Boosting of Redox-Active Polyimide Porous Organic Polymers with Multi-Walled Carbon Nanotubes towards Pseudocapacitive Energy Storage
Abstract
:1. Introduction
2. Materials and Methods
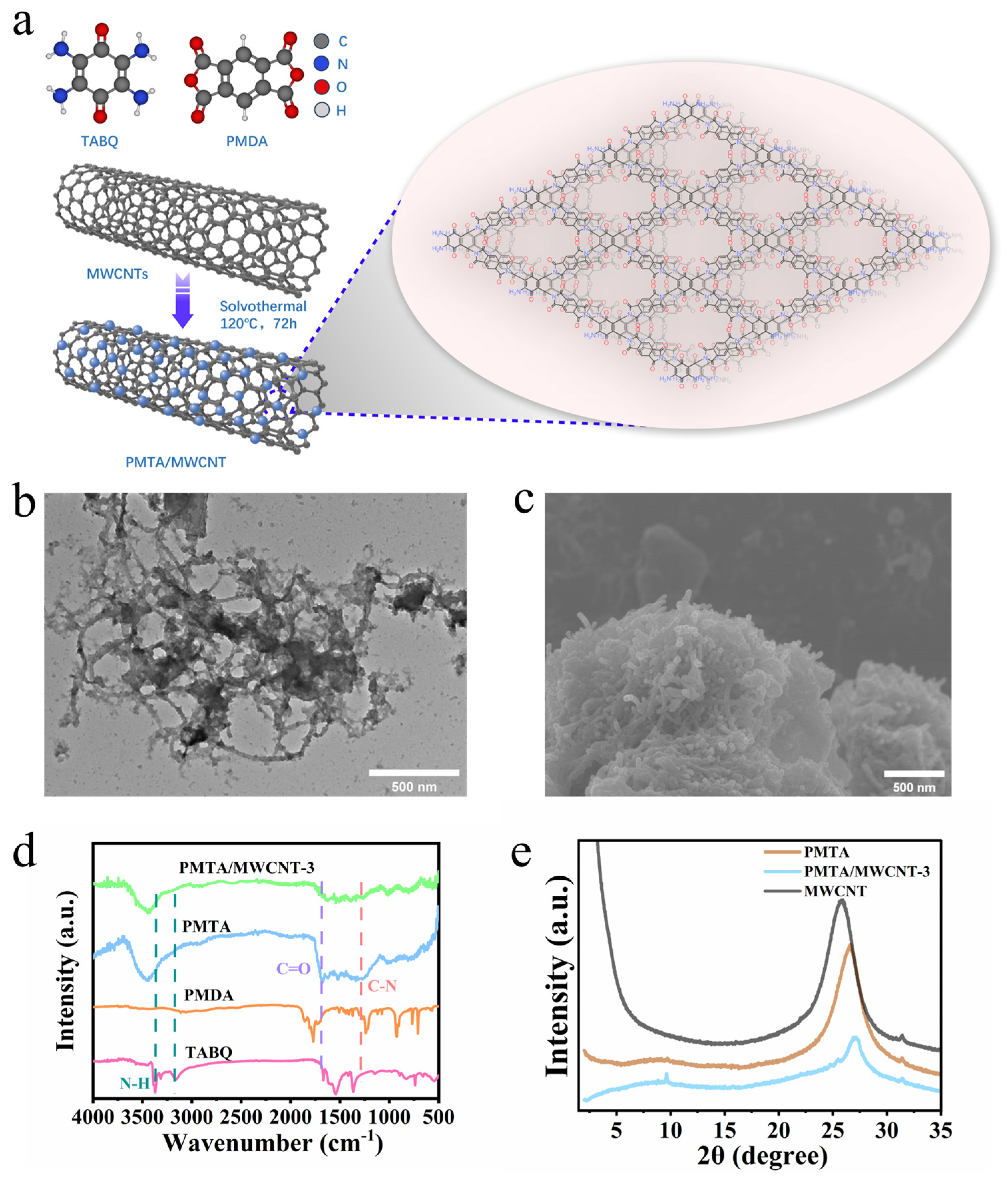
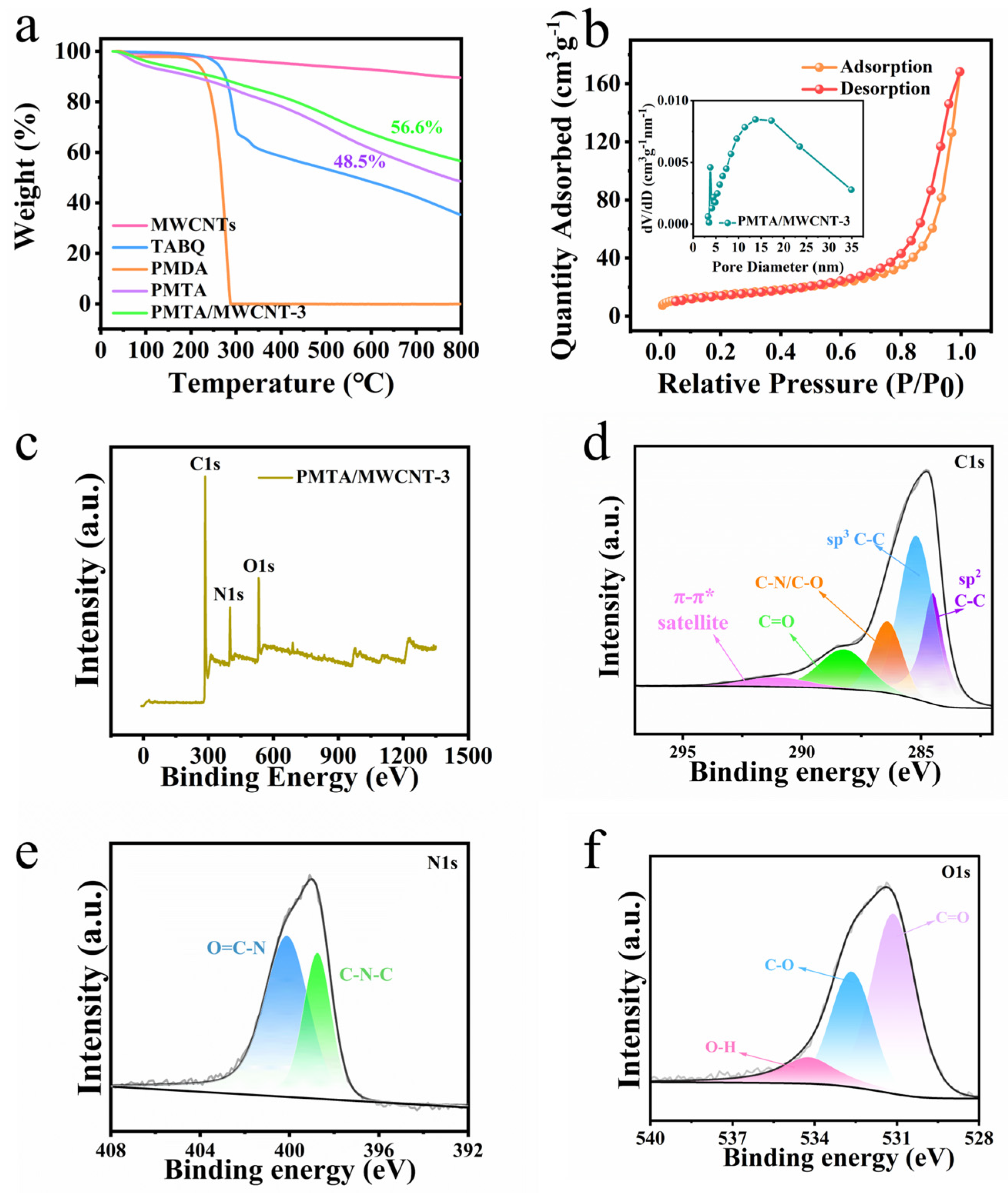
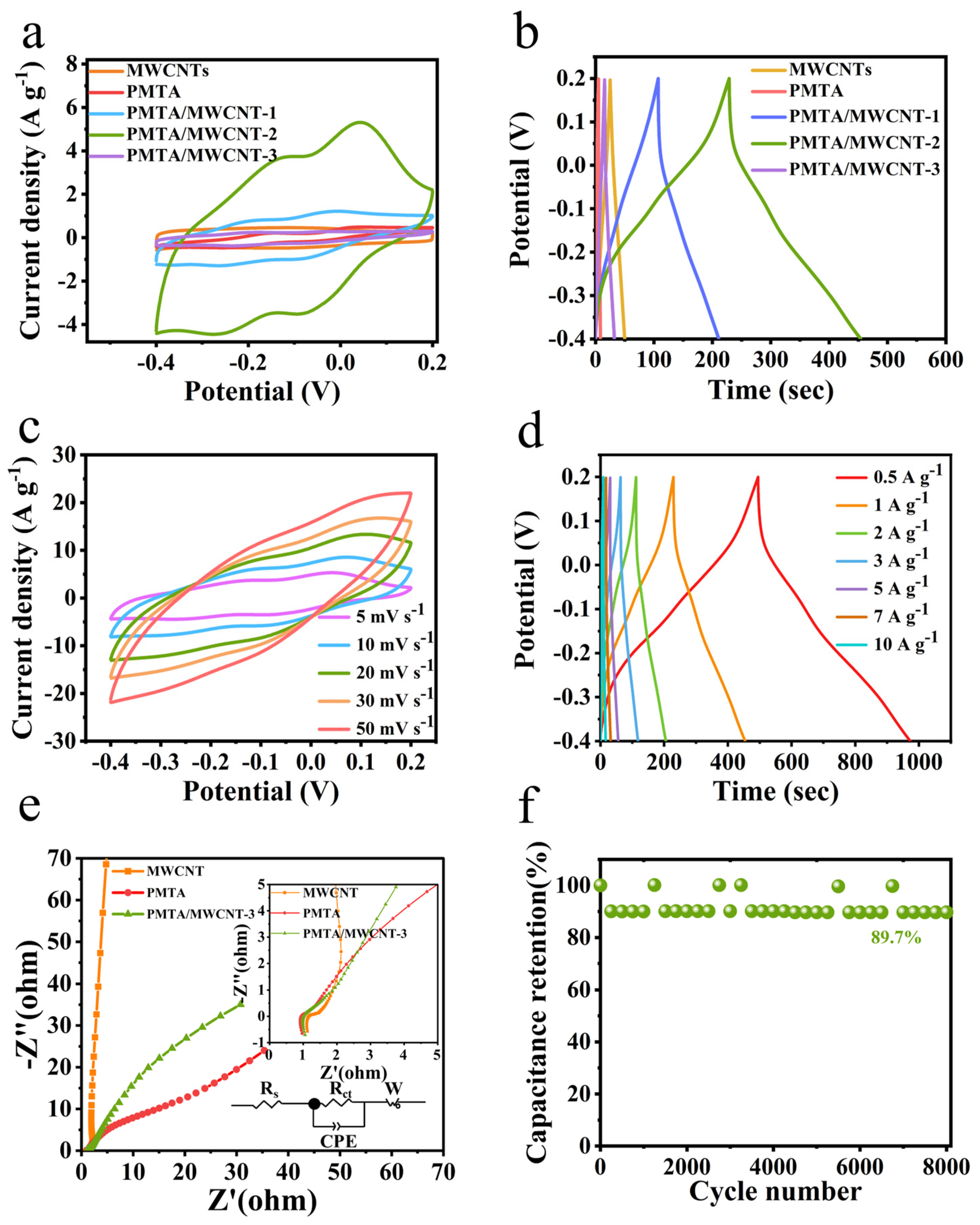
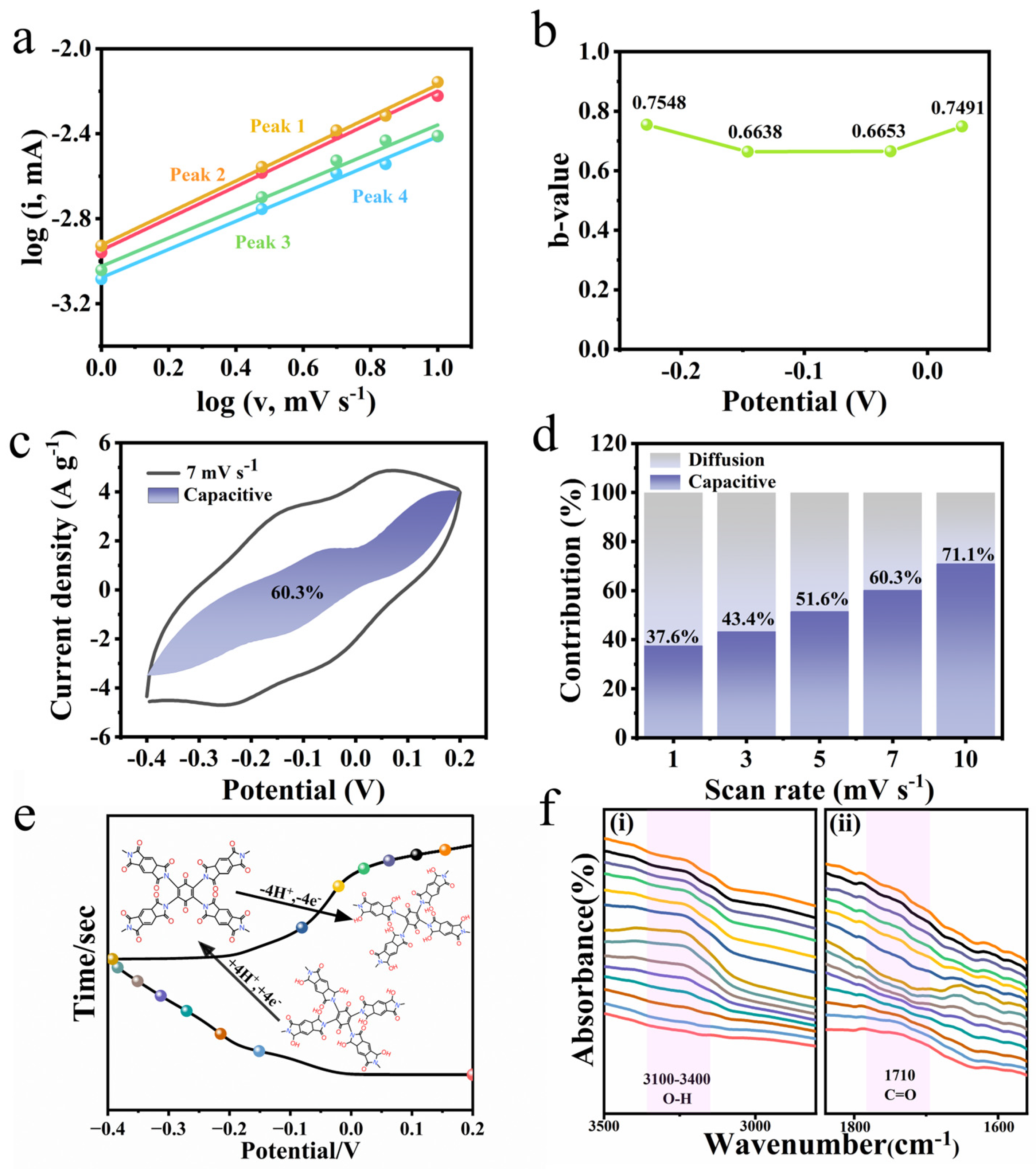
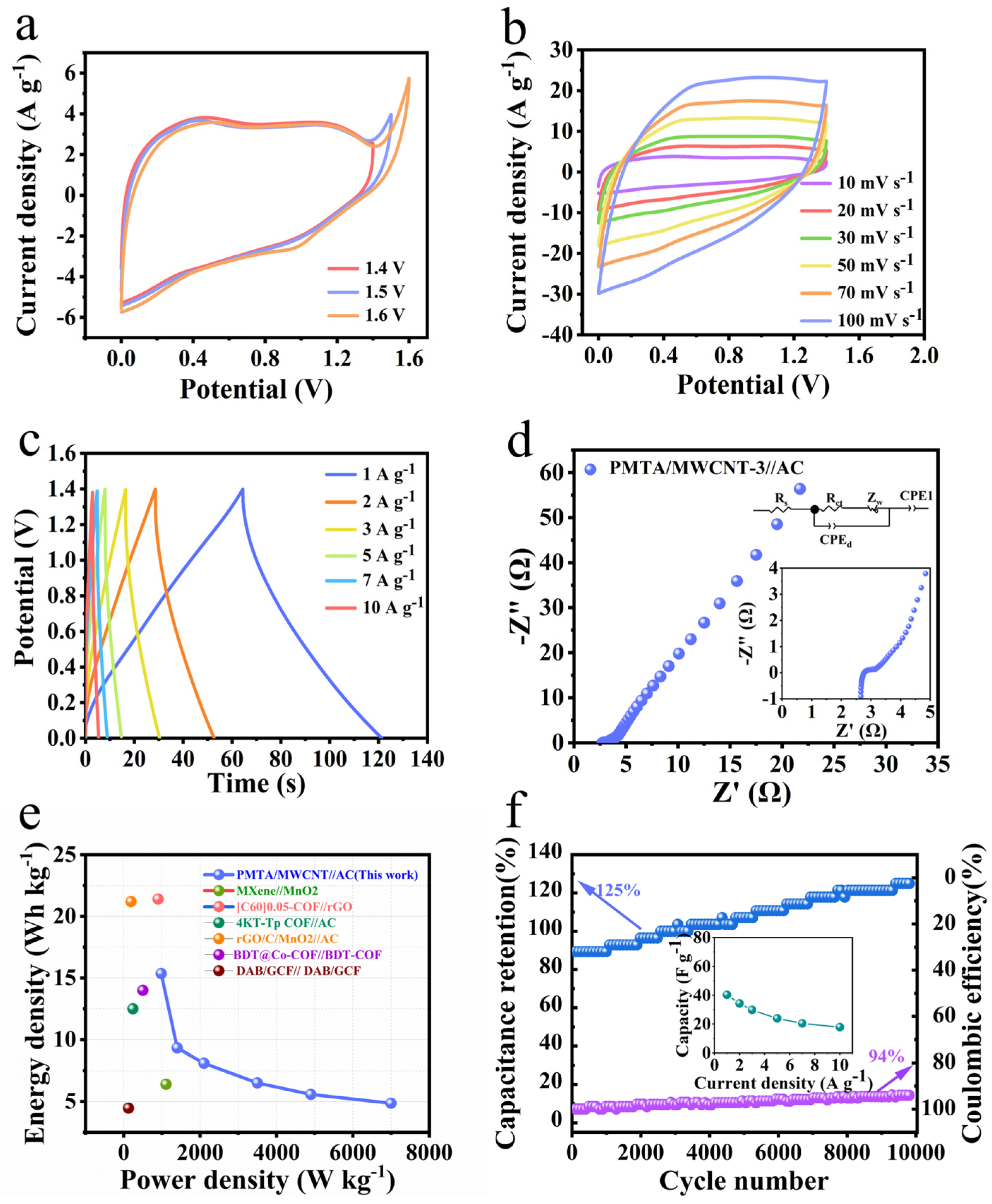
3. Results and Discussions
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Khattak, A.M.; Sin, H.; Ghazi, Z.A.; He, X.; Liang, B.; Khan, N.A.; Alanagh, H.R.; Iqbal, A.; Li, L.; Tang, Z. Controllable fabrication of redox-active conjugated microporous polymers on reduced graphene oxide for high performance faradaic energy storage. J. Mater. Chem. A 2018, 6, 18827–18832. [Google Scholar] [CrossRef]
- Liu, C.; Yan, X.; Hu, F.; Gao, G.; Wu, G.; Yang, X. Toward superior capacitive energy storage: Recent advances in pore engineering for dense electrodes. Adv. Mater. 2018, 30, e1705713. [Google Scholar] [CrossRef]
- Gan, Z.; Yin, J.; Xu, X.; Cheng, Y.; Yu, T. Nanostructure and advanced energy storage: Elaborate material designs lead to high-rate pseudocapacitive ion storage. ACS Nano 2022, 16, 5131–5152. [Google Scholar] [CrossRef]
- Okubo, M.; Sugahara, A.; Kajiyama, S.; Yamada, A. Mxene as a charge storage host. Acc. Chem. Res. 2018, 51, 591–599. [Google Scholar] [CrossRef]
- Ding, W.; Xiao, L.Y.; Lv, L.P.; Wang, Y. Redox-active organic electrode materials for supercapacitors. Batter. Supercaps 2023, 6, e202300278. [Google Scholar] [CrossRef]
