Recent Advances of Stretchable Nanomaterial-Based Hydrogels for Wearable Sensors and Electrophysiological Signals Monitoring
Abstract
1. Introduction
2. Nanomaterials for Preparing Hydrogels
2.1. Carbon Nanomaterials
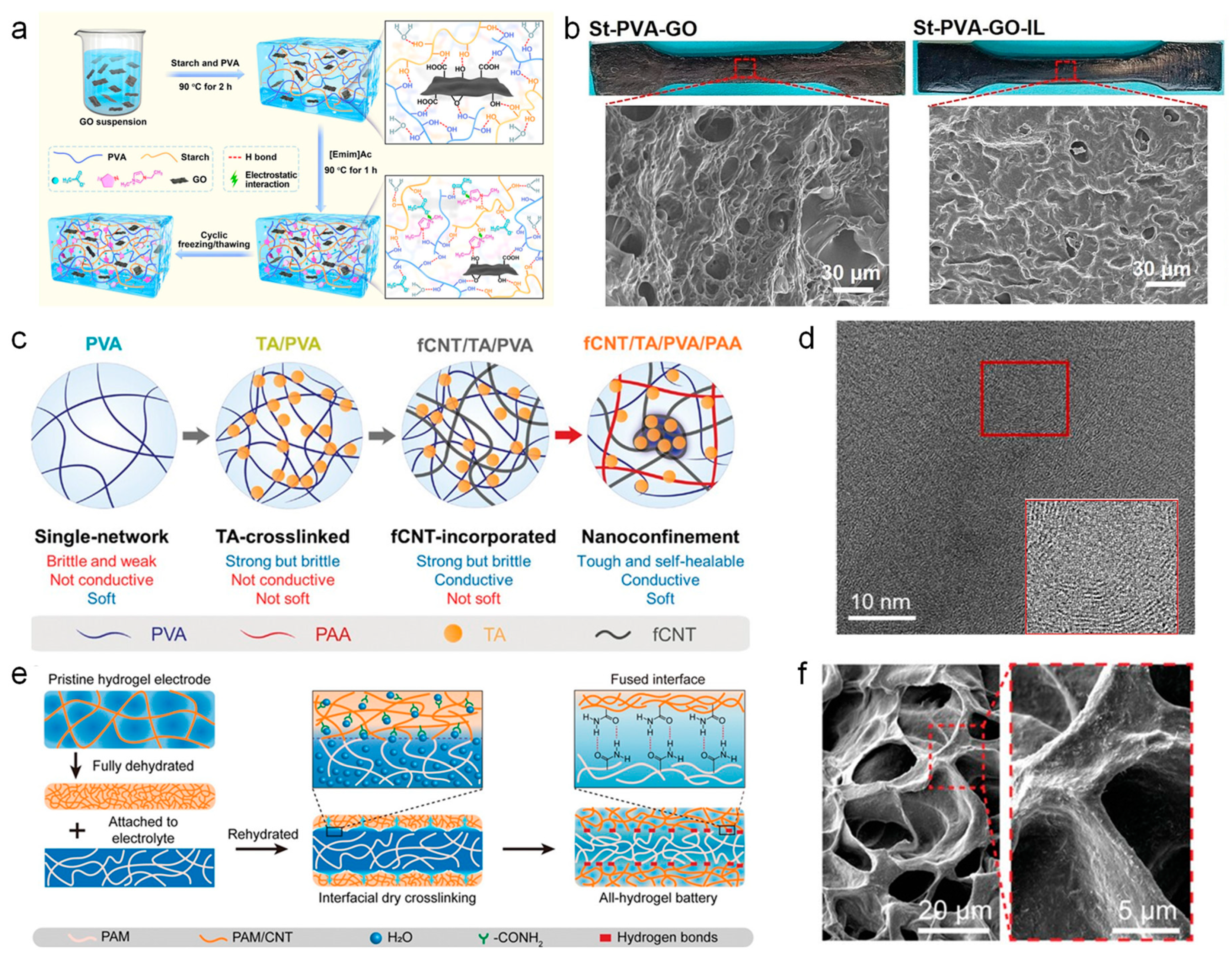
2.2. Nano Metal Materials
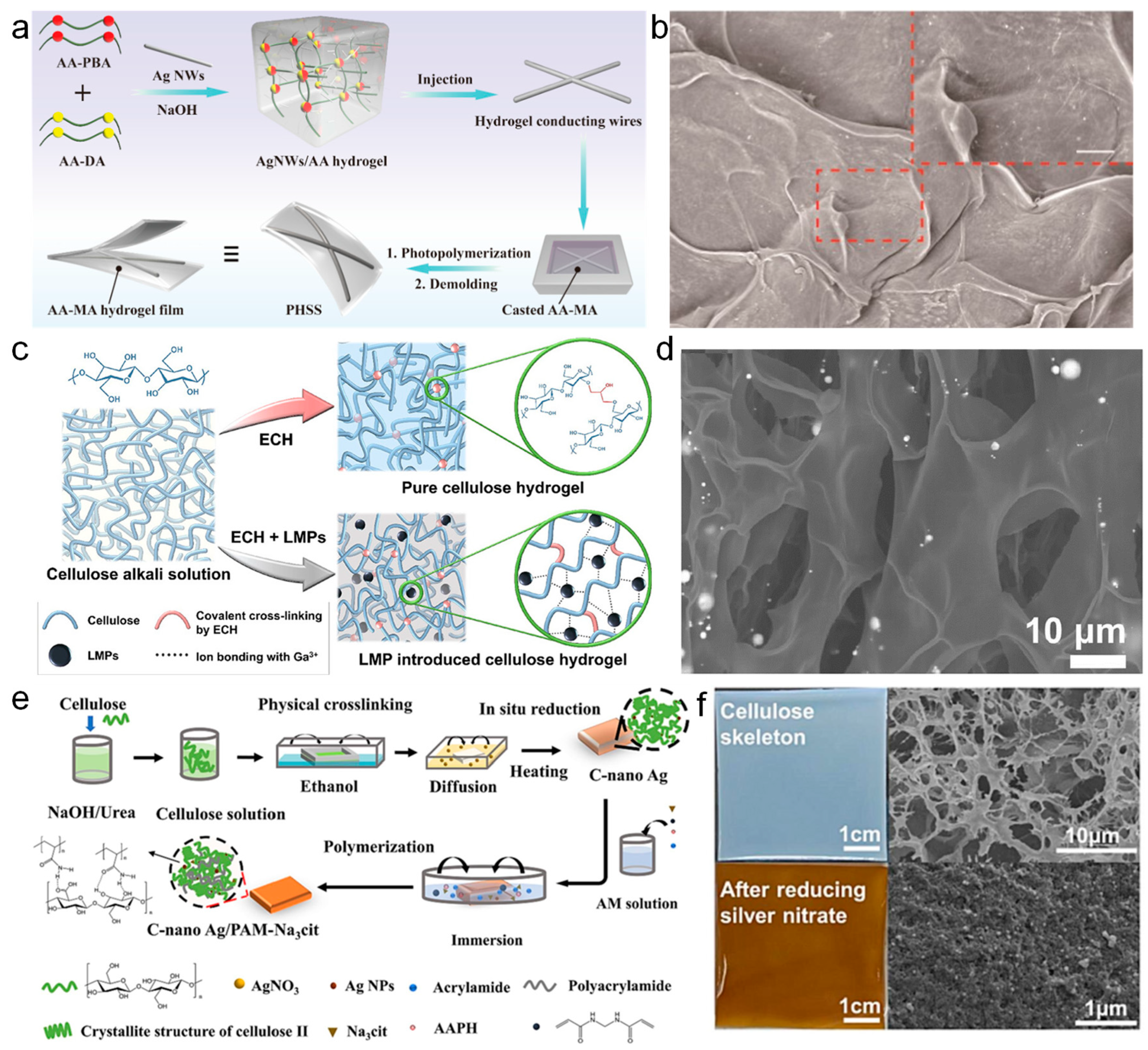
2.3. MXene Nanomaterials

3. Properties of Composite Hydrogel Wearable Sensor
3.1. Conductivity
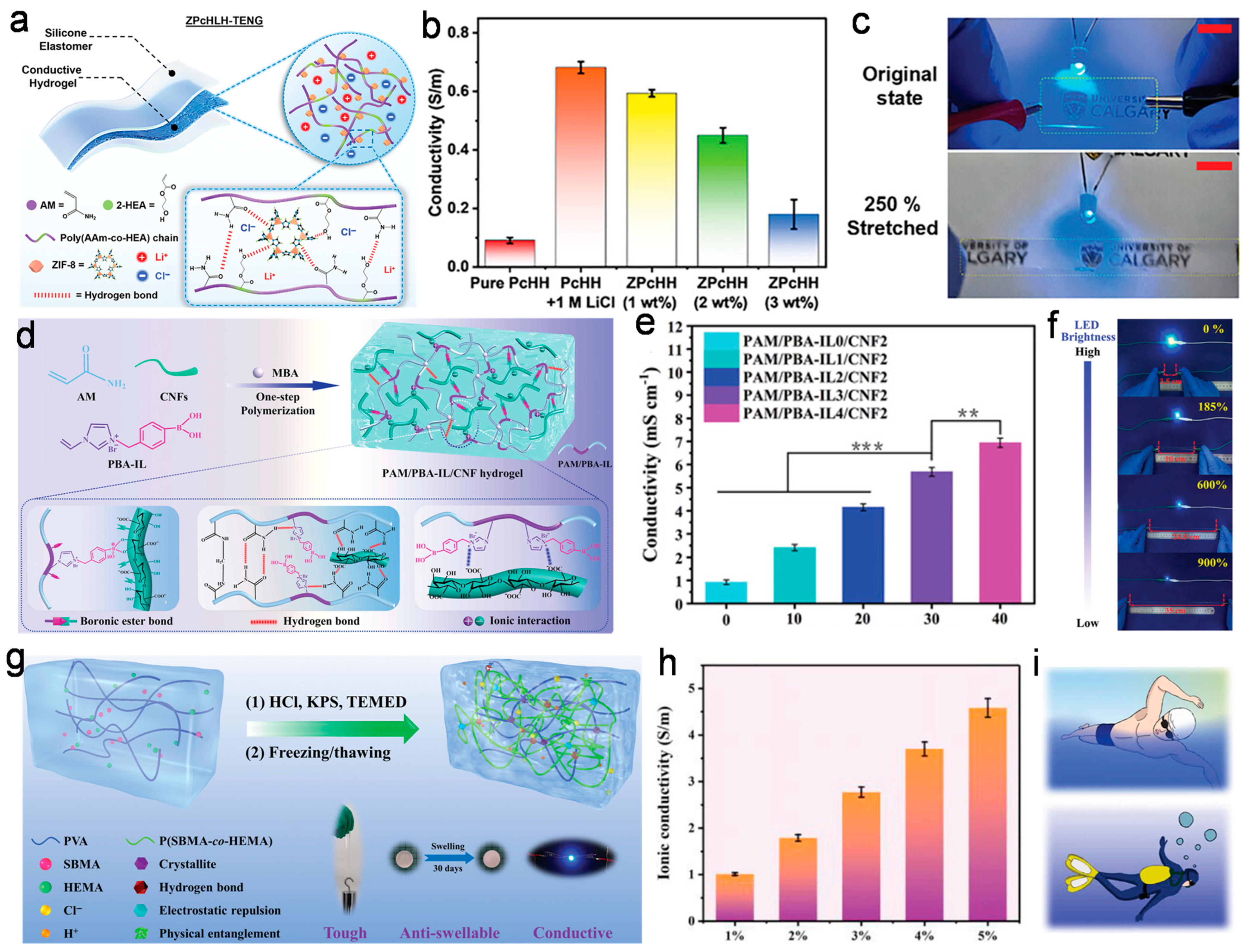
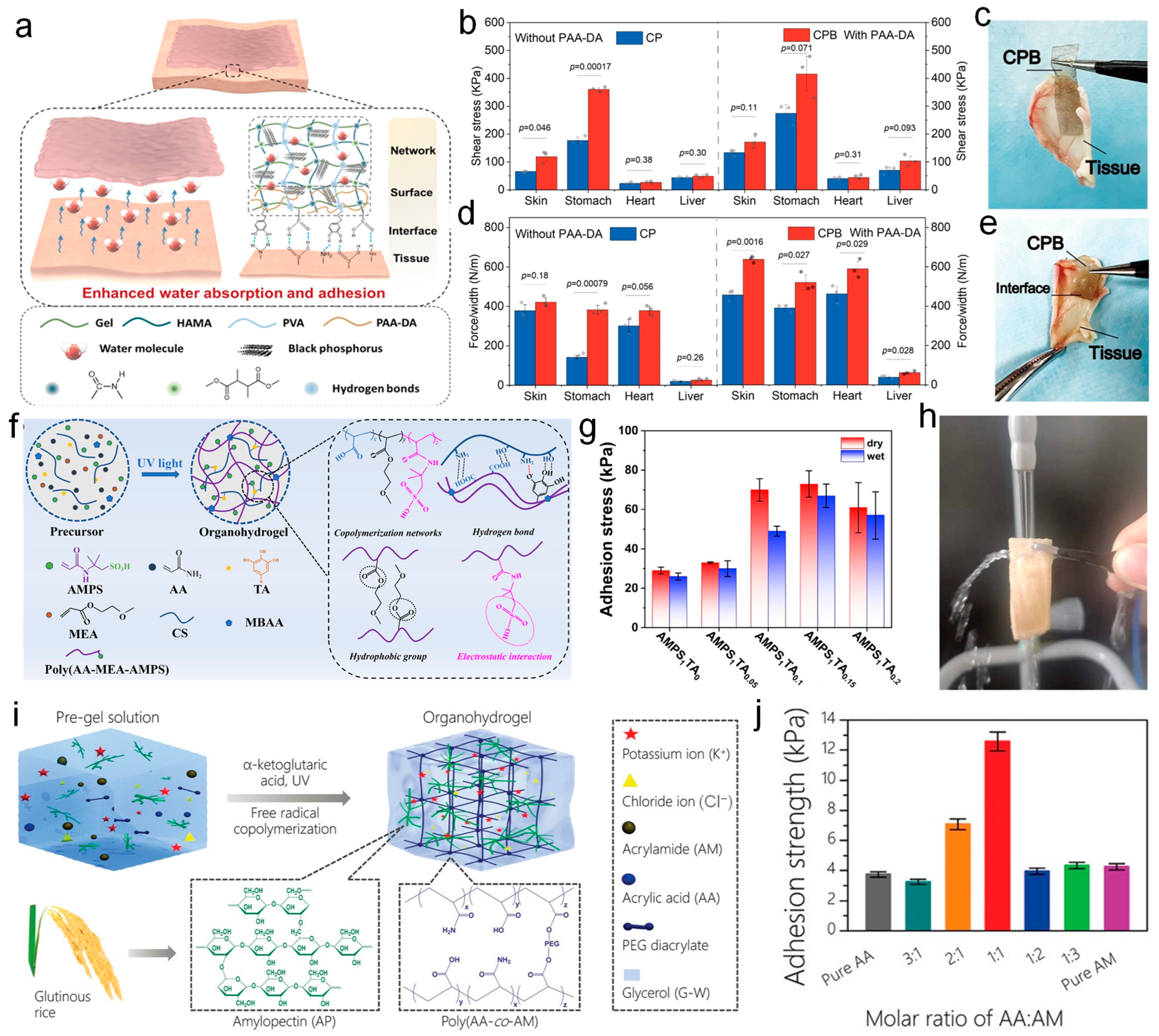
3.2. Adhesion Performance
3.3. Mechanical Properties
4. Electrophysiological Monitoring Applications
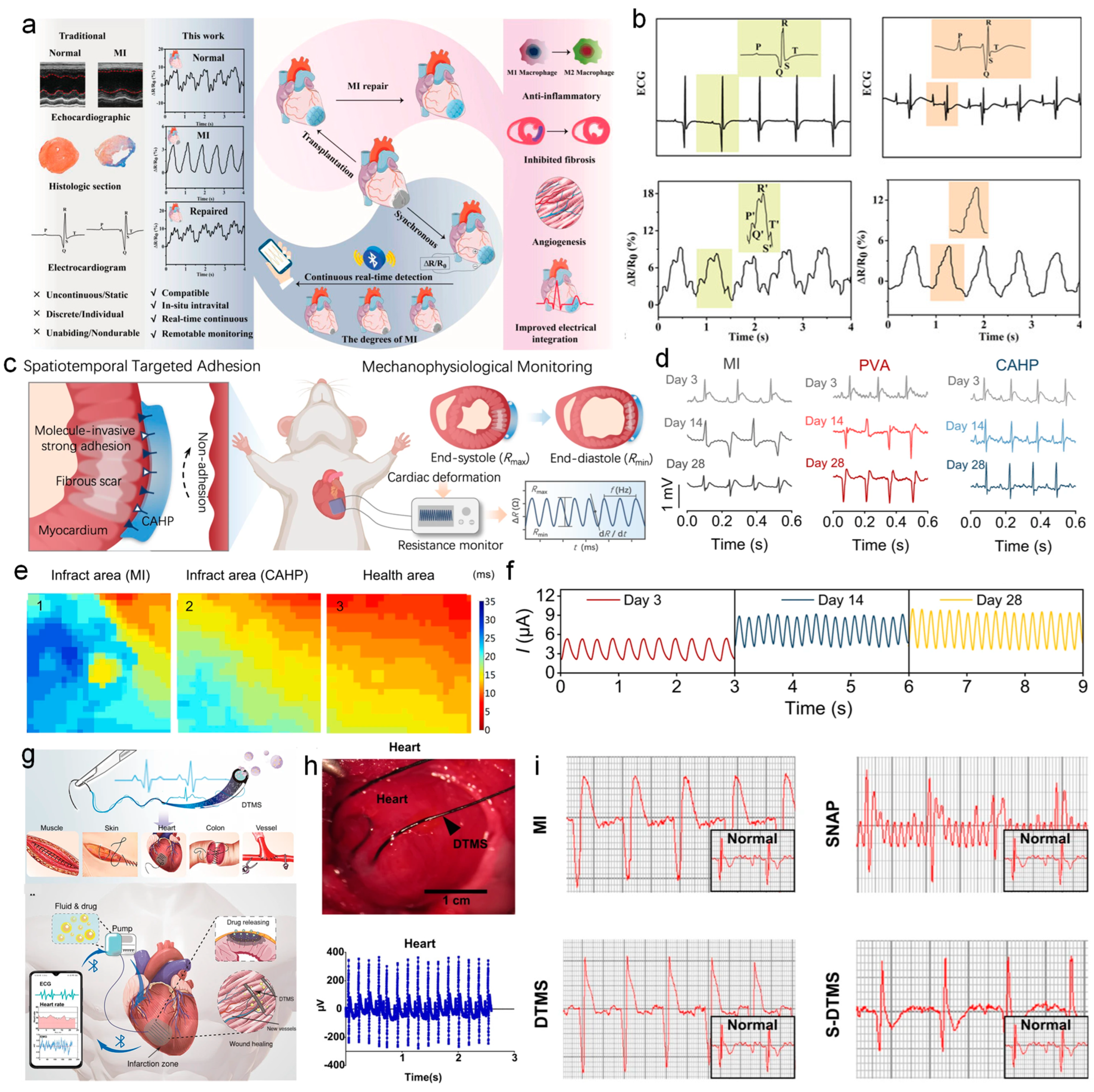
4.1. ECG Monitoring
4.2. EMG Monitoring
4.3. EEG and EOG Monitoring
5. Conclusions and Future Prospects
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Yu, X.; Zhang, H.; Wang, Y.; Fan, X.; Li, Z.; Zhang, X.; Liu, T. Highly Stretchable, Ultra-Soft, and Fast Self-Healable Conductive Hydrogels Based on Polyaniline Nanoparticles for Sensitive Flexible Sensors. Adv. Funct. Mater. 2022, 32, 2004366. [Google Scholar] [CrossRef]
- Liu, P.; Zhang, Y.; Guan, Y.; Zhang, Y. Peptide-Crosslinked, Highly Entangled Hydrogels with Excellent Mechanical Properties but Ultra-Low Solid Content. Adv. Mater. 2023, 35, 2210021. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Shen, S.; Lin, R.; Huang, J.; Pu, C.; Chen, P.; Duan, Q.; You, X.; Xu, C.; Yan, B.; et al. Highly Stretchable and Biocompatible Wrinkled Nanoclay-Composite Hydrogel with Enhanced Sensing Capability for Precise Detection of Myocardial Infarction. Adv. Mater. 2023, 35, 2209497. [Google Scholar] [CrossRef]
- Han, Q.; Zhang, C.; Guo, T.; Tian, Y.; Song, W.; Lei, J.; Li, Q.; Wang, A.; Zhang, M.; Bai, S.; et al. Hydrogel Nanoarchitectonics of a Flexible and Self-Ad hesive Electrode for Long-Term Wireless Electroencephalogram Recording and High-Accuracy Sustained Attention Evaluation. Adv. Mater. 2023, 35, 2209606. [Google Scholar] [CrossRef]
- Xia, X.; Liang, Q.; Sun, X.; Yu, D.; Huang, X.; Mugo, S.M.; Chen, W.; Wang, D.; Zhang, Q. Intrinsically Electron Conductive, Antibacterial, and Anti-swelling Hydrogels as Implantable Sensors for Bioelectronics. Adv. Funct. Mater. 2022, 32, 2208024. [Google Scholar] [CrossRef]
- Yang, G.; Zhu, K.; Guo, W.; Wu, D.; Quan, X.; Huang, X.; Liu, S.; Li, Y.; Fang, H.; Qiu, Y.; et al. Adhesive and Hydrophobic Bilayer Hydrogel Enabled On-Skin Biosensors for High-Fidelity Classification of Human Emotion. Adv. Funct. Mater. 2022, 32, 2200457. [Google Scholar] [CrossRef]
- Feng, T.; Ling, D.; Li, C.; Zheng, W.; Zhang, S.; Li, C.; Emel’yanov, A.; Pozdnyakov, A.S.; Lu, L.; Mao, Y. Stretchable on-skin touchless screen sensor enabled by ionic hydrogel. Nano Res. 2024, 17, 4462–4470. [Google Scholar] [CrossRef]
- Yang, C.; Hu, J.; Liu, L.; Wu, S.; Pan, M.; Liu, Y.; Wang, H.; Li, P.; Zhang, Q.; Qiu, W.; et al. An underwater vest containing an antioxidant MXene hydrogel for sensitive recognition of fish locomotion. Microsyst. Nanoeng. 2024, 10, 41. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Li, J.; Xiao, X.; Wang, J.; Li, Y.; Li, K.; Li, Z.; Yang, H.; Wang, Q.; Yang, J.; et al. Topographic design in wearable MXene sensors with in-sensor machine learning for full-body avatar reconstruction. Nat. Commun. 2022, 13, 5311. [Google Scholar] [CrossRef]
- Hu, Y.; Hou, C.; Du, J.; Fan, Q.; Fang, J.; Shi, Y.; Yang, G.; An, J.; Liu, Y. Flexible Sensor Based on Fe3O4-COOH@Ti3C2Tx MXene Rapid-Gelating Hydrogel for Human Motion Monitoring. Adv. Mater. Interfaces. 2022, 9, 2200487. [Google Scholar] [CrossRef]
- Bai, J.; Gu, W.; Bai, Y.; Li, Y.; Yang, L.; Fu, L.; Li, S.; Li, T.; Zhang, T. Multifunctional Flexible Sensor Based on PU-TA@MXene Janus Architecture for Selective Direction Recognition. Adv. Mater. 2023, 35, 2302847. [Google Scholar] [CrossRef] [PubMed]
- Long, Y.; Wang, Z.; Xu, F.; Jiang, B.; Xiao, J.; Yang, J.; Wang, Z.L.; Hu, W. Mechanically Ultra-Robust, Elastic, Conductive, and Multifunctional Hybrid Hydrogel for a Triboelectric Nanogenerator and Flexible/Wearable Sensor. Small 2022, 18, 2203956. [Google Scholar] [CrossRef] [PubMed]
- Xu, S.; Zong, Y.; Ma, J.; Liu, L. A Multifunctional Skin-Like Sensor Based on Liquid Metal Activated Gelatin Organohydrogel. Adv. Mater. Interfaces 2022, 9, 2201212. [Google Scholar] [CrossRef]
- Guo, B.; Zhong, Y.; Song, X.; Chen, X.; Zhou, P.; Zhao, F.; Bai, J. 3D Printing of Mechanically Elastic, Self-Adhesive, and Biocompatible Organohydrogels for Wearable and Breathable Strain Sensors. Adv. Mater. Technol. 2022, 8, 2201078. [Google Scholar] [CrossRef]
- Cao, L.; Zhao, Z.; Li, J.; Yi, Y.; Wei, Y. Gelatin-Reinforced Zwitterionic Organohydrogel with Tough, Self-Adhesive, Long-Term Moisturizing and Antifreezing Properties for Wearable Electronics. Biomacromolecules 2022, 23, 1278–1290. [Google Scholar] [CrossRef]
- He, K.; Cai, P.; Ji, S.; Tang, Z.; Fang, Z.; Li, W.; Yu, J.; Su, J.; Luo, Y.; Zhang, F.; et al. An Antidehydration Hydrogel Based on Zwitterionic Oligomers for Bioelectronic Interfacing. Adv. Mater. 2023, 36, 2311255. [Google Scholar] [CrossRef]
- Liu, Z.; Liu, J.; He, S.; Wu, X.; Wei, X.; Shao, W. Self-Healing and Self-Adhesion Conductive Hydrogels Reinforced by Carboxylated Carbon Nanotubes for High-Performance Wearable Strain Sensors. ACS Appl. Nano Mater. 2024, 7, 7653–7662. [Google Scholar] [CrossRef]
- Ma, S.; Xue, P.; Valenzuela, C.; Zhang, X.; Chen, Y.; Liu, Y.; Yang, L.; Xu, X.; Wang, L. Highly Stretchable and Conductive MXene-Encapsulated Liquid Metal Hydrogels for Bioinspired Self-Sensing Soft Actuators. Adv. Funct. Mater. 2023, 34, 2309899. [Google Scholar] [CrossRef]
- Ni, Q.; Lou, Q.; Shen, C.; Zheng, G.; Song, R.; Hao, J.; Liu, J.; Zhu, J.; Zang, J.; Dong, L.; et al. Sensitive humidity sensor based on moisture-driven energy generation. Nano Res. 2024, 17, 5578–5586. [Google Scholar] [CrossRef]
- Liu, H.; Qin, J.; Yang, X.; Lv, C.; Huang, W.; Li, F.; Zhang, C.; Wu, Y.; Dong, L.; Shan, C. Highly sensitive humidity sensors based on hexagonal boron nitride nanosheets for contactless sensing. Nano Res. 2023, 16, 10279–10286. [Google Scholar] [CrossRef]
- Wichterle, O.; Lim, D. Hydrophilic gels for biological use. Nature 1960, 185, 117–118. [Google Scholar] [CrossRef]
- Cao, J.; Yang, X.; Rao, J.; Mitriashkin, A.; Fan, X.; Chen, R.; Cheng, H.; Wang, X.; Goh, J.; Leo, H.L.; et al. Stretchable and Self-Adhesive PEDOT:PSS Blend with High Sweat Tolerance as Conformal Biopotential Dry Electrodes. ACS Appl. Mater. Interfaces 2022, 14, 39159–39171. [Google Scholar] [CrossRef]
