SnO2-Based Interfacial Engineering towards Improved Perovskite Solar Cells
Abstract
1. Introduction
2. Experimental Section
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Chin, Y.C.; Daboczi, M.; Henderson, C.; Luke, J.; Kim, J.S. Suppressing PEDOT: PSS Doping-Induced Interfacial Recombination Loss in Perovskite Solar Cells. ACS Energy Lett. 2022, 7, 560–568. [Google Scholar] [CrossRef]
- Wang, A. Research on High-Efficiency and High-Stability Tin-Based Perovskite Solar Cells Fabricated Based on Blade-Coating. 2023. Available online: https://kns.cnki.net/kcms2/article/abstract?v=xpM8-w1VMS-KGm44RzbUD-k5gKqcTB80Zyr2mgKzw9se9Q9Uyqkx-t6P63CbQ8m5uZgBk1f6dVRQAtMp7wRcc7oGwW1ZKYhwy8MNhtdWO3x0gkzDM-6AUkwGmfeyRh0kAAtgEgtgoI6bI_lMChjSGSFYl6crD8PKyPg78ksf-gSfliKPow3iqC8D4uXTs6KjsWmJfJaiReanNn27T6Nt_46D66_rlaAJf6CdDX62athk2mYqK-gFZwS_siRgjQFYcT7h1uHiQ56ct4MYdJP6xOVfN7u76tzLogABdu373xo4sY_kIAtb5A==&uniplatform=NZKPT&language=CHS (accessed on 26 August 2024).
- You, S.; Eickemeyer, F.T.; Gao, J.; Yum, J.-H.; Zheng, X.; Ren, D.; Xia, M.; Guo, R.; Rong, Y.; Zakeeruddin, S.M.; et al. Bifunctional hole-shuttle molecule for improved interfacial energy level alignment and defect passivation in perovskite solar cells. Nat. Energy 2023, 8, 515–525. [Google Scholar] [CrossRef]
- Ru, P.; Bi, E.; Zhang, Y.; Wang, Y.; Kong, W.; Sha, Y.; Tang, W.; Zhang, P.; Wu, Y.; Chen, W.; et al. High Electron Affinity Enables Fast Hole Extraction for Efficient Flexible Inverted Perovskite Solar Cells. Adv. Energy Mater. 2020, 10, 1903487. [Google Scholar] [CrossRef]
- Du, J. Study on Interfacial Passivation of Methylamine Lead Iodide Perovskite Solar Cells. 2022. Available online: https://kns.cnki.net/kcms2/article/abstract?v=xpM8-w1VMS_QrOqtMKf5yZQvTDNBAQ1INRdidICYN33XL5otgsV87ZXaBvlKWrJAzYZABIBc0LeFtV1Us5eYPKGqIc2ow2NtzQptt9K_CPC1aonQkbu5XqvWS5uGVpBBfxMsdYwM5AWZ4BBMEcGuGRTxf4SPIYRcrf6X_SDs8U3tJpcqD1Y5yII12-Q91cZh5uNEa0QY-WCcl1w0puBI4c7puNa7bIQ9ogPl1rEbek_YiBTlI5I12IahQE6l7qEZCTYCE6p1fIWirzcTDqPkBFCgeLcD0laH5dIZZT8rLGuqX492u85qkw==&uniplatform=NZKPT&language=CHS (accessed on 26 August 2024).
- Lan, Z.; Huang, H.; Du, S.; Lu, Y.; Sun, C.; Yang, Y.; Zhang, Q.; Suo, Y.; Qu, S.; Wang, M.; et al. Cascade Reaction in Organic Hole Transport Layer Enables Efficient Perovskite Solar Cells. Angew. Chem. Int. Ed. 2024, 63, e202402840. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Sina, M.; Parikh, P.; Uekert, T.; Shahbazian, B.; Devaraj, A.; Meng, Y.S. Role of 4-tert-Butylpyridine as a Hole Transport Layer Morphological Controller in Perovskite Solar Cells. Nano Lett. 2016, 16, 5594–5600. [Google Scholar] [CrossRef]
- Wu, T.; Zhuang, R.; Zhao, R.; Zhao, R.; Zhu, L.; Liu, G.; Wang, R.; Zhao, K.; Hua, Y. Understanding the Effects of Fluorine Substitution in Lithium Salt on Photovoltaic Properties and Stability of Perovskite Solar Cells. ACS Energy Lett. 2021, 6, 2218–2228. [Google Scholar] [CrossRef]
- Meng, X. Research on Interface and Stability of Perovskite Solar Cells. 2022. Available online: https://kns.cnki.net/kcms2/article/abstract?v=xpM8-w1VMS_-INBGTNPdh24wC-k4UiIhshRdRYr50iAMp8AnW6TVoQJMGlF4Cw2VxAme7pPj1sit-6VGtJtrHu3DhSXEhyuf_4BmWjpHGrR1B038794Hb2SMcYjoGqCE2fqFmus9Mh73Ab3DMeCOfAhJB10p39AyuGIY2BwRJD5qIDL7YuSdpw3XK5KlqycU5FOVW0Vuo1w0KCFheGOQ40Q_SfyS3F7OqtelGJTWKcVHegBqg6ZKu8sBO1BhqxmMmC8sUHH-djvobHu0wXlPDH4THBPwczB8G0xSwfWl9LHoaaE2e5A7tw==&uniplatform=NZKPT&language=CHS (accessed on 26 August 2024).
