Effects of Scutellaria baicalensis Extract-Induced Exosomes on the Periodontal Stem Cells and Immune Cells under Fine Dust
Abstract
:1. Introduction
2. Materials and Methods
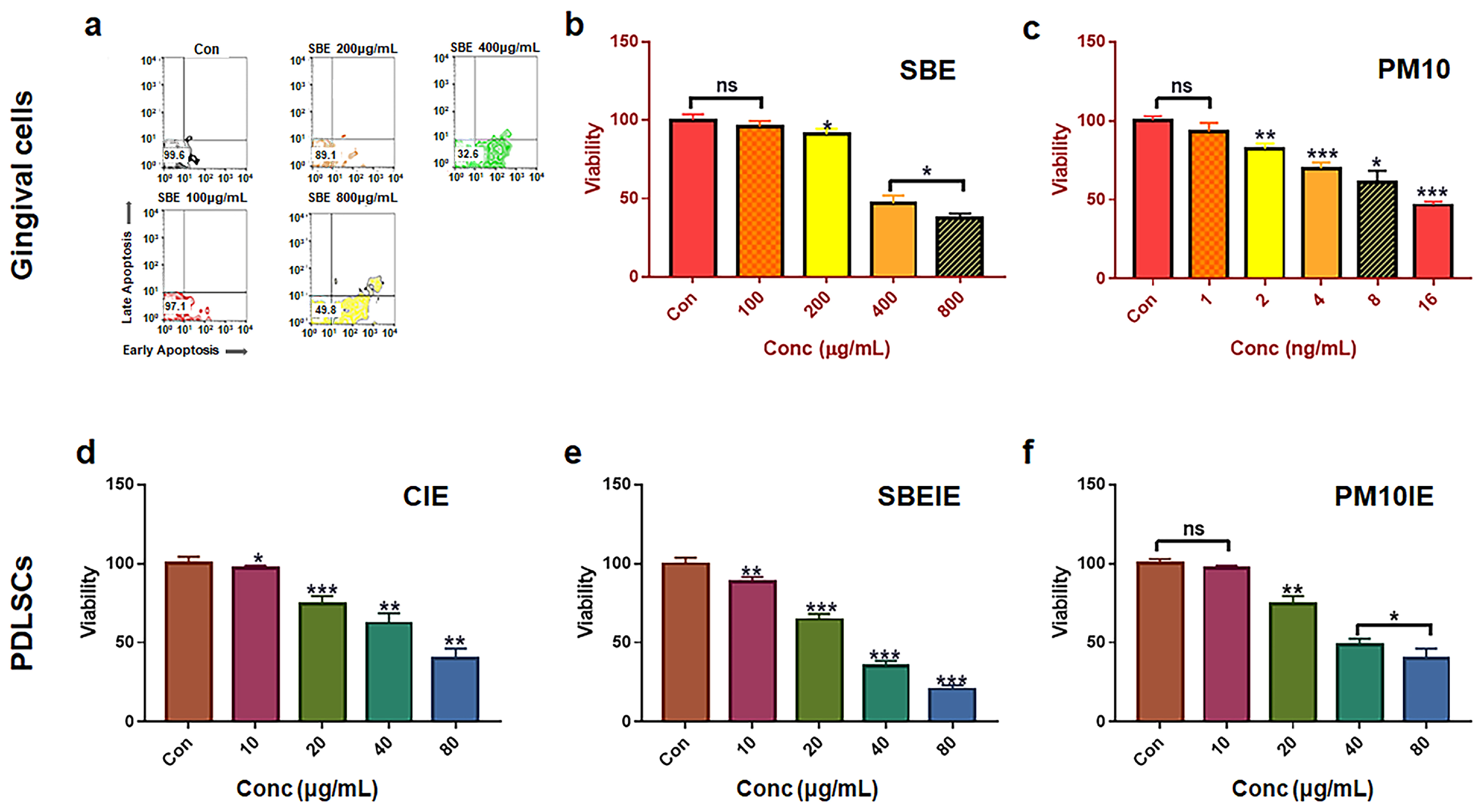
2.1. Cell Culture and Cytotoxicity Test for Establishing Treating Dosage