- Liu, W.; Xie, R.; Zhu, J.; Wu, J.; Hui, J.; Zheng, X.; Huo, F.; Fan, D. A temperature responsive adhesive hydrogel for fabrication of flexible electronic sensors. npj Flex. Electron. 2022, 6, 68. [Google Scholar] [CrossRef]
- Bao, R.; Wang, C.; Dong, L.; Shen, C.; Zhao, K.; Pan, C. CdS nanorods/organic hybrid LED array and the piezo-phototronic effect of the device for pressure mapping. Nanoscale 2016, 8, 8078–8082. [Google Scholar] [CrossRef] [PubMed]
- Chang, S.; Deng, Y.; Li, N.; Wang, L.; Shan, C.-X.; Dong, L. Continuous synthesis of ultra-fine fiber for wearable mechanoluminescent textile. Nano Res. 2023, 16, 9379–9386. [Google Scholar] [CrossRef]
- Li, X.; Zhu, P.; Zhang, S.; Wang, X.; Luo, X.; Leng, Z.; Zhou, H.; Pan, Z.; Mao, Y. A Self-Supporting, Conductor-Exposing, Stretchable, Ultrathin, and Recyclable Kirigami-Structured Liquid Metal Paper for Multifunctional E-Skin. ACS Nano 2022, 16, 5909–5919. [Google Scholar] [CrossRef]
- Wang, C.; Li, J.; Fang, Z.; Hu, Z.; Wei, X.; Cao, Y.; Han, J.; Li, Y. Temperature-Stress Bimodal Sensing Conductive Hydrogel-Liquid Metal by Facile Synthesis for Smart Wearable Sensor. Macromol. Rapid Commun. 2021, 43, 2100543. [Google Scholar] [CrossRef]
- Liu, F.; Xie, D.; Lv, F.; Shen, L.; Tian, Z.; Zhao, J. Additive Manufacturing of Stretchable Polyurethane/Graphene/Multiwalled Carbon Nanotube-Based Conducting Polymers for Strain Sensing. ACS Appl. Nano Mater. 2023, 6, 4522–4531. [Google Scholar] [CrossRef]
- Huang, Y.; Liu, B.; Zhang, W.; Qu, G.; Jin, S.; Li, X.; Nie, Z.; Zhou, H. Highly sensitive active-powering pressure sensor enabled by integration of double-rough surface hydrogel and flexible batteries. NPJ Flex. Electron. 2022, 6, 92. [Google Scholar] [CrossRef]
- Cheng, J.; You, L.; Cai, X.; Yang, J.; Chen, H.; Shi, X.; Wu, J.; Wang, J.; Xiong, C.; Wang, S. Fermentation-Inspired Gelatin Hydrogels with a Controllable Supermacroporous Structure and High Ductility for Wearable Flexible Sensors. ACS Appl. Mater. Interfaces 2022, 14, 26338–26349. [Google Scholar] [CrossRef] [PubMed]
- Xue, Y.; Li, J.; Jiang, T.; Han, Q.; Jing, Y.; Bai, S.; Yan, X. Biomimetic Conductive Hydrogel Scaffolds with Anisotropy and Electrical Stimulation for In Vivo Skeletal Muscle Reconstruction. Adv. Healthc. Mater. 2023, 13, 2302180. [Google Scholar] [CrossRef]
- Ge, S.J.; Liu, S.N.; Gu, Z.Z.; Xu, H. A Skin-Inspired Multifunctional Conductive Hydrogel with High Stretchable, Adhesive, Healable, and Decomposable Properties for Highly Sensitive Dual-Sensing of Temperature and Strain. Small Methods 2023, 7, 2300749. [Google Scholar] [CrossRef] [PubMed]
- He, Y.; Li, Q.; Chen, P.; Duan, Q.; Zhan, J.; Cai, X.; Wang, L.; Hou, H.; Qiu, X. A smart adhesive Janus hydrogel for non-invasive cardiac repair and tissue adhesion prevention. Nat. Commun. 2022, 13, 7666. [Google Scholar] [CrossRef]
- Xia, H.; Wang, L.; Zhang, H.; Wang, Z.; Zhu, L.; Cai, H.; Ma, Y.; Yang, Z.; Zhang, D. MXene/PPy@PDMS sponge-based flexible pressure sensor for human posture recognition with the assistance of a convolutional neural network in deep learning. Microsyst. Nanoeng. 2023, 9, 155. [Google Scholar] [CrossRef]
- Qin, Z.; Zhao, G.; Zhang, Y.; Gu, Z.; Tang, Y.; Aladejana, J.T.; Ren, J.; Jiang, Y.; Guo, Z.; Peng, X.; et al. A Simple and Effective Physical Ball-Milling Strategy to Prepare Super-Tough and Stretchable PVA@MXene@PPy Hydrogel for Flexible Capacitive Electronics. Small 2023, 19, 2303038. [Google Scholar] [CrossRef] [PubMed]
- Chong, J.; Sung, C.; Nam, K.S.; Kang, T.; Kim, H.; Lee, H.; Park, H.; Park, S.; Kang, J. Highly conductive tissue-like hydrogel interface through template-directed assembly. Nat. Commun. 2023, 14, 2206. [Google Scholar] [CrossRef]
- Tang, H.; Li, Y.; Chen, B.; Chen, X.; Han, Y.; Guo, M.; Xia, H.-Q.; Song, R.; Zhang, X.; Zhou, J. In Situ Forming Epidermal Bioelectronics for Daily Monitoring and Comprehensive Exercise. ACS Nano 2022, 16, 17931–17947. [Google Scholar] [CrossRef]
- Chun, K.Y.; Seo, S.; Han, C.S. A Wearable All-Gel Multimodal Cutaneous Sensor Enabling Simultaneous Single-Site Monitoring of Cardiac-Related Biophysical Signals. Adv. Mater. 2022, 34, 2110082. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Yang, J.; Wang, H.; Wang, C.; Gu, Y.; Xu, Y.; Lee, S.; Yokota, T.; Haick, H.; Someya, T. A 10-micrometer-thick nanomesh-reinforced gas-permeable hydrogel skin sensor for long-term electrophysiological monitoring. Sci. Adv. 2024, 10, eadj5389. [Google Scholar] [CrossRef]
- Lan, L.; Ping, J.; Li, H.; Wang, C.; Li, G.; Song, J.; Ying, Y. Skin-Inspired All-Natural Biogel for Bioadhesive Interface. Adv. Mater. 2024, 36, 2401151. [Google Scholar] [CrossRef]
- Yu, C.; Shi, M.; He, S.; Yao, M.; Sun, H.; Yue, Z.; Qiu, Y.; Liu, B.; Liang, L.; Zhao, Z.; et al. Chronological adhesive cardiac patch for synchronous mechanophysiological monitoring and electrocoupling therapy. Nat. Commun. 2023, 14, 6226. [Google Scholar] [CrossRef] [PubMed]
- Bao, B.; Zeng, Q.; Li, K.; Wen, J.; Zhang, Y.; Zheng, Y.; Zhou, R.; Shi, C.; Chen, T.; Xiao, C.; et al. Rapid fabrication of physically robust hydrogels. Nat. Mater. 2023, 22, 1253–1260. [Google Scholar] [CrossRef] [PubMed]
- Kailasa, S.K.; Joshi, D.J.; Kateshiya, M.R.; Koduru, J.R.; Malek, N.I. Review on the biomedical and sensing applications of nanomaterial-incorporated hydrogels. Mater. Today Chem. 2022, 23, 100746. [Google Scholar] [CrossRef]
- Shin, M.; Lim, J.; An, J.; Yoon, J.; Choi, J.W. Nanomaterial-based biohybrid hydrogel in bioelectronics. Nano Converg. 2023, 10, 8. [Google Scholar] [CrossRef]
- Yang, Y.; Wu, N.; Li, B.; Liu, W.; Pan, F.; Zeng, Z.; Liu, J. Biomimetic Porous MXene Sediment-Based Hydrogel for High-Performance and Multifunctional Electromagnetic Interference Shielding. ACS Nano 2022, 16, 15042–15052. [Google Scholar] [CrossRef]
- Zhang, Z.; Luo, Y.; Li, Y.; Ding, S.; Liu, K.; Luo, B. Flexible Hybrid Wearable Sensors for Pressure and Thermal Sensing Based on a Double-Network Hydrogel. ACS Appl. Bio Mater. 2023, 6, 5114–5123. [Google Scholar] [CrossRef] [PubMed]
- Chen, K.; Wang, F.; Hu, Y.; Liu, M.; Liu, P.; Yu, Y.; Feng, Q.; Xiao, X. Highly Stretchable, Sensitive, and Durable Ag/Tannic Acid@Graphene Oxide-Composite Hydrogel for Wearable Strain Sensors. ACS Appl. Polym. Mater. 2022, 4, 2036–2046. [Google Scholar] [CrossRef]
