Metal Bionanohybrids against Microbiologically Influenced Corrosion (MIC) Consortia
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Culture Media
2.3. Synthesis of Copper Bionanohybrids
2.4. Synthesis of Copper–Silver Bionanohybrids
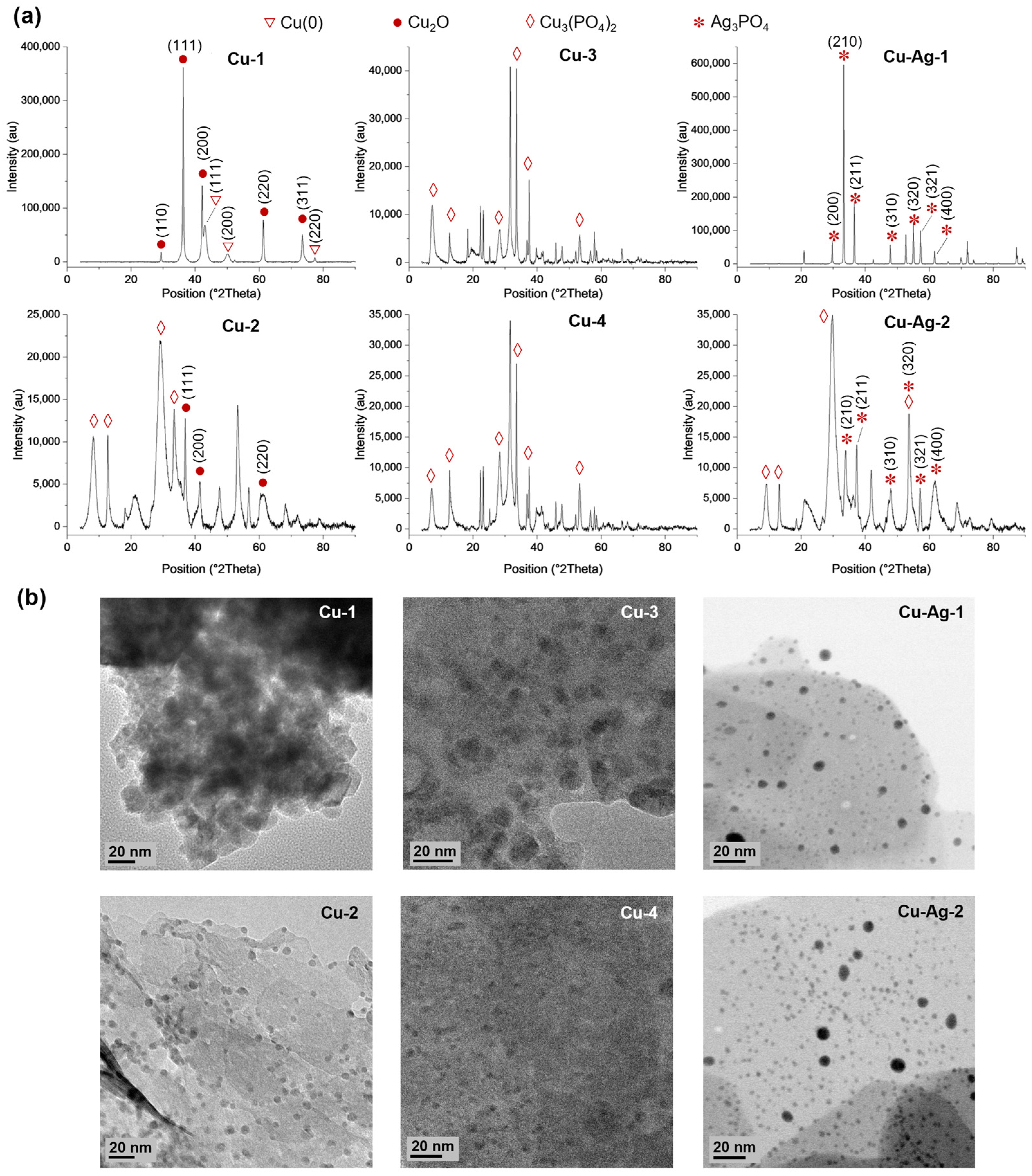
2.5. Bionanohybrid Characterization Techniques
2.6. Bacteria Growth
2.7. Cell Counts
2.8. Antibacterial Activity of Bionanohybrids
2.9. Coupons Experiments
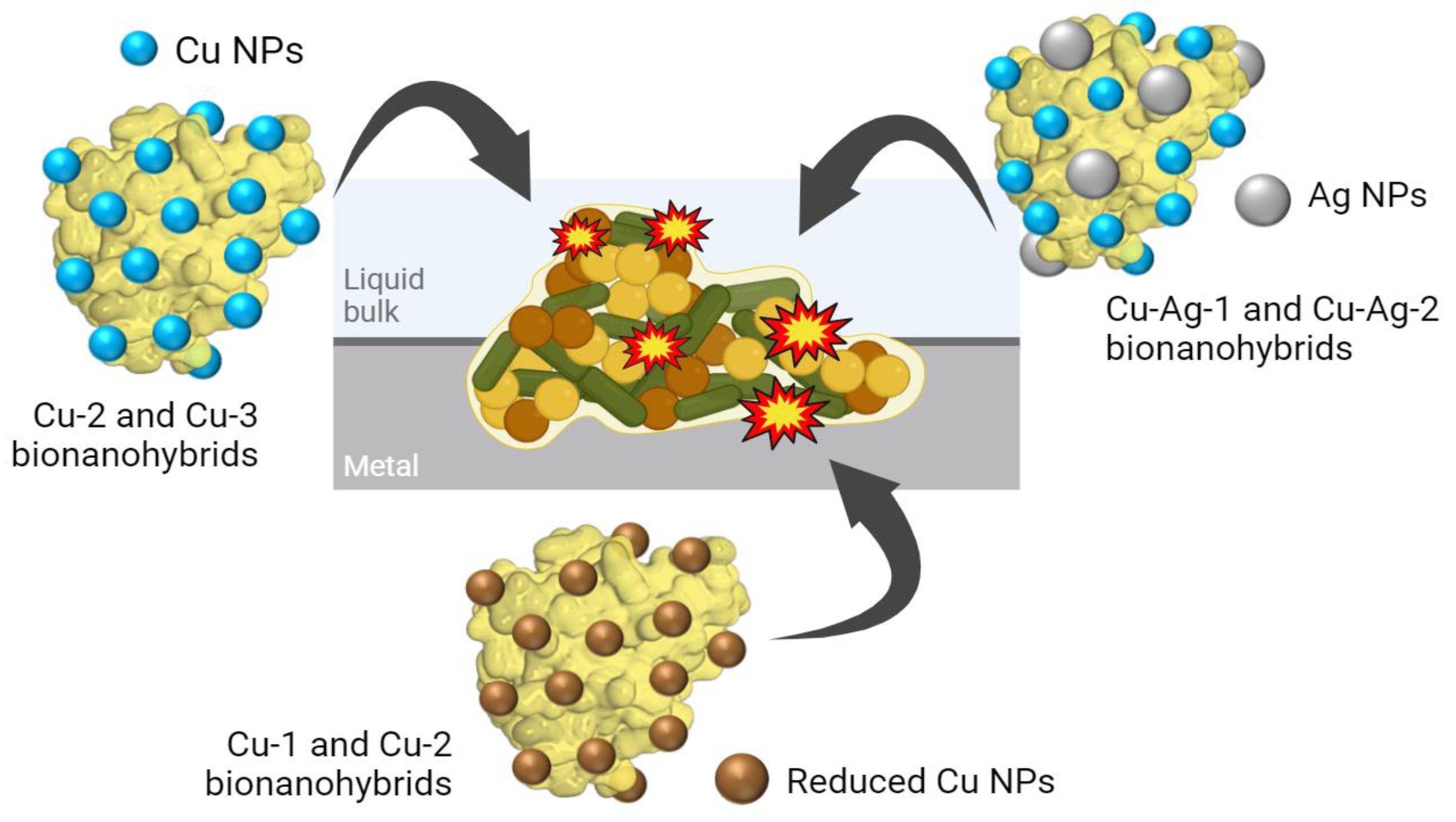
3. Results and Discussion
3.1. Synthesis and Characterization of Bionanohybrids
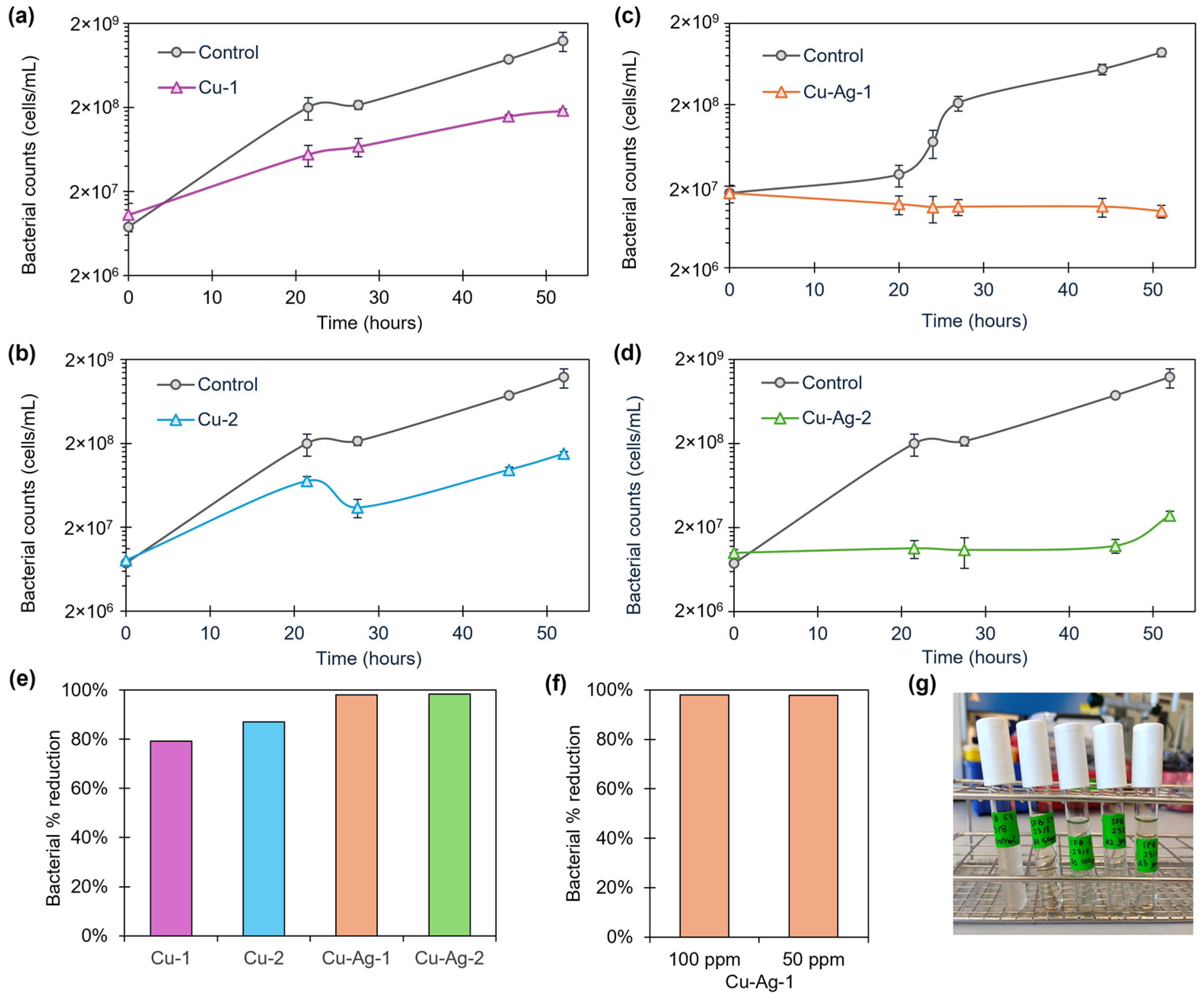
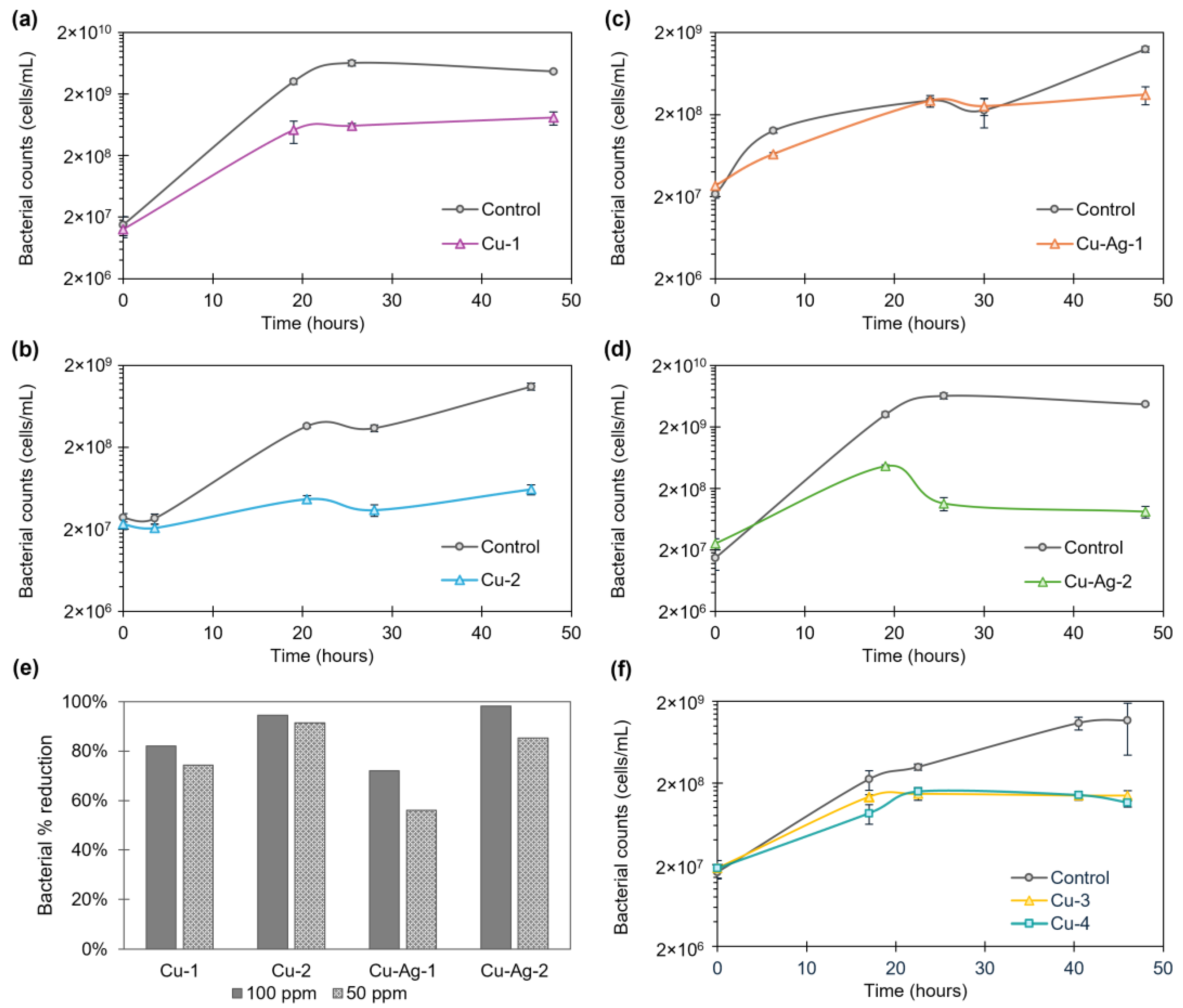
3.2. Antibacterial Activity of Bionanohybrids
3.2.1. Slime-Forming Bacteria
3.2.2. Sulfate-Reducing Bacteria
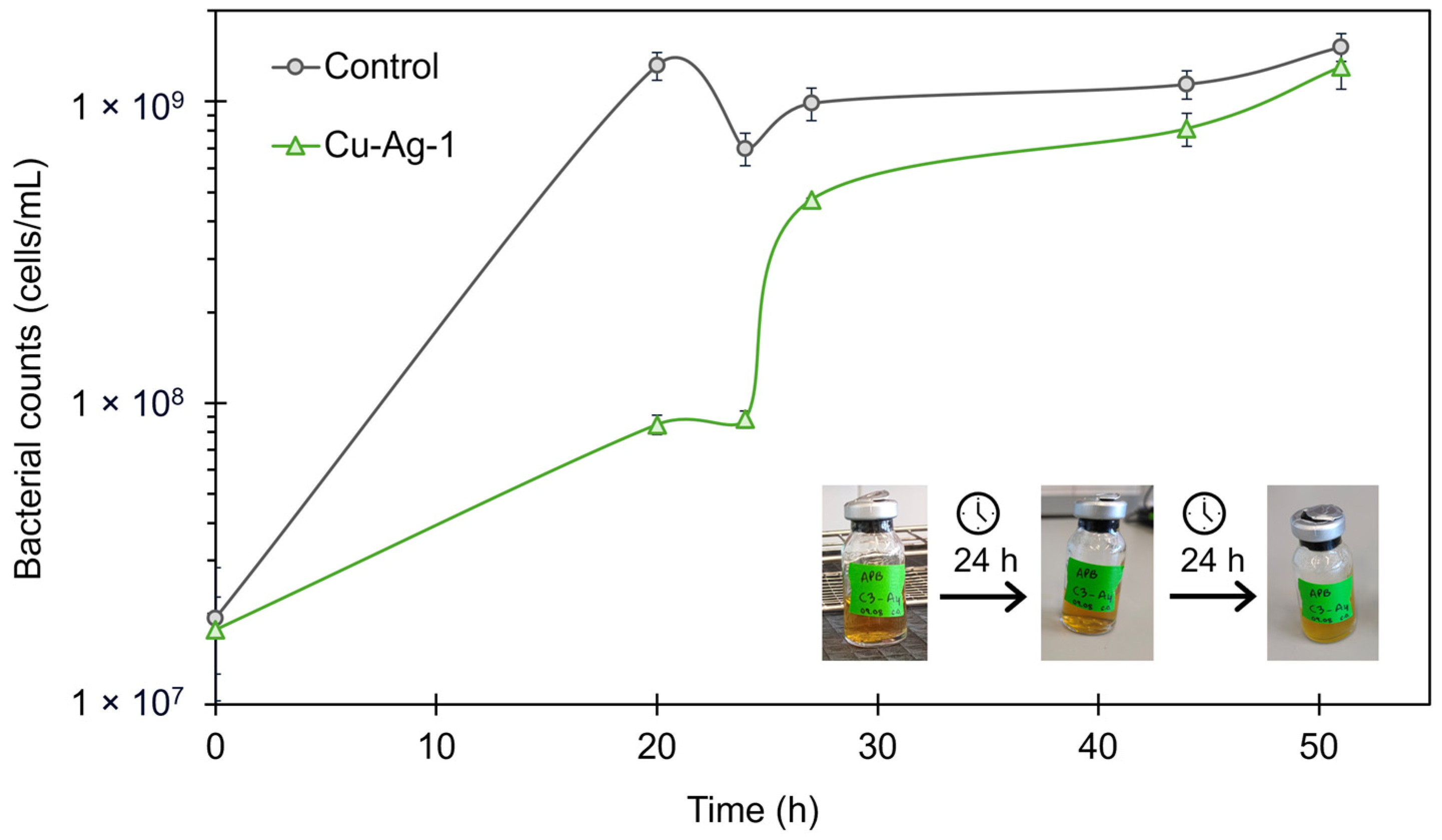
3.2.3. Acid-Producing Bacteria
3.3. Coupons Experiments
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Knisz, J.; Eckert, R.; Gieg, L.M.; Koerdt, A.; Lee, J.S.; Silva, E.R.; Skovhus, T.L.; An Stepec, B.A.; Wade, S.A. Microbiologically Influenced Corrosion—More than Just Microorganisms. FEMS Microbiol. Rev. 2023, 47, fuad041. [Google Scholar] [CrossRef]
