Bright Yellow Luminescence from Mn2+-Doped Metastable Zinc Silicate Nanophosphor with Facile Preparation and Its Practical Application
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Nanophosphor Fabrication
2.3. Characterization and Instruments
3. Results and Discussion
3.1. Luminescence Characteristics
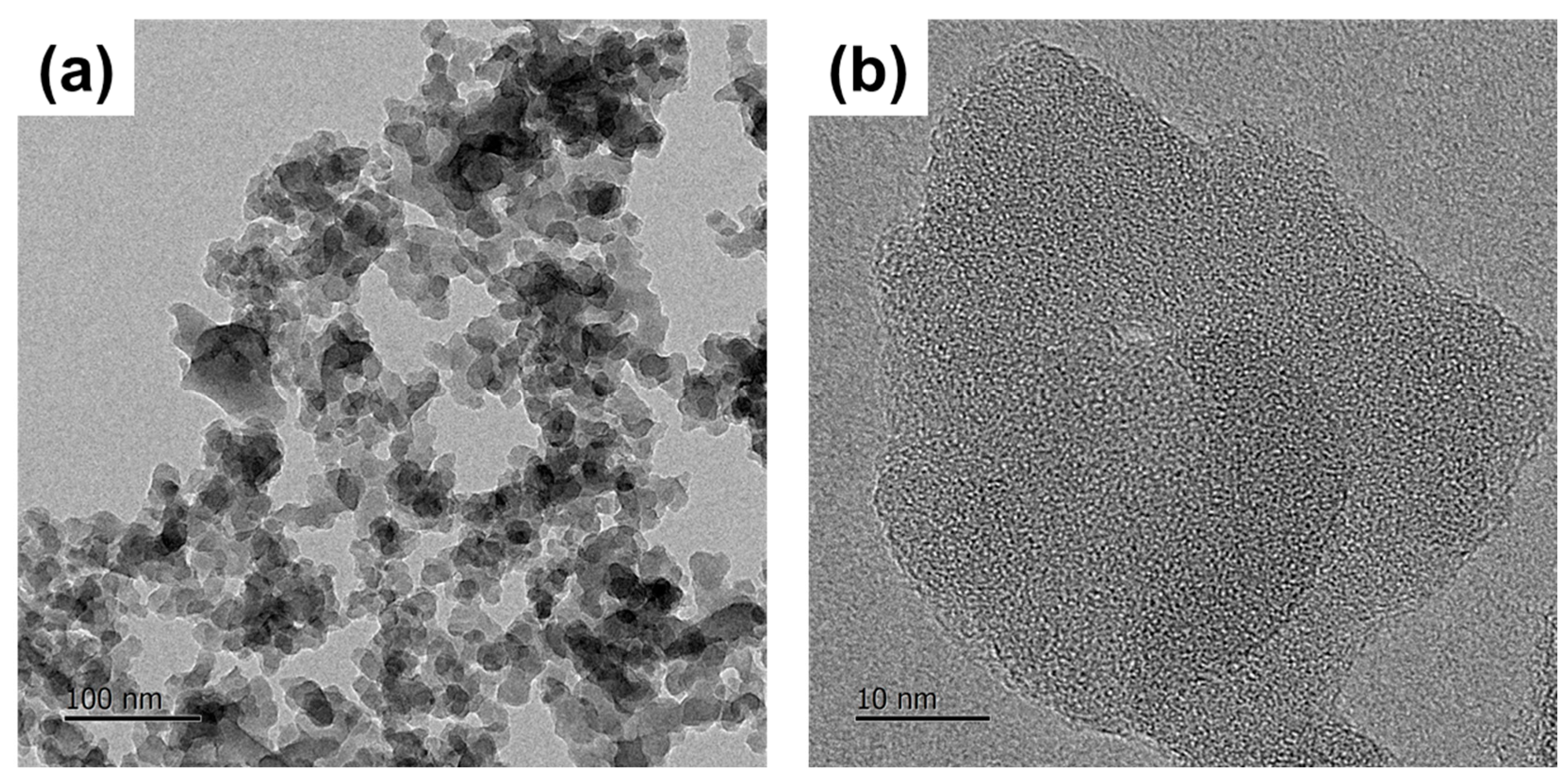
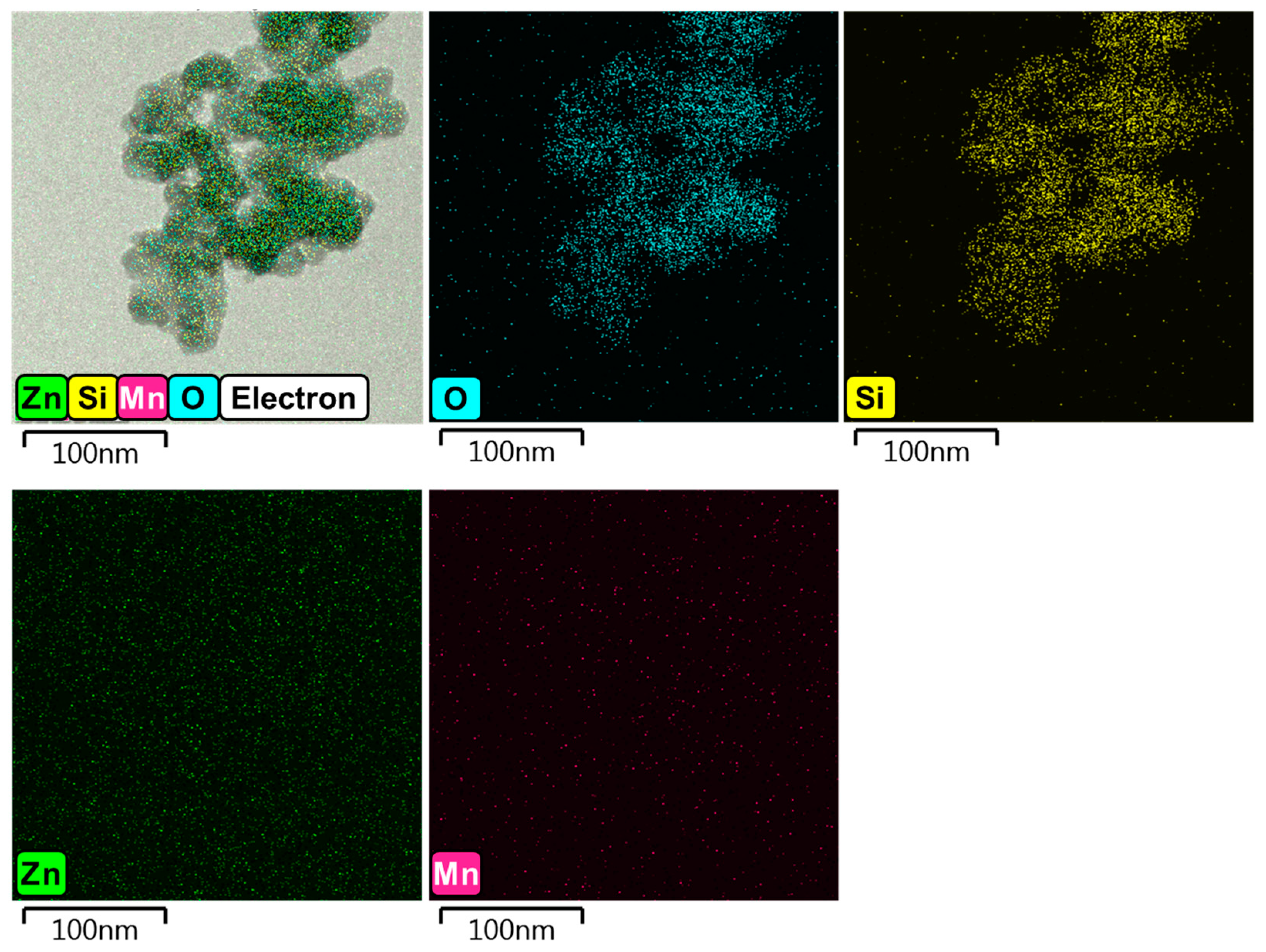
3.2. Crystallography Interpretation
3.3. Practical Application
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Chen, H.; Wei, M.; He, Y.; Abed, J.; Teale, S.; Sargent, E.H.; Yang, Z. Germanium Silicon Oxide Achieves Multi-Coloured Ultra-Long Phosphorescence and Delayed Fluorescence at High Temperature. Nat. Commun. 2022, 13, 4438. [Google Scholar] [CrossRef]
- Priya, R.; Pandey, O.P.; Dhoble, S.J. Review on the Synthesis, Structural and Photo-Physical Properties of Gd2O3 Phosphors for Various Luminescent Applications. Opt. Laser Technol. 2021, 135, 106663. [Google Scholar] [CrossRef]
- Mini Krishna, K.; Jayaraj, M.K. Oxide Luminescent Materials. In Nanostructured Metal Oxides and Devices. Materials Horizons: From Nature to Nanomaterials; Jayaraj, M., Ed.; Springer: Singapore, 2020. [Google Scholar] [CrossRef]
- Jahanbazi, F.; Mao, Y. Recent Advances on Metal Oxide-Based Luminescence Thermometry. J. Mater. Chem. C 2021, 9, 1641–16439. [Google Scholar] [CrossRef]
- Masai, H.; Yanagida, T. Radiation-Induced Luminescence in Oxide Glasses. Jpn. J. Appl. Phys. 2022, 62, 010606. [Google Scholar] [CrossRef]
