossRef]
- Ma, H.; Jiang, M.; Hou, Z.; Li, T.; Zhang, X.; Gao, Y.; Peng, J.; Li, Y.; Wang, J.-G. Medium-mediated high-crystalline Prussian blue toward exceptionally boosted sodium energy storage. Energy Storage Mater. 2024, 70, 103411. [Google Scholar] [CrossRef]
- Liu, S.; Fang, H.; Dai, E.; Yang, B.; Yao, Y.; Ma, W.; Dai, Y. Effect of carbon content on properties of LiMn0.8Fe0.19Mg0.01PO4/C composite cathode for lithium ion batteries. Electrochim. Acta 2014, 116, 97–102. [Google Scholar] [CrossRef]
- Lee, K.S.; Lee, K.J.; Kang, Y.S.; Shin, T.J.; Sung, Y.E.; Ahn, D. Effects of Ag-embedment on electronic and ionic conductivities of LiMnPO4 and its performance as a cathode for lithium-ion batteries. Nanoscale 2015, 7, 13860–13867. [Google Scholar] [CrossRef]
- Wu, L.; Lu, J.; Wei, G.; Wang, P.; Ding, H.; Zheng, J.; Li, X.; Zhong, S. Synthesis and electrochemical properties of xLiMn0.9Fe0.1PO4·yLi3V2(PO4)3/C composite cathode materials for lithium–ion batteries. Electrochim. Acta 2014, 146, 288–294. [Google Scholar] [CrossRef]
- Gao, Y.; Wang, X.; Ma, J.; Wang, Z.; Chen, L. Selecting substituent elements for Li-rich Mn-based cathode materials by density functional theory (DFT) calculations. Chem. Mater. 2015, 27, 3456–3461. [Google Scholar] [CrossRef]
- Chen, W.; Fang, H. Lattice modulation improving surface passivation of LiMnPO4 for stable cycling at high temperatures. Prog. Solid State Chem. 2024, 74, 100460. [Google Scholar] [CrossRef]
- Yang, R.; Chang, L.; Luo, S.; Bi, X.; Yang, W.; Cai, K.; Hou, Z. Acritical revelation of lithium ferromanganese phosphate (LMFP) performance in a Mn-rich cathode for Li-ion batteries using Fe equivalents to occupy a Mn site. J. Mater. Chem. C 2024, 12, 4961–4976. [Google Scholar] [CrossRef]
- Wang, Y.; Deng, Y.; Liu, Y.; Sun, X.; Wang, Y.; Liu, H.; Zhou, H.; He, P. Electrochemical Performance and In Situ Phase Transition Analysis of Iron-Doped Lithium Manganese Phosphate. Energy Fuels 2024, 38, 12265–12273. [Google Scholar] [CrossRef]
- Liang, L.; Sun, X.; Zhang, J.; Hou, L.; Sun, J.; Liu, Y.; Wang, S.; Yuan, C. In situ synthesis of hierarchical core double-shell Ti-doped LiMnPO4@NaTi2(PO4)3@C/3D graphene cathode with high-rate capability and long cycle life for lithium-ion batteries. Adv. Energy Mater. 2019, 9, 1802847. [Google Scholar] [CrossRef]
- Wang, D.; Buqa, H.; Crouzet, M.; Deghenghi, G.; Drezen, T.; Exnar, I.; Kwon, N.-H.; Miners, J.H.; Poletto, L.; Grätzel, M. High-performance, nano-structured LiMnPO4 synthesized via a polyol method. J. Power Sources 2009, 189, 624–628. [Google Scholar] [CrossRef]
- Schnepp, Z.; Yang, W.; Antonietti, M.; Giordano, C. Biotemplating of metal carbide microstructures: The magnetic leaf. Angew. Chem. Int. Ed. 2010, 49, 6564–6566. [Google Scholar] [CrossRef] [PubMed]
