Enhancing Slurry Stability and Surface Flatness of Silicon Wafers through Organic Amine-Catalyzed Synthesis Silica Sol
Abstract
:1. Introduction
2. Materials and Methods
2.1. Experimental Details
2.1.1. Synthesis of Silica Nanoparticles Using Organic Amines as Catalysts
2.1.2. Determination of Hydroxyl Content of Silica Nanoparticles
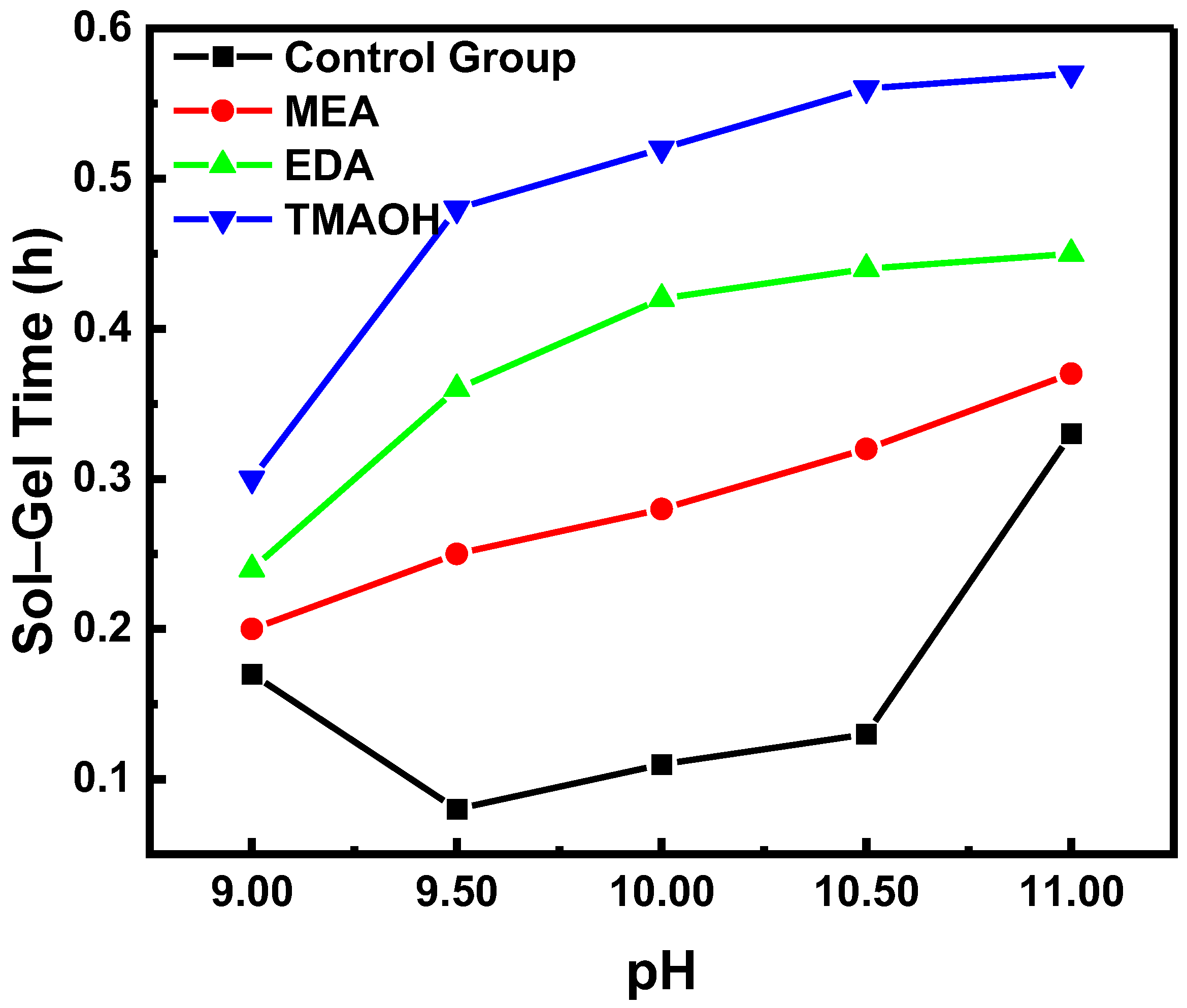
2.1.3. Sol–Gel Experiment
2.1.4. CMP Experiment
2.2. Characterization Methods
2.2.1. Characterizations of Silica Particles
2.2.2. Characterizations of Silicon Polishing
3. Results and Discussion
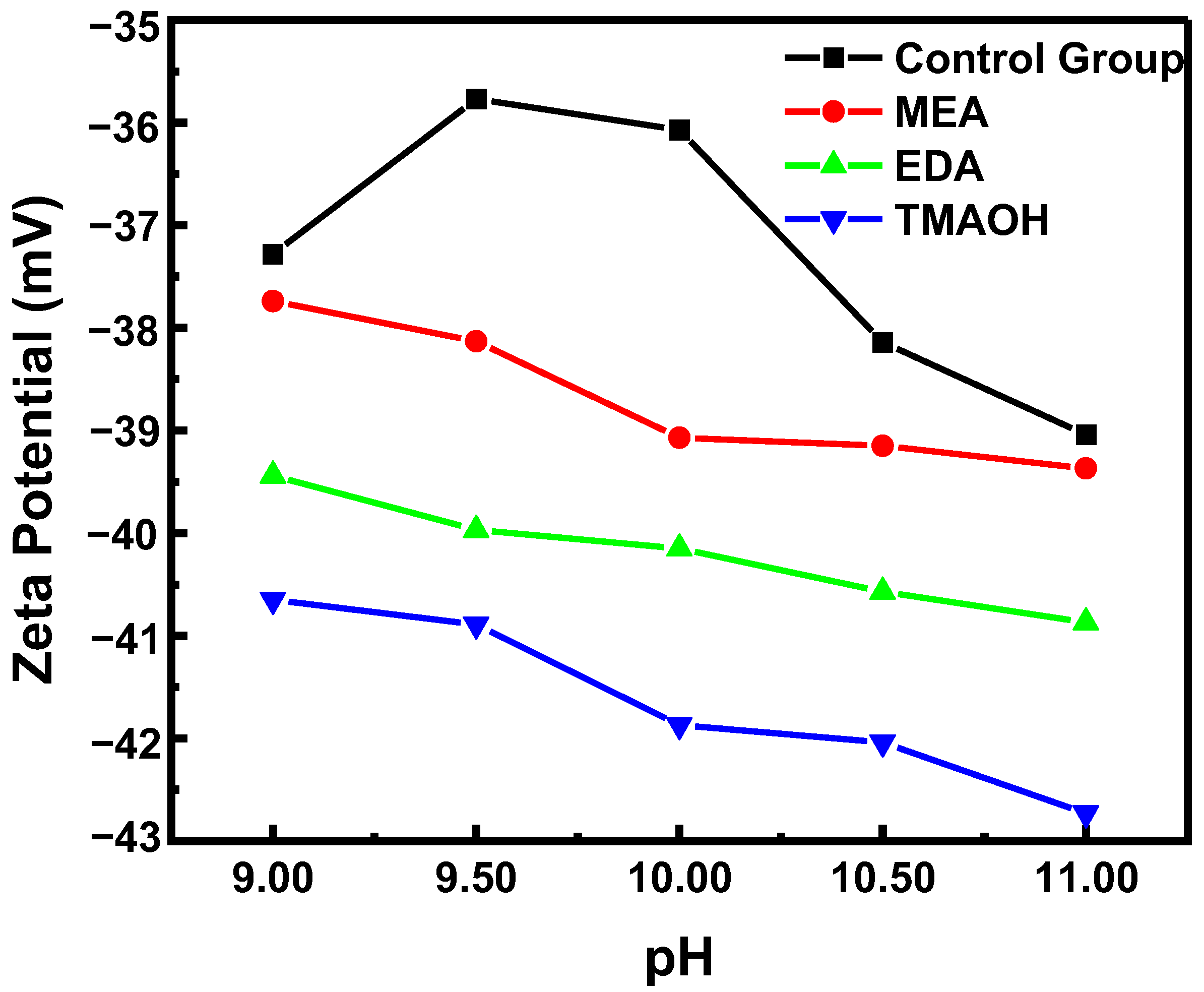
3.1. Stability Analysis of Organic Amine-Catalyzed Silica
3.2. Analysis of Silanol Groups of Silica Particles
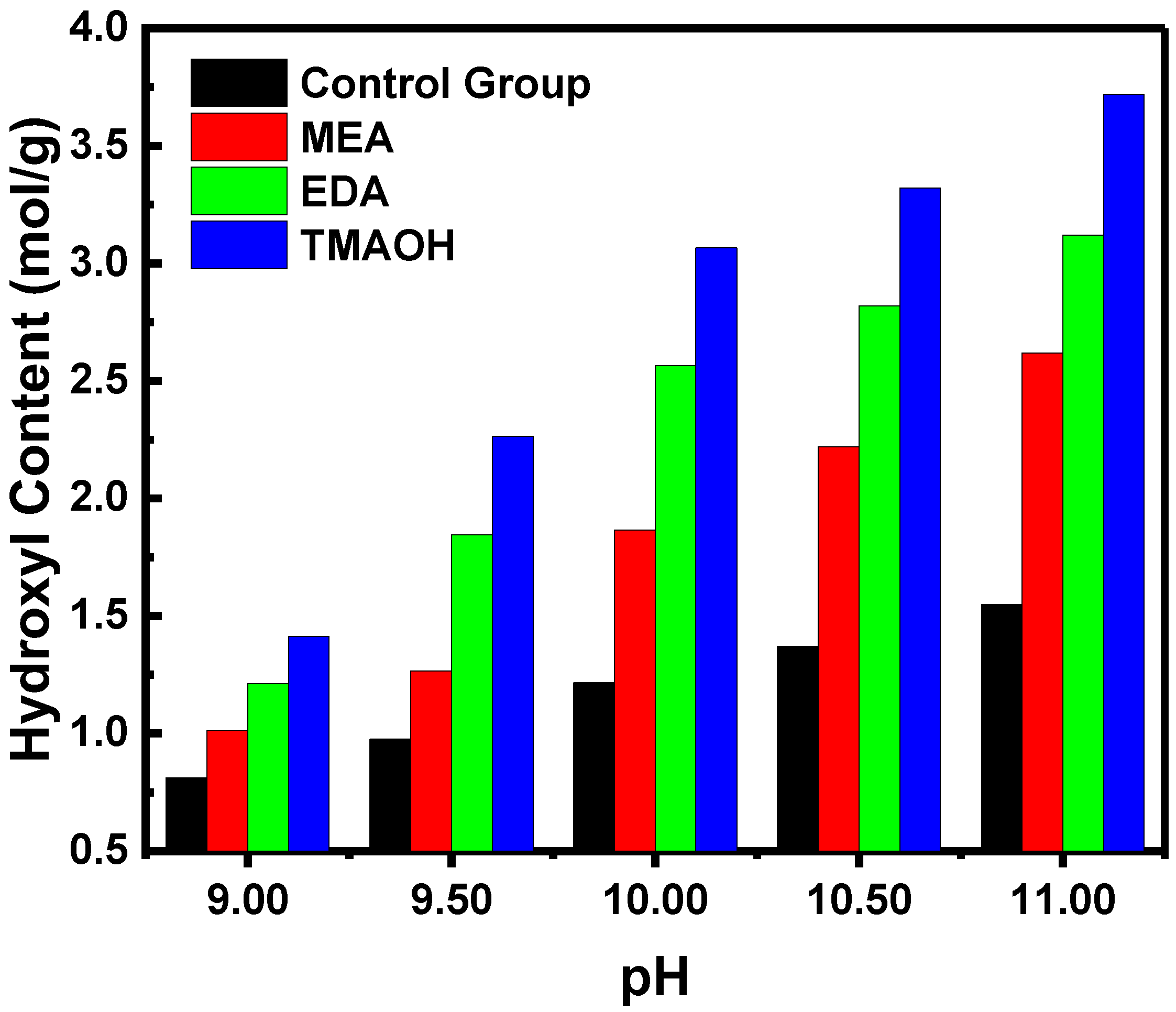
3.2.1. Quantification Testing of Hydroxyl Content
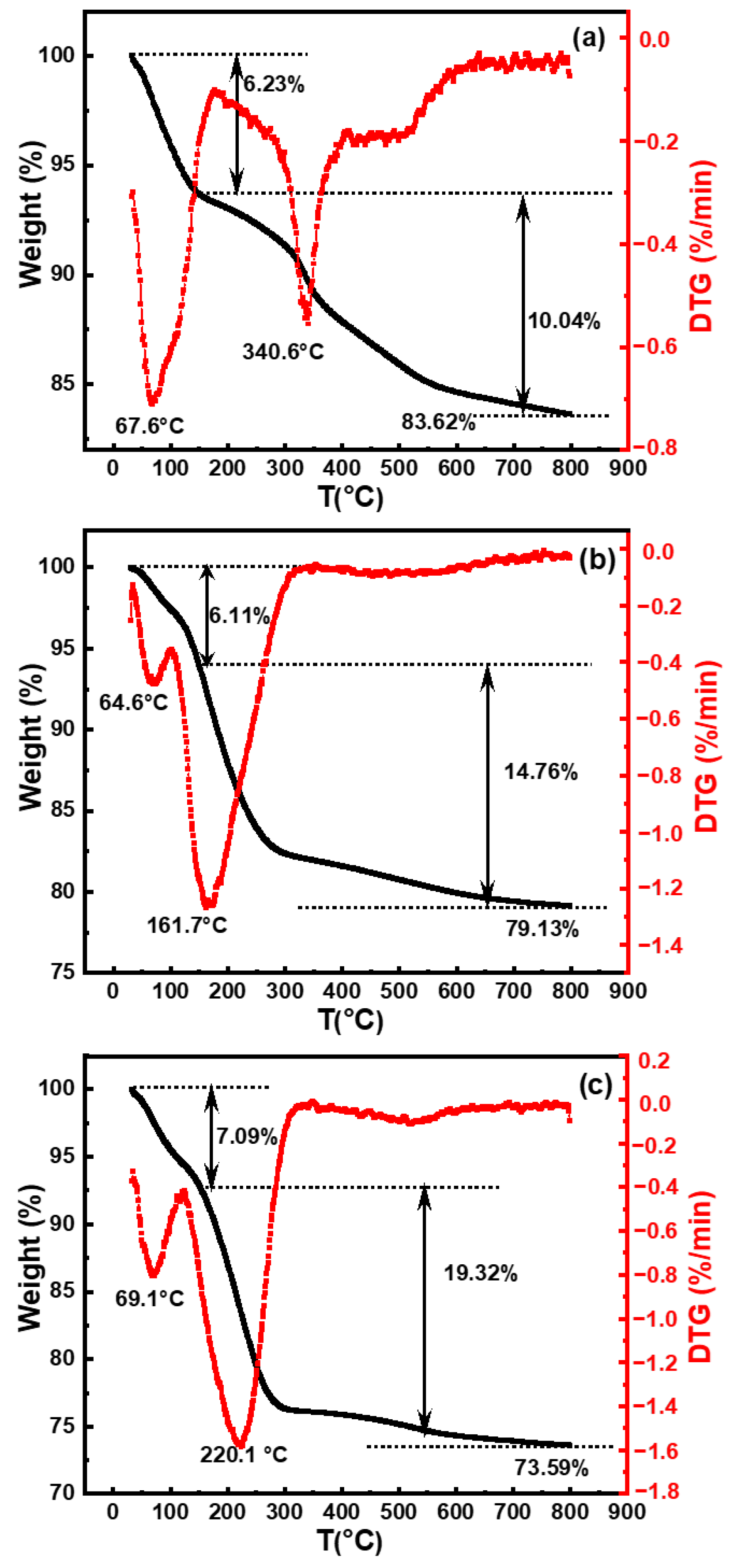
3.2.2. TGA
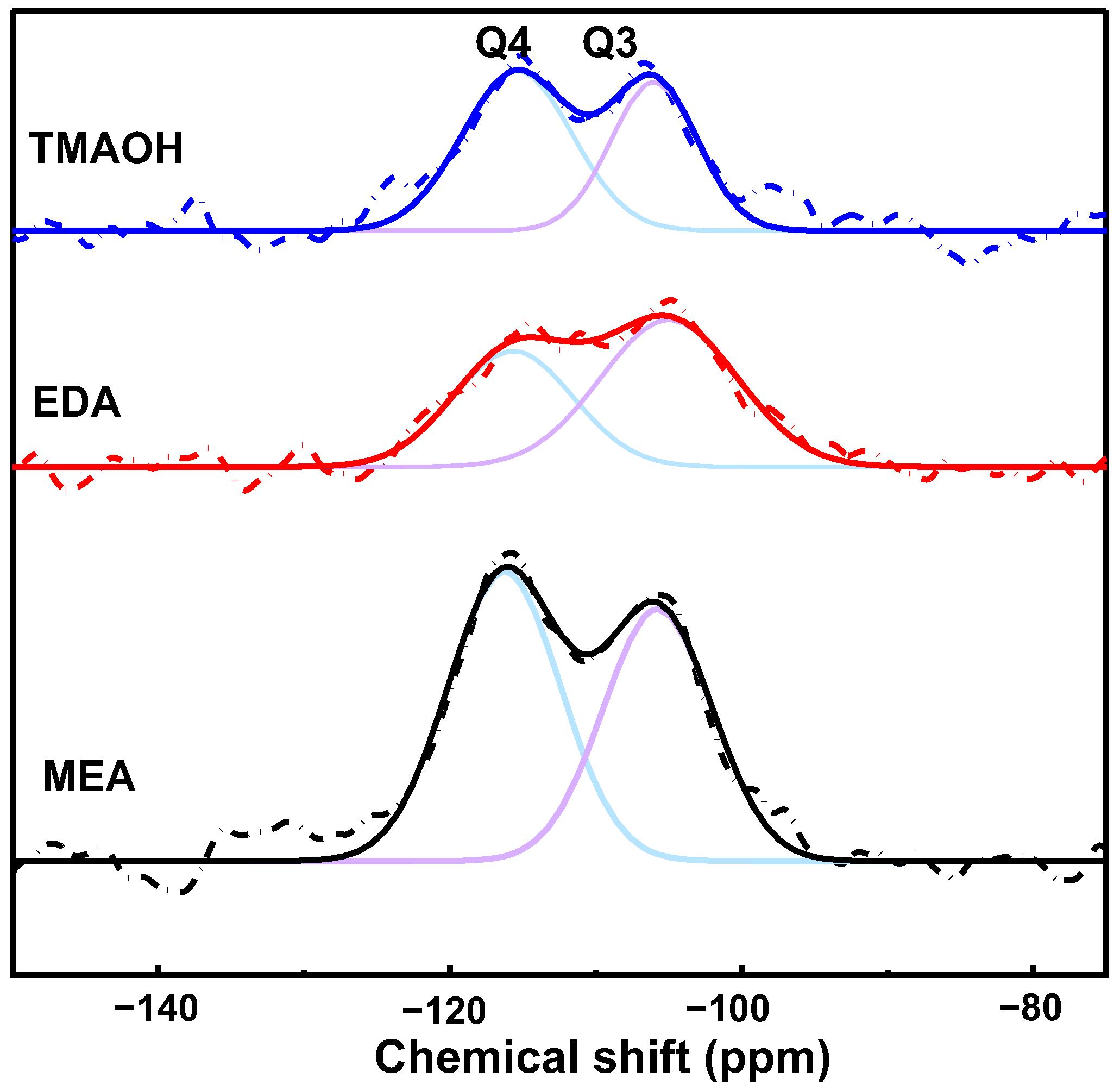
3.2.3. The 29Si Solid-State NMR Analysis
3.2.4. Micropore and Internal Hydroxyls Analysis
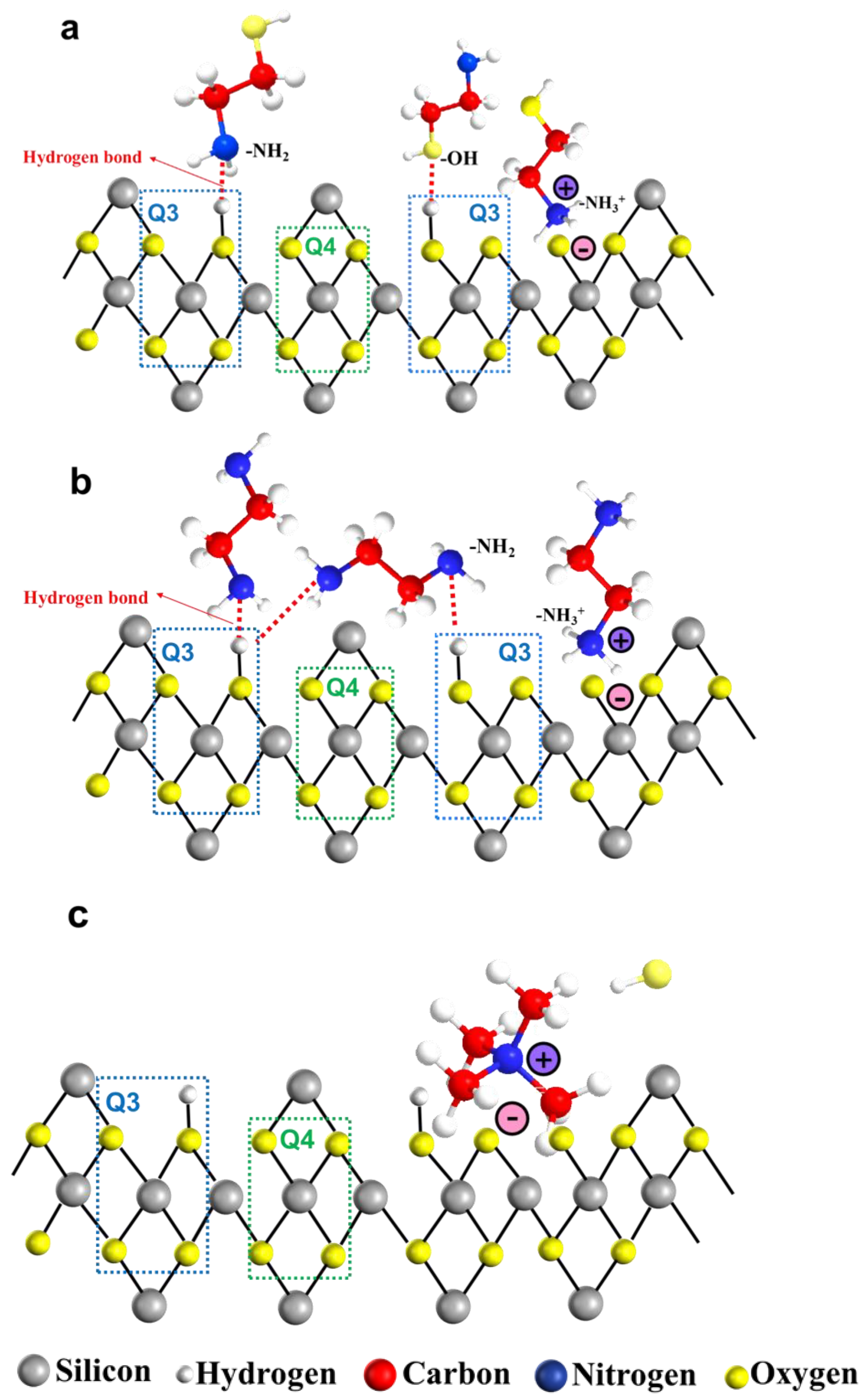
3.2.5. The Adsorption between Organic Amine and Silanol Groups