- Suo, H.; Guo, D.; Zhao, P.; Zhang, X.; Wang, Y.; Zheng, W.; Li, P.; Yin, T.; Guan, L.; Wang, Z.; et al. Ultrasensitive Colorimetric Luminescence Thermometry by Progressive Phase Transition. Adv. Sci. 2023, 11, 2305241. [Google Scholar] [CrossRef]
- Park, J.; Jang, S.C.; Lee, S.M.; Kang, M.; Chung, K.; Kim, H. Oxide Thin-Film Transistors Based On I-Line Stepper Process for High PPI Displays. J. Inf. Disp. 2022, 24, 103–108. [Google Scholar] [CrossRef]
- Karazhanov, S.Z.; Ravindran, P.; Fjellvåg, H.; Svensson, B.G. Electronic Structure and Optical Properties of ZnSiO3 and Zn2SiO4. J. Appl. Phys. 2009, 106, 123701. [Google Scholar] [CrossRef]
- Kang, T.; Afandi, M.M.; Kang, H.; Park, J.; Kim, J. Lower-Voltage Electroluminescence of Green Zinc Silicate on SiOx Interface in Metal-Oxide-Semiconductor Structure. Phys. Status Solidi (A) 2021, 218, 2100440. [Google Scholar] [CrossRef]
- Syono, Y.; Akimoto, S.; Matsui, Y. High Pressure Transformations in Zinc Silicates. J. Solid State Chem. 1971, 3, 369–380. [Google Scholar] [CrossRef]
- Liu, X.; Kanzaki, M.; Xue, X. Crystal Structures of Zn2SiO4 III and IV Synthesized at 6.5–8 GPa and 1273 K. Phys. Chem. Miner. 2013, 40, 467–478. [Google Scholar] [CrossRef]
- Singh, V.; Bhatia, K.; Rao, A.S.; Joo, J.B. Trivalent Gd Incorporated Zn2SiO4 Phosphor Material for EPR and Luminescence Investigations. Int. J. Mater. Res. 2024, 115, 438–445. [Google Scholar] [CrossRef]
- Jiang, X.; Gao, X.; Li, L.; Zhou, P.; Wang, S.; Liu, T.; Zhou, J.; Zhang, H.; Huang, K.; Li, Y.; et al. Enhancement of Light and X-ray Charging in Persistent Luminescence Nanoparticle Scintillators Zn2SiO4:Mn2+, Yb3+, Li+. ACS Appl. Mater. Interfaces 2023, 15, 21228–21238. [Google Scholar] [CrossRef]
