Superior Single-Entity Electrochemistry Performance of Capping Agent-Free Gold Nanoparticles Compared to Citrate-Capped Gold Nanoparticles
Abstract
:1. Introduction
2. Materials and Methods
2.1. Reagent
2.2. Preparation of Citrate-Capped Au NP
2.3. Preparation of “Capping Agent-Free” Au NP
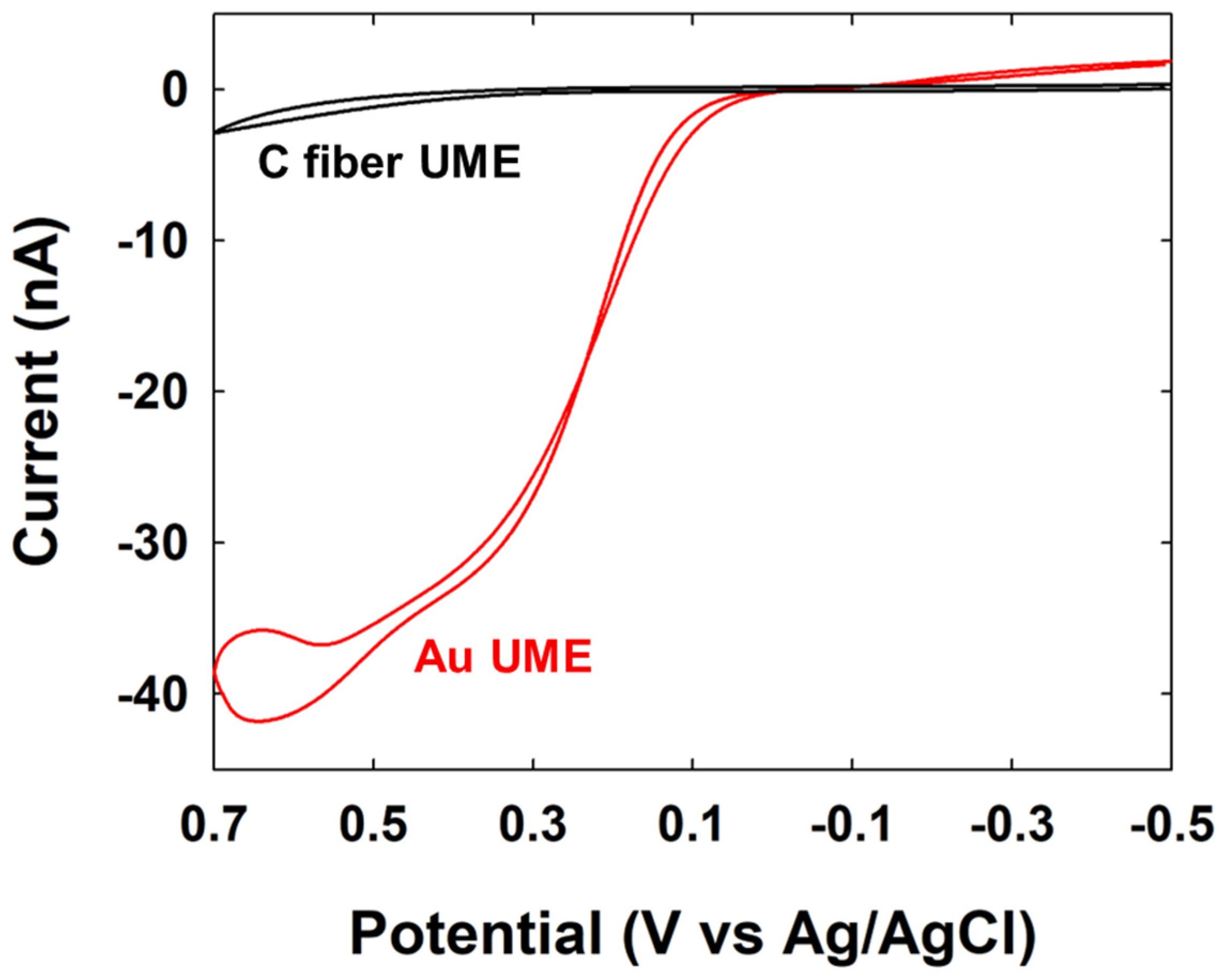
2.4. Preparation of UME
2.5. Instrumentation
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Baker, L.A. Perspective and Prospectus on Single-Entity Electrochemistry. J. Am. Chem. Soc. 2018, 140, 15549–15559. [Google Scholar] [CrossRef] [PubMed]
- Lu, S.M.; Li, M.Y.; Long, Y.T. Dynamic chemistry interactions: Controlled single-entity electrochemistry. J. Phys. Chem. Lett. 2022, 13, 4653–4659. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Wahab, O.; Jallow, A.; O’Dell, Z.; Pungsrisai, T.; Sridhar, S.; Vernon, K.; Willets, K.; Baker, L. Recent Developments in Single-Entity Electrochemistry. Anal. Chem. 2024, 96, 8036–8055. [Google Scholar] [CrossRef] [PubMed]
- Lemay, S.G.; Moazzenzade, T. Single-entity electrochemistry for digital biosensing at ultralow concentrations. Anal. Chem. 2021, 93, 9023–9031. [Google Scholar] [CrossRef] [PubMed]
- Ding, Q.; Sun, Z.; Ma, W. Probing conformational kinetics of catalase with and without magnetic field by single-entity collision electrochemistry. Sci. Bull. 2023, 68, 2564–2573. [Google Scholar] [CrossRef]
- Liu, Z.; Amin, H.M.A.; Peng, Y.; Corva, M.; Pentcheva, R.; Tschulik, K. Facet-Dependent Intrinsic Activity of Single Co3O4 Nanoparticles for Oxygen Evolution Reaction. Adv. Funct. Mater. 2023, 33, 2210945. [Google Scholar] [CrossRef]
- Saw, E.N.; Kanokkanchana, K.; Amin, H.M.A.; Tschulik, K. Unravelling Anion Solvation in Water-Alcohol Mixtures by Single Entity Electrochemistry. ChemElectroChem 2022, 9, e202101435. [Google Scholar] [CrossRef]