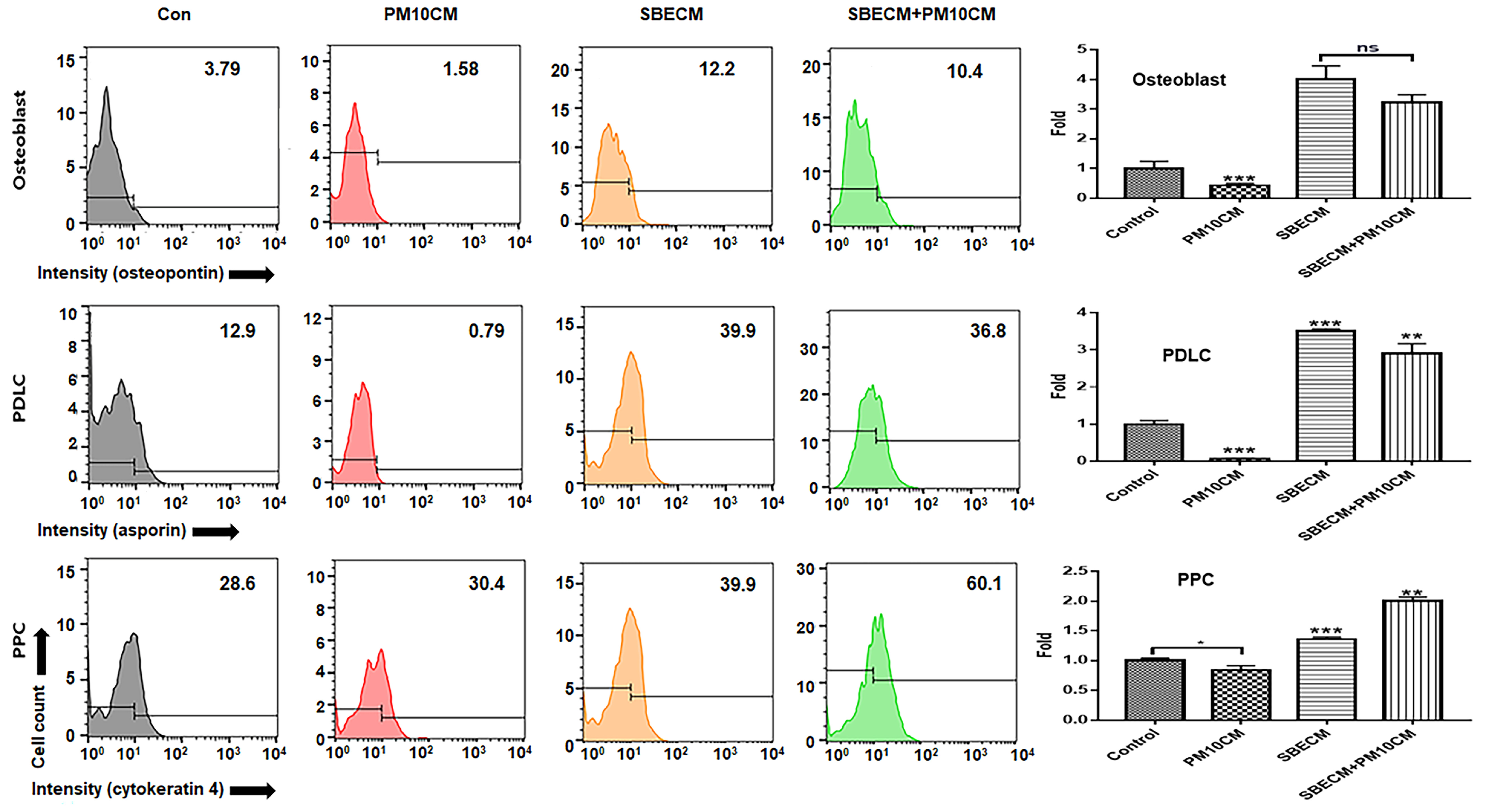
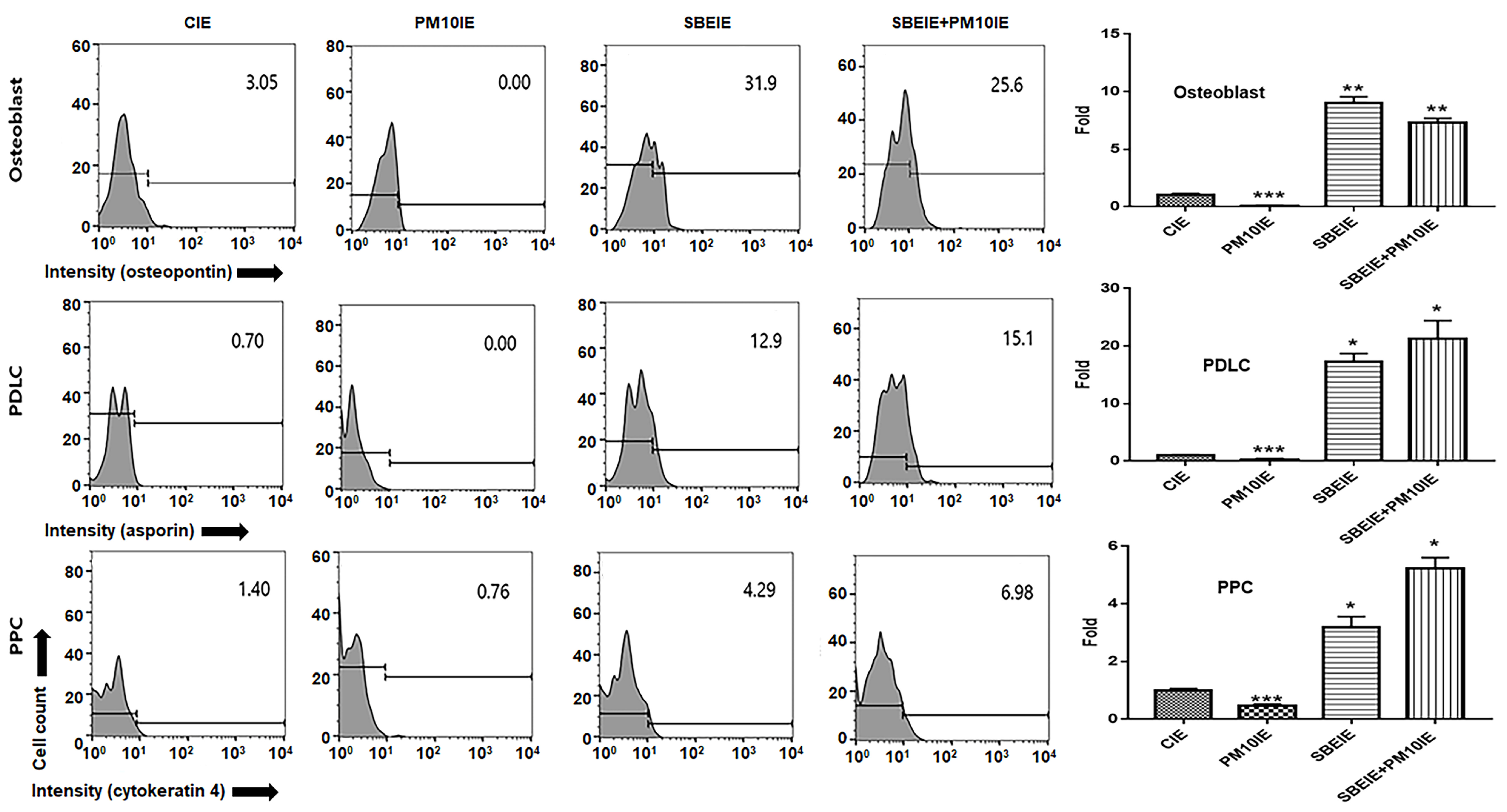
2.2. Analysis for Differentiating Patterns of PDLSCs under the Biomaterials
2.3. Localization of PLDC Marker Using Immunocytochemistry
