Deepening the Understanding of Carbon Active Sites for ORR Using Electrochemical and Spectrochemical Techniques
Abstract
1. Introduction
2. Experimental
2.1. Materials and Reagents
2.2. Purification and Thermal Treatment of the MWCNTs
2.3. Physicochemical Characterization
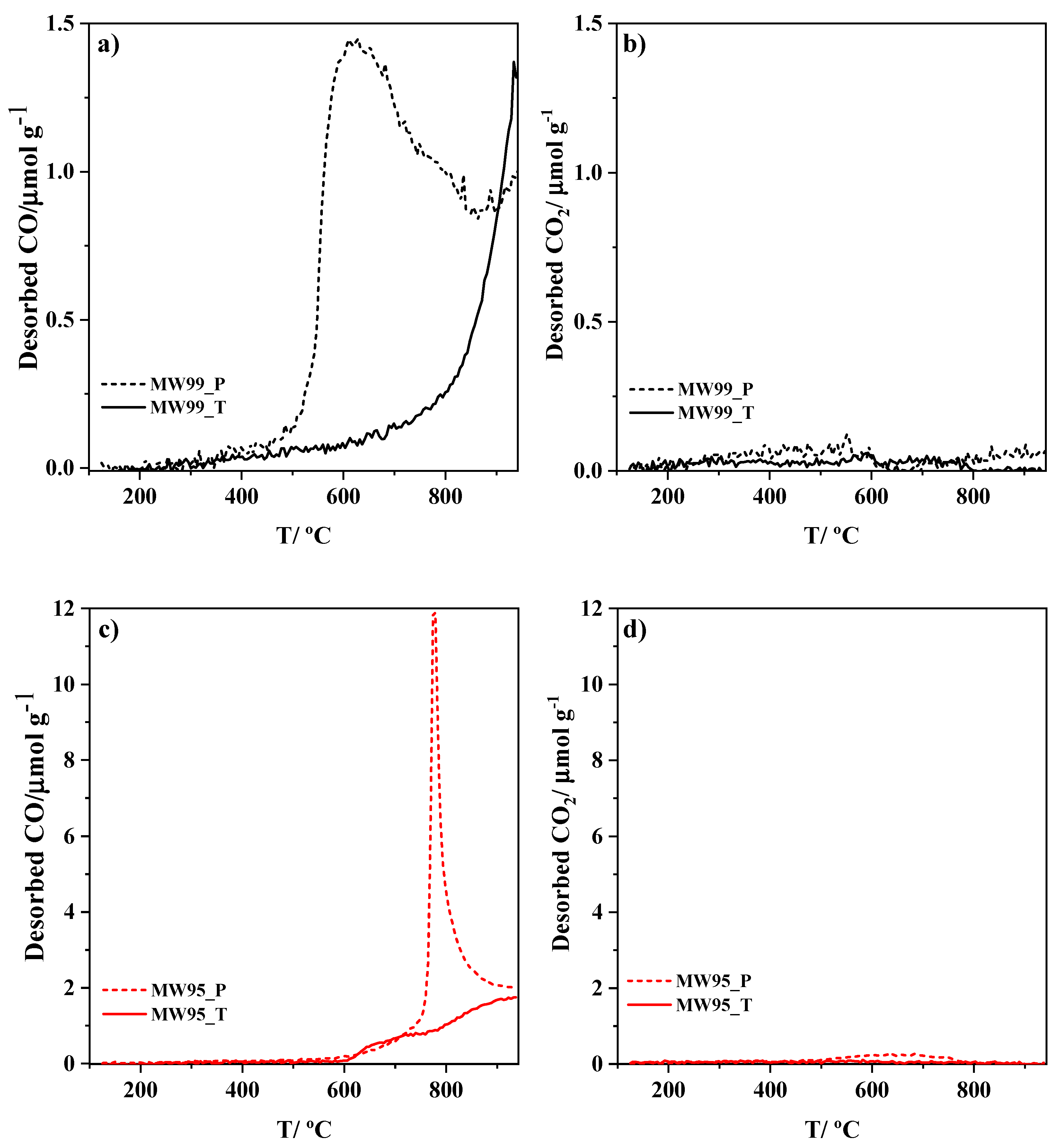
2.4. Carbon-Oxygen Gasification Characterization
2.5. Electrochemical Characterization
3. Results and Discussion
3.1. TEM Analysis of the Materials
3.2. Characterization of the Surface Chemistry and Bulk Composition
3.3. Porous Texture and Carbon-Oxygen Gasification Properties
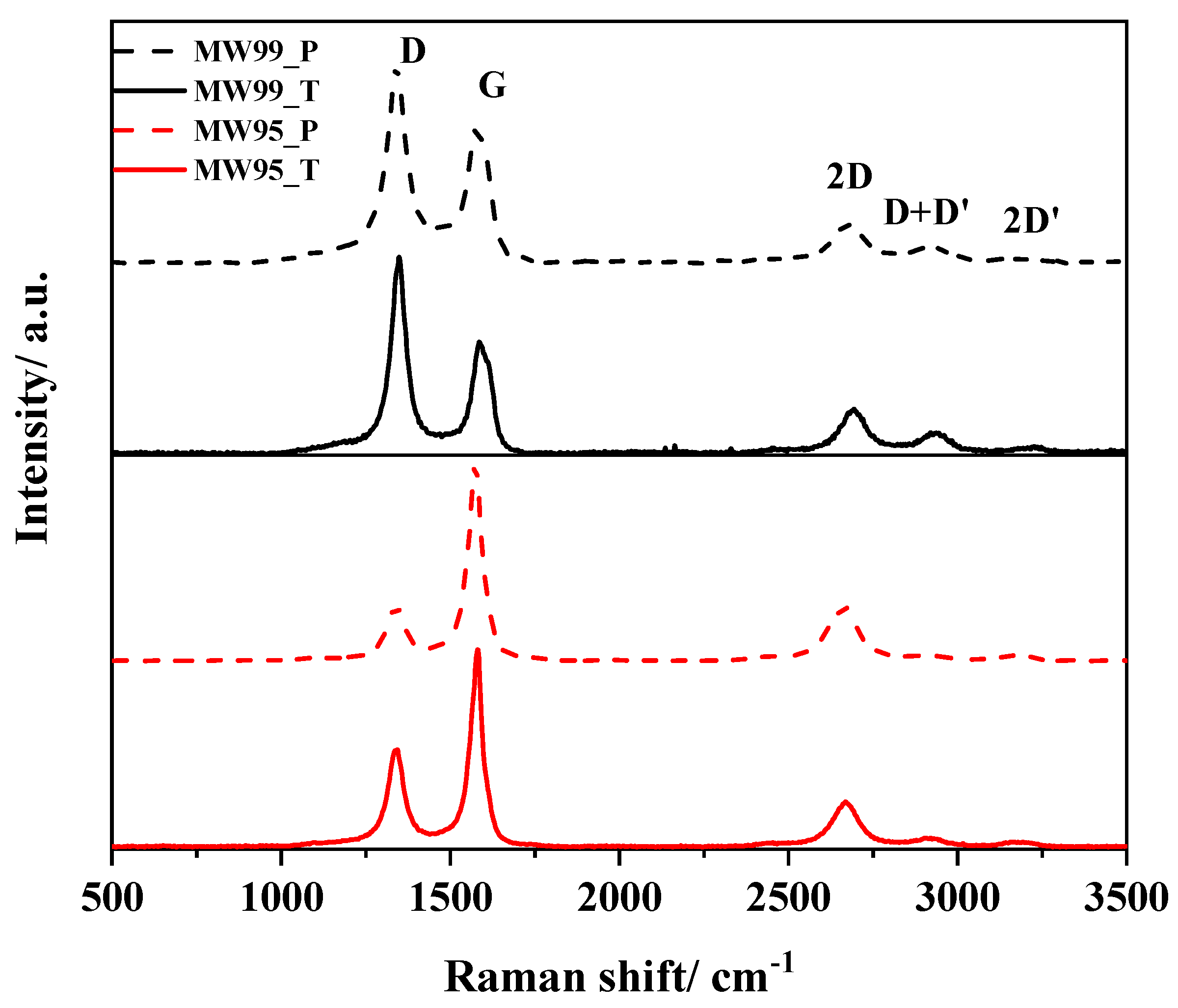
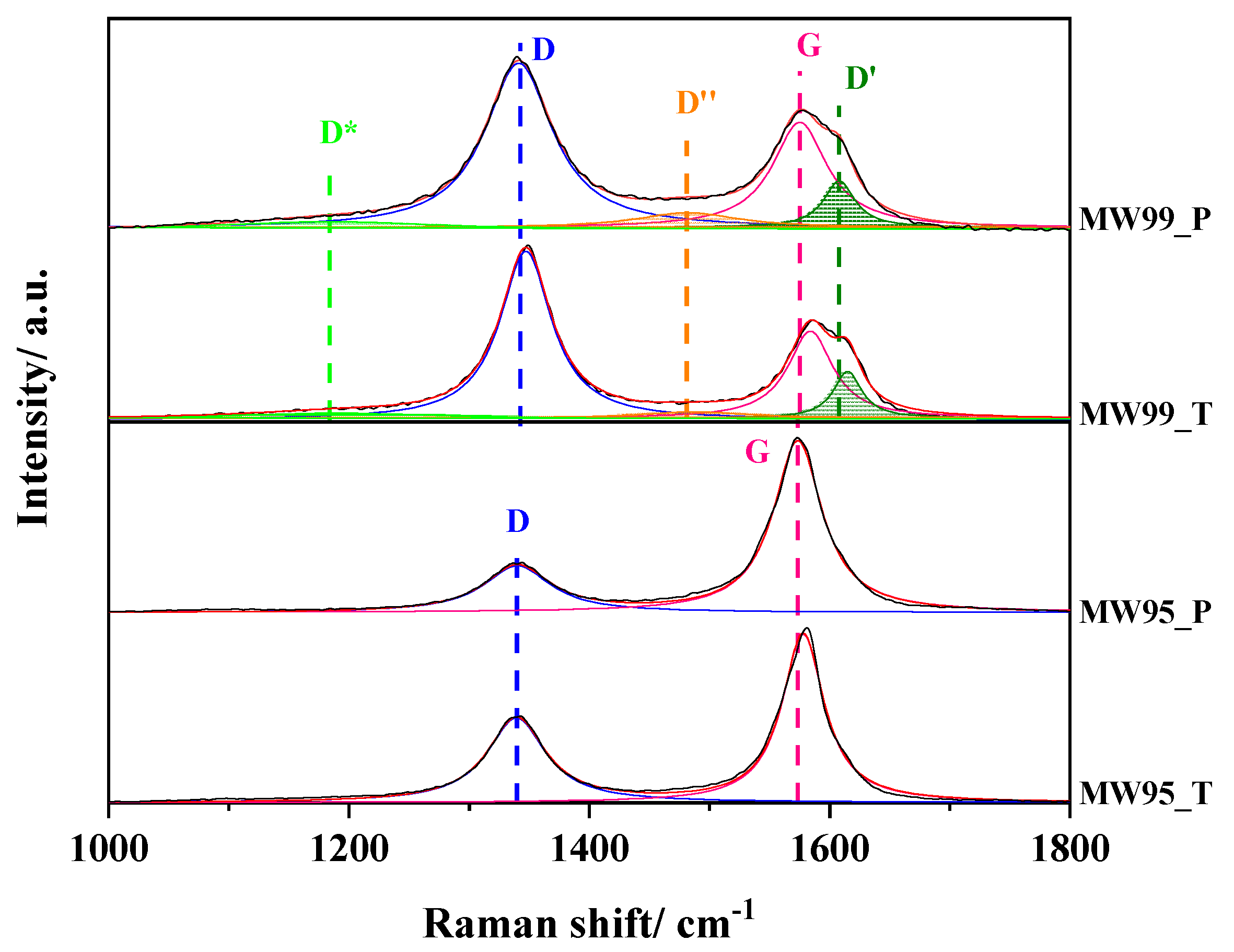
3.4. RAMAN Spectroscopy
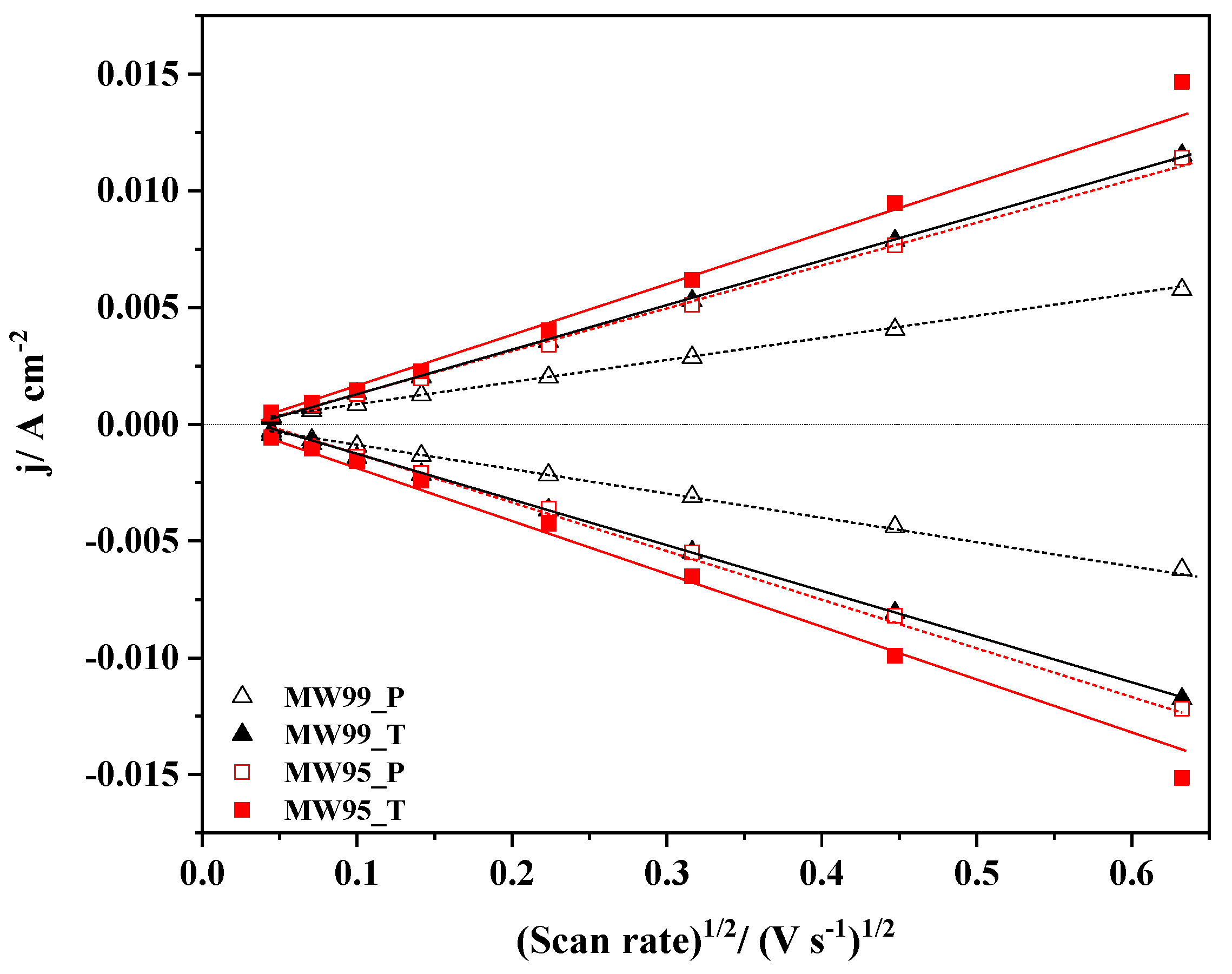
3.5. Electrochemical Characterization
3.6. Electrocatalytic Activity for ORR
4. Importance of Surface Chemical Analysis and Electrochemical and Spectrochemical Results in the ORR Performance
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Koolen, C.D.; Luo, W.; Züttel, A. From Single Crystal to Single Atom Catalysts: Structural Factors Influencing the Performance of Metal Catalysts for CO2 Electroreduction. ACS Catal. 2023, 13, 948–973. [Google Scholar] [CrossRef]
- Alaswad, A.; Baroutaji, A.; Achour, H.; Carton, J.; Al Makky, A.; Olabi, A.G. Developments in fuel cell technologies in the transport sector. Int. J. Hydrogen Energy 2016, 41, 16499–16508. [Google Scholar] [CrossRef]
- Andújar, J.M.; Segura, F. Fuel cells: History and updating. A walk along two centuries. Renew. Sustain. Energy Rev. 2009, 13, 2309–2322. [Google Scholar] [CrossRef]
- Debe, M.K. Electrocatalyst approaches and challenges for automotive fuel cells. Nature 2012, 486, 43–51. [Google Scholar] [CrossRef] [PubMed]
- Hussain, S.; Erikson, H.; Kongi, N.; Sarapuu, A.; Solla-Gullón, J.; Maia, G.; Kannan, A.M.; Alonso-Vante, N.; Tammeveski, K. Oxygen reduction reaction on nanostructured Pt-based electrocatalysts: A review. Int. J. Hydrogen Energy 2020, 45, 31775–31797. [Google Scholar] [CrossRef]
- Zadick, A.; Dubau, L.; Sergent, N.; Berthomé, G.; Chatenet, M. Huge Instability of Pt/C Catalysts in Alkaline Medium. ACS Catal. 2015, 5, 4819–4824. [Google Scholar] [CrossRef]
- Flores-Lasluisa, J.X.; Huerta, F.; Cazorla-Amorós, D.; Morallón, E. Transition metal oxides with perovskite and spinel structures for electrochemical energy production applications. Environ. Res. 2022, 214, 113731. [Google Scholar] [CrossRef] [PubMed]
- Shao, M.; Chang, Q.; Dodelet, J.-P.; Chenitz, R. Recent Advances in Electrocatalysts for Oxygen Reduction Reaction. Chem. Rev. 2016, 116, 3594–3657. [Google Scholar] [CrossRef]