- Xiao, X.; Fan, F.-R.F.; Zhou, J.; Bard, A.J. Current transient in single nanoparticle collision events. J. Am. Chem. Soc. 2008, 130, 16669–16677. [Google Scholar] [CrossRef]
- Xiao, X.; Bard, A.J. Observing single nanoparticle collisions at an ultramicroelectrode by electrocatalytic amplification. J. Am. Chem. Soc. 2007, 129, 9610–9612. [Google Scholar] [CrossRef]
- Park, S.; Kim, K.J.; Kwon, S.J. Investigation of electrocatalytic activity of palladium nanoparticle for ammonia borane oxidation via single-entity electrochemistry. Bull. Korean Chem. Soc. 2024, 45, 81–88. [Google Scholar] [CrossRef]
- Quinn, B.M.; Van’t Hof, P.G.; Lemay, S.G. Time-resolved electrochemical detection of discrete adsorption events. J. Am. Chem. Soc. 2004, 126, 8360–8361. [Google Scholar] [CrossRef] [PubMed]
- Liu, E.Z.; Popescu, S.R.; Eden, A.; Chung, J.; Roehrich, B.; Sepunaru, L. The role of applied potential on particle sizing precision in single-entity blocking electrochemistry. Electrochim. Acta 2023, 472, 143397. [Google Scholar] [CrossRef] [PubMed]
- Kwon, S.J.; Fan, F.-R.F.; Bard, A.J. Observing iridium oxide (IrOx) single nanoparticle collisions at ultramicroelectrodes. J. Am. Chem. Soc. 2010, 132, 13165–13167. [Google Scholar] [CrossRef] [PubMed]
- Mun, S.K.; Lee, S.; Kim, D.Y.; Kwon, S.J. Various current responses of single silver nanoparticle collisions on a gold ultramicroelectrode depending on the collision conditions. Chem. Asian J. 2017, 12, 2434–2440. [Google Scholar] [CrossRef]
- Bonezzi, J.; Boika, A. Deciphering the magnitude of current steps in electrochemical blocking collision experiments and its implications. Electrochim. Acta 2017, 236, 252–259. [Google Scholar] [CrossRef]
- Faraday, M. The Bakerian lecture: Experimental relations of gold (and other metals) to light. Philos. Trans. R. Soc. Lond. 1857, 147, 145–181. [Google Scholar]