- Xia, Y.; Zhang, W.; Xiao, Z.; Huang, H.; Zeng, H.; Chen, X.; Chen, F.; Gan, Y.; Tao, X. Biotemplated fabrication of hierarchically porous NiO/C composite from lotus pollen grains for lithium-ion batteries. J. Mater. Chem. 2012, 22, 9209. [Google Scholar] [CrossRef]
- Cao, Z.; Zhu, G.; Zhang, R.; Chen, S.; Sang, M.; Jia, J.; Yang, M.; Li, X.; Yang, S. Biological phytic acid guided formation of monodisperse large-sized carbon@ LiFePO4/graphene composite microspheres for high-performance lithium-ion battery cathodes. Chem. Eng. J. 2018, 351, 382–390. [Google Scholar] [CrossRef]
- Zhao, Q.; Zhang, Y.; Meng, Y.; Wang, Y.; Ou, J.; Guo, Y.; Xiao, D. Phytic acid derived LiFePO4 beyond theoretical capacity as high-energy density cathode for lithium ion battery. Nano Energy 2017, 34, 408–420. [Google Scholar] [CrossRef]
- Li, J.; Luo, S.-h.; Ding, X.; Wang, Q.; He, P. NaCl-template assisted synthesis of 3D honeycomb-like LiMnPO4/C with high rate and stable performance as lithium-ion battery cathodes. ACS Sustain. Chem. Eng. 2018, 6, 16683–16691. [Google Scholar] [CrossRef]
- Zhang, Z.; Hu, G.; Cao, Y.; Duan, J.; Du, K.; Peng, Z. Hierarchical LiMnPO4 assembled from nanosheets via a solvothermal method as a high performance cathode material. RSC Adv. 2015, 5, 81461–81467. [Google Scholar] [CrossRef]
- Yang, H.; Fu, C.; Sun, Y.; Wang, L.; Liu, T. Fe-doped LiMnPO4@C nanofibers with high Li-ion diffusion coefficient. Carbon 2020, 158, 102–109. [Google Scholar] [CrossRef]
- Qin, Z.; Zhou, X.; Xia, Y.; Tang, C.; Liu, Z. Morphology controlled synthesis and modification of high-performance LiMnPO4 cathode materials for Li-ion batteries. J. Mater. Chem. 2012, 22, 21144. [Google Scholar] [CrossRef]
- Kim, J.; Park, K.-Y.; Park, I.; Yoo, J.-K.; Hong, J.; Kang, K. Thermal stability of Fe-Mn binary olivine cathodes for Li rechargeable batteries. J. Mater. Chem. 2012, 22, 11964. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, Y.; Hu, C.; Hou, Z.; Wei, C.; Wang, J.-G. Green Phytic Acid-Assisted Synthesis of LiMn1-xFexPO4/C Cathodes for High-Performance Lithium-Ion Batteries. Nanomaterials 2024, 14, 1360. https://doi.org/10.3390/nano14161360
Li Y, Hu C, Hou Z, Wei C, Wang J-G. Green Phytic Acid-Assisted Synthesis of LiMn1-xFexPO4/C Cathodes for High-Performance Lithium-Ion Batteries. Nanomaterials. 2024; 14(16):1360. https://doi.org/10.3390/nano14161360
Chicago/Turabian StyleLi, Yueying, Chenlu Hu, Zhidong Hou, Chunguang Wei, and Jian-Gan Wang. 2024. "Green Phytic Acid-Assisted Synthesis of LiMn1-xFexPO4/C Cathodes for High-Performance Lithium-Ion Batteries" Nanomaterials 14, no. 16: 1360. https://doi.org/10.3390/nano14161360
APA StyleLi, Y., Hu, C., Hou, Z., Wei, C., & Wang, J.-G. (2024). Green Phytic Acid-Assisted Synthesis of LiMn1-xFexPO4/C Cathodes for High-Performance Lithium-Ion Batteries. Nanomaterials, 14(16), 1360. https://doi.org/10.3390/nano14161360