- Ding, W.; Xiao, L.Y.; Wang, Y.; Lv, L.P. Redox-active “structural pillar” molecular doping strategy towards high-performance polyaniline-based flexible supercapacitors. Chem. Eng. J. 2024, 495, 153505. [Google Scholar] [CrossRef]
- Fleischmann, S.; Mitchell, J.B.; Wang, R.; Zhan, C.; Jiang, D.E.; Presser, V.; Augustyn, V. Pseudocapacitance: From fundamental understanding to high power energy storage materials. Chem. Rev. 2020, 120, 6738–6782. [Google Scholar] [CrossRef]
- Mohamed, M.G.; EL-Mahdy, A.F.M.; Kotp, M.G.; Kuo, S.-W. Advances in porous organic polymers: Syntheses, structures, and diverse applications. Mater. Adv. 2022, 3, 707. [Google Scholar] [CrossRef]
- Wang, D.G.; Li, N.; Hu, Y.; Wan, S.; Song, M.; Yu, G.; Jin, Y.; Wei, W.; Han, K.; Kuang, G.C.; et al. Highly fluoro-substituted covalent organic framework and its application in lithium-sulfur batteries. ACS Appl. Mater. Interfaces 2018, 10, 42233–42240. [Google Scholar] [CrossRef] [PubMed]
- Zhou, H.; Zhu, S.; Hibino, M.; Honma, I. Electrochemical capacitance of self-ordered mesoporous carbon. J. Power Sources 2003, 122, 219–223. [Google Scholar] [CrossRef]
- Wu, J.; Huang, L.; Wang, S.; Li, X.; Wen, L.; Li, X.; Feng, T.; Li, P.; Fang, Z.; Wu, M.; et al. Ionogel electrolyte with dynamic metal-ligand interactions enabled self-healable supercapacitor with high energy density. Energy Storage Mater. 2023, 57, 549–556. [Google Scholar] [CrossRef]