- Xie, J.; Xie, Y. Transition Metal Nitrides for Electrocatalytic Energy Conversion: Opportunities and Challenges. Chem.—Eur. J. 2016, 22, 3588–3598. [Google Scholar] [CrossRef]
- Yin, S.; Yi, H.; Liu, M.; Yang, J.; Yang, S.; Zhang, B.W.; Chen, L.; Cheng, X.; Huang, H.; Huang, R.; et al. An in situ exploration of how Fe/N/C oxygen reduction catalysts evolve during synthesis under pyrolytic conditions. Nat. Commun. 2024, 15, 6229. [Google Scholar] [CrossRef] [PubMed]
- Quílez-Bermejo, J.; Morallón, E.; Cazorla-Amorós, D. Metal-free heteroatom-doped carbon-based catalysts for ORR: A critical assessment about the role of heteroatoms. Carbon 2020, 165, 434–454. [Google Scholar] [CrossRef]
- He, Z.; Wei, P.; Xu, T.; Guo, Z.; Han, J.; Akasaka, T.; Guo, K.; Lu, X. Defective porous carbon microrods derived from fullerenes (C70) as high-performance electrocatalysts for the oxygen reduction reaction. Nanoscale 2022, 14, 473–481. [Google Scholar] [CrossRef] [PubMed]
- Quílez-Bermejo, J.; Strutyński, K.; Melle-Franco, M.; Morallón, E.; Cazorla-Amorós, D. On the Origin of the Effect of pH in Oxygen Reduction Reaction for Nondoped and Edge-Type Quaternary N-Doped Metal-Free Carbon-Based Catalysts. ACS Appl. Mater. Interfaces 2020, 12, 54815–54823. [Google Scholar] [CrossRef]
- Yang, L.; Jiang, S.; Zhao, Y.; Zhu, L.; Chen, S.; Wang, X.; Wu, Q.; Ma, J.; Ma, Y.; Hu, Z. Boron-doped carbon nanotubes as metal-free electrocatalysts for the oxygen reduction reaction. Angew. Chem.—Int. Ed. 2011, 50, 7132–7135. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Xia, Z. Mechanisms of oxygen reduction reaction on nitrogen-doped graphene for fuel cells. J. Phys. Chem. C 2011, 115, 11170–11176. [Google Scholar] [CrossRef]
- Preuss, K.; Siwoniku, A.M.; Bucur, C.I.; Titirici, M.M. The Influence of Heteroatom Dopants Nitrogen, Boron, Sulfur, and Phosphorus on Carbon Electrocatalysts for the Oxygen Reduction Reaction. Chempluschem 2019, 84, 457–464. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Zou, X.; Yan, X.; Brown, C.L.; Chen, Z.; Zhu, G.; Yao, X. Defect-driven oxygen reduction reaction (ORR) of carbon without any element doping. Inorg. Chem. Front. 2016, 3, 417–421. [Google Scholar] [CrossRef]
- Huang, B.; Liu, Y.; Guo, Q.; Fang, Y.; Titirici, M.M.; Wang, X.; Xie, Z. Porous carbon nanosheets from biological nucleobase precursor as efficient pH-independent oxygen reduction electrocatalyst. Carbon 2020, 156, 179–186. [Google Scholar] [CrossRef]
- Jiang, Y.; Yang, L.; Sun, T.; Zhao, J.; Lyu, Z.; Zhuo, O.; Wang, X.; Wu, Q.; Ma, J.; Hu, Z. Significant Contribution of Intrinsic Carbon Defects to Oxygen Reduction Activity. ACS Catal. 2015, 5, 6707–6712. [Google Scholar] [CrossRef]