- Jaafar, S.H.; Zaid, M.H.M.; Matori, K.A.; Yaakob, Y.; Mustapha, H.M. Synthesis of Eu3+-Doped ZnO/Zn2SiO4 Composite Phosphor for Potent Optoelectronic Applications. Braz. J. Phys. 2022, 56, 6. [Google Scholar] [CrossRef]
- Diao, C.; Yang, C. Synthesis of High Efficiency Zn2SiO4:Mn2+ Green Phosphors Using Nano-Particles. Ceram. Int. 2010, 36, 1653–1657. [Google Scholar] [CrossRef]
- Naeimi, A.; Arabi, A.M.; Merajifar, V. A Novel Approach to the Synthesis of Zn2SiO4:Mn Luminescent Nanoparticles. J. Mater. Sci. Mater. Electron. 2019, 30, 9123–9132. [Google Scholar] [CrossRef]
- Dimza, V.; Popov, A.I.; Lāce, L.; Kundzins, M.; Kundzins, K.; Antonova, M.; Livins, M. Effects of Mn Doping on Dielectric Properties of Ferroelectric Relaxor PLZT Ceramics. Curr. Appl. Phys. 2017, 17, 169–173. [Google Scholar] [CrossRef]
- Diana, P.; Sebastian, S.; Sivaganesh, D.; Raj, C.S.A.; Jacob, S.S.K.; AlAbdulaal, T.H.; Shkir, M. Unveiling the Luminescence of α-Zn2SiO4 Phosphor: Profound Influence of Sintering Temperatures. Radiat. Phys. Chem. 2023, 212, 111156. [Google Scholar] [CrossRef]
- Luchechko, A.; Zhydachevskyy, Y.; Sugak, D.; Kravets, O.; Martynyuk, N.; Popov, A.I.; Ubizskii, S.; Suchocki, A. Luminescence Properties and Decay Kinetics of Mn2+ and Eu3+ Co-Dopant Ions in MgGa2O4 Ceramics. Latv. J. Phys. Tech. Sci. 2019, 55, 43–51. [Google Scholar] [CrossRef]
- Novita, M.; Ristanto, S.; Saptaningrum, E.; Supriyadi, S.; Marlina, D.; Rondonuwu, F.S.; Chauhan, A.S.; Walker, B.; Ogasawara, K.; Piasecki, M.; et al. Study on Local-Structure of K2XF6 Crystals Doped with Mn4+ Ions by First-Principles Calculations. materials 2023, 16, 4046. [Google Scholar] [CrossRef]