- Eckert, R.B.; Skovhus, T.L. (Eds.) Failure Analysis of Microbiologically Influenced Corrosion. CRC Press: Boca Raton, FL, USA, 2021; ISBN 9780367356804. [Google Scholar]
- Javaherdashti, R. Microbiologically Influenced Corrosion (MIC). In Microbiologically Influenced Corrosion: An Engineering Insight; Javaherdashti, R., Ed.; Springer International Publishing: Cham, Switzerland, 2017; pp. 29–79. [Google Scholar]
- Amendola, R.; Acharjee, A. Microbiologically Influenced Corrosion of Copper and Its Alloys in Anaerobic Aqueous Environments: A Review. Front. Microbiol. 2022, 13, 806688. [Google Scholar] [CrossRef] [PubMed]
- Blackwood, D. An Electrochemist Perspective of Microbiologically Influenced Corrosion. Corros. Mater. Degrad. 2018, 1, 59–76. [Google Scholar] [CrossRef]
- Yuan, S.; Liang, B.; Zhao, Y.; Pehkonen, S.O. Surface Chemistry and Corrosion Behaviour of 304 Stainless Steel in Simulated Seawater Containing Inorganic Sulphide and Sulphate-Reducing Bacteria. Corros. Sci. 2013, 74, 353–366. [Google Scholar] [CrossRef]
- Byrnes, T. Pipeline Coatings. In Trends in Oil and Gas. Corrosion Research and Technologies: Production and Transmission; El-Sherik, A.M., Ed.; Woodhead Publishing: Sawston, UK, 2017; pp. 563–591. [Google Scholar] [CrossRef]