- Xiang, Q.; Yin, W.; Liu, Y.; Yu, D.; Wang, X.; Li, S.; Chen, C. A study of defect-rich carbon spheres as a metal-free electrocatalyst for an efficient oxygen reduction reaction. J. Mater. Chem. A Mater. 2017, 5, 24314–24320. [Google Scholar] [CrossRef]
- Shen, A.; Zou, Y.; Wang, Q.; Dryfe, R.A.W.; Huang, X.; Dou, S.; Dai, L.; Wang, S. Oxygen reduction reaction in a droplet on graphite: Direct evidence that the edge is more active than the basal plane. Angew. Chem.—Int. Ed. 2014, 53, 10804–10808. [Google Scholar] [CrossRef]
- Zhong, G.; Wang, H.; Yu, H.; Wang, H.; Peng, F. Chemically drilling carbon nanotubes for electrocatalytic oxygen reduction reaction. Electrochim. Acta 2016, 190, 49–56. [Google Scholar] [CrossRef]
- Gara, M.; Compton, R.G. Activity of carbon electrodes towards oxygen reduction in acid: A comparative study. New J. Chem. 2011, 35, 2647–2652. [Google Scholar] [CrossRef]
- Zhong, G.; Wang, H.; Yu, H.; Peng, F. The effect of edge carbon of carbon nanotubes on the electrocatalytic performance of oxygen reduction reaction. Electrochem. Commun. 2014, 40, 5–8. [Google Scholar] [CrossRef]
- Jiang, S.; Zhang, Z.; Yang, N.; Li, L.; Wei, Z. Probing the Interaction between Nitrogen Dopants and Edge Structures of Doped Graphene Catalysts for the Highly Efficient Oxygen Reduction Reaction. J. Phys. Chem. C 2022, 126, 19113–19121. [Google Scholar] [CrossRef]
- Radovic, L.R.; Bockrath, B. On the chemical nature of graphene edges: Origin of stability and potential for magnetism in carbon materials. J. Am. Chem. Soc. 2005, 127, 5917–5927. [Google Scholar] [CrossRef] [PubMed]
- Radovic, L.R.; Salgado-Casanova, A.J.A.; Mora-Vilches, C.V. On the active sites for the oxygen reduction reaction catalyzed by graphene-based materials. Carbon 2020, 156, 389–398. [Google Scholar] [CrossRef]
- Zhao, H.; Sun, C.; Jin, Z.; Wang, D.-W.; Yan, X.; Chen, Z.; Zhu, G.; Yao, X. Carbon for the oxygen reduction reaction: A defect mechanism. J. Mater. Chem. A Mater. 2015, 3, 11736–11739. [Google Scholar] [CrossRef]
- Wang, X.; Jia, Y.; Mao, X.; Zhang, L.; Liu, D.; Song, L.; Yan, X.; Chen, J.; Yang, D.; Zhou, J.; et al. A Directional Synthesis for Topological Defect in Carbon. Chem 2020, 6, 2009–2023. [Google Scholar] [CrossRef]
- Zhao, X.; Yu, X.; Xin, S.; Chen, S.; Bao, C.; Xu, W.; Xue, J.; Hui, B.; Zhang, J.; She, X.; et al. Enhanced oxygen reduction reaction for Zn-air battery at defective carbon fibers derived from seaweed polysaccharide. Appl. Catal. B 2022, 301, 120785. [Google Scholar] [CrossRef]
- Waki, K.; Wong, R.A.; Oktaviano, H.S.; Fujio, T.; Nagai, T.; Kimoto, K.; Yamada, K. Non-nitrogen doped and non-metal oxygen reduction electrocatalysts based on carbon nanotubes: Mechanism and origin of ORR activity. Energy Environ. Sci. 2014, 7, 1950–1958. [Google Scholar] [CrossRef]