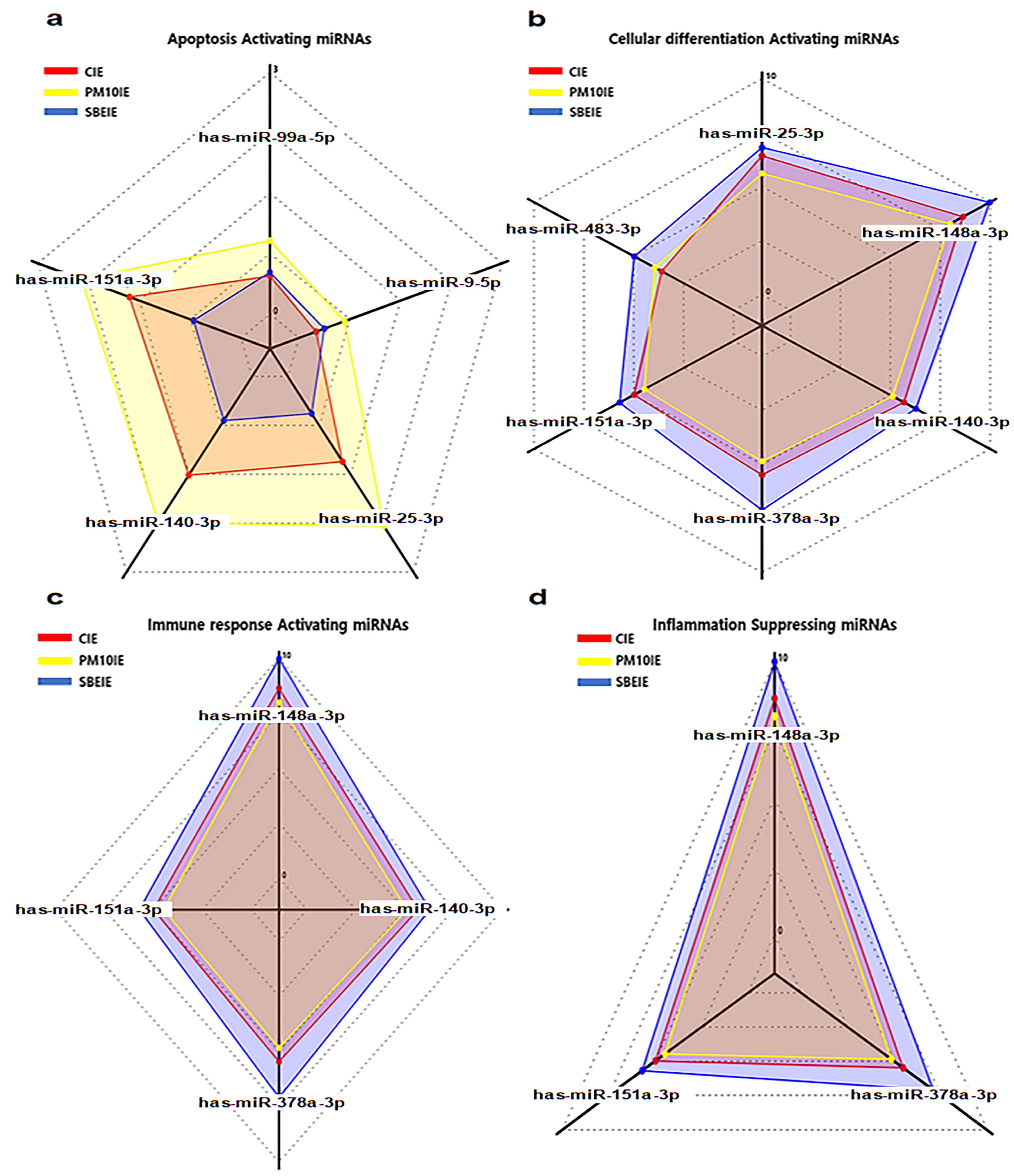
2.4. Profiling of microRNAs in SBEIE
2.5. Evaluating for the Levels of Osteogenic Markers
2.6. Imagine Analysis of Osteoblast Cells
2.7. Evaluating for Bacterial and Viral Phagocytosis
2.8. Evaluating of Cytokines in Immune Cells