- Pormohammad, A.; Turner, R.J. Silver Antibacterial Synergism Activities with Eight Other Metal(Loid)-Based Antimicrobials against Escherichia Coli, Pseudomonas Aeruginosa, and Staphylococcus Aureus. Antibiotics 2020, 9, 853. [Google Scholar] [CrossRef]
- Sánchez-López, E.; Gomes, D.; Esteruelas, G.; Bonilla, L.; Lopez-Machado, A.L.; Galindo, R.; Cano, A.; Espina, M.; Ettcheto, M.; Camins, A.; et al. Metal-Based Nanoparticles as Antimicrobial Agents: An Overview. Nanomaterials 2020, 10, 292. [Google Scholar] [CrossRef]
- Wei, T.; Yu, Q.; Chen, H. Responsive and Synergistic Antibacterial Coatings: Fighting against Bacteria in a Smart and Effective Way. Adv. Healthc. Mater. 2019, 8, 1801381. [Google Scholar] [CrossRef]
- Kalajahi, S.T.; Misra, A.; Koerdt, A. Nanotechnology to mitigate microbiologically influenced corrosion (MIC). Front. Nanotechnol. 2024, 6, 1340352. [Google Scholar] [CrossRef]
- Jileugha, C.; Ezealisiji, K.M.; Ezejiofor, A.N.; Orisakweet, O.E. Microbiologically Influenced Corrosion: Uncovering Mechanisms and Discovering Inhibitor—Metal and Metal Nanoparticles as Promising Biocorrosion Inhibitors. J. Bio Tribo Corros. 2021, 7, 109. [Google Scholar] [CrossRef]
- Gautam, S.; Das, D.K.; Kaur, J.; Kumar, A.; Ubaidullah, M.; Hasan, M.; Yadav, K.K.; Gupta, R.K. Transition metal-based nanoparticles as potential antimicrobial agents: Recent advancements, mechanistic, challenges, and future prospects. Discov. Nano 2023, 18, 84. [Google Scholar] [CrossRef]
- Benavente, R.; Lopez-Tejedor, D.; del Puerto Morales, M.; Perez-Rizquez, C.; Palomo, J.M. The Enzyme-Induced Formation of Iron Hybrid Nanostructures with Different Morphologies. Nanoscale 2020, 12, 12917–12927. [Google Scholar] [CrossRef] [PubMed]
- Filice, M.; Marciello, M.; del Puerto Morales, M.; Palomo, J.M. Synthesis of Heterogeneous Enzyme–Metal Nanoparticle Biohybrids in Aqueous Media and Their Applications in C–C Bond Formation and Tandem Catalysis. Chem. Comm. 2013, 49, 6876. [Google Scholar] [CrossRef] [PubMed]
- Losada-Garcia, N.; Vazquez-Calvo, A.; Ortega-Alarcon, D.; Abian, O.; Velazquez-Campoy, A.; Domingo-Calap, P.; Alcami, A.; Palomo, J.M. Nanostructured Biohybrid Material with Wide-Ranging Antiviral Action. Nano Res. 2023, 16, 11455–11463. [Google Scholar] [CrossRef]