- Tang, C.; Wang, H.F.; Chen, X.; Li, B.Q.; Hou, T.Z.; Zhang, B.; Zhang, Q.; Titirici, M.M.; Wei, F. Topological Defects in Metal-Free Nanocarbon for Oxygen Electrocatalysis. Adv. Mater. 2016, 28, 6845–6851. [Google Scholar] [CrossRef] [PubMed]
- Gabe, A.; Ruiz-Rosas, R.; Morallón, E.; Cazorla-Amorós, D. Understanding of oxygen reduction reaction by examining carbon-oxygen gasification reaction and carbon active sites on metal and heteroatoms free carbon materials of different porosities and structures. Carbon 2019, 148, 430–440. [Google Scholar] [CrossRef]
- Wang, L.; Ambrosi, A.; Pumera, M. “Metal-free” catalytic oxygen reduction reaction on heteroatom-doped graphene is caused by trace metal impurities. Angew. Chem.—Int. Ed. 2013, 52, 13818–13821. [Google Scholar] [CrossRef] [PubMed]
- Quintero-Jaime, A.F.; Cazorla-Amorós, D.; Morallón, E. Effect of surface oxygen groups in the electrochemical modification of multi-walled carbon nanotubes by 4-amino phenyl phosphonic acid. Carbon 2020, 165, 328–339. [Google Scholar] [CrossRef]
- Laine, N.R.; Vastola, F.J.; Walker, P.L. The Importance of Active Surface Area in the Carbon-Oxygen Reaction 1,2. J. Phys. Chem. 1963, 67, 2030–2034. [Google Scholar] [CrossRef]
- Jenkins, R.G.; Nandi, S.P.; Walker, P.L. Reactivity of heat-treated coals in air at 500 °C. Fuel 1973, 52, 288–293. [Google Scholar] [CrossRef]
- Xue, Y.; Miao, H.; Sun, S.; Wang, Q.; Li, S.; Liu, Z. (La1−xSrx)0.98MnO3 perovskite with A-site deficiencies toward oxygen reduction reaction in aluminum-air batteries. J. Power Sources 2017, 342, 192–201. [Google Scholar] [CrossRef]
- Esconjauregui, S.; Whelan, C.M.; Maex, K. The reasons why metals catalyze the nucleation and growth of carbon nanotubes and other carbon nanomorphologies. Carbon 2009, 47, 659–669. [Google Scholar] [CrossRef]
- Flahaut, E.; Peigney, A.; Bacsa, W.S.; Bacsa, R.R.; Laurent, C. CCVD synthesis of carbon nanotubes from (Mg,Co,Mo)O catalysts: Influence of the proportions of cobalt and molybdenum. J. Mater. Chem. 2004, 14, 646–653. [Google Scholar] [CrossRef]
- Bendjemil, B.; Borowiak-Palen, E.; Graff, A.; Pichler, T.; Guerioune, M.; Fink, J.; Knupfer, M. Elimination of metal catalyst and carbon-like impurities from single-wall carbon nanotube raw material. Appl. Phys. A Mater. Sci. Process. 2004, 78, 311–314. [Google Scholar] [CrossRef]
- Kwan, Y.C.G.; Ng, G.M.; Huan, C.H.A. Identification of functional groups and determination of carboxyl formation temperature in graphene oxide using the XPS O 1s spectrum. Thin Solid Films 2015, 590, 40–48. [Google Scholar] [CrossRef]