3.3. Application of Silica Sol with pH = 10.50 in Silicon Wafer Polishing
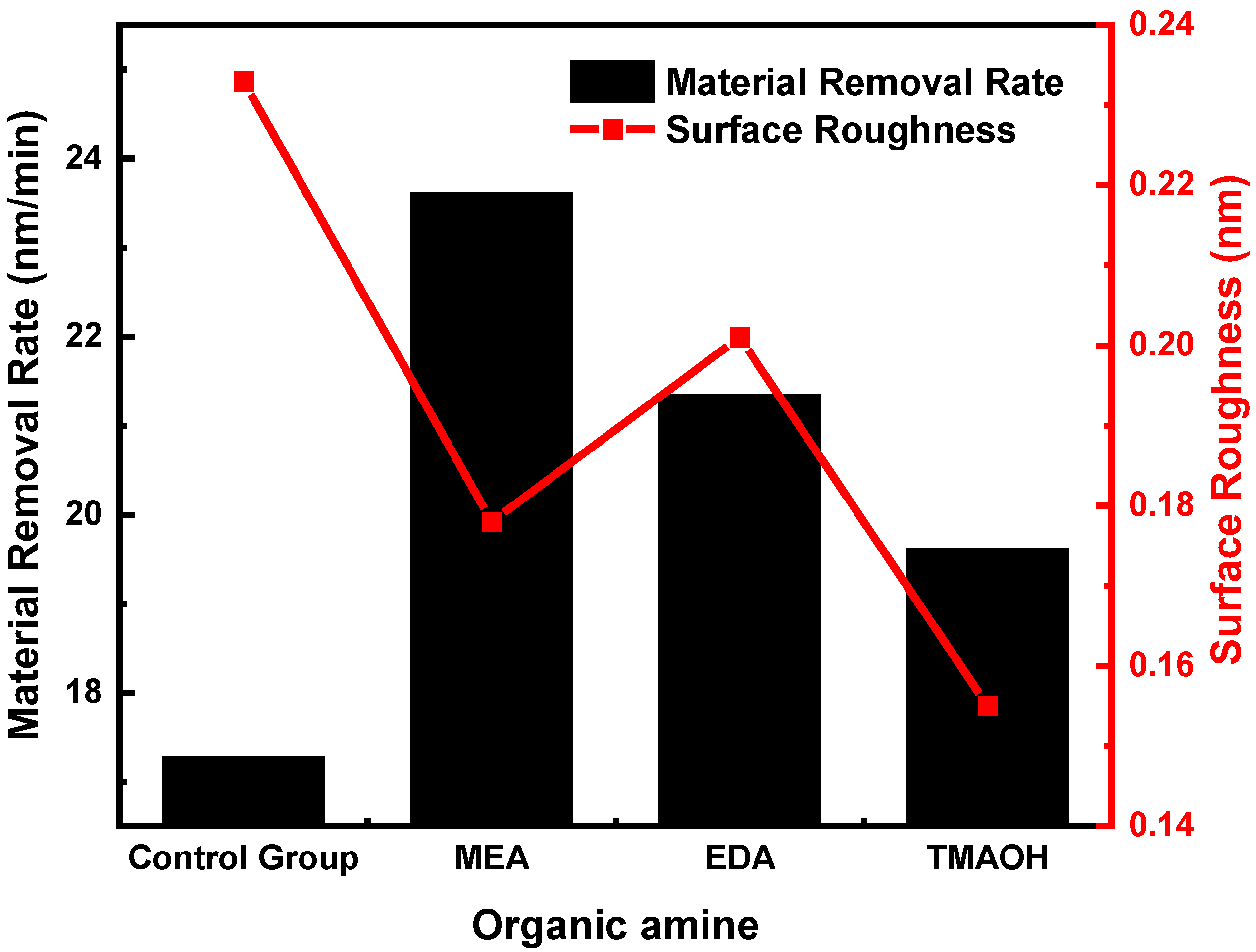
3.3.1. Material Removal Rate and Surface Morphology Analysis
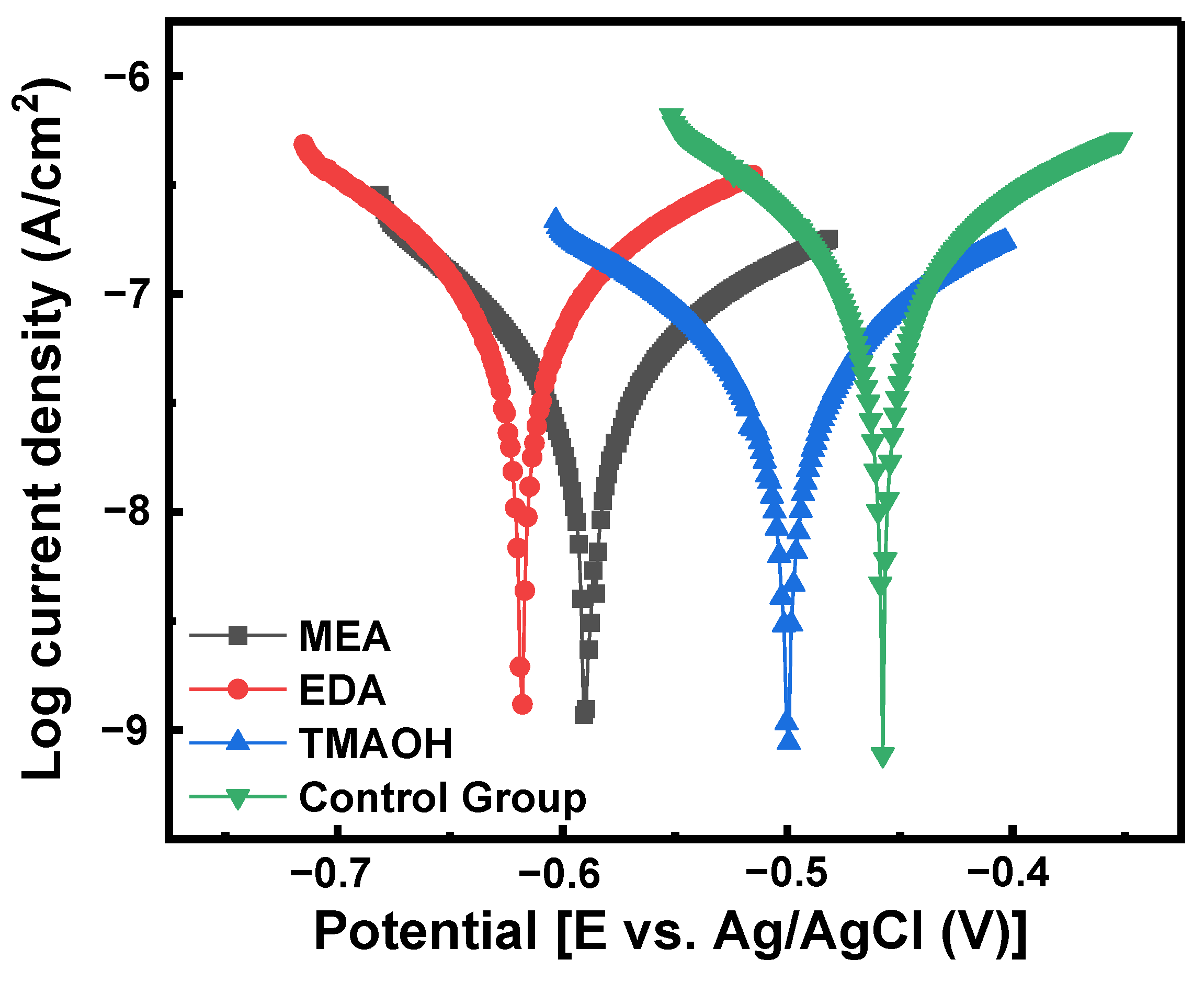
3.3.2. Electrochemical Analysis
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Zhang, D.; Lu, J.J.Q. 3D Integration Technologies: An Overview. In Materials for Advanced Packaging; Lu, D., Wong, C.P., Eds.; Springer International Publishing: Cham, Switzerland, 2017; pp. 1–26. [Google Scholar]
- Bae, J.Y.; Han, M.H.; Lee, S.J.; Kim, E.S.; Lee, K.; Lee, G.S.; Park, J.H.; Park, J.G. Silicon Wafer CMP Slurry Using a Hydrolysis Reaction Accelerator with an Amine Functional Group Remarkably Enhances Polishing Rate. Nanomaterials 2022, 12, 3893. [Google Scholar] [CrossRef]
- Koyanagi, M.; Fukushima, T.; Tanaka, T. High-Density Through Silicon Vias for 3-D LSIs. Proc. IEEE 2009, 97, 49–59. [Google Scholar] [CrossRef]
- Li, G.; Xiao, C.; Zhang, S.; Sun, R.; Wu, Y. An experimental investigation of silicon wafer thinning by sequentially using constant-pressure diamond grinding and fixed-abrasive chemical mechanical polishing. J. Mater. Process. Technol. 2022, 301, 117453. [Google Scholar] [CrossRef]