- Ortega-Nieto, C.; Losada-Garcia, N.; Pessela, B.C.; Domingo-Calap, P.; Palomo, J.M. Design and Synthesis of Copper Nanobiomaterials with Antimicrobial Properties. ACS Bio Med Chem Au 2023, 3, 349–358. [Google Scholar] [CrossRef]
- Rodríguez-Otero, A.; Losada-García, N.; Guerra-Rodríguez, S.; Palomo, J.M.; Rodríguez-Chueca, J. Antibacterial Effect of Metal-Enzyme Hybrid Nanomaterials. J. Environ. Chem. Eng. 2023, 11, 110499. [Google Scholar] [CrossRef]
- Godoy-Gallardo, M.; Eckhard, U.; Delgado, L.M.; de Roo Puente, Y.J.D.; Hoyos-Nogués, M.; Gil, F.J.; Perez, R.A. Antibacterial Approaches in Tissue Engineering Using Metal Ions and Nanoparticles: From Mechanisms to Applications. Bioact. Mater. 2021, 6, 4470–4490. [Google Scholar] [CrossRef] [PubMed]
- Shaikh, S.; Nazam, N.; Rizvi, S.M.D.; Ahmad, K.; Baig, M.H.; Lee, E.J.; Choi, I. Mechanistic Insights into the Antimicrobial Actions of Metallic Nanoparticles and Their Implications for Multidrug Resistance. Int. J. Mol. Sci. 2019, 20, 2468. [Google Scholar] [CrossRef]
