Effect of Sulfur Modification on Structural and Electrochemical Performance of Pitch-Based Carbon Materials for Lithium/Sodium Ion Batteries
Abstract
:1. Introduction
2. Experimental
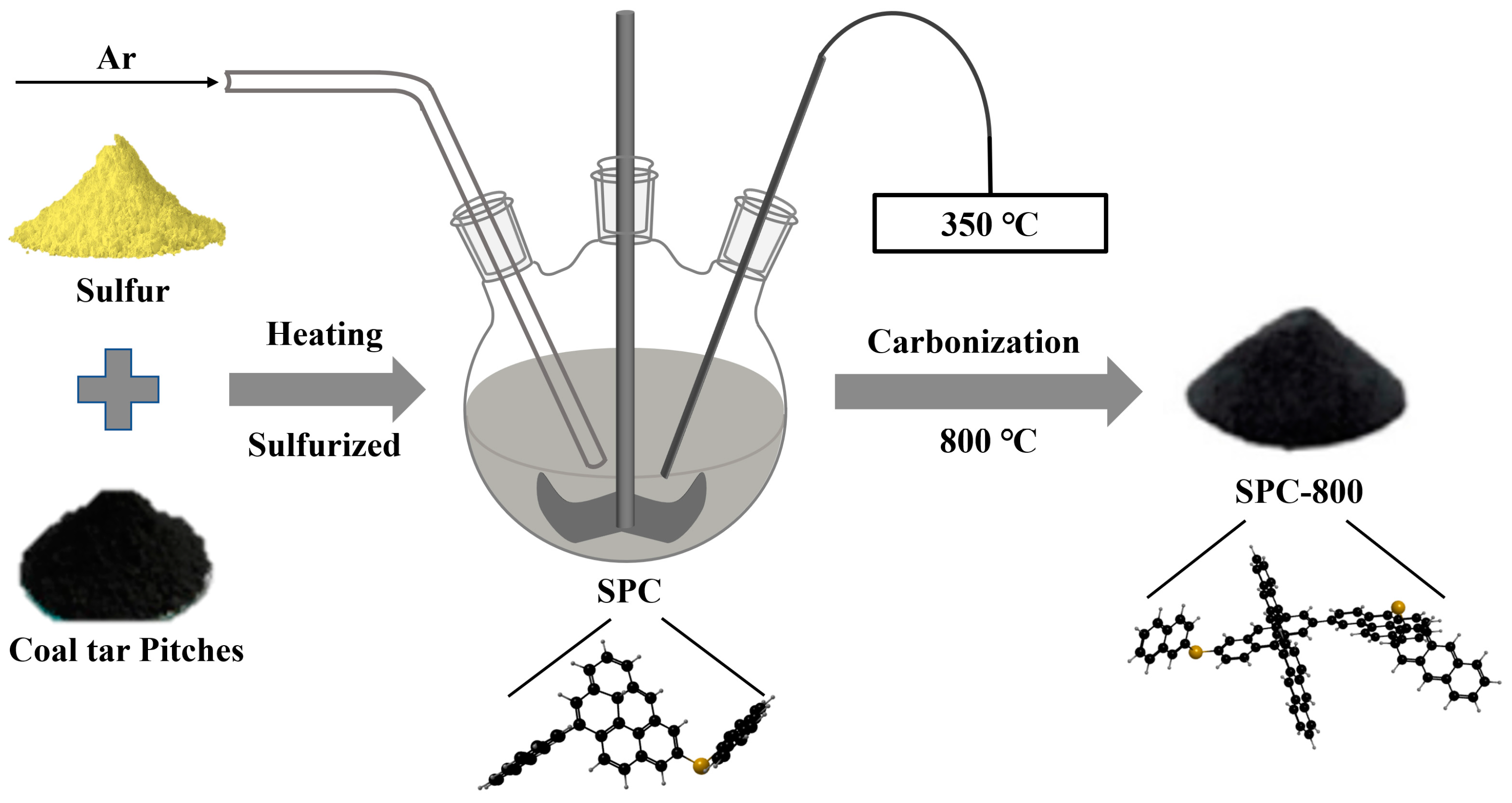
2.1. Materials Preparation
2.2. Materials Characterization
2.3. Electrochemical Characterization
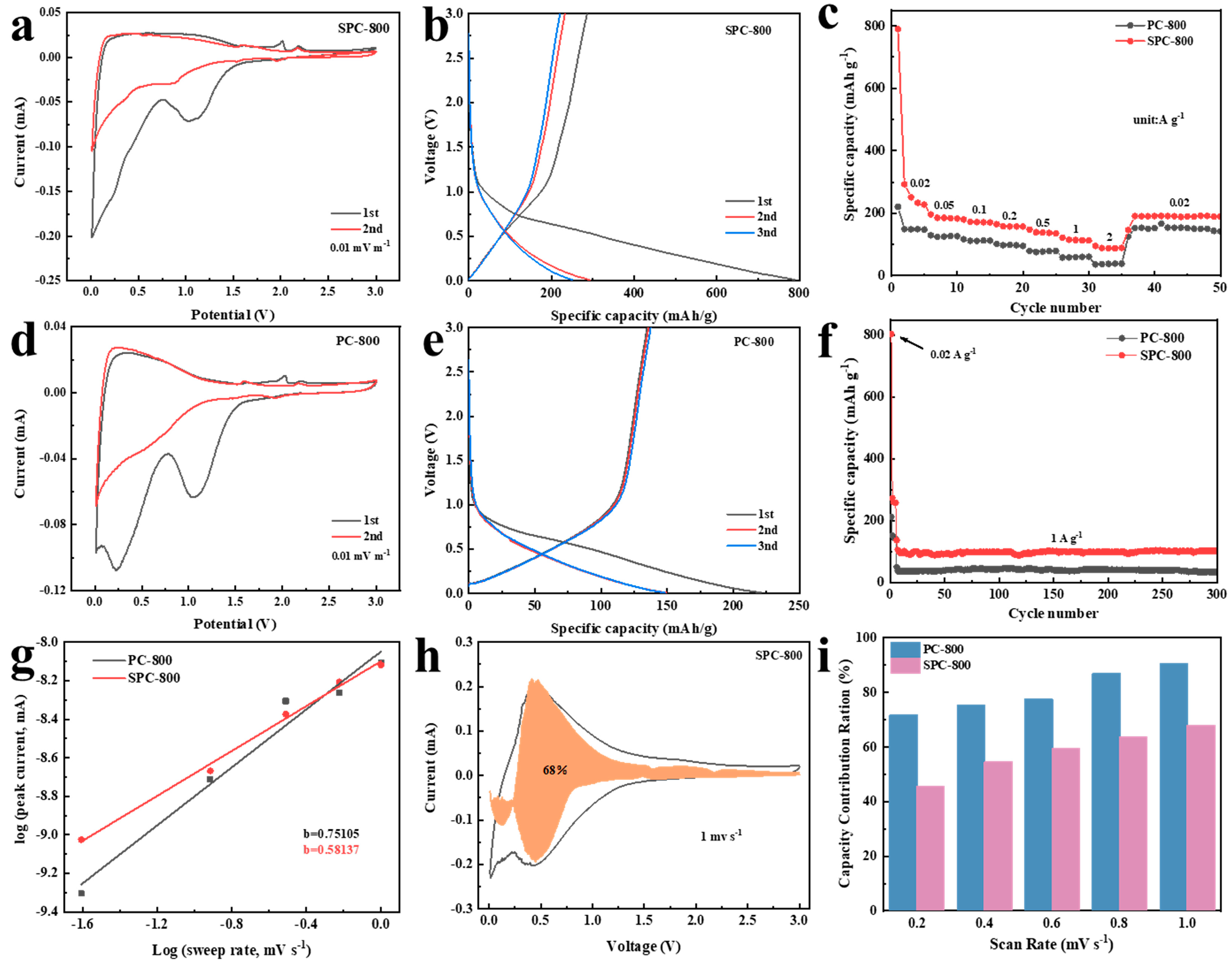
3. Results and Discussion
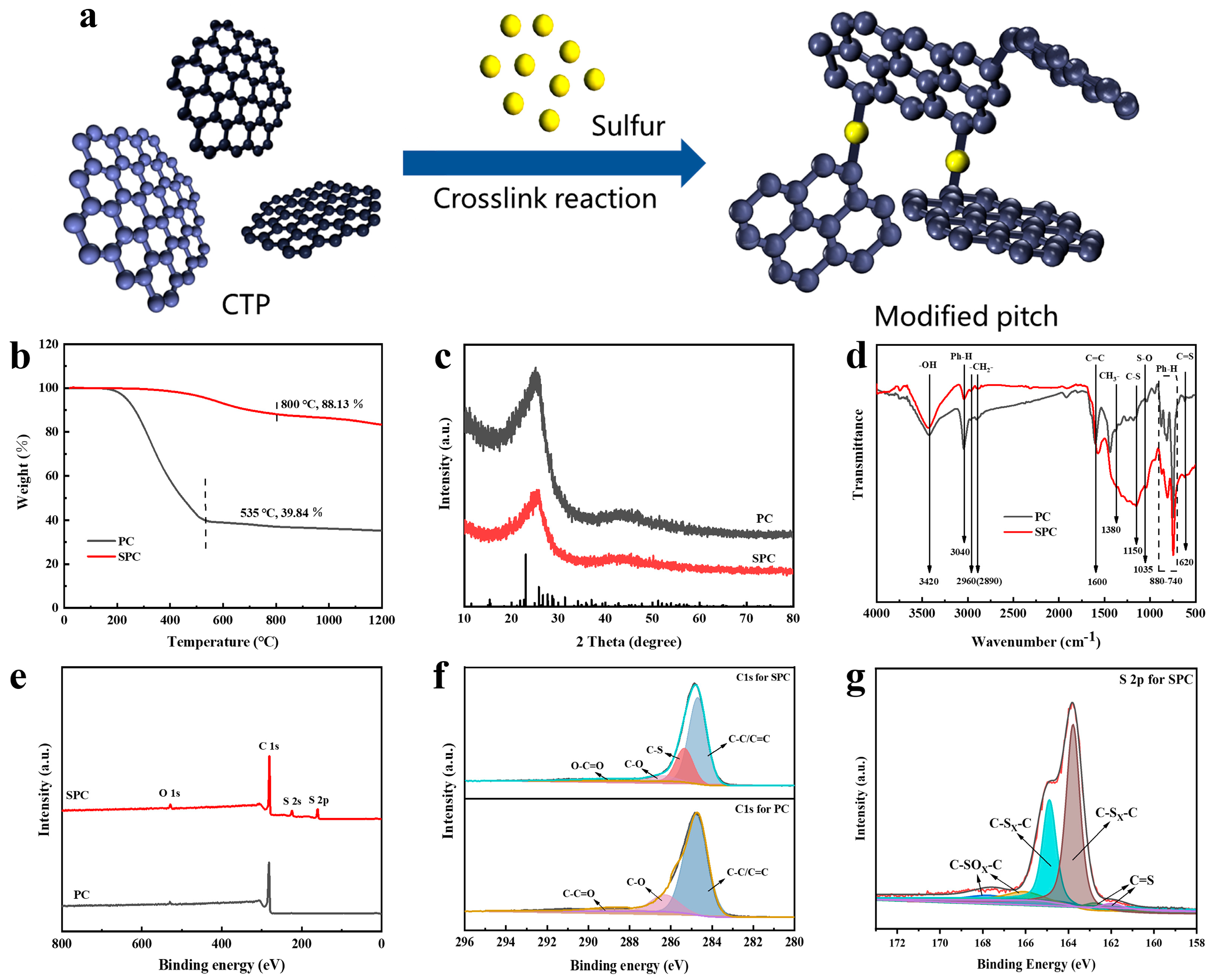
3.1. Structural Characterization
3.2. Electrochemical Performance
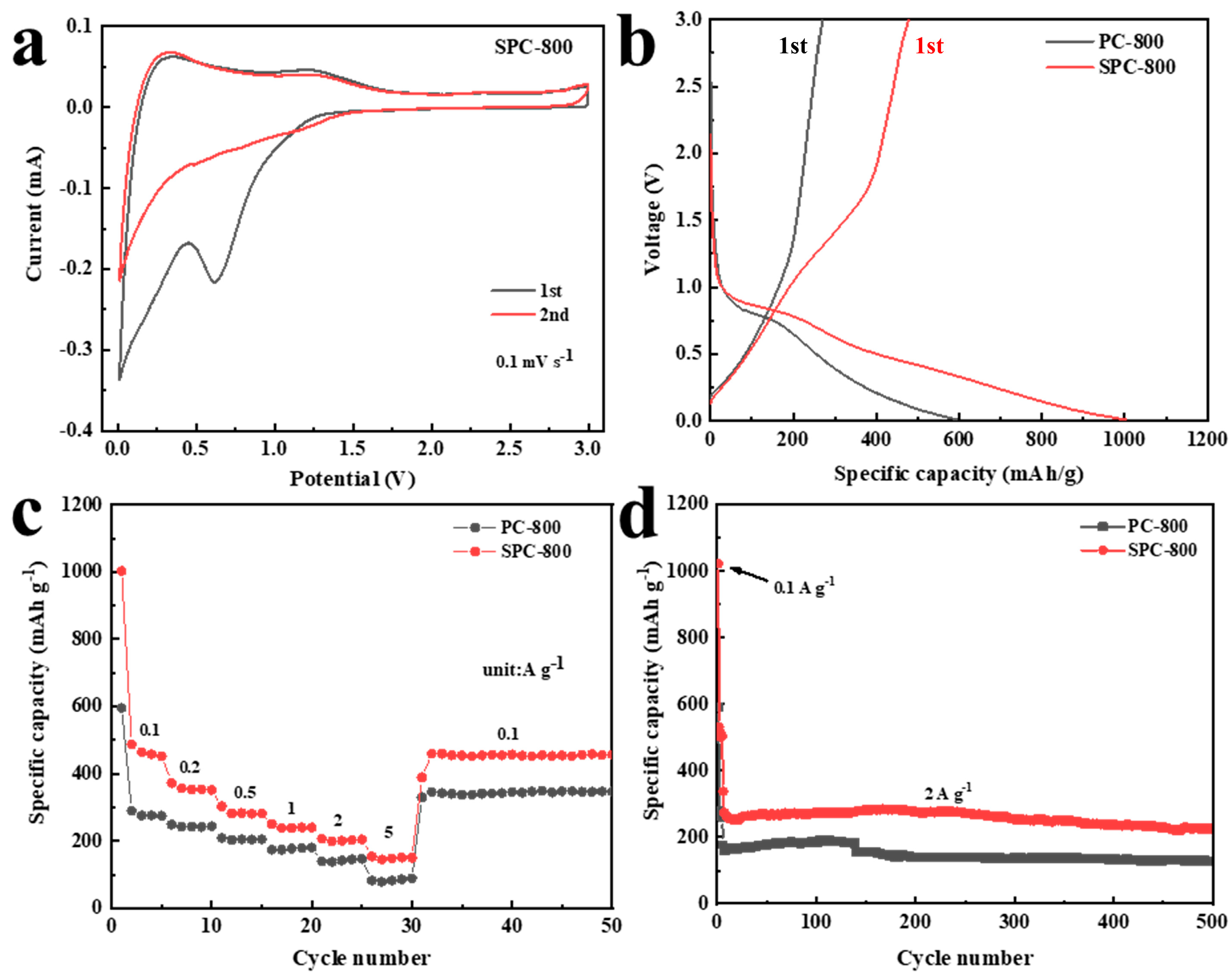
3.2.1. Electrochemical Performance of LIBs
3.2.2. Electrochemical Performance of SIBs
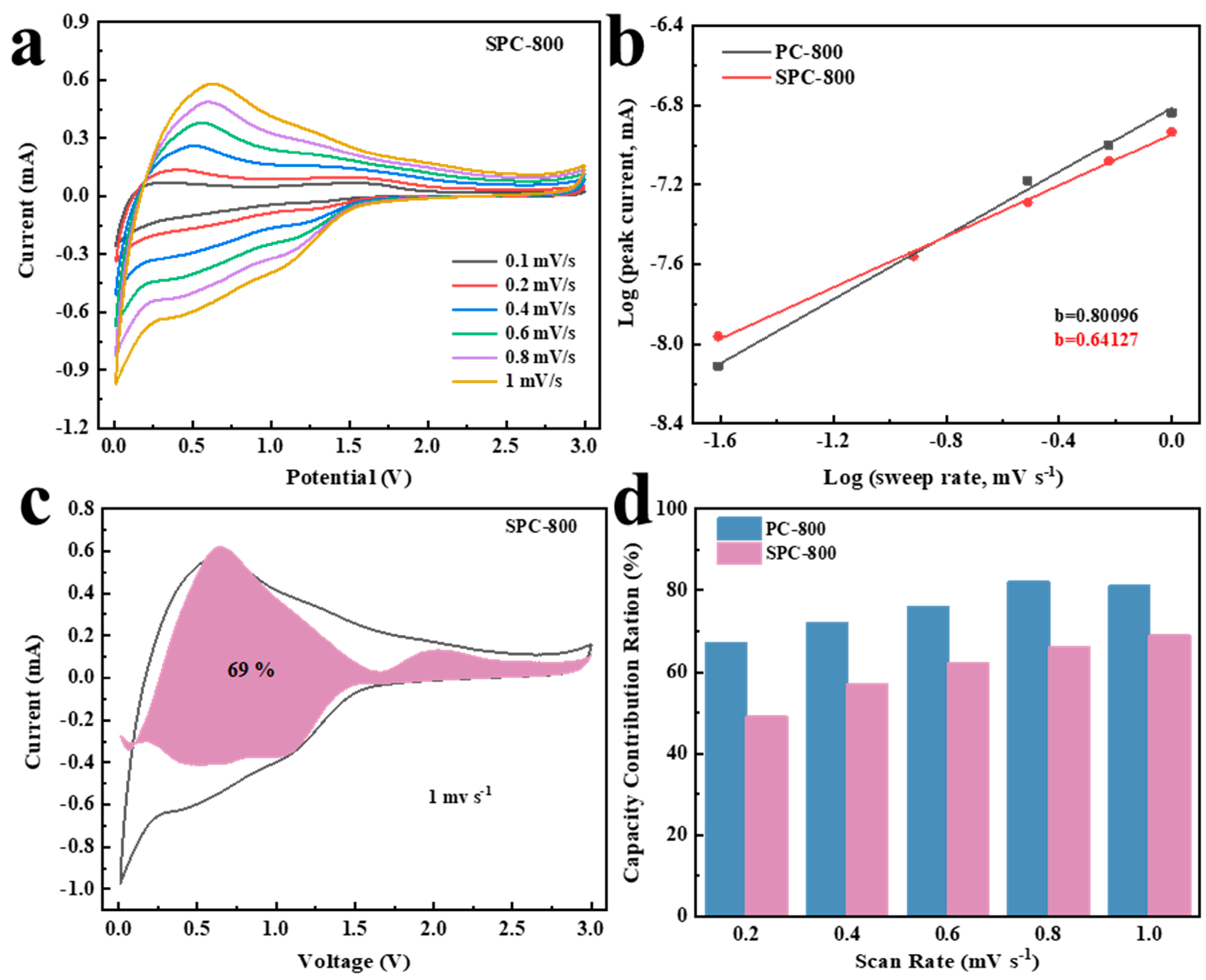
3.3. Relationship between Structure and Electrochemical Performance