- Kwon, Y.C.; Lee, S.H.; Lee, J. 25.4 A 20 nm 6 GB Function-In-Memory DRAM, Based on HBM2 with a 1.2TFLOPS Programmable Computing Unit Using Bank-Level Parallelism, for Machine Learning Applications. In Proceedings of the 2021 IEEE International Solid-State Circuits Conference (ISSCC), San Francisco, CA, USA, 13–22 February 2021; Volume 64, pp. 350–352. [Google Scholar]
- Yoshida, K. Abrasive properties of nano silica particles prepared by a sol–gel method for polishing silicon wafers. J. Sol-Gel Sci. Technol. 2007, 43, 9–13. [Google Scholar] [CrossRef]
- Stöber, W.; Fink, A.; Bohn, E. Controlled growth of monodisperse silica spheres in the micron size range. J. Colloid Interface Sci. 1968, 26, 62–69. [Google Scholar] [CrossRef]
- Green, D.L.; Jayasundara, S.; Lam, Y.F.; Harris, M.T. Chemical reaction kinetics leading to the first Stober silica nanoparticles—NMR and SAXS investigation. J. Non-Cryst. Solids 2003, 315, 166–179. [Google Scholar] [CrossRef]
- Malay, O.; Yilgor, I.; Menceloglu, Y.Z. Effects of solvent on TEOS hydrolysis kinetics and silica particle size under basic conditions. J. Sol-Gel Sci. Technol. 2013, 67, 351–361. [Google Scholar] [CrossRef]
- Bourebrab, M.A.; Oben, D.T.; Durand, G.G.; Taylor, P.G.; Bruce, J.I.; Bassindale, A.R.; Taylor, A. Influence of the initial chemical conditions on the rational design of silica particles. J. Sol-Gel Sci. Technol. 2018, 88, 430–441. [Google Scholar] [CrossRef]
- Bari, A.H.; Jundale, R.B.; Kulkarni, A.A. Understanding the role of solvent properties on reaction kinetics for synthesis of silica nanoparticles. Chem. Eng. J. 2020, 398, 125427. [Google Scholar] [CrossRef]
- Xu, Y.; Liu, R.; Wu, D.; Sun, Y.; Gao, H.; Yuan, H.; Deng, F. Ammonia-catalyzed hydrolysis kinetics of mixture of tetraethoxysilane with methyltriethoxysilane by 29Si NMR. J. Non-Cryst. Solids 2005, 351, 2403–2413. [Google Scholar] [CrossRef]
- Xu, Y.; Sun, X.; Wu, D.; Sun, Y.; Yang, Y.; Yuan, H.; Deng, F.; Wu, Z. Ammonia Catalyzed Hydrolysis-Condensation Kinetics of Tetraethoxysilane/Dimethyldiethoxysilane Mixtures Studied by 29 Si NMR and SAXS. J. Solut. Chem. 2007, 36, 327–344. [Google Scholar] [CrossRef]