- Ho, S.F.; Contarini, S.; Rabalais, J.W. Ion-beam-induced chemical changes in the oxyanions (Moyn-) and oxides (Mox) where M = chromium, molybdenum, tungsten, vanadium, niobium and tantalum. J. Phys. Chem. 1987, 91, 4779–4788. [Google Scholar] [CrossRef]
- Tan, B.J.; Klabunde, K.J.; Sherwood, P.M.A. XPS studies of solvated metal atom dispersed (SMAD) catalysts. Evidence for layered cobalt-manganese particles on alumina and silica. J. Am. Chem. Soc. 1991, 113, 855–861. [Google Scholar] [CrossRef]
- Szymański, G.S.; Karpiński, Z.; Biniak, S.; Świa, A. The effect of the gradual thermal decomposition of surface oxygen species on the chemical and catalytic properties of oxidized activated carbon. Carbon 2002, 40, 2627–2639. [Google Scholar] [CrossRef]
- Figueiredo, J.L.; Pereira, M.F.R.; Freitas, M.M.A.; Orfao, J.J.M. Modification of the surface chemistry of activated carbons. Carbon 1999, 37, 1379–1389. [Google Scholar] [CrossRef]
- Figueiredo, J.L.; Pereira, M.F.R. The role of surface chemistry in catalysis with carbons. Catal. Today 2010, 150, 2–7. [Google Scholar] [CrossRef]
- Vix-Guterl, C.; Couzi, M.; Dentzer, J.; Trinquecoste, M.; Delhaes, P. Surface Characterizations of Carbon Multiwall Nanotubes: Comparison between Surface Active Sites and Raman Spectroscopy. J. Phys. Chem. B 2004, 108, 19361–19367. [Google Scholar] [CrossRef]
- Vix-Guterl, C.; Dentzer, J.; Ehrburger, P.; Méténier, K.; Bonnamy, S.; Béguin, F. Surface properties and microtexture of catalytic multi-walled carbon nanotubes. Carbon 2001, 39, 318–320. [Google Scholar] [CrossRef]
- Tao, L.; Wang, Q.; Dou, S.; Ma, Z.; Huo, J.; Wang, S.; Dai, L. Edge-rich and dopant-free graphene as a highly efficient metal-free electrocatalyst for the oxygen reduction reaction. Chem. Commun. 2016, 52, 2764–2767. [Google Scholar] [CrossRef] [PubMed]
- Bîru, E.I.; Iovu, H. Graphene Nanocomposites Studied by Raman Spectroscopy. In Raman Spectroscopy; IntechOpen: London, UK, 2018. [Google Scholar] [CrossRef]
- López-Díaz, D.; Holgado, M.L.; García-Fierro, J.L.; Velázquez, M.M. Evolution of the Raman Spectrum with the Chemical Composition of Graphene Oxide. J. Phys. Chem. C 2017, 121, 20489–20497. [Google Scholar] [CrossRef]
- Vollebregt, S.; Ishihara, R.; Tichelaar, F.D.; Hou, Y.; Beenakker, C.I.M. Influence of the growth temperature on the first and second-order Raman band ratios and widths of carbon nanotubes and fibers. Carbon 2012, 50, 3542–3554. [Google Scholar] [CrossRef]
- Cançado, L.G.; Jorio, A.; Ferreira, E.H.M.; Stavale, F.; Achete, C.A.; Capaz, R.B.; Moutinho, M.V.O.; Lombardo, A.; Kulmala, T.S.; Ferrari, A.C. Quantifying defects in graphene via Raman spectroscopy at different excitation energies. Nano Lett. 2011, 11, 3190–3196. [Google Scholar] [CrossRef] [PubMed]