- Yu, S.; Xiong, Z.; Zhou, H.; Zhang, Q.; Wang, Z.; Ma, F.; Qu, Z.; Zhao, Y.; Chu, X.; Zhang, X.; et al. Homogenized NiOx nanoparticles for improved hole transport in inverted perovskite solar cells. Science 2023, 382, 1399–1404. [Google Scholar] [CrossRef]
- Kim, J.I.Y.; Lee, J.-W.; Jung, H.S.; Shin, H.; Park, N.-G. High-Efficiency Perovskite Solar Cells. Chem. Rev. 2020, 120, 7867–7918. [Google Scholar] [CrossRef]
- Nath, B.; Behera, S.K.; Kumar, J.; Hemmerle, A.; Fontaine, P.; Ramamurthy, P.C.; Mahapatra, D.R.; Hegde, G. Understanding the Heterointerfaces in Perovskite Solar Cells via Hole Selective Layer Surface Functionalization. Adv. Mater. 2024, 36, 2307547. [Google Scholar] [CrossRef]
- Cho, K.T.; Paek, S.; Grancini, G.; Roldán-Carmona, C.; Gao, P.; Lee, Y.; Nazeeruddin, M.K. Highly efficient perovskite solar cells with a compositionally engineered perovskite/hole transporting material interface. Energy Environ. Sci. 2017, 10, 621. [Google Scholar] [CrossRef]
- Zhu, M.; Duan, Y.; Liu, N.; Li, H.; Li, J.; Du, P.; Tan, Z.; Niu, G.; Gao, L.; Huang, Y.; et al. Electrohydro dynamically Printed High-Resolution Full-Color Hybrid Perovskites. Adv. Funct. Mater. 2019, 29, 1903294. [Google Scholar] [CrossRef]
- Yoon, H.; Kang, S.M.; Lee, J.K.; Choi, M. Hysteresis-free low-temperature-processed planar perovskite solar cells with 19.1% efficiency. Energy Environ. Sci. 2016, 9, 2262. [Google Scholar] [CrossRef]
- Ke, W.; Fang, G.; Liu, Q.; Xiong, L.; Qin, P.; Tao, H.; Wang, J.; Lei, H.; Li, B.; Wan, J.; et al. Low-Temperature Solution-Processed Tin Oxide as an Alternative Electron Transporting Layer for Efficient Perovskite Solar Cells. J. Am. Chem. Soc. 2015, 137, 6730–6733. [Google Scholar] [CrossRef] [PubMed]