- Dong, H.; Han, Y.; Yang, W. Preparation of fractal-like silica particles based on Stöber method by using tetrabutylammonium hydroxide as co-catalyst. Colloids Surf. A Physicochem. Eng. Asp. 2021, 626, 127091. [Google Scholar] [CrossRef]
- Meier, M.; Ungerer, J.; Klinge, M.; Nirschl, H. Synthesis of nanometric silica particles via a modified Stöber synthesis route. Colloids Surf. A Physicochem. Eng. Asp. 2018, 538, 559–564. [Google Scholar] [CrossRef]
- Yokoi, T.; Iwama, M.; Watanabe, R.; Sakamoto, Y.; Terasaki, O.; Kubota, Y.; Kondo, J.N.; Okubo, T.; Tatsumi, T. Synthesis of well-ordered nanospheres with uniform mesopores assisted by basic amino acids. Stud. Surf. Sci. Catal. 2007, 170, 1774–1780. [Google Scholar]
- Yokoi, T.; Wakabayashi, J.; Otsuka, Y.; Fan, W.; Iwama, M.; Watanabe, R.; Aramaki, K.; Shimojima, A.; Tatsumi, T.; Okubo, T. Mechanism of formation of uniform-sized silica nanospheres catalyzed by basic amino acids. Chem. Mater. 2009, 21, 3719–3729. [Google Scholar] [CrossRef]