- Sun, P.; Li, Q. Tension-Responsive Graphene Oxide Conductive Hydrogel with Robust Mechanical Properties and High Sensitivity for Human Motion Monitoring. Macromol. Mater. Eng. 2022, 308, 2200529. [Google Scholar] [CrossRef]
- Ma, F.; Wu, Y.; Dai, S.; Lin, P.; Sun, J.; Dong, L. A soft-contact hybrid electromagnetic-triboelectric nanogenerator for self-powered water splitting towards hydrogen production. Nano Res. 2024, 17, 6567–6574. [Google Scholar] [CrossRef]
- Zhang, N.; Qin, C.; Feng, T.; Li, J.; Yang, Z.; Sun, X.; Liang, E.; Mao, Y.; Wang, X. Non-contact cylindrical rotating triboelectric nanogenerator for harvesting kinetic energy from hydraulics. Nano Res. 2020, 13, 1903–1907. [Google Scholar] [CrossRef]
- Mao, Y.; Zhang, N.; Tang, Y.; Wang, M.; Chao, M.; Liang, E. A paper triboelectric nanogenerator for self-powered electronic systems. Nanoscale 2017, 9, 14499–14505. [Google Scholar] [CrossRef] [PubMed]
- Mattos, B.D.; Zhu, Y.; Tardy, B.L.; Beaumont, M.; Ribeiro, A.C.R.; Missio, A.L.; Otoni, C.G.; Rojas, O.J. Versatile Assembly of Metal-Phenolic Network Foams Enabled by Tannin-Cellulose Nanofibers. Adv Mater. 2023, 35, e2209685. [Google Scholar] [CrossRef] [PubMed]
- Muchlis, A.M.G.; Yang, C.; Tsai, Y.T.; Ummartyotin, S.; Lin, C.C. Multiresponsive Self-Healing Lanthanide Fluorescent Hydrogel for Smart Textiles. ACS Appl Mater Interfaces 2023, 15, 46085–46097. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Yao, C.; Huang, W.-Y.; Liu, C.-L.; Zhang, Y. Wearable Sensor Based on a Tough Conductive Gel for Real-Time and Remote Human Motion Monitoring. ACS Appl. Mater. Interfaces 2024, 16, 11957–11972. [Google Scholar] [CrossRef]
- Lin, M.; Zheng, Z.; Yang, L.; Luo, M.; Fu, L.; Lin, B.; Xu, C. A High-Performance, Sensitive, Wearable Multifunctional Sensor Based on Rubber/CNT for Human Motion and Skin Temperature Detection. Adv. Mater. 2021, 34, 2107309. [Google Scholar] [CrossRef]
- Han, L.; Qu, R.; Chen, D.; Liu, L.; Xu, H.; Zhang, Z.; Zhao, Y.; Song, X. Carbon Nanotube Anchored Organic Hydrogel for Soft Sensors. Macromol. Mater. Eng. 2022, 307, 2100890. [Google Scholar] [CrossRef]
- Yuan, S.; Bai, J.; Li, S.; Ma, N.; Deng, S.; Zhu, H.; Li, T.; Zhang, T. A Multifunctional and Selective Ionic Flexible Sensor with High Environmental Suitability for Tactile Perception. Adv. Funct. Mater. 2023, 34, 2309626. [Google Scholar] [CrossRef]
- He, S.; Sun, X.; Qin, Z.; Dong, X.; Zhang, H.; Shi, M.; Yao, F.; Zhang, H.; Li, J. Non-Swelling and Anti-Fouling MXene Nanocomposite Hydrogels for Underwater Strain Sensing. Adv. Mater. Technol. 2021, 7, 2101343. [Google Scholar] [CrossRef]
- Wang, Z.; Xu, X.; Tan, R.; Zhang, S.; Zhang, K.; Hu, J. Hierarchically Structured Hydrogel Composites with Ultra-High Conductivity for Soft Electronics. Adv. Funct. Mater. 2023, 34, 2312667. [Google Scholar] [CrossRef]
- Kumar, A.; Rakesh Kumar, R.K.; Shaikh, M.O.; Lu, C.-H.; Yang, J.-Y.; Chang, H.-L.; Chuang, C.-H. Ultrasensitive Strain Sensor Utilizing a AgF-AgNW Hybrid Nanocomposite for Breath Monitoring and Pulmonary Function Analysis. ACS Appl. Mater. Interfaces. 2022, 14, 55402–55413. [Google Scholar] [CrossRef]
- Zhou, H.; Jin, Z.; Gao, Y.; Wu, P.; Lai, J.; Li, S.; Jin, X.; Liu, H.; Chen, W.; Wu, Y.; et al. Thermoresponsive, magnetic, adhesive and conductive nanocomposite hydrogels for wireless and non-contact flexible sensors. Colloid Surf. A-Physicochem. Eng. Asp. 2022, 636, 128113. [Google Scholar] [CrossRef]
- Xiao, S.; Lao, Y.; Liu, H.; Li, D.; Wei, Q.; Ye, L.; Lu, S. A nanocomposite hydrogel loaded with Ag nanoparticles reduced by aloe vera polysaccharides as an antimicrobial multifunctional sensor. Int. J. Biol. Macromol. 2024, 267, 131541. [Google Scholar] [CrossRef] [PubMed]
- Qin, H.; Sun, M.; Li, P.; Li, J.; Zhang, Z.; Dai, S.; Huang, M.; Lu, B.; Pan, X.; Wu, L. Soft, Stretchable, and Conductive Hydrogel Based on Liquid Metal for Accurately Facial Expression Monitoring. Adv. Mater. Technol. 2023, 8, 2300406. [Google Scholar] [CrossRef]
- Zhang, Q.; Lu, H.; Yun, G.; Gong, L.; Chen, Z.; Jin, S.; Du, H.; Jiang, Z.; Li, W. A Laminated Gravity-Driven Liquid Metal-Doped Hydrogel of Unparalleled Toughness and Conductivity. Adv. Funct. Mater. 2023, 34, 2308113. [Google Scholar] [CrossRef]
- Dong, L.; Wang, M.; Wu, J.; Zhu, C.; Shi, J.; Morikawa, H. Stretchable, Adhesive, Self-Healable, and Conductive Hydrogel-Based Deformable Triboelectric Nanogenerator for Energy Harvesting and Human Motion Sensing. ACS Appl. Mater. Interfaces 2022, 14, 9126–9137. [Google Scholar] [CrossRef] [PubMed]
- Guo, X.; Xing, T.; Feng, J. Simultaneously Stretchable and Compressible Flexible Strain Sensors Based on Carbon Nanotube Composites for Motion Monitoring and Human–Computer Interactions. ACS Appl. Nano Mater. 2022, 5, 18427–18437. [Google Scholar] [CrossRef]
- Salimiyan, N.; Gholami, M.; Sedghi, R. Preparation of degradable, biocompatible, conductive and multifunctional chitosan/thiol-functionalized graphene nanocomposite hydrogel via click chemistry for human motion sensing. Chem. Eng. J. 2023, 471, 144648. [Google Scholar] [CrossRef]
- Song, Y.; Xing, L.; Zou, X.; Zhang, C.; Huang, Z.; Liu, W.; Wang, J. A chitosan-based conductive double network hydrogel doped by tannic acid-reduced graphene oxide with excellent stretchability and high sensitivity for wearable strain sensors. Int. J. Biol. Macromol. 2024, 258, 128861. [Google Scholar] [CrossRef]
- Yu, Y.; Zhao, X.; Ye, L. A novel biocompatible wearable sensors based on poly (vinyl alcohol)/graphene oxide hydrogel with superior self-adhesion, flexibility and sensitivity. Compos. Struct. 2023, 309, 116768. [Google Scholar] [CrossRef]
- Lin, F.; Qiu, Y.; Zheng, X.; Duanmu, Z.; Lu, Q.; Huang, B.; Tang, L.; Lu, B. One-pot mechanochemical assembly of lignocellulose nanofiber/graphite nanocomposites for wearable electronic devices. Chem. Eng. J. 2022, 437, 135286. [Google Scholar] [CrossRef]
- Wang, X.; Deng, Y.; Jiang, P.; Chen, X.; Yu, H. Low-hysteresis, pressure-insensitive, and transparent capacitive strain sensor for human activity monitoring. Microsyst. Nanoeng. 2022, 8, 113. [Google Scholar] [CrossRef]
- Wu, J.; Huang, W.; Wu, Z.; Yang, X.; Kottapalli, A.G.P.; Xie, X.; Zhou, Y.; Tao, K. Hydrophobic and Stable Graphene-Modified Organohydrogel Based Sensitive, Stretchable, and Self-Healable Strain Sensors for Human-Motion Detection in Various Scenarios. ACS Mater. Lett. 2022, 4, 1616–1629. [Google Scholar] [CrossRef]