- Myung, C.W.; Lee, G.; Kim, K.S. La-doped BaSnO3 electron transport layer for perovskite solar cells. J. Mater. Chem. A 2018, 6, 23071. [Google Scholar] [CrossRef]
- Li, Z.; Wang, L.; Liu, R.; Fan, Y.; Meng, H.; Shao, Z.; Cui, G.; Pang, S. Spontaneous Interface Ion Exchange: Passivating Surface Defects of Perovskite Solar Cells with Enhanced Photovoltage. Adv. Energy Mater. 2019, 9, 1902142. [Google Scholar] [CrossRef]
- Tan, H.; Jain, A.; Voznyy, O.; Lan, X.; García de Arquer, F.P.; Fan, J.Z.; Quintero-Bermudez, R.; Yuan, M.; Zhang, B.; Zhao, Y.; et al. Efficient and stable solution-processed planar perovskite solar cells via contact passivation. Science 2017, 355, 722–726. [Google Scholar] [CrossRef]
- Han, J.; Kwon, H.; Kim, E.; Kim, D.W.; Son, H.J.; Kim, D.H. Interfacial engineering of a ZnO electron transporting layer using self-assembled monolayers for high performance and stable perovskite solar cells. J. Mater. Chem. A 2020, 8, 2105. [Google Scholar] [CrossRef]
- Dou, J.; Zhang, Y.; Wang, Q.; Abate, A.; Li, Y.; Wei, M. Highly efficient Zn2SnO4 perovskite solar cells through band alignment engineering. Chem. Commun. 2019, 55, 14673. [Google Scholar] [CrossRef]
- Jiang, Q.; Zhang, L.; Wang, H.; Yang, X.; Meng, J.; Liu, H.; Yin, Z.; Wu, J.; Zhang, X.; You, J. Enhanced electron extraction using SnO2 for high-efficiency planar-structure HC(NH2)2PbI3-based perovskite solar cells. Nat. Energy 2017, 2, 16177. [Google Scholar] [CrossRef]
- Luan, Y.; Yi, X.; Mao, P.; Wei, Y.; Zhuang, J.; Chen, N.; Lin, T.; Li, C.; Wang, J. High-Performance Planar Perovskite Solar Cells with Negligible Hysteresis Using 2,2,2-Trifluoroethanol-Incorporated SnO2. Science 2019, 28, 433–441. [Google Scholar] [CrossRef]