2.9. Statistical Analysis
3. Results
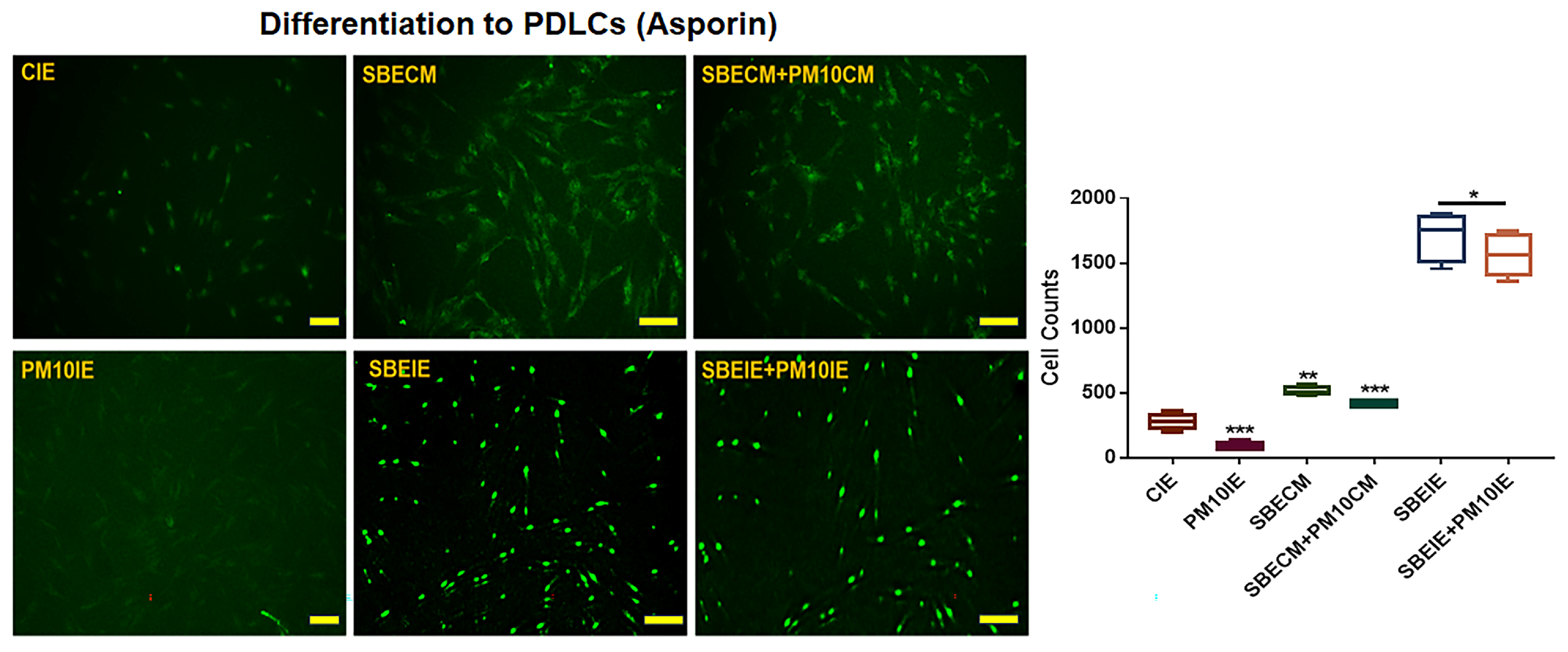
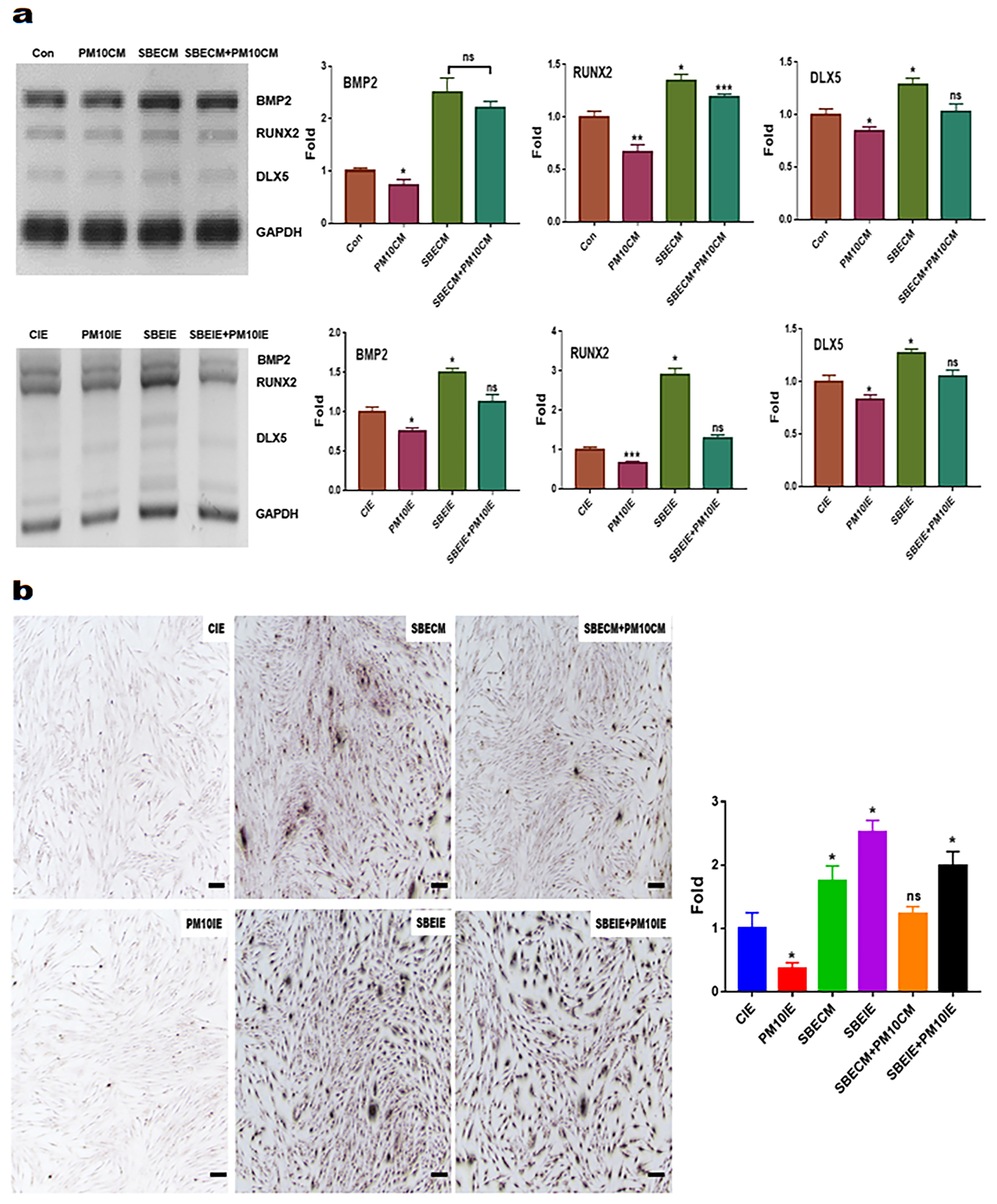
3.1. Activation for Differentiation of PDLSCs by SBEIE
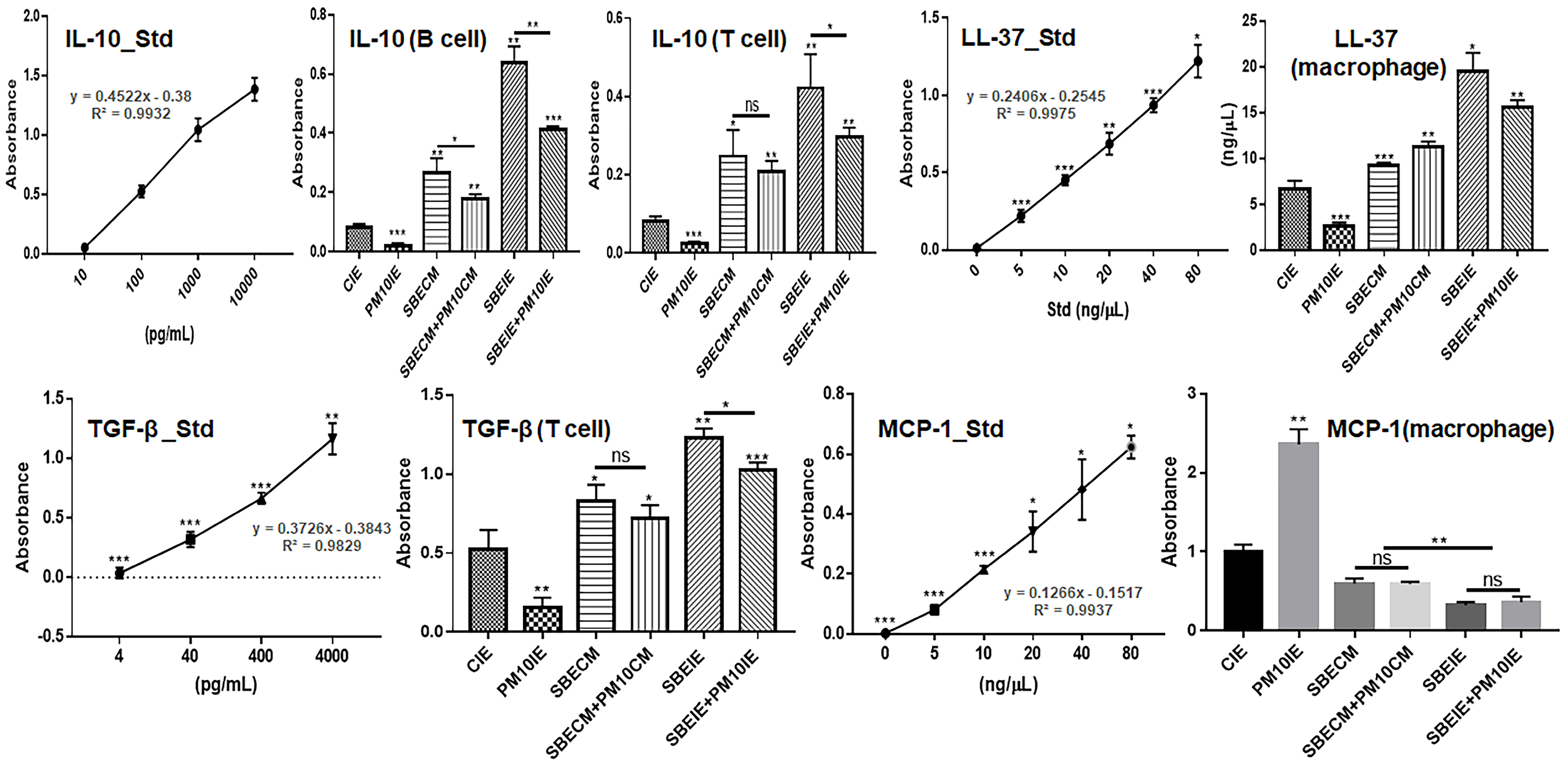
3.2. Activation of Immune System
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Shirmohamadi, A.; Agricultural Research Service; United States Department of Agriculture. Research and Development of Controlled Release Formulations of Pesticides; International Atomic Energy Agency: Vienna, Austria, 1994; p. 139.