- Zaida, A.; Bar-Ziv, E.; Radovic, L.R.; Lee, Y.J. Further development of Raman Microprobe spectroscopy for characterization of char reactivity. Proc. Combust. Inst. 2007, 31, 1881–1887. [Google Scholar] [CrossRef]
- Yao, N.; Lordi, V.; Ma SX, C.; Dujardin, E.; Krishnan, A.; Treacy MM, J.; Ebbesen, T.W. Structure and oxidation patterns of carbon nanotubes. J. Mater. Res. 1998, 13, 2432–2437. [Google Scholar] [CrossRef]
- Eckmann, A.; Felten, A.; Mishchenko, A.; Britnell, L.; Krupke, R.; Novoselov, K.S.; Casiraghi, C. Probing the Nature of Defects in Graphene by Raman Spectroscopy. Nano Lett. 2012, 12, 3925–3930. [Google Scholar] [CrossRef] [PubMed]
- Xu, T.; Sun, L. Structural Defects in Graphene; Elsevier Ltd.: Amsterdam, The Netherlands, 2018. [Google Scholar] [CrossRef]
- Kuznetsov, V.L.; Bokova-Sirosh, S.N.; Moseenkov, S.I.; Ishchenko, A.V.; Krasnikov, D.V.; Kazakova, M.A.; Romanenko, A.I.; Tkachev, E.N.; Obraztsova, E.D. Raman spectra for characterization of defective CVD multi-walled carbon nanotubes. Phys. Status Solidi B Basic Res. 2014, 251, 2444–2450. [Google Scholar] [CrossRef]
- Sharma, R.; Chadha, N.; Saini, P. Determination of defect density, crystallite size and number of graphene layers in graphene analogues using X-ray diffraction and Raman spectroscopy. Indian J. Pure Appl. Phys. 2017, 55, 625–629. [Google Scholar]
- Salinas-Torres, D.; Huerta, F.; Montilla, F.; Morallón, E. Study on electroactive and electrocatalytic surfaces of single walled carbon nanotube-modified electrodes. Electrochim. Acta 2011, 56, 2464–2470. [Google Scholar] [CrossRef]
- Nicholson, R.S. Theory and Application of Cyclic Voltammetry for Measurement of Electrode Reaction Kinetics. Anal. Chem. 1965, 37, 1351–1355. [Google Scholar] [CrossRef]
- Lavagnini, I.; Antiochia, R.; Magno, F. An extended method for the practical evaluation of the standard rate constant from cyclic voltammetric data. Electroanalysis 2004, 16, 505–506. [Google Scholar] [CrossRef]
- Mahé, E.; Devilliers, D.; Comninellis, C. Electrochemical reactivity at graphitic micro-domains on polycrystalline boron doped diamond thin-films electrodes. Electrochim. Acta 2005, 50, 2263–2277. [Google Scholar] [CrossRef]
- Pacios, M.; del Valle, M.; Bartroli, J.; Esplandiu, M.J. Electrochemical behavior of rigid carbon nanotube composite electrodes. J. Electroanal. Chem. 2008, 619–620, 117–124. [Google Scholar] [CrossRef]
- Kinkelin, S.J.; Röder, F.; Vogel, K.; Steimecke, M.; Bron, M. A fundamental study on cyclic voltammetry at porous carbon thin-film electrodes. Electrochim. Acta 2024, 488, 144183. [Google Scholar] [CrossRef]