- Calvano, C.D.; Picca, R.A.; Bonerba, E.; Tantillo, G.; Cioffi, N.; Palmisano, F. MALDI-TOF Mass Spectrometry Analysis of Proteins and Lipids in Escherichia coli Exposed to Copper Ions and Nanoparticles. J. Mass. Spectrom. 2016, 51, 828–840. [Google Scholar] [CrossRef]
- Wang, L.; Hu, C.; Shao, L. The Antimicrobial Activity of Nanoparticles: Present Situation and Prospects for the Future. Int. J. Nanomed. 2017, 12, 1227–1249. [Google Scholar] [CrossRef]
- Kędziora, A.; Speruda, M.; Krzyżewska, E.; Rybka, J.; Łukowiak, A.; Bugla-Płoskońska, G. Similarities and Differences between Silver Ions and Silver in Nanoforms as Antibacterial Agents. Int. J. Mol. Sci. 2018, 19, 444. [Google Scholar] [CrossRef] [PubMed]
- Roy, A.; Bulut, O.; Some, S.; Mandal, A.K.; Yilmaz, M.D. Green Synthesis of Silver Nanoparticles: Biomolecule-Nanoparticle Organizations Targeting Antimicrobial Activity. RSC Adv. 2019, 9, 2673–2702. [Google Scholar] [CrossRef]
- McCarthy, C.; Losada-Garcia, N.; Palomo, J.M. Direct Synthesis of Phenols from Phenylboronic Acids in Aqueous Media Catalyzed by a Cu(0)-Nanoparticles Biohybrid. ChemistrySelect 2020, 5, 7492–7496. [Google Scholar] [CrossRef]