- Li, H.-B.; Chen, F. Isolation and purification of baicalein, wogonin and oroxylin A from the medicinal plant Scutellaria baicalensis by high-speed counter-current chromatography. J. Chromatogr. A 2005, 1074, 107–110. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Q.; Chen, X.-Y.; Martin, C. Scutellaria baicalensis, the golden herb from the garden of Chinese medicinal plants. Sci. Bull. 2016, 61, 1391–1398. [Google Scholar] [CrossRef]
- Duan, C.; Matsumura, S.; Kariya, N.; Nishimura, M.; Shimono, T. In vitro antibacterial activities of Scutellaria baicalensis Georgi against cariogenic bacterial. Pediatr. Dent. J. 2007, 17, 58–64. [Google Scholar] [CrossRef]
- Li, M.; Wang, Y.; Xue, J.; Xu, Q.; Zhang, Y.; Liu, J.; Xu, H.; Guan, Z.; Bian, C.; Zhang, G. Baicalin can enhance odonto/osteogenic differentiation of inflammatory dental pulp stem cells by inhibiting the NF-κB and β-catenin/Wnt signaling pathways. Mol. Biol. Rep. 2023, 50, 4435–4446. [Google Scholar] [CrossRef] [PubMed]
- Ming, J.; Zhuoneng, L.; Guangxun, Z. Protective role of flavonoid baicalin from Scutellaria baicalensis in periodontal disease pathogenesis: A literature review. Complement. Ther. Med. 2018, 38, 11–18. [Google Scholar] [CrossRef]
- Huang, T.; Liu, Y.; Zhang, C. Pharmacokinetics and bioavailability enhancement of baicalin: A review. Eur. J. Drug Metab. Pharmacokinet. 2019, 44, 159–168. [Google Scholar] [CrossRef] [PubMed]
- Shah, M.; Mubin, S.; Hassan, S.S.u.; Tagde, P.; Ullah, O.; Rahman, M.H.; Al-Harrasi, A.; Rehman, N.U.; Murad, W. Phytochemical profiling and bio-potentiality of genus scutellaria: Biomedical approach. Biomolecules 2022, 12, 936. [Google Scholar] [CrossRef]
- Bartel, D.P. Metazoan micrornas. Cell 2018, 173, 20–51. [Google Scholar] [CrossRef]
- Montecalvo, A.; Larregina, A.T.; Shufesky, W.J.; Beer Stolz, D.; Sullivan, M.L.; Karlsson, J.M.; Baty, C.J.; Gibson, G.A.; Erdos, G.; Wang, Z. Mechanism of transfer of functional microRNAs between mouse dendritic cells via exosomes. Blood J. Am. Soc. Hematol. 2012, 119, 756–766. [Google Scholar] [CrossRef]
- Lytle, J.R.; Yario, T.A.; Steitz, J.A. Target mRNAs are repressed as efficiently by microRNA-binding sites in the 5′ UTR as in the 3′ UTR. Proc. Natl. Acad. Sci. USA 2007, 104, 9667–9672. [Google Scholar] [CrossRef]
- Tan, F.; Li, X.; Wang, Z.; Li, J.; Shahzad, K.; Zheng, J. Clinical applications of stem cell-derived exosomes. Signal Transduct. Target. Ther. 2024, 9, 17. [Google Scholar] [CrossRef]
- Mukherjee, A.; Bisht, B.; Dutta, S.; Paul, M.K. Current advances in the use of exosomes, liposomes, and bioengineered hybrid nanovesicles in cancer detection and therapy. Acta Pharmacol. Sin. 2022, 43, 2759–2776. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Chen, Z.; Shan, D.; Wu, Y.; Zhao, Y.; Li, C.; Shu, Y.; Linghu, X.; Wang, B. Adverse effects of exposure to fine particles and ultrafine particles in the environment on different organs of organisms. J. Environ. Sci. 2024, 135, 449–473. [Google Scholar] [CrossRef] [PubMed]
- Combes, A.; Franchineau, G. Fine particle environmental pollution and cardiovascular diseases. Metabolism 2019, 100, 153944. [Google Scholar] [CrossRef]
- Zhang, Z.; Dong, B.; Li, S.; Chen, G.; Yang, Z.; Dong, Y.; Wang, Z.; Ma, J.; Guo, Y. Exposure to ambient particulate matter air pollution, blood pressure and hypertension in children and adolescents: A national cross-sectional study in China. Environ. Int. 2019, 128, 103–108. [Google Scholar] [CrossRef]
- Javadinejad, S.; Dara, R.; Jafary, F. Health impacts of extreme events. Saf. Extrem. Environ. 2020, 2, 171–181. [Google Scholar] [CrossRef]
- Xu, H.; Jia, Y.; Sun, Z.; Su, J.; Liu, Q.S.; Zhou, Q.; Jiang, G. Environmental pollution, a hidden culprit for health issues. Eco-Environ. Health 2022, 1, 31–45. [Google Scholar] [CrossRef]
- Mishra, R.; Krishnamoorthy, P.; Gangamma, S.; Raut, A.A.; Kumar, H. Particulate matter (PM10) enhances RNA virus infection through modulation of innate immune responses. Environ. Pollut. 2020, 266, 115148. [Google Scholar] [CrossRef]