- Takesue, M.; Hayashi, H.; Smith, R.L., Jr. Thermal and Chemical Methods for Producing Zinc Silicate (willemite): A Review. Prog. Cryst. Growth Chacact. Mater. 2009, 55, 98–124. [Google Scholar] [CrossRef]
- Hafeez, M.; Ali, A.; Manzoor, S.; Bhatti, A.S. Anomalous Optical and Magnetic Behavior of Multi-Phase Mn-Doped Zn2SiO4 Nanowires: A New Class of Dilute Magnetic Semiconductors. Nanoscale 2014, 6, 14845–14855. [Google Scholar] [CrossRef]
- Afandi, M.M.; Baek, G.; Kim, J. Lower-Voltage Electroluminescent Device with Metastable Nanostructure for Silicon Photonics. Next Mater. 2024, 4, 100203. [Google Scholar] [CrossRef]
- Afandi, M.M.; Park, H.; Lee, S.; Kim, J. Yellow Electroluminescence from Metastable Zinc Silicate in an Electrolyte-Assisted Silicon Semiconductor Structure. J. Lumin. 2024, 275, 120794. [Google Scholar] [CrossRef]
- Kang, T.; Kang, H.; Park, S.; Deressa, G.; Park, J.; Kim, J. Critical Synthesis Parameters of β-Phase Zn2SiO4:Mn2+ Phosphor and its Metastability. Mater. Today Commun. 2021, 26, 101798. [Google Scholar] [CrossRef]
- Santra, B.; Pal, S.; Saha, S.; Kanjilal, A. Tailoring Structural, Chemical, and Photocatalytic Properties of ZnO@β-SiC Composites: The Effect of Annealing Temperature and Environment. ACS Omega 2023, 8, 24113–24124. [Google Scholar] [CrossRef] [PubMed]
- Ye, R.; Jia, G.; Deng, D.; Hua, Y.; Cui, Z.; Zhao, S.; Huang, L.; Wang, H.; Li, C.; Xu, S. Controllable Synthesis and Tunable Colors of α- and β-Zn2SiO4:Mn2+ Nanocrystals for UV and Blue Chip Excited White LEDs. J. Phys. Chem. C 2011, 115, 10851–10858. [Google Scholar] [CrossRef]