- Carcouet, C.C.; van de Put, M.W.; Mezari, B.; Magusin, P.C.; Laven, J.; Bomans, P.H.; Friedrich, H.; Esteves, A.C.; Sommerdijk, N.A.; van Benthem, R.A.; et al. Nucleation and growth of monodisperse silica nanoparticles. Nano Lett. 2014, 14, 1433–1438. [Google Scholar] [CrossRef]
- Xing, Y.; Wu, Z.; Li, Y.; Gong, J.; Zhang, Z.; Liu, W.; Song, Z. Understanding the stability behavior of colloidal silica in different alkali environments. J. Nanopart. Res. 2024, 26, 92. [Google Scholar] [CrossRef]
- Jiang, B.; Guan, J.; Zhao, P.; Chen, Y.; Zhang, Z. Effect of Polyoxyethylene-Based Nonionic Surfactants on Chemical–Mechanical Polishing Performance of Monocrystalline Silicon Wafers. Crystals 2024, 14, 460. [Google Scholar] [CrossRef]
- Dong, A.; Huang, J.; Lan, S.; Wang, T.; Xiao, L.; Wang, W.; Zhao, T.; Zheng, X.; Liu, F.; Gao, G.; et al. Synthesis of N-halamine-functionalized silica-polymer core-shell nanoparticles and their enhanced antibacterial activity. Nanotechnology 2011, 22, 295602. [Google Scholar] [CrossRef]