- Li, X.; Zhang, X.; Xu, J.; Duan, Z.; Xu, Y.; Zhang, X.; Zhang, L.; Wang, Y.; Chu, P.K. Potassium-Rich Iron Hexacyanoferrate/Carbon Cloth Electrode for Flexible and Wearable Potassium-Ion Batteries. Adv. Sci. 2023, 11, 2301713. [Google Scholar] [CrossRef]
- Liu, Y.; Zhuo, F.; Zhou, J.; Kuang, L.; Tan, K.; Lu, H.; Cai, J.; Guo, Y.; Cao, R.; Fu, Y.; et al. Machine-Learning Assisted Handwriting Recognition Using Graphene Oxide-Based Hydrogel. ACS Appl. Mater. Interfaces 2022, 14, 54276–54286. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Song, H.; Chen, L.; Li, W.; Yang, D.; Cheng, P.; Duan, H. 3D Printed Ultrasensitive Graphene Hydrogel Self-Adhesive Wearable Devices. ACS Appl. Electron. Mater. 2022, 4, 5199–5207. [Google Scholar] [CrossRef]
- Li, W.; Wu, S.; Li, S.; Zhong, X.; Zhang, X.; Qiao, H.; Kang, M.; Chen, J.; Wang, P.; Tao, L.-Q. Gesture Recognition System Using Reduced Graphene Oxide-Enhanced Hydrogel Strain Sensors for Rehabilitation Training. ACS Appl. Mater. Interfaces 2023, 15, 45106–45115. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, Q.; Chen, J.; Zheng, Y.; Lu, X.; Chen, Q. Rapid gelation of dual physical network hydrogel with ultra-stretchable, antifreezing, moisturing for stable and sensitive response. J. Appl. Polym. Sci. 2023, 140, e53566. [Google Scholar] [CrossRef]
- Xu, J.; Huang, H.; Sun, C.; Yu, J.; Wang, M.; Dong, T.; Wang, S.; Chen, X.; Cui, T.; Li, J. Flexible Accelerated-Wound-Healing Antibacterial Hydrogel-Nanofiber Scaffold for Intelligent Wearable Health Monitoring. ACS Appl. Mater. Interfaces 2023, 16, 5438–5450. [Google Scholar] [CrossRef] [PubMed]
- Lv, A.; Lv, X.; Tian, S.; Xie, T.; Xu, X.; Sun, S. Tough, Self-Healing, and Antimicrobial Hydrogel Sensors Based on Hydrogen-Bonded, Cross-linked Chitosan and MWCNTs. ACS Appl. Polym. Mater. 2023, 5, 6452–6462. [Google Scholar] [CrossRef]
- Xu, P.; Wang, S.; Lin, A.; Min, H.-K.; Zhou, Z.; Dou, W.; Sun, Y.; Huang, X.; Tran, H.; Liu, X. Conductive and elastic bottlebrush elastomers for ultrasoft electronics. Nat. Commun. 2023, 14, 623. [Google Scholar] [CrossRef]
- Cheng, J.; Shang, J.; Yang, S.; Dou, J.; Shi, X.; Jiang, X. Wet-Adhesive Elastomer for Liquid Metal-Based Conformal Epidermal Electronics. Adv. Funct. Mater. 2022, 32, 2200444. [Google Scholar] [CrossRef]
- Min, H.; Baik, S.; Kim, J.; Lee, J.; Bok, B.G.; Song, J.H.; Kim, M.S.; Pang, C. Tough Carbon Nanotube-Implanted Bioinspired Three-Dimensional Electrical Adhesive for Isotropically Stretchable Water-Repellent Bioelectronics. Adv. Funct. Mater. 2021, 32, 2107285. [Google Scholar] [CrossRef]
- Liu, X.; Huang, Z.; Ye, C.; Luo, Z.; Chen, L.; Yao, X.; Liang, F.; Yang, T.; Bi, H.; Wang, C.; et al. Graphene-Based Hydrogel Strain Sensors with Excellent Breathability for Motion Detection and Communication. Macromol. Mater. Eng. 2022, 307, 2200001. [Google Scholar] [CrossRef]
- Lu, Y.; Yang, G.; Wang, S.; Zhang, Y.; Jian, Y.; He, L.; Yu, T.; Luo, H.; Kong, D.; Xianyu, Y.; et al. Stretchable graphene–hydrogel interfaces for wearable and implantable bioelectronics. Nat. Electron. 2023, 7, 51–65. [Google Scholar] [CrossRef]
- Zhu, P.; Zhang, B.; Wang, H.; Wu, Y.; Cao, H.; He, L.; Li, C.; Luo, X.; Li, X.; Mao, Y. 3D printed triboelectric nanogenerator as self-powered human-machine interactive sensor for breathing-based language expression. Nano Res. 2022, 15, 7460–7467. [Google Scholar] [CrossRef]
- Li, X.; Zhang, S.; Li, X.; Lu, L.; Cui, B.; Yuan, C.; Guo, L.; Yu, B.; Chai, Q. Starch/polyvinyl alcohol with ionic liquid/graphene oxide enabled highly tough, conductive and freezing-resistance hydrogels for multimodal wearable sensors. Carbohydr. Polym. 2023, 320, 121262. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.; Shah, L.A.; Rahman, T.U.; Yoo, H.-M.; Ye, D.; Vacharasin, J. Hydrophobically Associated Functionalized CNT-Reinforced Double-Network Hydrogels as Advanced Flexible Strain Sensors. ACS Appl. Polym. Mater. 2022, 4, 7397–7407. [Google Scholar] [CrossRef]
- Lee, C.-Y.; Lin, Y.-T.; Hong, S.-H.; Wang, C.-H.; Jeng, U.S.; Tung, S.-H.; Liu, C.-L. Mixed Ionic–Electronic Conducting Hydrogels with Carboxylated Carbon Nanotubes for High Performance Wearable Thermoelectric Harvesters. ACS Appl. Mater. Interfaces 2023, 15, 56072–56083. [Google Scholar] [CrossRef]
- Abodurexiti, A.; Maimaitiyiming, X. Carbon Nanotubes-Based 3D Printing Ink for Multifunctional “Artificial Epidermis” with Long-Term Environmental Stability. Macromol. Chem. Phys. 2022, 223, 2100486. [Google Scholar] [CrossRef]
- Liu, D.; Zhu, P.; Zhang, F.; Li, P.; Huang, W.; Li, C.; Han, N.; Mu, S.; Zhou, H.; Mao, Y. Intrinsically stretchable polymer semiconductor based electronic skin for multiple perceptions of force, temperature, and visible light. Nano Res. 2023, 16, 1196–1204. [Google Scholar] [CrossRef]
- Zhu, P.; Mu, S.; Huang, W.; Sun, Z.; Lin, Y.; Chen, K.; Pan, Z.; Haghighi, M.G.; Sedghi, R.; Wang, J.; et al. Soft multifunctional neurological electronic skin through intrinsically stretchable synaptic transistor. Nano Res. 2024, 17, 6550–6559. [Google Scholar] [CrossRef]
- Yuan, G.; Lv, S.; Zhang, R.; Liu, Z.; Zhao, Y.; Dong, Y.; Li, Z.; Gong, W.; Sun, Y. Flexible, Wearable, and Ultralow-Power-Consumption Electronic Skins Based on a Thermally Reduced Graphene Oxide/Carbon Nanotube Composite Film. ACS Appl. Electron. Mater. 2023, 5, 4451–4461. [Google Scholar] [CrossRef]
- Yu, G.; Zhang, Y.; Wang, Q.; Dan, N.; Chen, Y.; Li, Z.; Dan, W.; Wang, Y. Wearable and Flexible Hydrogels for Strain Sensing and Wound Electrical Stimulation. Ind. Eng. Chem. Res. 2023, 62, 5468–5481. [Google Scholar] [CrossRef]
- Park, J.; Kim, J.Y.; Heo, J.H.; Kim, Y.; Kim, S.A.; Park, K.; Lee, Y.; Jin, Y.; Shin, S.R.; Kim, D.W.; et al. Intrinsically Nonswellable Multifunctional Hydrogel with Dynamic Nanoconfinement Networks for Robust Tissue-Adaptable Bioelectronics. Adv. Sci. 2023, 10, 2207237. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Ke, L.; Zhang, X.; Xu, F.; Hu, Y.; Lin, H.; Zhu, J. Breathable and Wearable Strain Sensors Based on Synergistic Conductive Carbon Nanotubes/Cotton Fabrics for Multi-directional Motion Detection. ACS Appl. Mater. Interfaces 2022, 14, 25753–25762. [Google Scholar] [CrossRef]