- Jiang, Q.; Zhang, X.; You, J. SnO2: A Wonderful Electron Transport Layer for Perovskite Solar Cells. Small 2018, 14, 1801154. [Google Scholar] [CrossRef]
- Jeong, S.; Seo, S.; Park, H.; Shin, H. Atomic layer deposition of a SnO2 electron-transporting layer for planar perovskite solar cells with a power conversion efficiency of 18.3%. Chem. Commun. 2019, 55, 2433–2436. [Google Scholar] [CrossRef]
- Yoo, J.J.; Seo, G.; Chua, M.R.; Park, T.G.; Lu, Y.; Rotermund, F.; Kim, Y.-K.; Moon, C.S.; Jeon, N.J.; Correa-Baena, J.-P.; et al. Efficient perovskite solar cells via improved carrier management. Nature 2021, 590, 587–593. [Google Scholar] [CrossRef]
- Jung, K.H.; Seo, J.Y.; Lee, S.; Shin, H.; Park, N.G. Solution-processed SnO2 thin film for a hysteresis-free planar perovskite solar cell with a power conversion efficiency of 19.2%. J. Mater. Chem. A 2017, 5, 24790–24803. [Google Scholar] [CrossRef]
- Jeong, J.; Kim, M.; Seo, J.; Lu, H.; Ahlawat, P.; Mishra, A.; Yang, Y.; Hope, M.A.; Eickemeyer, F.T.; Kim, M.; et al. Pseudo-halide anion engineering for α-FAPbI3 perovskite solar cells. Nature 2021, 592, 381–385. [Google Scholar] [CrossRef]
- Kaur, H.; Siwal, S.S.; Saini, R.V.; Singh, N.; Thakur, V.K. Significance of an Electrochemical Sensor and Nanocomposites: Toward the Electrocatalytic Detection of Neurotransmitters and Their Importance within the Physiological System. ACS Nanosci. Au 2023, 3, 1–27. [Google Scholar] [CrossRef] [PubMed]
- Filho, M.A.M.; Farmer, W.; Hsiao, C.L.; dos Santos, R.B.; Hultman, L.; Birch, J.; Ankit, K.; Gueorguiev, G.K. Density Functional Theory-Fed Phase Field Model for Semiconductor Nanostructures: The Case of Self-Induced Core−Shell InAlN Nanorods. Cryst. Growth Des. 2024, 24, 4717–4727. [Google Scholar] [CrossRef] [PubMed]