- Cao, Y.; Xu, Q.; Sun, Y.; Shi, J.; Xu, Y.; Tang, Y.; Chen, X.; Yang, S.; Jiang, Z.; Um, H.D.; et al. Steering lithium and potassium storage mechanism in covalent organic frameworks by incorporating transition metal single atoms. Proc. Natl. Acad. Sci. USA 2024, 121, e2315407121. [Google Scholar] [CrossRef]
- Zan, G.; Li, S.; Chen, P.; Dong, K.; Wu, Q.; Wu, T. Mesoporous cubic nanocages assembled by coupled monolayers with 100% theoretical capacity and robust cycling. ACS Cent. Sci. 2024, 10, 1283–1294. [Google Scholar] [CrossRef]
- Troschke, E.; Oschatz, M.; Ilic, I.K. Schiff-bases for sustainable battery and supercapacitor electrodes. Exploration 2021, 1, 20210128. [Google Scholar] [CrossRef]
- Li, J.F.; Dong, Z.H.; Chen, R.; Wu, Q.S.; Zan, G.T. Advanced nickel-based composite materials for supercapacitor electrodes. Ionics 2024, 30, 1833–1855. [Google Scholar] [CrossRef]
- Yang, Y.-X.; Ge, K.-K.; Ur Rehman, S.; Bi, H. Nanocarbon-based electrode materials applied for supercapacitors. Rare Met. 2022, 41, 3957–3975. [Google Scholar] [CrossRef]
- Yue, T.; Shen, B.; Gao, P. Carbon material/mno2 as conductive skeleton for supercapacitor electrode material: A review. Renew. Sustain. Energy Rev. 2022, 158, 112131. [Google Scholar] [CrossRef]
- Yalovega, G.E.; Brzhezinskaya, M.; Dmitriev, V.O.; Shmatko, V.A.; Ershov, I.V.; Ulyankina, A.A.; Chernysheva, D.V.; Smirnova, N.V. Interfacial interaction in meo(x)/mwnts (me-cu, ni) nanostructures as efficient electrode materials for high-performance supercapacitors. Nanomaterials 2024, 14, 947. [Google Scholar] [CrossRef]