- Xie, Y.; Lv, X.; Li, Y.; Lv, A.; Sui, X.; Tian, S.; Jiang, L.a.; Li, R.; Sun, S. Carbon Nanotubes and Silica@polyaniline Core–Shell Particles Synergistically Enhance the Toughness and Electrical Conductivity in Hydrophobic Associated Hydrogels. Langmuir 2023, 39, 1299–1308. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Wang, Y.; Shi, Z.; Tan, D.; Yang, X.; Xiong, L.; Li, G.; Lei, Y.; Xue, L. Fast Self-Assembly of Photonic Crystal Hydrogel for Wearable Strain and Temperature Sensor. Small Methods 2022, 6, 2200461. [Google Scholar] [CrossRef] [PubMed]
- Ye, T.; Wang, J.; Jiao, Y.; Li, L.; He, E.; Wang, L.; Li, Y.; Yun, Y.; Li, D.; Lu, J.; et al. A Tissue-Like Soft All-Hydrogel Battery. Adv. Mater. 2021, 34, 2105120. [Google Scholar] [CrossRef]
- Hang, T.; Chen, Y.; Yin, F.; Shen, J.; Li, X.; Li, Z.; Zheng, J. Highly stretchable polyvinyl alcohol composite conductive hydrogel sensors reinforced by cellulose nanofibrils and liquid metal for information transmission. Int. J. Biol. Macromol. 2024, 258, 128855. [Google Scholar] [CrossRef]
- Hao, X.P.; Zhang, C.W.; Zhang, X.N.; Hou, L.X.; Hu, J.; Dickey, M.D.; Zheng, Q.; Wu, Z.L. Healable, Recyclable, and Multifunctional Soft Electronics Based on Biopolymer Hydrogel and Patterned Liquid Metal. Small 2022, 18, 2201643. [Google Scholar] [CrossRef]
- Hwang, C.; Choi, M.-H.; Kim, H.-E.; Jeong, S.-H.; Park, J.-U. Reactive oxygen species-generating hydrogel platform for enhanced antibacterial therapy. NPG Asia Mater. 2022, 14, 72. [Google Scholar] [CrossRef]
- Hao, F.; Sun, S.; Xu, Y.; Maimaitiyiming, X. 3D printing of flexible sensors based on polyvinyl alcohol/carboxylated chitosan/sodium alginate/silver nanowire high-strength hydrogels. Polymer 2024, 290, 126594. [Google Scholar] [CrossRef]
- Zhou, L.; Li, Y.; Xiao, J.; Chen, S.-W.; Tu, Q.; Yuan, M.-S.; Wang, J. Liquid Metal-Doped Conductive Hydrogel for Construction of Multifunctional Sensors. Anal. Chem. 2023, 95, 3811–3820. [Google Scholar] [CrossRef] [PubMed]
- Yuan, X.; Guo, C.; Wang, Z.; Jiang, H.; He, Y.; Xu, J.; Guo, B. Liquid Metal–Hydrogel Biosensor for Behavior and Sweat Monitoring. ACS Appl. Electron. Mater. 2023, 5, 1420–1428. [Google Scholar] [CrossRef]
- Bai, Y.; Lang, S.; Du, Y.; Hu, Q.; Li, X.; Liu, G. Metallic-Polyphenolic Nanoparticles Reinforced Cationic Guar Gum Hydrogel for Effectively Treating Burn Wound. Macromol. Biosci. 2023, 24, 2300396. [Google Scholar] [CrossRef] [PubMed]
- Sun, H.; Fang, X.; Fang, Z.; Zhao, L.; Tian, B.; Verma, P.; Maeda, R.; Jiang, Z. An ultrasensitive and stretchable strain sensor based on a microcrack structure for motion monitoring. Microsyst. Nanoeng. 2022, 8, 111. [Google Scholar] [CrossRef]
- Rahmani, P.; Shojaei, A.; Sakorikar, T.; Wang, M.; Mendoza-Apodaca, Y.; Dickey, M.D. Liquid Metal Nanoparticles Physically Hybridized with Cellulose Nanocrystals Initiate and Toughen Hydrogels with Piezoionic Properties. ACS Nano 2024, 18, 8038–8050. [Google Scholar] [CrossRef]
- Wang, T.; Song, J.; Liu, R.; Chan, S.Y.; Wang, K.; Su, Y.; Li, P.; Huang, W. Motion Detecting, Temperature Alarming, and Wireless Wearable Bioelectronics Based on Intrinsically Antibacterial Conductive Hydrogels. ACS Appl. Mater. Interfaces 2022, 14, 14596–14606. [Google Scholar] [CrossRef]
- Zhao, M.; Zhang, W.; Sun, P.; Shi, L.; Liu, Y.; Zhang, X.; Zhang, L.; Han, W.; Chen, P. Skin-Inspired Packaging of Injectable Hydrogel Sensors Enabled by Photopolymerizable and Swellable Hydrogels toward Sustainable Electronics. ACS Sustain. Chem. Eng. 2022, 10, 6657–6666. [Google Scholar] [CrossRef]
- Wei, Z.; Wang, Y.; Cai, C.; Zhang, Y.; Guo, S.; Fu, Y.; Tan, S.C. Dual-Network Liquid Metal Hydrogel with Integrated Solar-Driven Evaporation, Multi-Sensory Applications, and Electricity Generation via Enhanced Light Absorption and Bénard–Marangoni Effect. Adv. Funct. Mater. 2022, 32, 2206287. [Google Scholar] [CrossRef]
- Lee, J.H.; Nam, I.-W.; Hyun, J.E.; Ahn, H.; Yeo, S.Y.; Lim, T. Conductive and Robust Cellulose Hydrogel Generated by Liquid Metal for Biomedical Applications. ACS Appl. Polym. Mater. 2023, 6, 49–58. [Google Scholar] [CrossRef]
- Rajati, H.; Alvandi, H.; Rahmatabadi, S.S.; Hosseinzadeh, L.; Arkan, E. A nanofiber-hydrogel composite from green synthesized AgNPs embedded to PEBAX/PVA hydrogel and PA/Pistacia atlantica gum nanofiber for wound dressing. Int. J. Biol. Macromol. 2023, 226, 1426–1443. [Google Scholar] [CrossRef]
- Jian, J.; Xie, Y.; Gao, S.; Sun, Y.; Lai, C.; Wang, J.; Wang, C.; Chu, F.; Zhang, D. A skin-inspired biomimetic strategy to fabricate cellulose enhanced antibacterial hydrogels as strain sensors. Carbohydr. Polym. 2022, 294, 119760. [Google Scholar] [CrossRef] [PubMed]
- Guan, L.; Liu, H.; Ren, X.; Wang, T.; Zhu, W.; Zhao, Y.; Feng, Y.; Shen, C.; Zvyagin, A.V.; Fang, L.; et al. Balloon Inspired Conductive Hydrogel Strain Sensor for Reducing Radiation Damage in Peritumoral Organs During Brachytherapy. Adv. Funct. Mater. 2022, 32, 2112281. [Google Scholar] [CrossRef]
- Li, M.; Zhang, Y.; Lian, L.; Liu, K.; Lu, M.; Chen, Y.; Zhang, L.; Zhang, X.; Wan, P. Flexible Accelerated-Wound-Healing Antibacterial MXene-Based Epidermic Sensor for Intelligent Wearable Human-Machine Interaction. Adv. Funct. Mater. 2022, 32, 2208141. [Google Scholar] [CrossRef]
- Chae, A.; Murali, G.; Lee, S.Y.; Gwak, J.; Kim, S.J.; Jeong, Y.J.; Kang, H.; Park, S.; Lee, A.S.; Koh, D.Y.; et al. Highly Oxidation-Resistant and Self-Healable MXene-Based Hydrogels for Wearable Strain Sensor. Adv. Funct. Mater. 2023, 33, 2213382. [Google Scholar] [CrossRef]
- Zhang, Y.; Xu, Z.; Yuan, Y.; Liu, C.; Zhang, M.; Zhang, L.; Wan, P. Flexible Antiswelling Photothermal-Therapy MXene Hydrogel-Based Epidermal Sensor for Intelligent Human–Machine Interfacing. Adv. Funct. Mater. 2023, 33, 2300299. [Google Scholar] [CrossRef]
- Wang, Z.; Zhou, H.; Liu, D.; Chen, X.; Wang, D.; Dai, S.; Chen, F.; Xu, B.B. A Structural Gel Composite Enabled Robust Underwater Mechanosensing Strategy with High Sensitivity. Adv. Funct. Mater. 2022, 32, 2201396. [Google Scholar] [CrossRef]
- Zhuo, F.; Zhou, J.; Liu, Y.; Xie, J.; Chen, H.; Wang, X.; Luo, J.; Fu, Y.; Elmarakbi, A.; Duan, H. Kirigami-Inspired 3D-Printable MXene Organohydrogels for Soft Electronics. Adv. Funct. Mater. 2023, 33, 2308487. [Google Scholar] [CrossRef]
- Pu, J.; Gao, Y.; Geng, Z.; Zhang, Y.; Cao, Q.; Yang, J.; Zhao, X.; Wang, Y.; Wang, J.; Guan, C. Grafted MXene Assisted Bifunctional Hydrogel for Stable and Highly Sensitive Self-Powered Fibrous System. Adv. Funct. Mater. 2024, 34, 2304453. [Google Scholar] [CrossRef]