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Gao, M.Y.; Xue, Y.C.; Zhang, Y.T.; Zhu, C.X.; Yu, H.W.; Guo, X.M.; Sun, S.S.; Xiong, S.L.; Kong, Q.H.; Zhang, J.H. Growing Co-Ni-Se Nanosheets on 3D Carbon Frameworks as Advanced Dual Functional Electrodes for Supercapacitors and Sodium ion Batteries. Inorg. Chem. Front. 2022, 9, 3933–3942. [Google Scholar] [CrossRef]
- Wang, S.; Jiang, Y.J.; Hu, X.L. Ionogel-Based Membranes for Safe Lithium/Sodium Batteries. Adv. Mater. 2022, 34, 2200945. [Google Scholar] [CrossRef]
- Kulkarni, P.; Jung, H.Y.Y.; Ghosh, D.; Jalalah, M.; Alsaiari, M.; Harraz, F.A.; Balakrishna, R.G. A Comprehensive Review of Pre-lithiation/sodiation Additives for Li-ion and Na-ion Batteries. J. Energy Chem. 2023, 76, 479–494. [Google Scholar] [CrossRef]
- Wu, J.R.; Yang, T.; Song, Y.; Zhang, X.S.; Tian, X.D.; Liu, Z.J. Correlation between Microstructure, Optical Texture and Lithium Storage Performance in Pitch-based Materials. J. Alloys Compd. 2023, 945, 169367. [Google Scholar] [CrossRef]
- Lv, W.M.; Wen, F.S.; Xiang, J.Y.; Zhao, J.; Li, L.; Wang, L.M.; Liu, Z.Y.; Tian, Y.J. Peanut Shell Derived Hard Carbon as Ultralong Cycling Anodes for Lithium and Sodium Batteries. Electrochim. Acta 2015, 176, 533–541. [Google Scholar] [CrossRef]
- Xing, B.L.; Zhang, C.T.; Liu, Q.R.; Zhang, C.X.; Huang, G.X.; Guo, H.; Cao, J.L.; Cao, Y.J.; Yu, J.L.; Chen, Z.F. Green Synthesis of Porous Graphitic Carbons from Coal Tar Pitch Templated by Nano-CaCO3 for High-performance Lithium-ion Batteries. J. Alloys Compd. 2019, 795, 91–102. [Google Scholar] [CrossRef]