- Ryabova, A.S.; Napolskiy, F.S.; Poux, T.; Istomin, S.Y.; Bonnefont, A.; Antipin, D.M.; Baranchikov, A.Y.; Levin, E.E.; Abakumov, A.M.; Kéranguéven, G.; et al. Rationalizing the Influence of the Mn(IV)/Mn(III) Red-Ox Transition on the Electrocatalytic Activity of Manganese Oxides in the Oxygen Reduction Reaction. Electrochim. Acta 2016, 187, 161–172. [Google Scholar] [CrossRef]
- Mattick, V.F.; Jin, X.; Yang, T.; White, R.E.; Huang, K. Unraveling Oxygen Electrocatalysis Mechanisms on a Thin-Film Oxygen-Deficient Perovskite La 0.6 Sr 0.4 CoO 3−δ. ACS Appl. Energy Mater. 2018, 1, 3937–3946. [Google Scholar] [CrossRef]
- Tong, X.; Cherif, M.; Zhang, G.; Zhan, X.; Ma, J.; Almesrati, A.; Vidal, F.; Song, Y.; Claverie, J.P.; Sun, S. N, P-Codoped Graphene Dots Supported on N-Doped 3D Graphene as Metal-Free Catalysts for Oxygen Reduction. ACS Appl. Mater. Interfaces 2021, 13, 30512–30523. [Google Scholar] [CrossRef]
- Hou, Y.; Huang, T.; Wen, Z.; Mao, S.; Cui, S.; Chen, J. Metal-organic framework-derived nitrogen-doped core-shell-structured porous Fe/Fe3C@C nanoboxes supported on graphene sheets for efficient oxygen reduction reactions. Adv. Energy Mater. 2014, 4, 1400337. [Google Scholar] [CrossRef]
- Cao, R.; Thapa, R.; Kim, H.; Xu, X.; Kim, M.G.; Li, Q.; Park, N.; Liu, M.; Cho, J. Promotion of oxygen reduction by a bio-inspired tethered iron phthalocyanine carbon nanotube-based catalyst. Nat. Commun. 2013, 4, 2076. [Google Scholar] [CrossRef] [PubMed]
- Liu, K.; Li, J.; Wang, Q.; Wang, X.; Qian, D.; Jiang, J.; Li, J.; Chen, Z. Designed synthesis of LaCoO3/N-doped reduced graphene oxide nanohybrid as an efficient bifunctional electrocatalyst for ORR and OER in alkaline médium. J. Alloys Compd. 2017, 725, 260–269. [Google Scholar] [CrossRef]
- Plieth, W. Electrochemistry for Materials Science; Elsevier: Amsterdam, The Netherlands, 2008. [Google Scholar] [CrossRef]

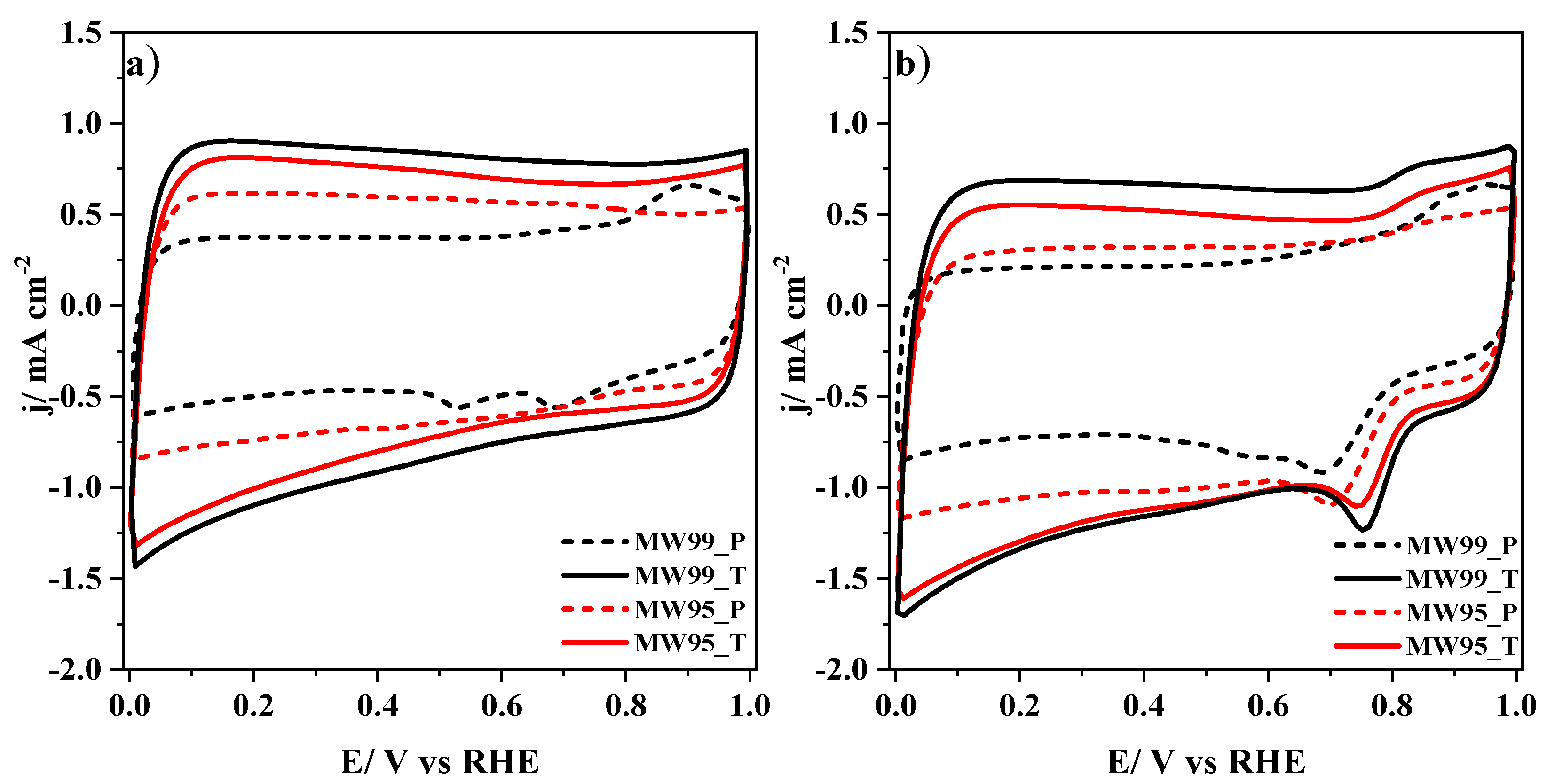


| Samples | XPS | EDX | Elemental Analysis | |||||
|---|---|---|---|---|---|---|---|---|
| C wt% | O wt% | N wt% | C wt% | O wt% | Other Elements wt% | C wt% | H wt% | |
| MW99_P | 98.1 | 1.8 | 0.1 | 97.6 | 1.3 | 1.1 * | 99.3 | 0.0 |
| MW99_T | 98.7 | 1.3 | 0.0 | 100.0 | 0.0 | 0.0 | 99.9 | 0.1 |
| MW95_P | 97.8 | 1.4 | 0.8 | 95.6 | 1.6 | 2.8 ** | 89.5 | 0.0 |
| MW95_T | 98.8 | 1.2 | 0.0 | 97.6 | 0.9 | 1.5 ** | 96.7 | 0.1 |
| Samples | CO/µmol g−1 | CO2/µmol g−1 | O/µmol g−1 | BET Surface Area/m2 g−1 | ASA/m2 g−1 | R550/g g−1 h−1 |
|---|---|---|---|---|---|---|
| MW99_P | 1370 | 105 | 1580 | 195 | 2.35 | 8.1 |
| MW99_T | 389 | 59 | 507 | 307 | 9.37 | 1.4 |
| MW95_P | 2220 | 217 | 2654 | 253 | 38.2 | 4.6 |