- El-Sherik, A.M. Trends in Oil and Gas Corrosion Research and Technologies: Production and Transmission; El-Sherik, A.M., Ed.; Woodhead Publishing: Sawston, UK, 2017; ISBN 978-0-08-101105-8. [Google Scholar]
- Lee, I.; Cheon, H.J.; Adhikari, M.D.; Tran, T.D.; Yeon, K.-M.; Kim, M.I.; Kim, J. Glucose Oxidase-Copper Hybrid Nanoflowers Embedded with Magnetic Nanoparticles as an Effective Antibacterial Agent. Int. J. Biol. Macromol. 2020, 155, 1520–1531. [Google Scholar] [CrossRef]
- Xiong, J.; Cai, X.; Ge, J. Enzyme–Metal Nanocomposites for Antibacterial Applications. Particuology 2022, 64, 134–139. [Google Scholar] [CrossRef]
- Kessler, A.; Hedberg, J.; Blomberg, E.; Odnevall, I. Reactive Oxygen Species Formed by Metal and Metal Oxide Nanoparticles in Physiological Media—A Review of Reactions of Importance to Nanotoxicity and Proposal for Categorization. Nanomaterials 2022, 12, 1922. [Google Scholar] [CrossRef]
- Lemire, J.; Harrison, J.; Turner, R. Antimicrobial activity of metals: Mechanisms, molecular targets and applications. Nat. Rev. Microbiol. 2013, 11, 371–384. [Google Scholar] [CrossRef] [PubMed]