- Xie, L.J.; Tang, C.; Bi, Z.H.; Song, M.X.; Fan, Y.F.; Yan, C.; Li, X.M.; Su, F.Y.; Zhang, Q.; Chen, C.M. Hard Carbon Anodes for Next-Generation Li-Ion Batteries: Review and Perspective. Adv. Energy Mater. 2021, 11, 2101650. [Google Scholar] [CrossRef]
- Jiang, M.C.; Sun, N.; Soomro, R.A.; Xu, B. The Recent Progress of Pitch-Based Carbon Anodes in Sodium-ion batteries. J. Energy Chem. 2021, 55, 34–47. [Google Scholar] [CrossRef]
- Lu, Y.X.; Zhao, C.L.; Qi, X.G.; Qi, Y.R.; Li, H.; Huang, X.J.; Chen, L.Q.; Hu, Y.S. Pre-Oxidation-Tuned Microstructures of Carbon Anodes Derived from Pitch for Enhancing Na Storage Performance. Adv. Energy Mater. 2018, 8, 1800108. [Google Scholar] [CrossRef]
- Daher, N.; Huo, D.; Davoisne, C.; Meunier, P.; Janot, R. Impact of Preoxidation Treatments on Performances of Pitch-Based Hard Carbons for Sodium-Ion Batteries. ACS Appl. Energy Mater. 2020, 3, 6501–6510. [Google Scholar] [CrossRef]