- Kim, J.W.; Aruchamy, G.; Kim, B.K. Recent advances in single-entity electrochemistry for metal nanoparticle, nanodroplet, and bio-entity analysis. TrAC Trends Anal. Chem. 2023, 169, 117358. [Google Scholar] [CrossRef]
- Bentley, C.L.; Kang, M.; Unwin, P.R. Time-resolved detection of surface oxide formation at individual gold nanoparticles: Role in electrocatalysis and new approach for sizing by electrochemical impacts. J. Am. Chem. Soc. 2016, 138, 12755–12758. [Google Scholar] [CrossRef] [PubMed]
- Peng, Y.Y.; Guo, D.; Ma, W.; Long, Y.T. Intrinsic electrocatalytic activity of gold nanoparticles measured by single entity electrochemistry. ChemElectroChem 2018, 5, 2982–2985. [Google Scholar] [CrossRef]
- Guo, J.; Pan, J.; Chang, S.; Wang, X.; Kong, N.; Yang, W.; He, J. Monitoring the dynamic process of formation of plasmonic molecular junctions during single nanoparticle collisions. Small 2018, 14, 1704164. [Google Scholar] [CrossRef]
- Suherman, A.L.; Zampardi, G.; Amin, H.M.A.; Young, N.P.; Compton, R.G. Tannic acid capped gold nanoparticles: Capping agent chemistry controls the redox activity. Phys. Chem. Chem. Phys. 2019, 21, 4444–4451. [Google Scholar] [CrossRef]
- Suherman, A.L.; Zampardi, G.; Kuss, S.; Tanner, E.E.L.; Amin, H.M.A.; Young, N.P.; Compton, R.G. Understanding gold nanoparticle dissolution in cyanide-containing solution via impact-chemistry. Phys. Chem. Chem. Phys. 2018, 20, 28300–28307. [Google Scholar] [CrossRef] [PubMed]
- Yadid, M.; Feiner, R.; Dvir, T. Gold nanoparticle-integrated scaffolds for tissue engineering and regenerative medicine. Nano Lett. 2019, 19, 2198–2206. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Lin, J.; Li, S.; Tian, H.; Zhang, D.; Xin, Q. Label-free detection of single living bacteria: Single-entity electrochemistry targeting metabolic products. Anal. Chem. 2023, 95, 13082–13090. [Google Scholar] [CrossRef] [PubMed]