- Cohen-Gerassi, D.; Messer, O.; Finkelstein-Zuta, G.; Aviv, M.; Favelukis, B.; Shacham-Diamand, Y.; Sokol, M.; Adler-Abramovich, L. Conductive Peptide-Based MXene Hydrogel as a Piezoresistive Sensor. Adv. Healthc. Mater. 2024, 14, 2303632. [Google Scholar] [CrossRef]
- Liu, Y.; Tian, G.; Du, Y.; Shi, P.; Li, N.; Li, Y.; Qin, Z.; Jiao, T.; He, X. Highly Stretchable, Low-Hysteresis, and Adhesive TA@MXene-Composited Organohydrogels for Durable Wearable Sensors. Adv. Funct. Mater. 2024, 34, 2315813. [Google Scholar] [CrossRef]
- Zhao, L.; Xu, H.; Liu, L.; Zheng, Y.; Han, W.; Wang, L. MXene-Induced Flexible, Water-Retention, Semi-Interpenetrating Network Hydrogel for Ultra-Stable Strain Sensors with Real-Time Gesture Recognition. Adv. Sci. 2023, 10, 2303922. [Google Scholar] [CrossRef]
- Cao, Z.; Zhu, Y.B.; Chen, K.; Wang, Q.; Li, Y.; Xing, X.; Ru, J.; Meng, L.G.; Shu, J.; Shpigel, N.; et al. Super-Stretchable and High-Energy Micro-Pseudocapacitors Based on MXene Embedded Ag Nanoparticles. Adv. Mater. 2024, 36, 2401271. [Google Scholar] [CrossRef] [PubMed]
- Ni, Y.; Zang, X.; Chen, J.; Zhu, T.; Yang, Y.; Huang, J.; Cai, W.; Lai, Y. Flexible MXene-Based Hydrogel Enables Wearable Human–Computer Interaction for Intelligent Underwater Communication and Sensing Rescue. Adv. Funct. Mater. 2023, 33, 2301127. [Google Scholar] [CrossRef]
- Zhang, S.; Chhetry, A.; Zahed, M.A.; Sharma, S.; Park, C.; Yoon, S.; Park, J.Y. On-skin ultrathin and stretchable multifunctional sensor for smart healthcare wearables. npj Flex. Electron. 2022, 6, 11. [Google Scholar] [CrossRef]
- Zhang, S.; Guo, F.; Gao, X.; Yang, M.; Huang, X.; Zhang, D.; Li, X.; Zhang, Y.; Shang, Y.; Cao, A. High-Strength, Antiswelling Directional Layered PVA/MXene Hydrogel for Wearable Devices and Underwater Sensing. Adv. Sci. 2024, 12, 2405880. [Google Scholar] [CrossRef]
- Bi, Y.; Sun, M.; Zhang, Y.; Sun, F.; Du, Y.; Wang, J.; Zhou, M.; Ma, C.B. Seconds Timescale Synthesis of Highly Stretchable Antibacterial Hydrogel for Skin Wound Closure and Epidermal Strain Sensor. Adv. Healthc. Mater. 2023, 13, e2302810. [Google Scholar] [CrossRef]
- Yang, M.; Cheng, Y.; Yue, Y.; Chen, Y.; Gao, H.; Li, L.; Cai, B.; Liu, W.; Wang, Z.; Guo, H.; et al. High-Performance Flexible Pressure Sensor with a Self-Healing Function for Tactile Feedback. Adv. Sci. 2022, 9, e2200507. [Google Scholar] [CrossRef]
- Zhu, C.; Shao, Z.; Chang, B.; Wang, Y.; Yu, T.; Huang, C.; Yang, Y.; Ding, X.; Liu, F.; Zhang, W. Dual-Network Conductive Hydrogels with Self-Healing, Ultrasensitive, and Antibacterial Activity toward Multifunctional Flexible Strain Sensor. ACS Appl. Polym. Mater. 2024, 6, 7020–7035. [Google Scholar] [CrossRef]
- Han, S.; Wu, Q.; Xu, Y.; Zhang, J.; Chen, A.; Chen, Y.; Huang, J.; Yang, X.; Guan, L. Multi-Functional Eutectic Hydrogel for 3D Printable Flexible Omnidirectional Strain Sensors. Adv. Mater. Technol. 2023, 8, 2301123. [Google Scholar] [CrossRef]
- Liu, J.; McKeon, L.; Garcia, J.; Pinilla, S.; Barwich, S.; Möbius, M.; Stamenov, P.; Coleman, J.N.; Nicolosi, V. Additive Manufacturing of Ti3C2-MXene-Functionalized Conductive Polymer Hydrogels for Electromagnetic-Interference Shielding. Adv. Mater. 2021, 34, 2106253. [Google Scholar] [CrossRef] [PubMed]
- Jiang, H.; Ou, C.; Zhang, D.; Hu, X.; Ma, Y.; Wang, M.; Huang, Y.; Xiao, L. Tough Ion-Conductive Hydrogel with Anti-Dehydration as a Stretchable Strain Sensor for Gesture Recognition. ACS Appl. Polym. Mater. 2023, 5, 6828–6841. [Google Scholar] [CrossRef]
- Zhang, W.; Huang, Y.; Zhou, H.; Nie, Z.; Jin, S. Anti-Freezing Wearable Tactile Sensors Prepared by Laser Processing of Crumpled Xanthan Gum-Based Hydrogels. Adv. Mater. Technol. 2023, 8, 2301196. [Google Scholar] [CrossRef]
- Zhao, X.; Wang, H.; Luo, J.; Ren, G.; Wang, J.; Chen, Y.; Jia, P. Ultrastretchable, Adhesive, Anti-freezing, Conductive, and Self-Healing Hydrogel for Wearable Devices. ACS Appl. Polym. Mater. 2022, 4, 1784–1793. [Google Scholar] [CrossRef]
- Zeng, L.; Gao, G. Stretchable Organohydrogel with Adhesion, Self-Healing, and Environment-Tolerance for Wearable Strain Sensors. ACS Appl. Mater. Interfaces 2023, 15, 28993–29003. [Google Scholar] [CrossRef] [PubMed]
- Xu, K.; Wang, L.; Shan, W.; Gao, K.; Wang, J.; Zhong, Q.; Zhou, W. Highly Stretchable and Self-Adhesive Wearable Biosensor Based on Nanozyme-Catalyzed Conductive Hydrogels. ACS Appl. Polym. Mater. 2024, 6, 2188–2200. [Google Scholar] [CrossRef]
- Ma, Y.; Zhang, D.; Wang, Z.; Zhang, H.; Xia, H.; Mao, R.; Cai, H.; Luan, H. Self-Adhesive, Anti-Freezing MXene-Based Hydrogel Strain Sensor for Motion Monitoring and Handwriting Recognition with Deep Learning. ACS Appl. Mater. Interfaces 2023, 15, 29413–29424. [Google Scholar] [CrossRef]
- Bai, Y.; Yan, S.; Wang, Y.; Wang, Q.; Duan, X. Facile Preparation of a Self-Adhesive Conductive Hydrogel with Long-Term Usability. ACS Appl. Mater. Interfaces 2023, 15, 48744–48753. [Google Scholar] [CrossRef]
- Lu, L.; Hu, G.; Liu, J.; Yang, B. 5G NB-IoT System Integrated with High-Performance Fiber Sensor Inspired by Cirrus and Spider Structures. Adv. Sci. 2024, 11, e2309894. [Google Scholar] [CrossRef]
- GhavamiNejad, P.; GhavamiNejad, A.; Zheng, H.; Dhingra, K.; Samarikhalaj, M.; Poudineh, M. A Conductive Hydrogel Microneedle-Based Assay Integrating PEDOT:PSS and Ag-Pt Nanoparticles for Real-Time, Enzyme-Less, and Electrochemical Sensing of Glucose. Adv. Healthc. Mater. 2022, 12, 2202362. [Google Scholar] [CrossRef] [PubMed]
- Lin, G.; Si, M.; Wang, L.; Wei, S.; Lu, W.; Liu, H.; Zhang, Y.; Li, D.; Chen, T. Dual-Channel Flexible Strain Sensors Based on Mechanofluorescent and Conductive Hydrogel Laminates. Adv. Opt. Mater. 2022, 10, 2102306. [Google Scholar] [CrossRef]
- Fu, D.; Huang, G.; Xie, Y.; Zheng, M.; Feng, J.; Kan, K.; Shen, J. Novel Uracil-Functionalized Poly(ionic liquid) Hydrogel: Highly Stretchable and Sensitive as a Direct Wearable Ionic Skin for Human Motion Detection. ACS Appl. Mater. Interfaces 2023, 15, 11062–11075. [Google Scholar] [CrossRef] [PubMed]
- Shen, Z.; Zhang, Z.; Zhang, N.; Li, J.; Zhou, P.; Hu, F.; Rong, Y.; Lu, B.; Gu, G. High-Stretchability, Ultralow-Hysteresis ConductingPolymer Hydrogel Strain Sensors for Soft Machines. Adv. Mater. 2022, 34, 2203650. [Google Scholar] [CrossRef]
- Rahman, M.T.; Rahman, M.S.; Kumar, H.; Kim, K.; Kim, S. Metal-Organic Framework Reinforced Highly Stretchable and Durable Conductive Hydrogel-Based Triboelectric Nanogenerator for Biomotion Sensing and Wearable Human-Machine Interfaces. Adv. Funct. Mater. 2023, 33, 2303471. [Google Scholar] [CrossRef]