- Chen, X.; Liu, J.; Zhou, J.; Wang, J.; Chen, C.; Song, Y.; Pan, J. Urban particulate matter (PM) suppresses airway antibacterial defence. Respir. Res. 2018, 19, 5. [Google Scholar] [CrossRef]
- Franzetti, A.; Gandolfi, I.; Gaspari, E.; Ambrosini, R.; Bestetti, G. Seasonal variability of bacteria in fine and coarse urban air particulate matter. Appl. Microbiol. Biotechnol. 2011, 90, 745–753. [Google Scholar] [CrossRef]
- Turner, M.C.; Andersen, Z.J.; Baccarelli, A.; Diver, W.R.; Gapstur, S.M.; Pope, C.A., III; Prada, D.; Samet, J.; Thurston, G.; Cohen, A. Outdoor air pollution and cancer: An overview of the current evidence and public health recommendations. CA A Cancer J. Clin. 2020, 70, 460–479. [Google Scholar] [CrossRef] [PubMed]
- Panagakos, F.; Scannapieco, F. Periodontal inflammation: From gingivitis to systemic disease. In Gingival Diseases Their Aetiology, Prevention and Treatment; IntechOpen: London, UK, 2011; pp. 155–168. [Google Scholar]
- Park, Y.; Shin, G.H.; Jin, G.S.; Jin, S.; Kim, B.; Lee, S.G. Effects of black jade on osteogenic differentiation of adipose derived stem cells under benzopyrene. Appl. Sci. 2021, 11, 1346. [Google Scholar] [CrossRef]
- Wada, Y.; Ikemoto, T.; Morine, Y.; Imura, S.; Saito, Y.; Yamada, S.; Shimada, M. The differences in the characteristics of insulin-producing cells using human adipose-tissue derived mesenchymal stem cells from subcutaneous and visceral tissues. Sci. Rep. 2019, 9, 13204. [Google Scholar] [CrossRef]
- Nydell Helkimo, A.; Rolander, B.; Koch, G. Oral health with focus on dental fear and dental caries in Swedish preschool child populations attending public dental health care: Trends over 30 years. Int. J. Dent. Hyg. 2024. epub ahead of print. [Google Scholar] [CrossRef] [PubMed]
- Amiruddin, W.M.; Sukri, S.; Al-Amsyar, S.; Rusli, N.; Mat, K.; Mohd, M.; Harun, H. Application of herbal plants in giant freshwater prawn: A review on its opportunities and limitation. In Proceedings of IOP Conference Series: Earth and Environmental Science; IOP Publishing, Bristol, UK, 2021; p. 012022. [Google Scholar]
- Patra, J.K.; Das, G.; Lee, S.; Kang, S.-S.; Shin, H.-S. Selected commercial plants: A review of extraction and isolation of bioactive compounds and their pharmacological market value. Trends Food Sci. Technol. 2018, 82, 89–109. [Google Scholar] [CrossRef]
- Gaviria, L.; Salcido, J.P.; Guda, T.; Ong, J.L. Current trends in dental implants. J. Korean Assoc. Oral Maxillofac. Surg. 2014, 40, 50. [Google Scholar] [CrossRef]
- Nevins, M.; Langer, B. The successful use of osseointegrated implants for the treatment of the recalcitrant periodontal patient. J. Periodontol. 1995, 66, 150–157. [Google Scholar] [CrossRef]
- Yin, H.; He, H.; Cao, X.; Shen, X.; Han, S.; Cui, C.; Zhao, J.; Wei, Y.; Chen, Y.; Xia, L. MiR-148a-3p regulates skeletal muscle satellite cell differentiation and apoptosis via the PI3K/AKT signaling pathway by targeting Meox2. Front. Genet. 2020, 11, 512. [Google Scholar] [CrossRef]
- Anastasio, C.; Donisi, I.; Colloca, A.; D’Onofrio, N.; Balestrieri, M.L. MiR-148a-3p/SIRT7 Axis Relieves Inflammatory-Induced Endothelial Dysfunction. Int. J. Mol. Sci. 2024, 25, 5087. [Google Scholar] [CrossRef]
- Figueredo, C.; Lira-Junior, R.; Love, R. T and B cells in periodontal disease: New functions in a complex scenario. Int. J. Mol. Sci. 2019, 20, 3949. [Google Scholar] [CrossRef]
- Lira-Junior, R.; Figueredo, C.M. Periodontal and inflammatory bowel diseases: Is there evidence of complex pathogenic interactions? World J. Gastroenterol. 2016, 22, 7963. [Google Scholar] [CrossRef] [PubMed]
- Friedrich, M.; Pracht, K.; Mashreghi, M.F.; Jäck, H.M.; Radbruch, A.; Seliger, B. The role of the miR-148/-152 family in physiology and disease. Eur. J. Immunol. 2017, 47, 2026–2038. [Google Scholar] [CrossRef]
- Li, Y.; Zhou, T.; Cheng, X.; Li, D.; Zhao, M.; Zheng, W.V. microRNA-378a-3p regulates the progression of hepatocellular carcinoma by regulating PD-L1 and STAT3. Bioengineered 2022, 13, 4730–4743. [Google Scholar] [CrossRef]