- An, N.; Guo, Z.; Xin, J.; He, Y.; Xie, K.; Sun, D.; Dong, X.; Hu, Z. Hierarchical porous covalent organic framework/graphene aerogel electrode for high-performance supercapacitors. J. Mater. Chem. A 2021, 9, 16824–16833. [Google Scholar] [CrossRef]
- Šimonová, Z.F.; Machotová, J.; Zelenka, J.; Vlček, M.; Šimon, P. Chemically imidized semi-alicyclic polyimides: The effect of catalyst type and imidization temperature. Polym. Biopolym. 2023, 58, 16. [Google Scholar] [CrossRef]
- Kong, X.; Wu, Z.; Stromme, M.; Xu, C. Ambient aqueous synthesis of imine-linked covalent organic frameworks (cofs) and fabrication of freestanding cellulose nanofiber@cof nanopapers. J. Am. Chem. Soc. 2024, 146, 742–751. [Google Scholar] [CrossRef] [PubMed]
- Luo, Z.; Liu, L.; Ning, J.; Lei, K.; Lu, Y.; Li, F.; Chen, J. A microporous covalent-organic framework with abundant accessible carbonyl groups for lithium-ion batteries. Angew. Chem. Int. Ed. 2018, 57, 9443–9446. [Google Scholar] [CrossRef] [PubMed]
- Ding, W.; Liu, J.; Ding, J.; Xiao, L.; Wang, Y.; Lv, L.-P. Activation of redox-active covalent organic frameworks enriched with imine and quinone sites towards high pseudocapacitance. J. Alloys Compd. 2024, 1002, 175234. [Google Scholar] [CrossRef]