- Lafleur, J.P.; Senkbeil, S.; Jensen, T.G.; Kutter, J.P. Gold nanoparticle-based optical microfluidic sensors for analysis of environmental pollutants. Lab. Chip. 2012, 12, 4651–4656. [Google Scholar] [CrossRef] [PubMed]
- Sen, T.; Patra, A. Recent advances in energy transfer processes in gold-nanoparticle-based assemblies. J. Phys. Chem. C 2012, 116, 17307–17317. [Google Scholar] [CrossRef]
- Tseng, R.J.; Huang, J.; Ouyang, J.; Kaner, R.B.; Yang. Polyaniline nanofiber/gold nanoparticle nonvolatile memory. Nano Lett. 2005, 5, 1077–1080. [Google Scholar] [CrossRef]
- Brust, M.; Bethell, D.; Kiely, C.J.; Schiffrin, D.J. Self-assembled gold nanoparticle thin films with nonmetallic optical and electronic properties. Langmuir 1998, 14, 5425–5429. [Google Scholar] [CrossRef]
- Amin, H.M.A.; El-Kady, M.F.; Atta, N.F.; Galal, A. Gold Nanoparticles Decorated Graphene as a High Performance Sensor for Determination of Trace Hydrazine Levels in Water. Electroanalysis 2018, 30, 1749–1758. [Google Scholar] [CrossRef]
- Kim, D.Y.; Lee, S.; Kwon, S.J. Detection of Single Au Nanoparticle Collisions Using Electrocatalytic Amplification Method. Bull. Korean Chem. Soc. 2015, 36, 678–681. [Google Scholar] [CrossRef]
- Bard, A.J.; Faulkner, L.R. Electrochemical Methods, Fundamentals and Applications, 2nd ed.; John Wiley & Sons: New York, NY, USA, 2001. [Google Scholar]
- Xiao, X.; Pan, S.; Jang, J.S.; Fan, F.R.F.; Bard, A.J. Single nanoparticle electrocatalysis: Effect of monolayers on particle and electrode on electron transfer. J. Phys. Chem. C 2009, 113, 14978–14982. [Google Scholar] [CrossRef]