- Ghashghaei, M.; Le, C.T.; Shaalan, H.; Escano, L.; Yue, M.; Arsalan, A.; Rouhi, A.; Nguyen, T.A.; Vu, L.P. miR-148a-3p and DDX6 functional link promotes survival of myeloid leukemia cells. Blood Adv. 2023, 7, 3846–3861. [Google Scholar] [CrossRef] [PubMed]
- Mukhtar, F.; Guarnieri, A.; Brancazio, N.; Falcone, M.; Di Naro, M.; Azeem, M.; Zubair, M.; Nicolosi, D.; Di Marco, R.; Petronio Petronio, G. The role of Mycobacterium tuberculosis exosomal miRNAs in host pathogen cross-talk as diagnostic and therapeutic biomarkers. Front. Microbiol. 2024, 15, 1441781. [Google Scholar] [CrossRef]
- Yadav, A.; Saini, V.; Arora, S. MCP-1: Chemoattractant with a role beyond immunity: A review. Clin. Chim. Acta 2010, 411, 1570–1579. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.; Anshita, D.; Ravichandiran, V. MCP-1: Function, regulation, and involvement in disease. Int. Immunopharmacol. 2021, 101, 107598. [Google Scholar] [CrossRef]
- Gupta, M.; Chaturvedi, R.; Jain, A. Role of monocyte chemoattractant protein-1 (MCP-1) as an immune-diagnostic biomarker in the pathogenesis of chronic periodontal disease. Cytokine 2013, 61, 892–897. [Google Scholar] [CrossRef] [PubMed]
- Bucki, R.; Leszczyńska, K.; Namiot, A.; Sokołowski, W. Cathelicidin LL-37: A multitask antimicrobial peptide. Arch. Immunol. Et Ther. Exp. 2010, 58, 15–25. [Google Scholar] [CrossRef]
- Kahlenberg, J.M.; Kaplan, M.J. Little peptide, big effects: The role of LL-37 in inflammation and autoimmune disease. J. Immunol. 2013, 191, 4895–4901. [Google Scholar] [CrossRef]
- Chen, X.; Zou, X.; Qi, G.; Tang, Y.; Guo, Y.; Si, J.; Liang, L. Roles and mechanisms of human cathelicidin LL-37 in cancer. Cell. Physiol. Biochem. 2018, 47, 1060–1073. [Google Scholar] [CrossRef] [PubMed]
- Sigurdardottir, S.L.; Thorleifsdottir, R.H.; Guzman, A.M.; Gudmundsson, G.H.; Valdimarsson, H.; Johnston, A. The anti-microbial peptide LL-37 modulates immune responses in the palatine tonsils where it is exclusively expressed by neutrophils and a subset of dendritic cells. Clin. Immunol. 2012, 142, 139–149. [Google Scholar] [CrossRef] [PubMed]
- Tokajuk, J.; Deptuła, P.; Piktel, E.; Daniluk, T.; Chmielewska, S.; Wollny, T.; Wolak, P.; Fiedoruk, K.; Bucki, R. Cathelicidin LL-37 in health and diseases of the oral cavity. Biomedicines 2022, 10, 1086. [Google Scholar] [CrossRef] [PubMed]
- Dubois-Camacho, K.; Diaz-Jimenez, D.; De la Fuente, M.; Quera, R.; Simian, D.; Martínez, M.; Landskron, G.; Olivares-Morales, M.; Cidlowski, J.A.; Xu, X. Inhibition of miR-378a-3p by inflammation enhances IL-33 levels: A novel mechanism of alarmin modulation in ulcerative colitis. Front. Immunol. 2019, 10, 2449. [Google Scholar] [CrossRef] [PubMed]

| Gene | Sequence (5′→3′) |
|---|---|
| GAPDH | Forward primer GGTCACCAGGGCTGCTTTTA Reverse primer CCCGTTCTCAGCCATGTAGT |
| DLX2 | Forward primer CTGCTTAGACCAGAGCAGCC Reverse primer CTGGAACGGAGCTTGGAAGT |
| RNX2 | Forward primer CGCCTCACAAACAACCACAG Reverse primer TCACTGTGCTGAAGAGGCTG |
| BMP2 | Forward primer CTGAAACAGAGACCCACCCC Reverse primer TGGTCACGGGGAATTTCGAG |
| Category | Apoptotic Activation | Differentiating Activation | Immune Activation | Anti-Inflammation |
|---|---|---|---|---|
| Dramatic Up-regulation | hsa-miR-151a-3p hsa-miR-140-3p hsa-miR-25-5p hsa-miR-99a-5p hsa-miR-9-5p | hsa-miR-183-3p hsa-miR-151a-3p hsa-miR-376a-3p hsa-miR-140-3p hsa-miR-148a-3p hsa-miR-25-3p | hsa-miR-148a-3p hsa-miR-151a-3p hsa-miR-378a-3p hsa-miR-140-3p | hsa-miR-148a-3p hsa-miR-151a-3p hsa-miR-378a-3p |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yun, M.; Kim, B. Effects of Scutellaria baicalensis Extract-Induced Exosomes on the Periodontal Stem Cells and Immune Cells under Fine Dust. Nanomaterials 2024, 14, 1396. https://doi.org/10.3390/nano14171396
Yun M, Kim B. Effects of Scutellaria baicalensis Extract-Induced Exosomes on the Periodontal Stem Cells and Immune Cells under Fine Dust. Nanomaterials. 2024; 14(17):1396. https://doi.org/10.3390/nano14171396
Chicago/Turabian StyleYun, Mihae, and Boyong Kim. 2024. "Effects of Scutellaria baicalensis Extract-Induced Exosomes on the Periodontal Stem Cells and Immune Cells under Fine Dust" Nanomaterials 14, no. 17: 1396. https://doi.org/10.3390/nano14171396