- Omri, K.; El Mir, L. Effect of Manganese Concentration on Photoluminescence Properties of Zn2SiO4:Mn Nanophosphor Material. Superlattices Microstruct. 2014, 70, 24–32. [Google Scholar] [CrossRef]
- Bertail, C.; Maron, S.; Buissette, V.; Le Mercier, T.; Gacoin, T.; Boilot, J. Structural and Photoluminescent Properties of Zn2SiO4:Mn2+ Nanoparticles Prepared by a Protected Annealing Process. Chem. Mater. 2011, 23, 2961–2967. [Google Scholar] [CrossRef]
- Lin, C.; Wang, J.; Zhao, X.; Zhu, E.; Long, N.; Rüssel, C. Competitive Crystallization of β-Zn2SiO4 and ZnO in an Aluminosilicate Glass. Ceram. Int. 2018, 44, 7209–7213. [Google Scholar] [CrossRef]
- Lee, S.; Jeon, B.; Kang, T.; Lee, W.; Malik, A.M.; Park, S.; Lim, J.; Park, B.; Jeong, Y.; Kim, J. Optical and Electrical Properties of Zn2SiO4:Mn2+ Powder Electroluminescent Device. J. Lumin. 2018, 196, 290–293. [Google Scholar] [CrossRef]
- Rivera-Enríquez, C.E.; Fernández-Osorio, A.; Chávez-Fernández, J. Luminescence properties of α- and β-Zn2SiO4:Mn nanoparticles prepared by a co-precipitation method. J. Alloys Compd. 2016, 688, 775–782. [Google Scholar] [CrossRef]
- Leverenz, H.W. An Introduction to Luminescence of Solids; John Wiley & Sons, Inc.: New York, NY, USA, 1950. [Google Scholar]
- Ma, Y.Y.; Hu, J.Q.; Song, E.H.; Ye, S.; Zhang, Q.Y. Regulation of Red to Near-Infrared Emission in Mn2+ Single Doped Magnesium Zinc Phosphate Solid-Solution Phosphors by Modification the Crystal Field. J. Mater. Chem. C 2015, 3, 12443. [Google Scholar] [CrossRef]