- Yao, X.; Zhang, S.; Qian, L.; Wei, N.; Nica, V.; Coseri, S.; Han, F. Super Stretchable, Self-Healing, Adhesive Ionic Conductive Hydrogels Based on Tailor-Made Ionic Liquid for High-Performance Strain Sensors. Adv. Funct. Mater. 2022, 32, 2204565. [Google Scholar] [CrossRef]
- Sun, Z.; Dong, C.; Chen, B.; Li, W.; Hu, H.; Zhou, J.; Li, C.; Huang, Z. Strong, Tough, and Anti-Swelling Supramolecular Conductive Hydrogels for Amphibious Motion Sensors. Small 2023, 19, 2303612. [Google Scholar] [CrossRef]
- Ren, J.; Liu, Y.; Wang, Z.; Chen, S.; Ma, Y.; Wei, H.; Lü, S. An Anti-Swellable Hydrogel Strain Sensor for Underwater Motion Detection. Adv. Funct. Mater. 2021, 32, 2107404. [Google Scholar] [CrossRef]
- Sahoo, S.D.; Vasudha, T.K.; Muthuvijayan, V.; Prasad, E. Chitosan-Based Self-Healable and Adhesive Hydrogels for Flexible Strain Sensor Application. ACS Appl. Polym. Mater. 2022, 4, 9176–9185. [Google Scholar] [CrossRef]
- Wang, S.; Zeng, H.; Gu, B.; Ya, H.; Huang, B.; Lin, B.; Xu, C.; Wei, Y.; Fu, L. Nacre-Mimetic Structure Multifunctional Ion-Conductive Hydrogel Strain Sensors with Ultrastretchability, High Sensitivity, and Excellent Adhesive Properties. ACS Appl. Mater. Interfaces 2024, 16, 21146–21160. [Google Scholar] [CrossRef]
- He, Z.; Zhou, Z.; Yuan, W. Highly Adhesive, Stretchable, and Antifreezing Hydrogel with Excellent Mechanical Properties for Sensitive Motion Sensors and Temperature-/Humidity-Driven Actuators. ACS Appl. Mater. Interfaces 2022, 14, 38205–38215. [Google Scholar] [CrossRef]
- Zhao, C.; Chen, Y.; Guo, M.; Wu, Y.; Li, Y.; Xiang, D.; Li, H.; Wang, L.; Sun, Z. Flexible, adhesive, strain-sensitive, and skin-matchable hydrogel strain sensors for human motion and handwritten signal monitoring. Polym. Adv. Technol. 2022, 34, 430–440. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, C.; Guo, A.; Yang, Y.; Nie, Y.; Liao, J.; Liu, B.; Zhou, Y.; Li, L.; Chen, Z.; et al. Black phosphorus boosts wet-tissue adhesion of composite patches by enhancing water absorption and mechanical properties. Nat. Commun. 2024, 15, 1618. [Google Scholar] [CrossRef] [PubMed]
- Shang, Z.; Liu, G.; Sun, Y.; Li, C.; Zhao, N.; Chen, Z.; Guo, R.; Zheng, Z.; Zhou, F.; Liu, W. Mussel-Inspired Wet-Adhesive Multifunctional Organohydrogel with Extreme Environmental Tolerance for Wearable Strain Sensor. ACS Appl. Mater. Interfaces 2023, 15, 44342–44353. [Google Scholar] [CrossRef]
- Zhou, H.; Lai, J.; Zheng, B.; Jin, X.; Zhao, G.; Liu, H.; Chen, W.; Ma, A.; Li, X.; Wu, Y. From Glutinous-Rice-Inspired Adhesive Organohydrogels to Flexible Electronic Devices Toward Wearable Sensing, Power Supply, and Energy Storage. Adv. Funct. Mater. 2021, 32, 2108423. [Google Scholar] [CrossRef]
- Xiong, X.; Chen, Y.; Wang, Z.; Liu, H.; Le, M.; Lin, C.; Wu, G.; Wang, L.; Shi, X.; Jia, Y.-G.; et al. Polymerizable rotaxane hydrogels for three-dimensional printing fabrication of wearable sensors. Nat. Commun. 2023, 14, 1331. [Google Scholar] [CrossRef]
- Li, J.; Carlos, C.; Zhou, H.; Sui, J.; Wang, Y.; Silva-Pedraza, Z.; Yang, F.; Dong, Y.; Zhang, Z.; Hacker, T.A. Stretchable piezoelectric biocrystal thin films. Nat. Commun. 2023, 14, 6562. [Google Scholar] [CrossRef]
- Go, Y.; Park, H.Y.; Zhu, Y.; Yoo, K.; Kwak, J.; Jin, S.H.; Yoon, J. Optically Transparent and Mechanically Robust Ionic Hydrogel Electrodes for Bright Electroluminescent Devices Achieving High Stretchability Over 1400%. Adv. Funct. Mater. 2023, 33, 2215193. [Google Scholar] [CrossRef]
- Wu, Y.; Zhang, Y.; Wu, H.; Wen, J.; Zhang, S.; Xing, W.; Zhang, H.; Xue, H.; Gao, J.; Mai, Y. Solvent-Exchange-Assisted Wet Annealing: A New Strategy for Superstrong, Tough, Stretchable, and Anti-Fatigue Hydrogels. Adv. Mater. 2023, 35, 2210624. [Google Scholar] [CrossRef]
- Liang, Q.; Xia, X.; Sun, X.; Yu, D.; Huang, X.; Han, G.; Mugo, S.M.; Chen, W.; Zhang, Q. Highly Stretchable Hydrogels as Wearable and Implantable Sensors for Recording Physiological and Brain Neural Signals. Adv. Sci. 2022, 9, 2201059. [Google Scholar] [CrossRef]
- Wang, X.; Zheng, S.; Xiong, J.; Liu, Z.; Li, Q.; Li, W.; Yan, F. Stretch-Induced Conductivity Enhancement in Highly Conductive and Tough Hydrogels. Adv. Mater. 2024, 36, 2313845. [Google Scholar] [CrossRef]
- Chong, Y.T.; Wang, X.; Cao, S.; Cui, F.; Zhu, Q.; Xu, J.; Wang, F. Anisotropic Pressure Sensors Fabricated by 3D Printing-Aligned Carbon Nanotube Composites. Adv. Eng. Mater. 2023, 25, 2300510. [Google Scholar] [CrossRef]
- Man, T.; Yu, G.; Zhu, F.; Huang, Y.; Wang, Y.; Su, Y.; Deng, S.; Pei, H.; Li, L.; Ye, H.; et al. Antidiabetic Close Loop Based on Wearable DNA-Hydrogel Glucometer and Implantable Optogenetic Cells. JACS Au. 2024, 4, 1500–1508. [Google Scholar] [CrossRef] [PubMed]
- Shirzadi, E.; Huynh, M.; GhavamiNejad, P.; Zheng, H.; Saini, A.; Bakhshandeh, F.; Keyvani, F.; Mantaila, D.; Rahman, F.A.; Quadrilatero, J.; et al. A PEDOT:PSS-Based Composite Hydrogel as a Versatile Electrode for Wearable Microneedle Sensing Platforms. Adv. Sens. Res. 2023, 3, 2300122. [Google Scholar] [CrossRef]
- Rauf, S.; Bilal, R.M.; Li, J.; Vaseem, M.; Ahmad, A.N.; Shamim, A. Fully Screen-Printed and Gentle-to-Skin Wet ECG Electrodes with Compact Wireless Readout for Cardiac Diagnosis and Remote Monitoring. ACS Nano. 2024, 18, 10074–10087. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Chen, Y.; Sun, Y.; Qin, Y.; Zhang, H.; Yu, X.; Liu, Y. Stretchable slide-ring supramolecular hydrogel for flexible electronic devices. Commun. Mater. 2022, 3, 2. [Google Scholar] [CrossRef]
- Wang, F.; Ma, M.; Fu, R.; Zhang, X. EEG-based detection of driving fatigue using a novel electrode. Sens. Actuator A-Phys. 2024, 365, 114895. [Google Scholar] [CrossRef]
- Li, N.; Wang, X.; Liu, Y.; Li, Y.; Li, J.; Qin, Z.; Jiao, T. Ultrastretchable, Self-Adhesive and conductive MXene nanocomposite hydrogel for body-surface temperature distinguishing and electrophysiological signal monitoring. Chem. Eng. J. 2024, 483, 149303. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, C.; Xue, J.; Huang, G.; Zheng, S.; Zhao, K.; Huang, J.; Wang, Y.; Zhang, Y.; Yin, T.; et al. Body Temperature Enhanced Adhesive, Antibacterial, and Recyclable Ionic Hydrogel for Epidermal Electrophysiological Monitoring. Adv. Healthc. Mater. 2022, 11, 2200653. [Google Scholar] [CrossRef]