- Lei, H.; Zhu, H.; Sun, S.H.; Zhu, Z.F.; Hao, J.C.; Lu, S.L.; Cai, Y.R.; Zhang, M.; Du, M.L. Synergistic integration of au nanoparticles, co-mof and mwcnt as biosensors for sensitive detection of low-concentration nitrite. Electrochim. Acta 2021, 365, 137375. [Google Scholar] [CrossRef]
- Liu, H.J.; Wang, J.; Wang, C.X.; Xia, Y.Y. Ordered hierarchical mesoporous/microporous carbon derived from mesoporous titanium-carbide/carbon composites and its electrochemical performance in supercapacitor. Adv. Energy Mater. 2011, 1, 1101–1108. [Google Scholar] [CrossRef]
- Shanavaz, H.; Prasanna, B.P.; Prashanth, M.K.; Jhaa, G.; Alharethy, F.; Raghu, M.S.; Jeon, B.-H.; Kumar, K.Y. Microwave assisted cobalt incorporated covalent organic frameworks as cathode material for asymmetric supercapacitor device. J. Alloys Compd. 2024, 970, 172634. [Google Scholar] [CrossRef]
- Brzhezinskaya, M.; Bauman, Y.I.; Maksimova, T.A.; Stoyanovskii, V.O.; Vedyagin, A.A.; Mishakov, I.V.; Shubin, Y.V.; Gerasimov, E.Y. One-pot functionalization of catalytically derived carbon nanostructures with heteroatoms for toxic-free environment. Appl. Surf. Sci. 2022, 590, 153055. [Google Scholar] [CrossRef]
- Sobaszek, M.; Brzhezinskaya, M.; Olejnik, A.; Mortet, V.; Alam, M.; Sawczak, M.; Ficek, M.; Gazda, M.; Weiss, Z.; Bogdanowicz, R. Highly Occupied Surface States at Deuterium-Grown Boron-Doped Diamond Interfaces for Efficient Photoelectrochemistry. Small 2023, 19, 2208265. [Google Scholar] [CrossRef]