- Afandi, M.M.; Kim, J. Strategies for Fourfold Enhancement of Narrowband Green AC-Driven Electroluminescence from Zinc Gallate Co-Doped with Ce3+ and Mn2+ Ions by Field-Enhanced Energy Transfer. J. Alloys Compd. 2023, 967, 171622. [Google Scholar] [CrossRef]
- Park, K.W.; Lim, H.S.; Park, S.W.; Deressa, G.; Kim, J.S. Strong Blue Absorption of Green Zn2SiO4:Mn2+ Phosphor by Doping Heavy Mn2+ Concentrations. Chem. Phys. Lett. 2015, 636, 141–145. [Google Scholar] [CrossRef]
- Krasnenko, T.I.; Zaitseva, N.A.; Ivanova, I.V.; Baklanava, I.V.; Samigullina, R.F.; Rotermel, M.V. The Effect of Mg Introduction on Structural and Luminescence Properties of Zn2SiO4:Mn Phosphor. J. Alloys Compd. 2020, 845, 156296. [Google Scholar] [CrossRef]
- Barthou, C.; Benoit, J.; Benalloul, P.; Morell, A. Mn2+ Concentration Effect on the Optical Properties of Zn2SiO4: Mn Phosphors. J. Electrochem. Soc. 1994, 141, 524. [Google Scholar] [CrossRef]
- Park, J.; Kim, J. Single-Crystalline Zn2SiO4:Mn2+ Luminescent Film on Amorphous Quartz Glass. J. Alloys Compd. 2021, 855, 157343. [Google Scholar] [CrossRef]
- Kang, Z.T.; Liu, Y.; Wagner, B.K.; Gilstrap, R.; Liu, M.; Summers, C.J. Luminescence Properties of Mn2+ Doped Zn2SiO4 Phosphor Films Synthesized by Combustion CVD. J. Lumin. 2006, 121, 595–600. [Google Scholar] [CrossRef]
- Lu, Q.; Wang, P.; Li, J. Structure and Luminescence Properties of Mn-doped Zn2SiO4 Prepared with Mesoporous Silica. Mater. Res. Bull. 2011, 46, 791–795. [Google Scholar] [CrossRef]
- Samigullina, R.F.; Krasnenko, T.I. Thermal Analysis and Mechanism of Formation of Zn2SiO4:Mn Phosphor under Heating of Synthetic Hemimorphite. Mater. Res. Bull. 2020, 129, 110890. [Google Scholar] [CrossRef]