- Tang, S.; Sha, D.; He, Z.; Chen, X.; Ma, Y.; Liu, C.; Yuan, Y. Environmentally Adaptable Organo–Ionic Gel-Based Electrodes for Real-Time On-Skin Electrocardiography Monitoring. Adv. Healthc. Mater. 2023, 12, 2300475. [Google Scholar] [CrossRef]
- Li, Y.; Zhao, Z.; Veronica, A.; Yu Yeung, S.; Hsing, I.M. Skin-Adherent Elastomer-Hydrogel Patch for Continuous 12-Lead Cardiac Ambulatory Monitoring during Physical Activities. Adv. Mater. Technol. 2023, 8, 2300326. [Google Scholar] [CrossRef]
- Leng, Z.; Zhu, P.; Wang, X.; Wang, Y.; Li, P.; Huang, W.; Li, B.; Jin, R.; Han, N.; Wu, J.; et al. Sebum-Membrane-Inspired Protein-Based Bioprotonic Hydrogel for Artificial Skin and Human-Machine Merging Interface. Adv. Funct. Mater. 2023, 33, 2211056. [Google Scholar] [CrossRef]
- Wang, F.; Xue, Y.; Chen, X.; Zhang, P.; Shan, L.; Duan, Q.; Xing, J.; Lan, Y.; Lu, B.; Liu, J. 3D Printed Implantable Hydrogel Bioelectronics for Electrophysiological Monitoring and Electrical Modulation. Adv. Funct. Mater. 2023, 34, 2314471. [Google Scholar] [CrossRef]
- Luo, J.; Sun, C.; Chang, B.; Zhang, B.; Li, K.; Li, Y.; Zhang, Q.; Wang, H.; Hou, C. On-Skin Paintable Water-Resistant Biohydrogel for Wearable Bioelectronics. Adv. Funct. Mater. 2024, 34, 2400884. [Google Scholar] [CrossRef]
- Shen, S.; Zhang, J.; Han, Y.; Pu, C.; Duan, Q.; Huang, J.; Yan, B.; You, X.; Lin, R.; Shen, X.; et al. A Core–Shell Nanoreinforced Ion-Conductive Implantable Hydrogel Bioelectronic Patch with High Sensitivity and Bioactivity for Real-Time Synchronous Heart Monitoring and Repairing. Adv. Healthc. Mater. 2023, 12, 2301990. [Google Scholar] [CrossRef] [PubMed]
- Xue, F.; Zhao, S.; Tian, H.; Qin, H.; Li, X.; Jian, Z.; Du, J.; Li, Y.; Wang, Y.; Lin, L.; et al. Two way workable microchanneled hydrogel suture to diagnose, treat and monitor the infarcted heart. Nat. Commun. 2024, 15, 864. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Ding, Q.; Luo, Y.; Wu, Z.; Yu, J.; Chen, H.; Zhou, Y.; Zhang, H.; Tao, K.; Chen, X.; et al. High-Performance Hydrogel Sensors Enabled Multimodal and Accurate Human-Machine Interaction System for Active Rehabilitation. Adv. Mater. 2023, 36, 2309868. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Ji, X.; Zhu, R.; Gu, J.; Liang, J. A Microphase-Separated Design toward an All-Round Ionic Hydrogel with Discriminable and Anti-Disturbance Multisensory Functions. Adv. Mater. 2024, 36, 2309508. [Google Scholar] [CrossRef]
- Roubert Martinez, S.; Le Floch, P.; Liu, J.; Howe, R.D. Pure Conducting Polymer Hydrogels Increase Signal-to-Noise of Cutaneous Electrodes by Lowering Skin Interface Impedance. Adv. Healthc. Mater. 2023, 12, 2202661. [Google Scholar] [CrossRef]
- Huang, X.; Chen, C.; Ma, X.; Zhu, T.; Ma, W.; Jin, Q.; Du, R.; Cai, Y.; Zhang, M.; Kong, D.; et al. In Situ Forming Dual-Conductive Hydrogels Enable Conformal, Self-Adhesive and Antibacterial Epidermal Electrodes. Adv. Funct. Mater. 2023, 33, 2302846. [Google Scholar] [CrossRef]
- Xue, H.; Wang, D.; Jin, M.; Gao, H.; Wang, X.; Xia, L.; Li, D.a.; Sun, K.; Wang, H.; Dong, X.; et al. Hydrogel electrodes with conductive and substrate-adhesive layers for noninvasive long-term EEG acquisition. Microsyst. Nanoeng. 2023, 9, 79. [Google Scholar] [CrossRef] [PubMed]
- Hao, Y.; Yan, Q.; Liu, H.; He, X.; Zhang, P.; Qin, X.; Wang, R.; Sun, J.; Wang, L.; Cheng, Y. A Stretchable, Breathable, And Self-Adhesive Electronic Skin with Multimodal Sensing Capabilities for Human-Centered Healthcare. Adv. Funct. Mater. 2023, 33, 2303881. [Google Scholar] [CrossRef]
- Ban, S.; Lee, Y.J.; Kwon, S.; Kim, Y.-S.; Chang, J.W.; Kim, J.-H.; Yeo, W.-H. Soft Wireless Headband Bioelectronics and Electrooculography for Persistent Human–Machine Interfaces. ACS Appl. Electron. Mater. 2023, 5, 877–886. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Wang, H.; Wang, B.; Miyata, H.; Wang, Y.; Nayeem, M.O.G.; Kim, J.J.; Lee, S.; Yokota, T.; Onodera, H. On-skin paintable biogel for long-term high-fidelity electroencephalogram recording. Sci. Adv. 2022, 8, eabo1396. [Google Scholar] [CrossRef]



| Filled Nanomaterial | Tensile Strain % | Breaking Strength | Electrical Conductivity | GF | Adhesion | Reference |
|---|---|---|---|---|---|---|
| Ag/TA@GO | 1250 | / | 0.15 S/m | 3.1 | Y | [47] |
| Graphene Oxide nanosheets | 1458 | 2.5 MPa | 4.3 S/m | 3.04 | Y | [54] |
| CNTs | 217 | 12.58 MPa | 0.071 S/m | 25.98 | N | [55] |
| mCNT-OH | 1600 | 556 kPa | 0.0031 S/cm | 6.39 | N | [56] |
| MXene | 400 | 0.93 MPa | 8.1 S/m | 1.12 | N | [57] |
| MXene | 730 | 0.54 MPa | 0.069 S/m | 4.42 | Y | [58] |
| AgNWs | 480 | 240 kPa | 1739 S/cm | / | Y | [59] |
| AgFs, AgNWs | 1000 | 5.42 MPa | 83.836 S/m | 87 | Y | [60] |
| Fe3O4 nanoparticles | 352 | 56 kPa | / | 4.21 | Y | [61] |
| Silver nanoparticles | 732.9 | 1267.6 kPa | 0.39 S/m | 6.8 | Y | [62] |
| LM/HA | 2700 | / | 116 S/m | 4.8 | Y | [63] |
| Filled Nanomaterial | Young’s Modulus | Elongation at Break | Toughness | Breaking Strength | Reference |
|---|---|---|---|---|---|
| fCNTs | 10–100 kPa | 1000% | 400–873 J/m3 | 121 kPa | [94] |
| LM | 49–98 kPa | 2000% | 1.8 MJ/m3 | 70 kPa | [107] |
| MXene | 1.41 MPa | 400% | 1.98 MJ/m3 | 0.93 MPa | [57] |
| Graphene | / | 2500% | 3.11 MJ/m3 | 0.27 MPa | [77] |
| AgNPs | 7.65 MPa | 126.92% | / | 20.7 ± 1.8 MPa | [112] |
| AgNWs | 19.8 kPa | 1800% | / | 0.35 MPa | [32] |
| CNTs/SiO2 | / | 3948.37% | 28.48 MJ/m3 | 1939.36 kPa | [96] |
| EGaIn/CNCs | / | 346.35% | / | 0.11 MPa | [110] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Duan, H.; Zhang, Y.; Zhang, Y.; Zhu, P.; Mao, Y. Recent Advances of Stretchable Nanomaterial-Based Hydrogels for Wearable Sensors and Electrophysiological Signals Monitoring. Nanomaterials 2024, 14, 1398. https://doi.org/10.3390/nano14171398
Duan H, Zhang Y, Zhang Y, Zhu P, Mao Y. Recent Advances of Stretchable Nanomaterial-Based Hydrogels for Wearable Sensors and Electrophysiological Signals Monitoring. Nanomaterials. 2024; 14(17):1398. https://doi.org/10.3390/nano14171398
Chicago/Turabian StyleDuan, Haiyang, Yilong Zhang, Yitao Zhang, Pengcheng Zhu, and Yanchao Mao. 2024. "Recent Advances of Stretchable Nanomaterial-Based Hydrogels for Wearable Sensors and Electrophysiological Signals Monitoring" Nanomaterials 14, no. 17: 1398. https://doi.org/10.3390/nano14171398
APA StyleDuan, H., Zhang, Y., Zhang, Y., Zhu, P., & Mao, Y. (2024). Recent Advances of Stretchable Nanomaterial-Based Hydrogels for Wearable Sensors and Electrophysiological Signals Monitoring. Nanomaterials, 14(17), 1398. https://doi.org/10.3390/nano14171398