- Lu, X.M.; Cao, Y.; Sun, Y.; Wang, H.; Sun, W.; Xu, Y.; Wu, Y.; Yang, C.; Wang, Y. Sp-carbon-conjugated organic polymer as multifunctional interfacial layers for ultra-long dendrite-free lithium metal batteries. Angew. Chem. Int. Ed. 2024, 63, e202320259. [Google Scholar] [CrossRef]
- Brzhezinskaya, M.; Belenkov, E.A.; Greshnyakov, V.A.; Yalovega, G.E.; Bashkin, I.O. New aspects in the study of carbon-hydrogen interaction in hydrogenated carbon nanotubes for energy storage applications. J. Alloys Compd. 2019, 792, 713. [Google Scholar] [CrossRef]
- Yu, J.; Chen, X.; Wang, H.-G.; Gao, B.; Han, D.; Si, Z. Conjugated ladder-type polymers with multielectron reactions as high-capacity organic anode materials for lithium-ion batteries. Sci. Chin. Mater. 2022, 65, 2354–2362. [Google Scholar] [CrossRef]
- Liu, J.; Yuan, Y.; Fang, H.; Xu, Y.; Sun, W.; Chen, S.; Wang, Y.; Lv, L.-P. Redox-active tetramino-benzoquinone π–π stacking and h-bonding onto multiwalled carbon nanotubes toward a high-performance asymmetric supercapacitor. ACS Appl. Energy Mater. 2022, 5, 8112–8122. [Google Scholar] [CrossRef]
- Shi, M.; Wang, R.; Li, L.; Chen, N.; Xiao, P.; Yan, C.; Yan, X. Redox-active polymer integrated with mxene for ultra-stable and fast aqueous proton storage. Adv. Funct. Mater. 2022, 33, 2209777. [Google Scholar] [CrossRef]
- Yao, M.; Guo, C.; Geng, Q.; Zhang, Y.; Zhao, X.; Zhao, X.; Wang, Y. Construction of anthraquinone-containing covalent organic frameworks/graphene hybrid films for a flexible high-performance microsupercapacitor. Ind. Eng. Chem. Res. 2022, 61, 7480–7488. [Google Scholar] [CrossRef]