- Lu, Q.; Yun, G. Facile One-Step Solid-State Reaction Route to Synthesize Ordered Mesoporous β-Zn2SiO4-SiO2 Nanocomposites. Ceram. Int. 2013, 39, 3533–3538. [Google Scholar] [CrossRef]
- Pradeep, A.; Priyadharini, P.; Chandrasekaran, G. Sol-Gel Route of Synthesis of Nanoparticles of MgFe2O4 and XRD, FTIR and VSM Study. J. Magn. Magn. Mater. 2008, 320, 2774–2779. [Google Scholar] [CrossRef]
- Kishore, K.; Chandan, A.K.; Hung, P.T. On the Enhanced Hardening Ability and Plasticity Mechanisms in Novel Mn-Added CoCrNi Medium Entropy Alloy during High-Pressure Torsion. J. Alloys Compd. 2022, 904, 163941. [Google Scholar] [CrossRef]
- Yu, X.; Wang, Y. Synthesis and VUV Spectral Properties of Nanoscaled Zn2SiO4:Mn2+ Green Phosphor. J. Phys. Chem. Solids 2009, 70, 1146–1149. [Google Scholar] [CrossRef]
- Joffin, N.; Caillier, B.; Dexpert-Ghys, J.; Verelst, M.; Baret, G.; Garcia, A.; Guillot, P.; Galy, J.; Mauricot, R.; Schamm, S. Elaboration by Spray Pyrolysis and Characterization in the VUV Range of Phosphor Particels with Spherical Shape and Micronic Size. J. Phys. D Appl. Phys. 2005, 38, 3261–3268. [Google Scholar] [CrossRef]
- Choi, S.; Seo, J.; Yun, Y.J.; Jung, H. Transparent Green-Emitting Luminescent Layer Using Spherical SiO2/Zn2SiO4:Mn2+ Core-Shell Nanophosphors. Electrochem. Solid State Lett. 2012, 15, J6–J9. [Google Scholar] [CrossRef]
- Hao, Y.; Wang, Y. Synthesis and Photoluminescence of New Phosphors M2(Mg, Zn)Si2O7:Mn2+ (M = Ca, Sr, Ba). Mater. Res. Bull. 2007, 42, 2219–2223. [Google Scholar] [CrossRef]