- Wang, S.; Kuai, L.; Huang, Y.; Yu, X.; Liu, Y.; Li, W.; Chen, L.; Geng, B. A Highly Efficient, Clean-Surface, Porous Platinum Electrocatalyst and the Inhibition Effect of Surfactants on Catalytic Activity. Chem. Eur. J. 2013, 19, 240–248. [Google Scholar] [CrossRef] [PubMed]
- Quinson, J.; Kunz, S.; Arenz, M. Surfactant-free colloidal syntheses of precious metal nanoparticles for improved catalysts. ACS Catal. 2023, 13, 4903–4937. [Google Scholar] [CrossRef]
- Li, D.; Wang, C.; Tripkovic, D.; Sun, S.; Markovic, N.M.; Stamenkovic, V.R. Surfactant removal for colloidal nanoparticles from solution synthesis: The effect on catalytic performance. ACS Catal. 2012, 2, 1358–1362. [Google Scholar] [CrossRef]
- Wall, M.A.; Harmsen, S.; Pal, S.; Zhang, L.; Arianna, G.; Lombardi, J.R.; Drain, C.M.; Kircher, M.F. Surfactant-Free Shape Control of Gold Nanoparticles Enabled by Unified Theoretical Framework of Nanocrystal Synthesis. Adv. Mater. 2017, 29, 1605622. [Google Scholar] [CrossRef] [PubMed]
- Bockris, J.M.; Oldfield, L.F. The oxidation-reduction reactions of hydrogen peroxide at inert metal electrodes and mercury cathodes. Trans. Faraday Soc. 1955, 51, 249–259. [Google Scholar] [CrossRef]
- Grabar, K.C.; Freeman, R.G.; Hommer, M.B.; Natan, M.J. Preparation and characterization of Au colloid monolayers. Anal. Chem. 1995, 67, 735–743. [Google Scholar] [CrossRef]
- Gubitosa, J.; Rizzi, V.; Laurenzana, A.; Scavone, F.; Frediani, E.; Fibbi, G.; Fanelli, F.; Sibillano, T.; Giannini, C.; Fini, P.; et al. The “end life” of the grape pomace waste become the new beginning: The development of a virtuous cycle for the green synthesis of gold nanoparticles and removal of emerging contaminants from water. Antioxidants 2022, 11, 994. [Google Scholar] [CrossRef]