- Alfei, S.; Schito, G.C.; Schito, A.M.; Zuccari, G. Reactive Oxygen Species (ROS)-Mediated Antibacterial Oxidative Therapies: Available Methods to Generate ROS and a Novel Option Proposal. Int. J. Mol.Sci. 2024, 25, 7182. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ortega-Nieto, C.; Salta, M.; Noël-Hermes, N.; Palomo, J.M. Metal Bionanohybrids against Microbiologically Influenced Corrosion (MIC) Consortia. Nanomaterials 2024, 14, 1376. https://doi.org/10.3390/nano14171376
Ortega-Nieto C, Salta M, Noël-Hermes N, Palomo JM. Metal Bionanohybrids against Microbiologically Influenced Corrosion (MIC) Consortia. Nanomaterials. 2024; 14(17):1376. https://doi.org/10.3390/nano14171376
Chicago/Turabian StyleOrtega-Nieto, Clara, Maria Salta, Nanni Noël-Hermes, and Jose M. Palomo. 2024. "Metal Bionanohybrids against Microbiologically Influenced Corrosion (MIC) Consortia" Nanomaterials 14, no. 17: 1376. https://doi.org/10.3390/nano14171376
APA StyleOrtega-Nieto, C., Salta, M., Noël-Hermes, N., & Palomo, J. M. (2024). Metal Bionanohybrids against Microbiologically Influenced Corrosion (MIC) Consortia. Nanomaterials, 14(17), 1376. https://doi.org/10.3390/nano14171376