- Geng, Q.; Wang, H.; Wu, Y.; Lv, L.P.; Chen, S.; Sun, W.; Wang, Y. Covalent-induced heterostructure of covalent-organic frameworks and mxene as advanced electrodes with motivated pseudocapacitance performance. ChemElectroChem 2022, 9, e202200340. [Google Scholar] [CrossRef]
- Ojha, M.; Wu, B.; Deepa, M. Nico metal-organic framework and porous carbon interlayer-based supercapacitors integrated with a solar cell for a stand-alone power supply system. ACS Appl. Mater. Interfaces 2020, 12, 42749–42762. [Google Scholar] [CrossRef] [PubMed]
- Ma, F.; Hu, Z.; Jiao, L.; Wang, X.; Yang, Y.; Li, Z.; He, Y. Synthesis and application of naphthalene diimide as an organic molecular electrode for asymmetric supercapacitors with high energy storage. Adv. Mater. Interfaces 2021, 8, 2002161. [Google Scholar] [CrossRef]
- An, N.; Guo, Z.; Guo, C.; Wei, M.; Sun, D.; He, Y.; Li, W.; Zhou, L.; Hu, Z.; Dong, X. A novel cof/mxene film electrode with fast redox kinetics for high-performance flexible supercapacitor. Chem. Eng. J. 2023, 458, 141434. [Google Scholar] [CrossRef]
- Li, M.; Liu, J.; Li, Y.; Xing, G.; Yu, X.; Peng, C.; Chen, L. Skeleton engineering of isostructural 2d covalent organic frameworks: Orthoquinone redox-active sites enhanced energy storage. CCS Chem. 2021, 3, 696–706. [Google Scholar] [CrossRef]