| MW95_T | 985 | 117 | 1219 | 465 | 13.1 | 5.8 |
| Samples | Bandwidth D + D′/cm−1 | ID/IG | ID/ID′ | I2D/ID | Lsp2/nm | LD/nm | nD × 1011/cm−2 |
|---|---|---|---|---|---|---|---|
| MW99_P | 120 | 1.42 | 3.23 | 0.35 | 13.35 | 11.63 | 4.26 |
| MW99_T | 106 | 1.94 | 3.61 | 0.23 | 9.78 | 9.95 | 5.81 |
| MW95_P | 83 | 0.29 | - | 0.97 | 66.43 | 25.94 | 0.86 |
| MW95_T | 100 | 0.49 | - | 0.45 | 38.45 | 19.74 | 1.48 |
| Sample | k0/cm s−1 | EASA/ m2 g−1 | /V (at −0.10 mA/cm2) | /V | (at 0.7 V) | /mA cm−2 (at 0.4 V) | Tafel Slope/mV dec−1 |
|---|---|---|---|---|---|---|---|
| MW99_P | 1.44 × 10−3 | 4.50 | 0.80 | 0.66 | 2.97 | −5.11 | 61 |
| MW99_T | 1.82 × 10−3 | 9.07 | 0.85 | 0.73 | 3.35 | −7.52 | 39 |
| MW95_P | 2.11 × 10−3 | 9.07 | 0.82 | 0.70 | 2.80 | −3.53 | 58 |
| MW95_T | 2.32 × 10−3 | 11.43 | 0.84 | 0.76 | 2.97 | −3.67 | 47 |
| Pt/C | - | - | 0.98 | 0.86 | 3.99 | −5.51 | 60 |
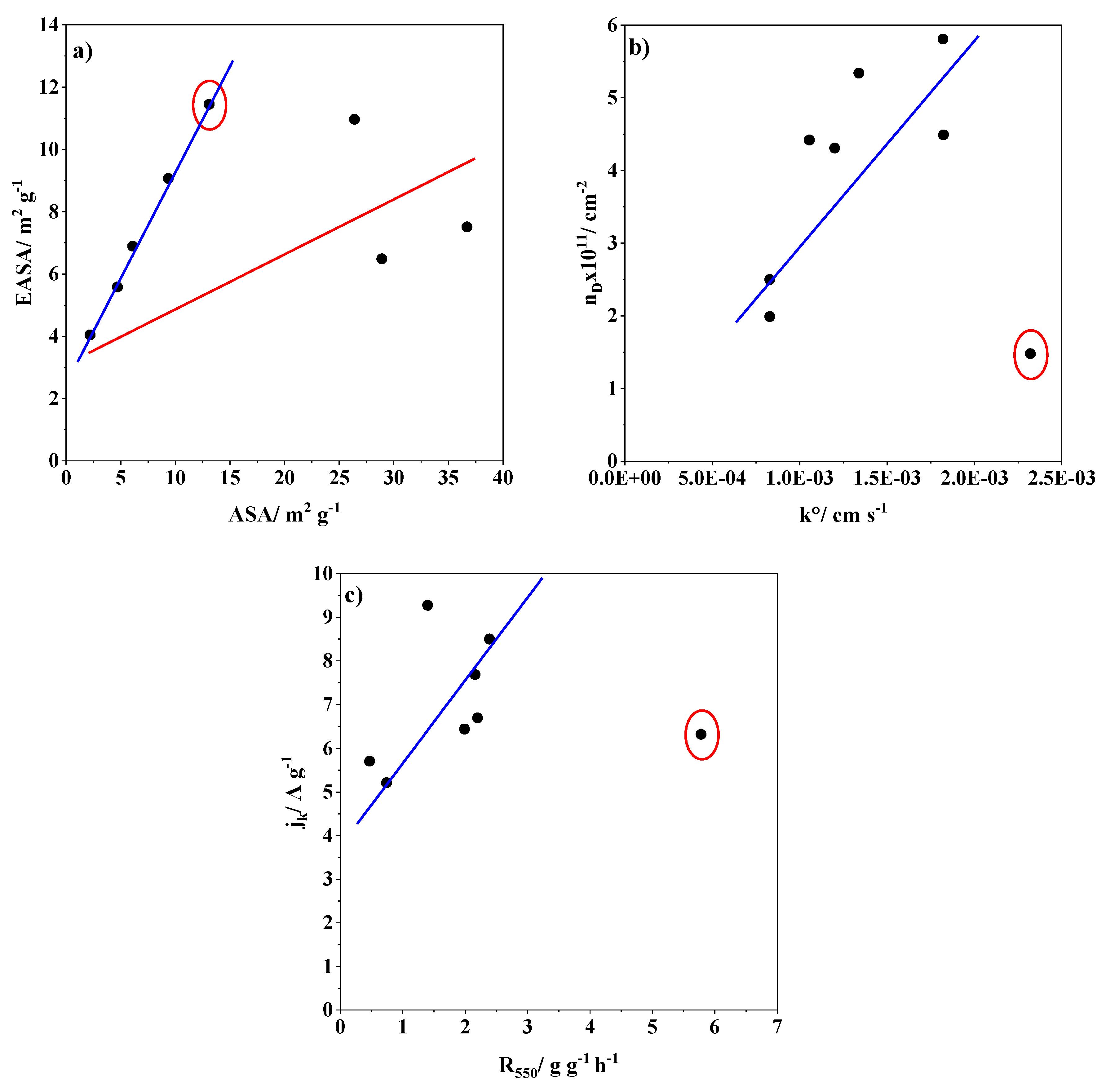
| Sample | kº/cm s−1 | EASA/m2 g−1 | jk/A g−1 | ASA/m2 g−1 | R550/g g−1 h−1 | nD × 1011/cm−2 |
|---|---|---|---|---|---|---|
| XC72 | 1.82 × 10−3 | 5.58 [33] | 5.21 | 4.7 [33] | 0.7 [33] | 4.49 |
| AC | 1.06 × 10−3 | 7.51 [33] | 7.69 | 36.7 [33] | 2.2 [33] | 4.42 |
| YPF | 1.20 × 10−3 | 10.97 [33] | 6.69 | 26.4 [33] | 2.2 [33] | 4.31 |
| CNF | 1.34 × 10−3 | 6.49 [33] | 6.44 | 28.9 [33] | 2.0 [33] | 5.34 |
| Herring | 8.29 × 10−3 | 4.04 [33] | 5.71 | 2.2 [33] | 0.5 [33] | 2.50 |
| SW | 8.29 × 10−3 | 6.89 [33] | 8.50 | 6.1 [33] | 2.4 [33] | 1.99 |
| MW95_T | 2.32 × 10−3 | 11.45 | 6.32 | 13.1 | 5.8 | 1.48 |
| MW99_T | 1.82 × 10−3 | 9.07 | 9.27 | 9.37 | 1.4 | 5.81 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Flores-Lasluisa, J.X.; Cazorla-Amorós, D.; Morallón, E. Deepening the Understanding of Carbon Active Sites for ORR Using Electrochemical and Spectrochemical Techniques. Nanomaterials 2024, 14, 1381. https://doi.org/10.3390/nano14171381
Flores-Lasluisa JX, Cazorla-Amorós D, Morallón E. Deepening the Understanding of Carbon Active Sites for ORR Using Electrochemical and Spectrochemical Techniques. Nanomaterials. 2024; 14(17):1381. https://doi.org/10.3390/nano14171381
Chicago/Turabian StyleFlores-Lasluisa, Jhony Xavier, Diego Cazorla-Amorós, and Emilia Morallón. 2024. "Deepening the Understanding of Carbon Active Sites for ORR Using Electrochemical and Spectrochemical Techniques" Nanomaterials 14, no. 17: 1381. https://doi.org/10.3390/nano14171381
APA StyleFlores-Lasluisa, J. X., Cazorla-Amorós, D., & Morallón, E. (2024). Deepening the Understanding of Carbon Active Sites for ORR Using Electrochemical and Spectrochemical Techniques. Nanomaterials, 14(17), 1381. https://doi.org/10.3390/nano14171381