- Gubitosa, J.; Rizzi, V.; Fini, P.; Laurenzana, A.; Fibbi, G.; Veiga-Villauriz, C.; Fanelli, F.; Fracassi, F.; Onzo, A.; Bianco, G.; et al. Biomolecules from snail mucus (Helix aspersa) conjugated gold nanoparticles, exhibiting potential wound healing and anti-inflammatory activity. Soft Matter 2020, 16, 10876–10888. [Google Scholar] [CrossRef]
- Pramanik, G.; Humpolickova, J.; Valenta, J.; Kundu, P.; Bals, S.; Bour, P.; Dracinsky, M.; Cigler, P. Gold nanoclusters with bright near-infrared photoluminescence. Nanoscale 2018, 10, 3792–3798. [Google Scholar] [CrossRef]
- Park, J.W.; Shumaker-Parry, J.S. Structural study of citrate layers on gold nanoparticles: Role of intermolecular interactions in stabilizing nanoparticles. J. Am. Chem. Soc. 2014, 136, 1907–1921. [Google Scholar] [CrossRef] [PubMed]
- Wei, X.; Popov, A.; Hernandez, R. Electric potential of citrate-capped gold nanoparticles is affected by poly (allylamine hydrochloride) and salt concentration. ACS Appl. Mater. Interfaces 2022, 14, 12538–12550. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.; Wang, W.; Shangguan, E.; Gao, S.; Liu, Y. Effects of gold nanoparticle morphologies on interactions with proteins. Mater. Sci. Eng. C 2020, 111, 110830. [Google Scholar] [CrossRef] [PubMed]
- Kwon, S.J.; Zhou, H.; Fan, F.R.F.; Vorobyev, V.; Zhang, B.; Bard, A.J. Stochastic electrochemistry with electrocatalytic nanoparticles at inert ultramicroelectrodes—Theory and experiments. Phys. Chem. Chem. Phys. 2011, 13, 5394–5402. [Google Scholar] [CrossRef]
- Kleijn, S.E.F.; Serrano-Bou, B.; Yanson, A.I.; Koper, M.T.M. Influence of hydrazine-induced aggregation on the electrochemical detection of platinum nanoparticles. Langmuir 2013, 29, 2054–2064. [Google Scholar] [CrossRef]
- Zheng, B.; Liu, X.; Wu, Y.; Yan, L.; Du, J.; Dai, J.; Dai, J.; Xiong, Q.; Guo, Y.; Xiao, D. Surfactant-free gold nanoparticles: Rapid and green synthesis and their greatly improved catalytic activities for 4-nitrophenol reduction. Inorg. Chem. Front. 2017, 4, 1268–1272. [Google Scholar] [CrossRef]