- Tamatani, M. Fluorescence in β–Al2O3–Like Materials of K, Ba and La Activated with Eu2+ and Mn2+. Jpn. J. Appl. Phys. 1974, 13, 950. [Google Scholar] [CrossRef]

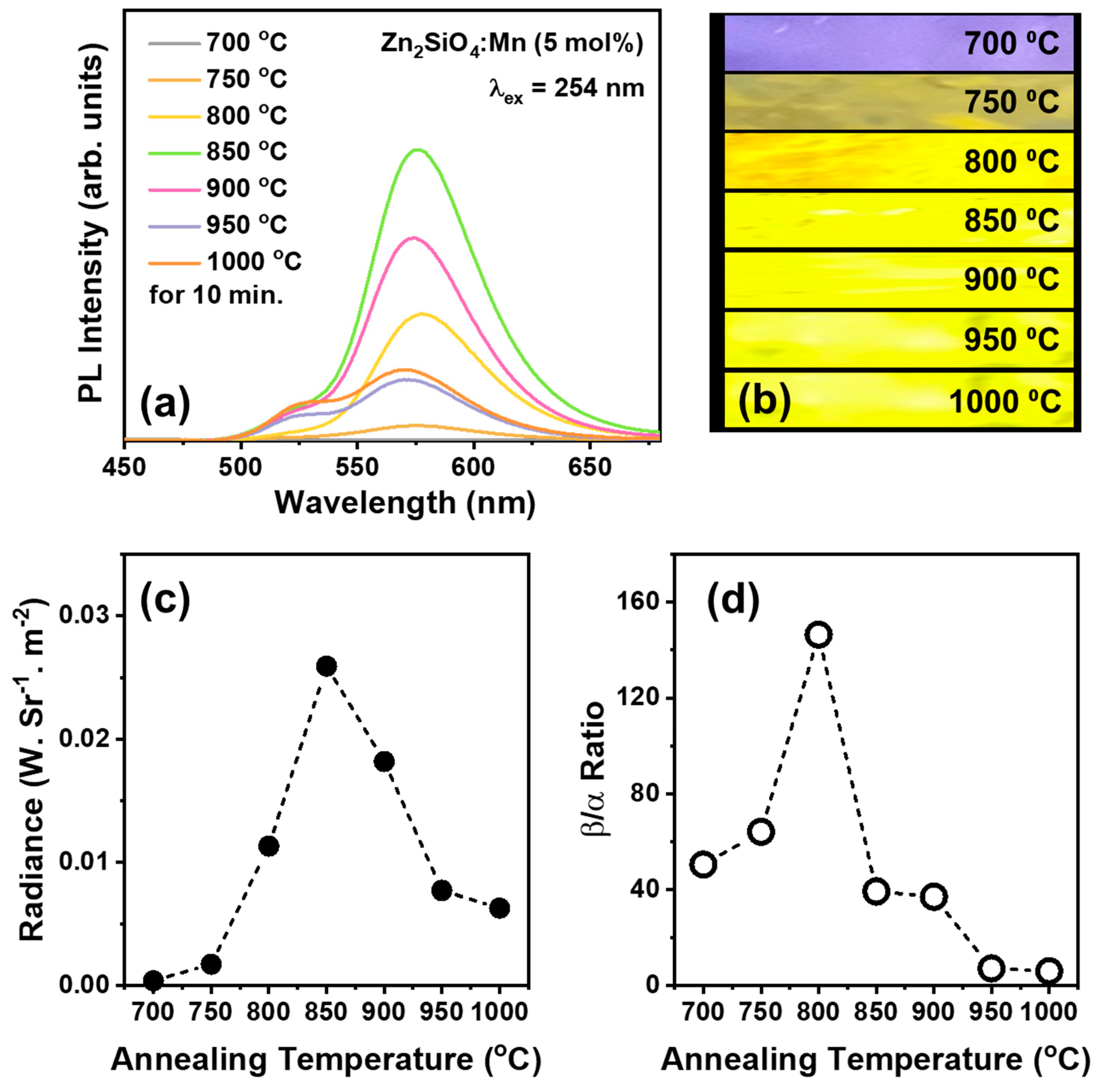
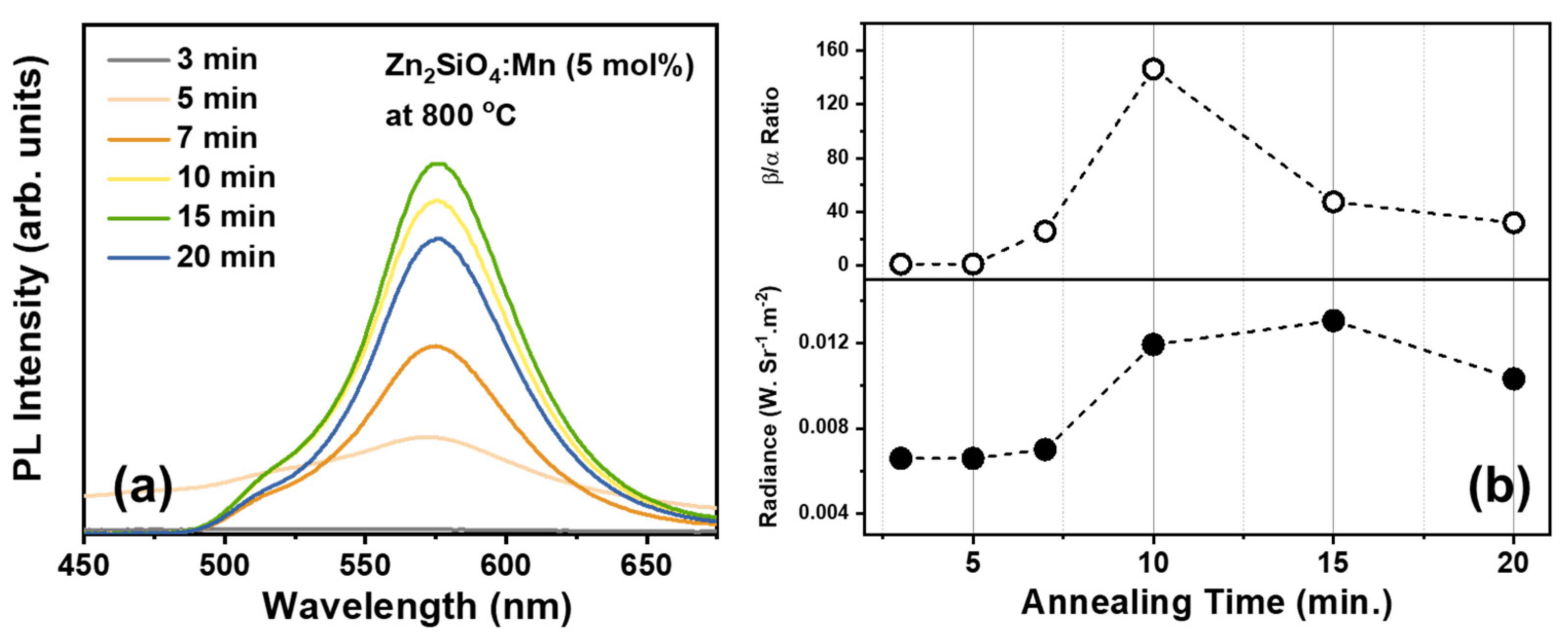
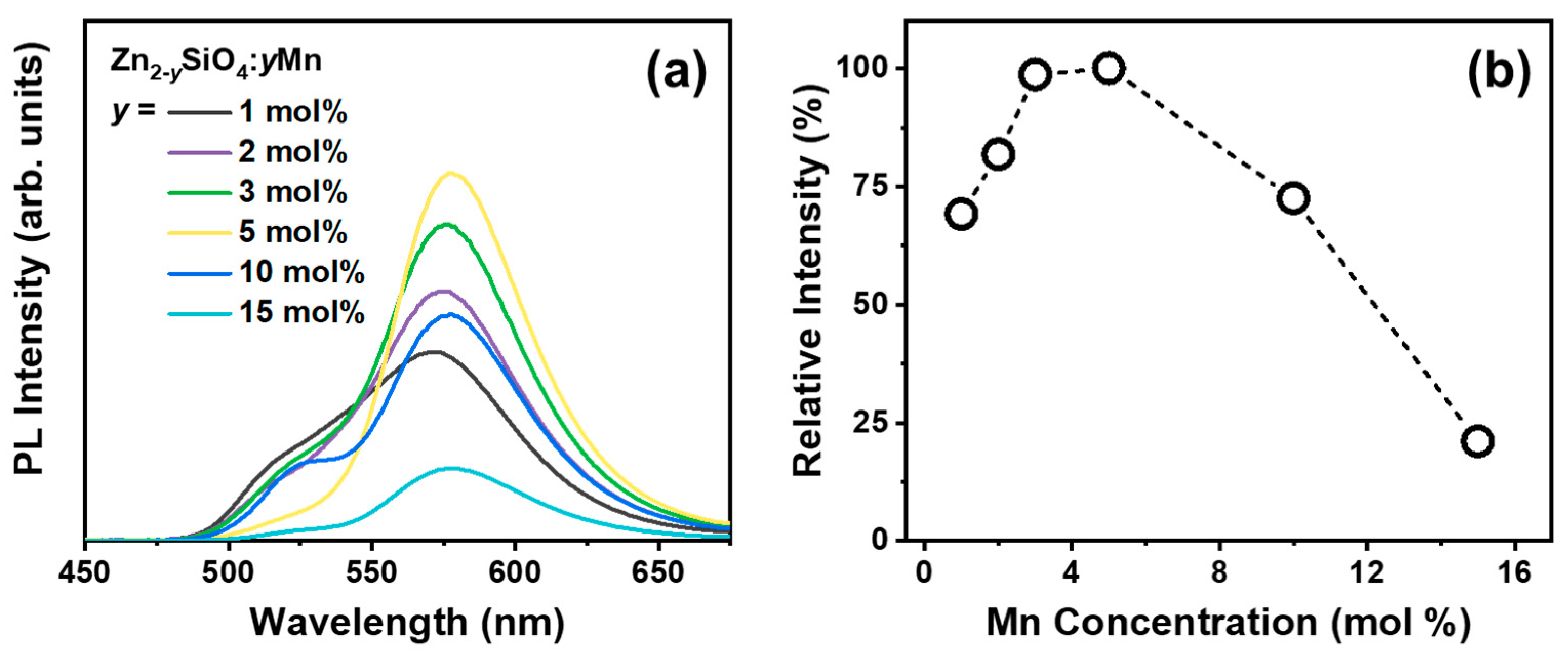
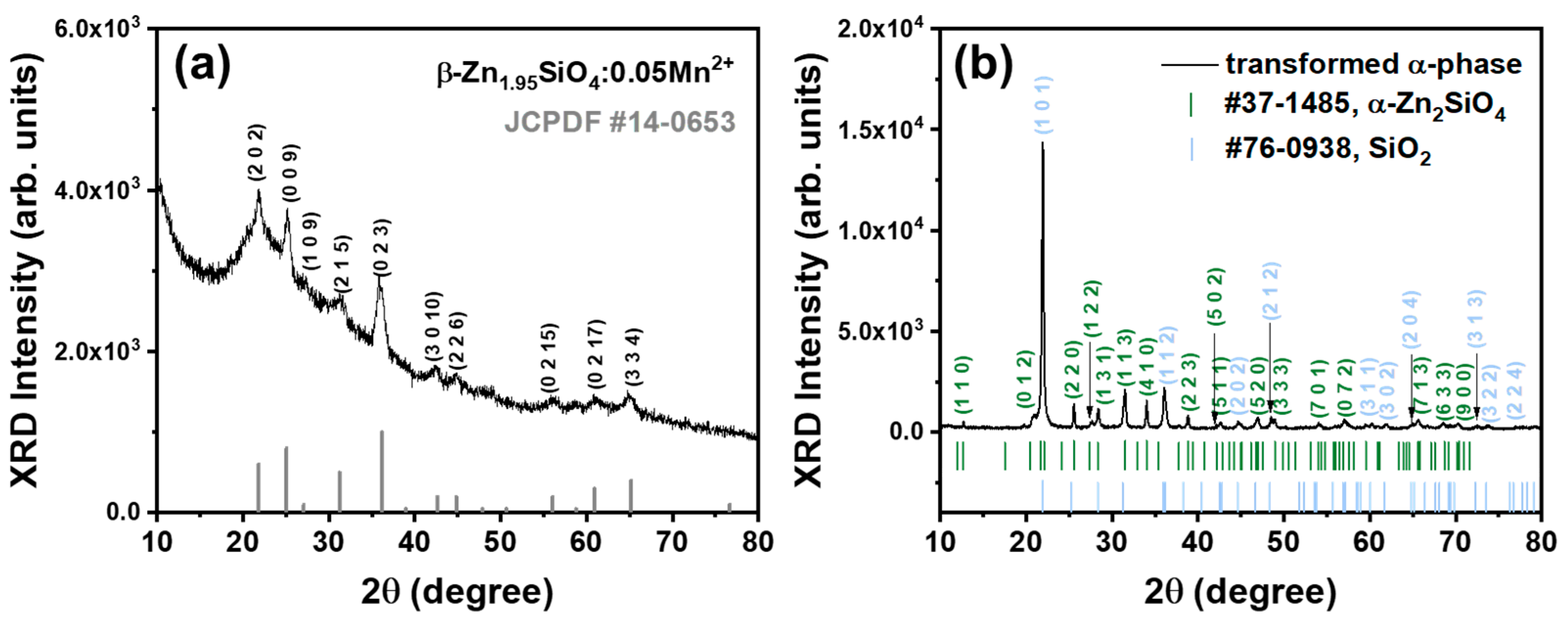

| Annealing Temperature (°C) | Emission Center (nm) | FWHM (nm) | Color Coordinate (x, y) |
|---|---|---|---|
| 700 | 566 | 80.01 | 0.3259, 0.4032 |
| 750 | 574 | 62.75 | 0.4628, 0.5007 |
| 800 | 577 | 54.51 | 0.5097, 0.4849 |
| 850 | 575 | 53.94 | 0.4967, 0.4974 |
| 900 | 574 | 53.64 | 0.4849, 0.5081 |
| 950 | 570 | 59.00 | 0.4460, 0.5398 |
| 1000 | 569 | 78.65 | 0.4346, 0.5499 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Afandi, M.M.; Byeon, S.; Kang, T.; Kang, H.; Kim, J. Bright Yellow Luminescence from Mn2+-Doped Metastable Zinc Silicate Nanophosphor with Facile Preparation and Its Practical Application. Nanomaterials 2024, 14, 1395. https://doi.org/10.3390/nano14171395
Afandi MM, Byeon S, Kang T, Kang H, Kim J. Bright Yellow Luminescence from Mn2+-Doped Metastable Zinc Silicate Nanophosphor with Facile Preparation and Its Practical Application. Nanomaterials. 2024; 14(17):1395. https://doi.org/10.3390/nano14171395
Chicago/Turabian StyleAfandi, Mohammad M., Sanghun Byeon, Taewook Kang, Hyeonwoo Kang, and Jongsu Kim. 2024. "Bright Yellow Luminescence from Mn2+-Doped Metastable Zinc Silicate Nanophosphor with Facile Preparation and Its Practical Application" Nanomaterials 14, no. 17: 1395. https://doi.org/10.3390/nano14171395