- Legrand, A.; Hommel, H.; Tuel, A.; Vidal, A.; Balard, H.; Papirer, E.; Levitz, P.; Czernichowski, M.; Erre, R.; Van Damme, H. Hydroxyls of silica powders. Adv. Colloid Interface Sci. 1990, 33, 91–330. [Google Scholar] [CrossRef]
- Bergna, H.; Roberts, W. Colloidal Silica: Fundamentals and Applications; CRC Press: Boca Raton, FL, USA, 2005. [Google Scholar]
- Pasqua, L.; Procopio, A.; Oliverio, M.; Paonessa, R.; Prete, R.; Nardi, M.; Casula, M.F.; Testa, F.; Nagy, J.B. Hybrid MCM-41 grafted by a general microwave-assisted procedure: A characterization study. J. Porous Mater. 2013, 20, 865–873. [Google Scholar] [CrossRef]
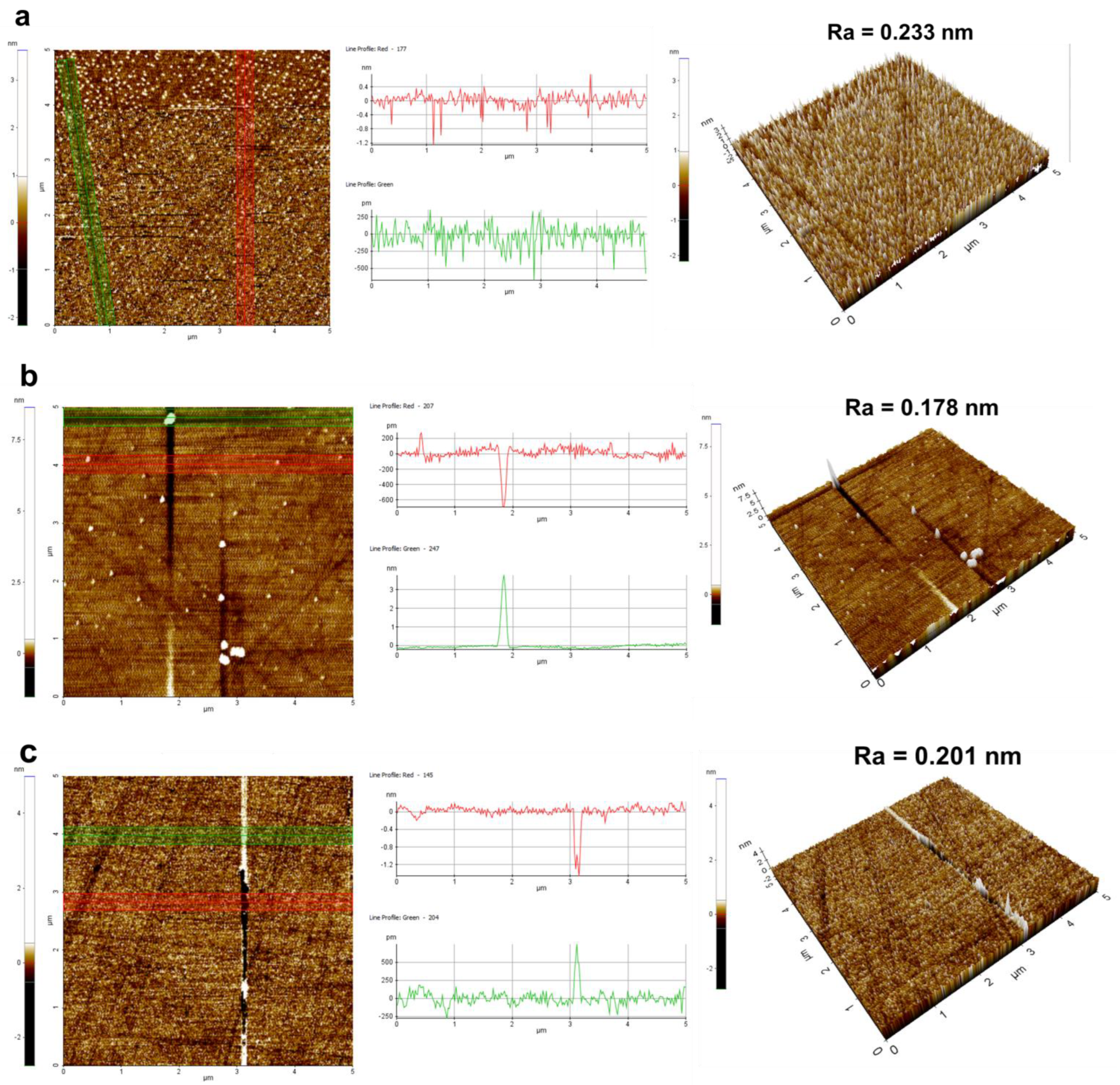
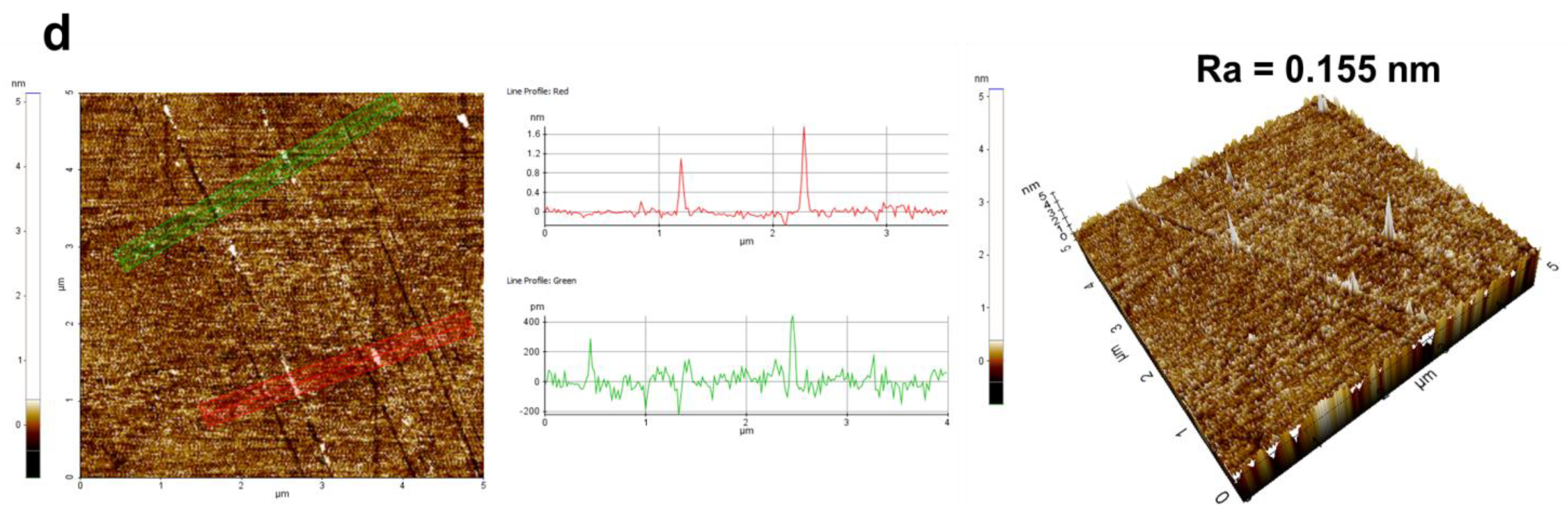
| Process Parameters | Value |
|---|---|
| Polishing time | 10 min |
| Pad/Head rotation speed | 40/40 rpm |
| Wafer rotation speed | 30 rpm |
| Slurry flow rate | 155 mL/min |
| Pressure/Down force | 2 psi |
| Parameter | Samples | ||
|---|---|---|---|
| MEA | EDA | TMAOH | |
| SBET a (m2/g) | 44.22 | 10.02 | 23.34 |
| Smicro b (m2/g) | 5.42 | 0.75 | 13.63 |
| Smicro/SBET (%) | 12.48 | 7.48 | 58.39 |
| Vt c (cm3/g) | 0.29 | 0.04 | 0.12 |
| Vmicro d (cm3/g) | 2.7 × 10−3 | 3.18 × 10−4 | 2.9 × 10−3 |