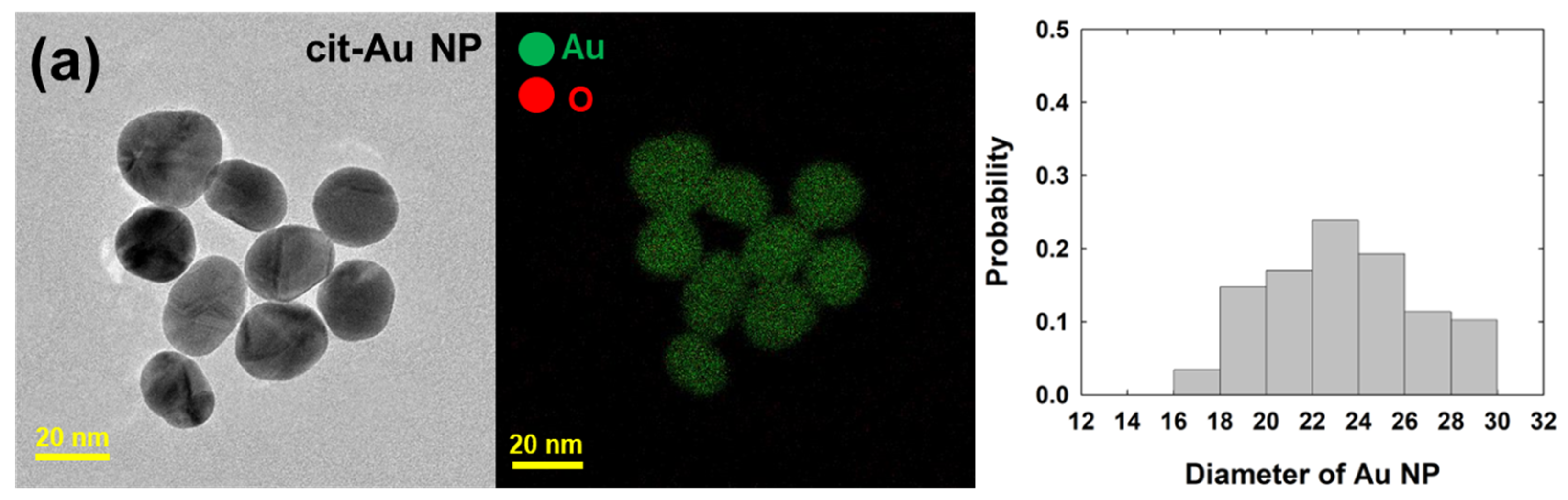
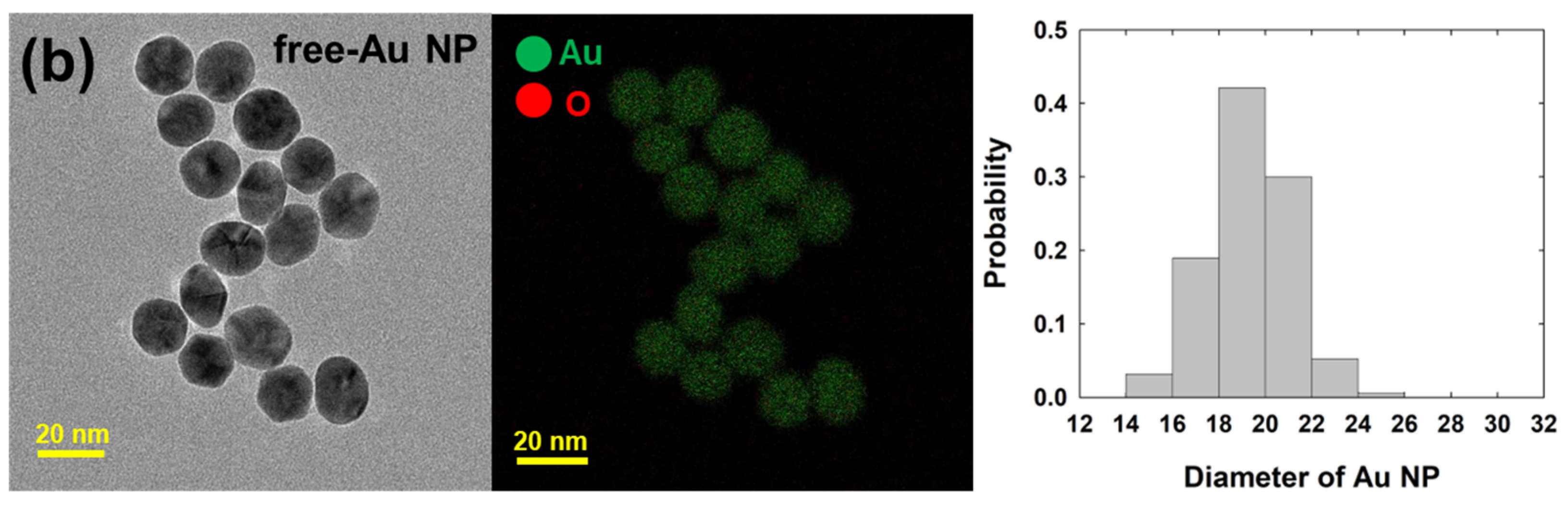
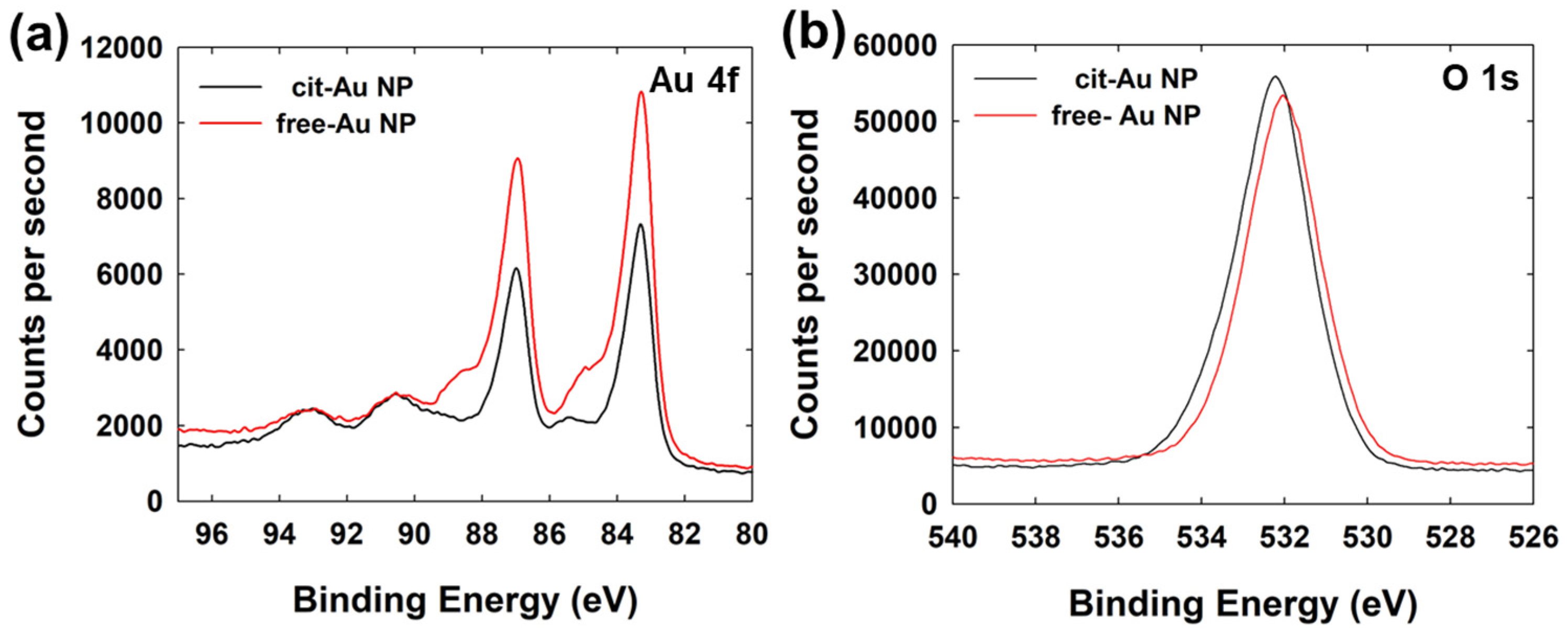



| Free-Au NPs | Cit-Au NPs | ||||
|---|---|---|---|---|---|
| Peak height (pA) | Frequency (s−1pM−1) | Peak height (pA) | Frequency (s−1pM−1) | ||
| Hydrazine Oxidation a | Theoretical value | 141 (±15) | 0.33 (±0.03) | 171 (±25) | 0.27 (±0.05) |
| Experimental value | 161 (±88) | 0.31 (±0.02) | 246 (±181) | 0.13 (±0.02) | |
| Glucose Oxidation b | Theoretical value | 25 (±3) | 0.33 (±0.03) | 31 (±5) | 0.27 (±0.05) |
| Experimental value | 6.4 (±5.2) | 0.05 (±0.04) | - | - | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Heo, D.; Kim, K.J.; Kwon, S.J. Superior Single-Entity Electrochemistry Performance of Capping Agent-Free Gold Nanoparticles Compared to Citrate-Capped Gold Nanoparticles. Nanomaterials 2024, 14, 1399. https://doi.org/10.3390/nano14171399
Heo D, Kim KJ, Kwon SJ. Superior Single-Entity Electrochemistry Performance of Capping Agent-Free Gold Nanoparticles Compared to Citrate-Capped Gold Nanoparticles. Nanomaterials. 2024; 14(17):1399. https://doi.org/10.3390/nano14171399
Chicago/Turabian StyleHeo, Dain, Ki Jun Kim, and Seong Jung Kwon. 2024. "Superior Single-Entity Electrochemistry Performance of Capping Agent-Free Gold Nanoparticles Compared to Citrate-Capped Gold Nanoparticles" Nanomaterials 14, no. 17: 1399. https://doi.org/10.3390/nano14171399
APA StyleHeo, D., Kim, K. J., & Kwon, S. J. (2024). Superior Single-Entity Electrochemistry Performance of Capping Agent-Free Gold Nanoparticles Compared to Citrate-Capped Gold Nanoparticles. Nanomaterials, 14(17), 1399. https://doi.org/10.3390/nano14171399