- Zhao, X.; Sajjad, M.; Zheng, Y.; Zhao, M.; Li, Z.; Wu, Z.; Kang, K.; Qiu, L. Covalent organic framework templated ordered nanoporous c60 as stable energy efficient supercapacitor electrode material. Carbon 2021, 182, 144–154. [Google Scholar] [CrossRef]
- Zhao, Y.; Xu, Q.; Shi, J.; Zhang, Y.; Xu, Z.; Wang, X.; Zang, L.; Sun, L. Covalent organic frameworks, reduced graphene oxide and ni12p5 ternary composite for high performance supercapacitors. J. Energy Storage 2024, 97, 112616. [Google Scholar] [CrossRef]
- Dong, Y.; Wang, Y.; Zhang, X.; Lai, Q.; Yang, Y. Carbon-based elastic foams supported redox-active covalent organic frameworks for flexible supercapacitors. Chem. Eng. J. 2022, 449, 137858. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhou, T.; Yuan, Y.; Xiao, L.; Ding, W.; Wang, Y.; Lv, L.-P. Boosting of Redox-Active Polyimide Porous Organic Polymers with Multi-Walled Carbon Nanotubes towards Pseudocapacitive Energy Storage. Nanomaterials 2024, 14, 1388. https://doi.org/10.3390/nano14171388
Zhou T, Yuan Y, Xiao L, Ding W, Wang Y, Lv L-P. Boosting of Redox-Active Polyimide Porous Organic Polymers with Multi-Walled Carbon Nanotubes towards Pseudocapacitive Energy Storage. Nanomaterials. 2024; 14(17):1388. https://doi.org/10.3390/nano14171388
Chicago/Turabian StyleZhou, Tian, Yu Yuan, Luyi Xiao, Wei Ding, Yong Wang, and Li-Ping Lv. 2024. "Boosting of Redox-Active Polyimide Porous Organic Polymers with Multi-Walled Carbon Nanotubes towards Pseudocapacitive Energy Storage" Nanomaterials 14, no. 17: 1388. https://doi.org/10.3390/nano14171388