| Vmicro/Vt | 0.93 | 0.79 | 2.41 |
| Slurry | Components (pH = 10.50) | Ecorr (V) | Icorr (A/cm2) | IE% |
|---|---|---|---|---|
| 1 | Control Group | −0.4577 | 3.7246 × 10−7 | / |
| 2 | SiO2 + MEA | −0.4999 | 4.5896 × 10−8 | 87 |
| 3 | SiO2 + EDA | −0.5679 | 4.1846 × 10−8 | 88 |
| 4 | SiO2 + TMAOH | −0.5892 | 3.7797 × 10−8 | 89 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xing, Y.; Wang, W.; Liu, W.; Song, Z. Enhancing Slurry Stability and Surface Flatness of Silicon Wafers through Organic Amine-Catalyzed Synthesis Silica Sol. Nanomaterials 2024, 14, 1371. https://doi.org/10.3390/nano14161371
Xing Y, Wang W, Liu W, Song Z. Enhancing Slurry Stability and Surface Flatness of Silicon Wafers through Organic Amine-Catalyzed Synthesis Silica Sol. Nanomaterials. 2024; 14(16):1371. https://doi.org/10.3390/nano14161371
Chicago/Turabian StyleXing, Yi, Weilei Wang, Weili Liu, and Zhitang Song. 2024. "Enhancing Slurry Stability and Surface Flatness of Silicon Wafers through Organic Amine-Catalyzed Synthesis Silica Sol" Nanomaterials 14, no. 16: 1371. https://doi.org/10.3390/nano14161371