- Yang, D.; Yang, R.; Zhang, C.; Ye, T.; Wang, K.; Hou, Y.; Zheng, L.; Priya, S.; Liu, S. Highest-Efficiency Flexible Perovskite Solar Module by Interface Engineering for Efficient Charge Transfer. Adv. Mater. 2023, 35, 2302484. [Google Scholar] [CrossRef]
- Lou, Q.; Lou, G.; Guo, H.; Sun, T.; Wang, C.; Chai, G.; Chen, X.; Yang, G.; Guo, Y.; Zhou, H. Enhanced Efficiency and Stability of n-i-p Perovskite Solar Cells by Incorporation of Fluorinated Graphene in the Spiro-OMeTAD Hole Transport Layer. Adv. Energy Mater. 2022, 12, 2201344. [Google Scholar] [CrossRef]

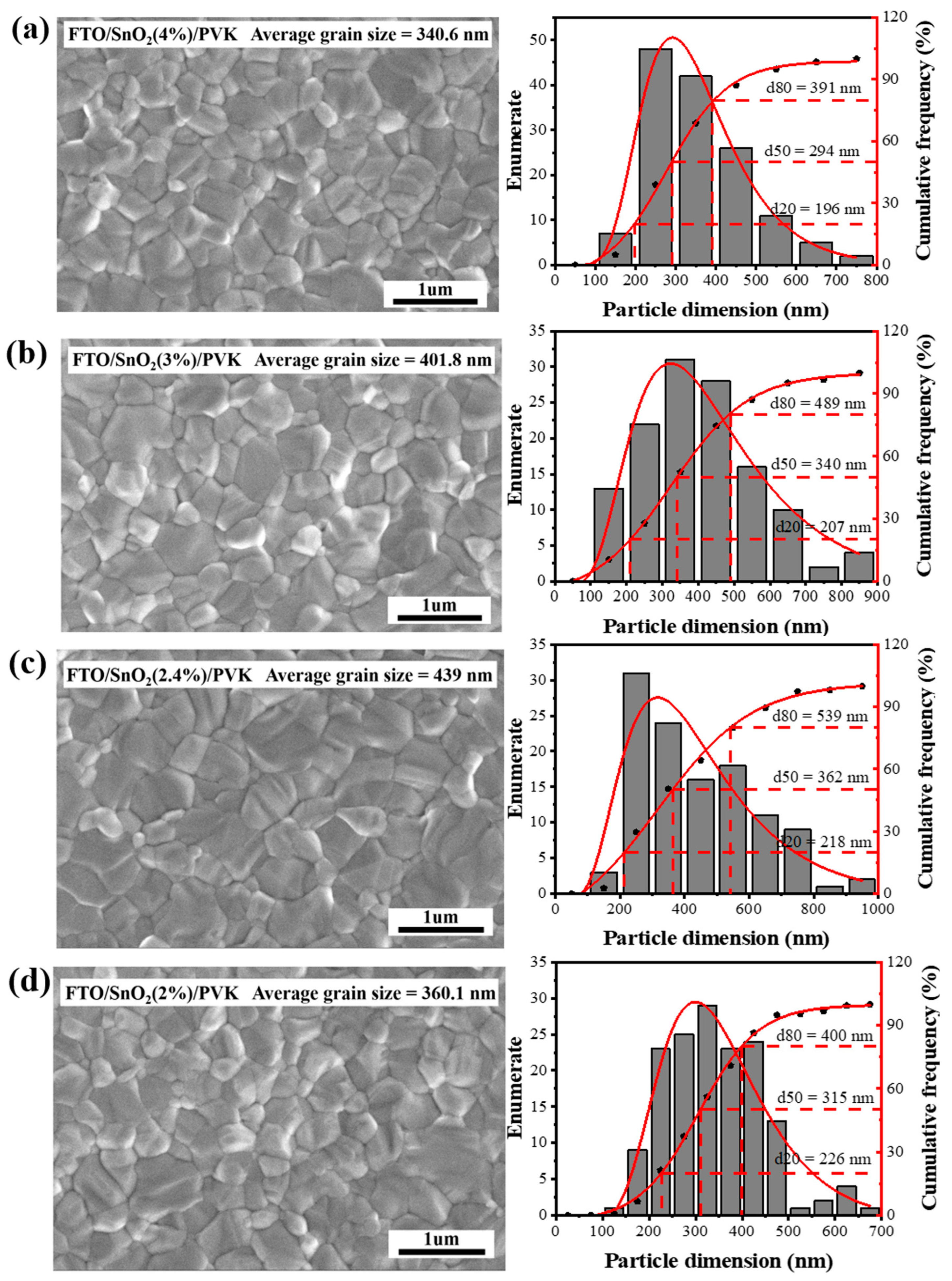

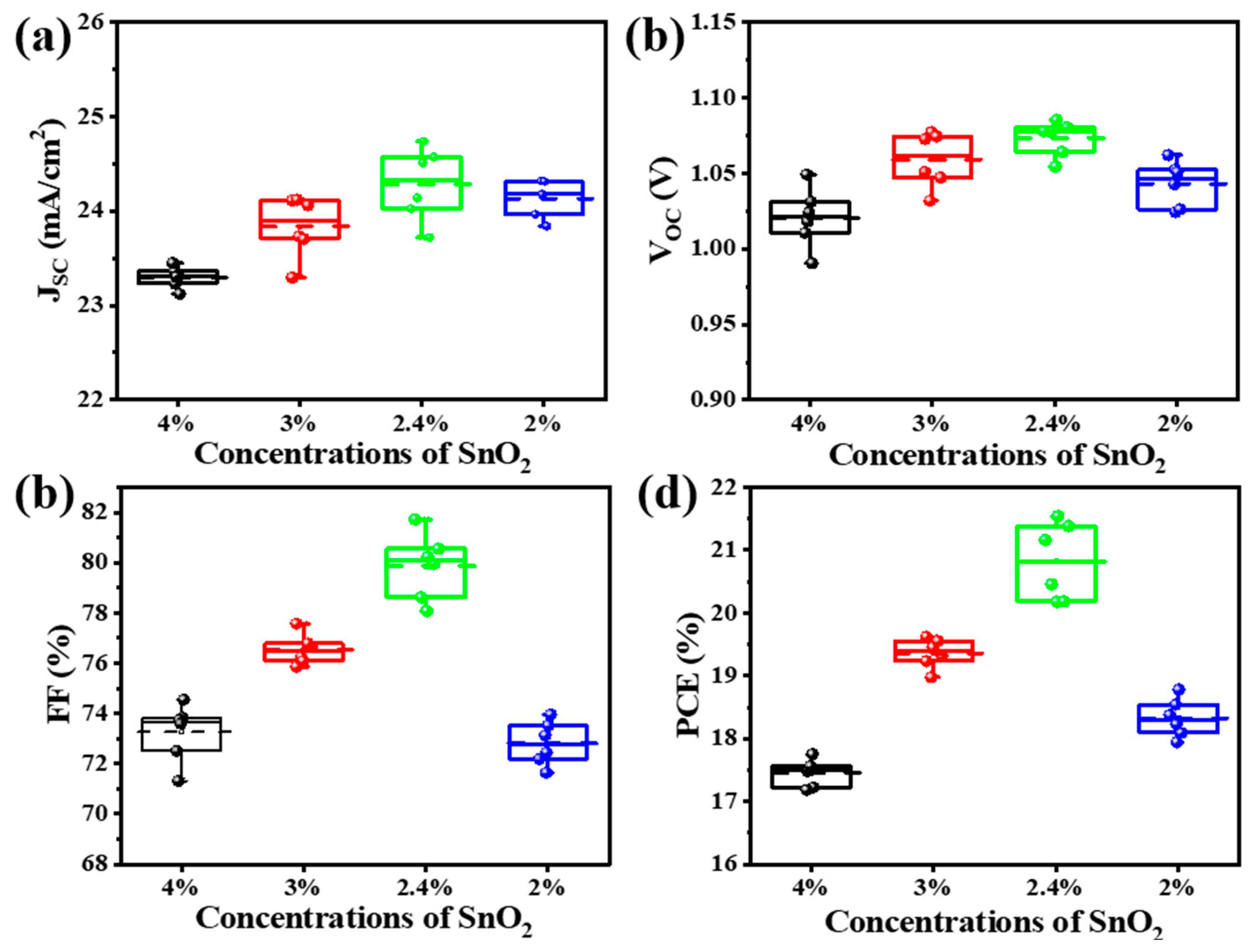
| Concentration Ratios | PCE (%) | VOC (V) | FF (%) | JSC (mA/cm2) |
|---|---|---|---|---|
| SnO2 (4%) | 17.40 | 1.02 | 73.26 | 23.29 |
| SnO2 (3%) | 19.34 | 1.06 | 76.54 | 23.84 |
| SnO2 (2.4%) | 20.27 | 1.07 | 79.9 | 24.29 |
| SnO2 (2%) | 18.28 | 1.04 | 72.82 | 24.14 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, B.; Liu, C.; Zhang, X. SnO2-Based Interfacial Engineering towards Improved Perovskite Solar Cells. Nanomaterials 2024, 14, 1406. https://doi.org/10.3390/nano14171406
Li B, Liu C, Zhang X. SnO2-Based Interfacial Engineering towards Improved Perovskite Solar Cells. Nanomaterials. 2024; 14(17):1406. https://doi.org/10.3390/nano14171406
Chicago/Turabian StyleLi, Bing’e, Chuangping Liu, and Xiaoli Zhang. 2024. "SnO2-Based Interfacial Engineering towards Improved Perovskite Solar Cells" Nanomaterials 14, no. 17: 1406. https://doi.org/10.3390/nano14171406
APA StyleLi, B., Liu, C., & Zhang, X. (2024). SnO2-Based Interfacial Engineering towards Improved Perovskite Solar Cells. Nanomaterials, 14(17), 1406. https://doi.org/10.3390/nano14171406