- Kong, F.N.; Yue, Y.; Li, Q.Y.; Ren, S.J. Sulfur-Doped Graphdiyne as a High-Capacity Anode Material for Lithium-Ion Batteries. Nanomaterials 2021, 11, 1161. [Google Scholar] [CrossRef] [PubMed]
- Zou, G.Q.; Wang, C.; Hou, H.S.; Wang, C.W.; Qiu, X.Q.; Ji, X.B. Controllable Interlayer Spacing of Sulfur-Doped Graphitic Carbon Nanosheets for Fast Sodium-Ion Batteries. Small 2017, 13, 1700762. [Google Scholar] [CrossRef]
- Li, X.Y.; Xue, C.J.; Liu, Y.P.; Zhao, J.L.; Zhang, J.W. Amorphous Structure and Sulfur Doping Synergistically Inducing Defect-rich Short Carbon Nanotubes as a Superior Anode Material in Lithium-ion Batteries. Electrochim. Acta 2023, 440, 141697. [Google Scholar] [CrossRef]
- Wan, H.R.; Hu, X.F. Sulfur-doped Honeycomb-like Carbon with Outstanding Electrochemical Performance as an Anode Material for Lithium and Sodium ion Batteries. J. Colloid Interface Sci. 2020, 558, 242–250. [Google Scholar] [CrossRef]
- Yin, R.L.; Wang, K.; Han, B.B.; Xu, G.Y.; Li, L.X.; An, B.G.; Ju, D.Y.; Chai, M.R.; Li, S.N.; Zhou, W.M. Structural Evaluation of Coal-Tar-Pitch-Based Carbon Materials and Their Na+ Storage Properties. Coatings 2021, 11, 948. [Google Scholar] [CrossRef]
- He, L.; Sun, Y.R.; Wang, C.L.; Guo, H.Y.; Guo, Y.Q.; Li, C.; Zhou, Y. High Performance Sulphur-doped Pitch-based Carbon Materials as Anode Materials for Sodium-ion Batteries. New Carbon Mater. 2020, 35, 420–427. [Google Scholar] [CrossRef]
- Afanador, L.; Ortega, S.; Gomez, R.; Nino-Gomez, M.E. Titanyl Sulfate Extracted from The Mineral Ilmenite as Mesoporous Catalyst for the Oleic Acid Esterification. Fuel 2012, 100, 43–47. [Google Scholar] [CrossRef]
- Wu, C.; Shi, L.Z.; Xue, S.G.; Li, W.C.; Jiang, X.X.; Rajendran, M.; Qian, Z.Y. Effect of Sulfur-iron Modified Biochar on the Available Cadmium and Bacterial Community Structure in Contaminated Soils. Sci. Total Environ. 2019, 647, 1158–1168. [Google Scholar] [CrossRef]
- Guo, H.Y.; Li, Y.Y.; Wang, C.L.; He, L.; Li, C.; Guo, Y.Q.; Zhou, Y. Effect of the Air Oxidation Stabilization of Pitch on the Microstructure and Sodium of Hard Carbons. New Carbon Mater. 2021, 36, 1073–1080. [Google Scholar] [CrossRef]
- Zhao, G.; Yu, D.; Zhang, H.; Sun, F.; Li, J.; Zhu, L.; Sun, L.; Yu, M.; Besenbacher, F.; Sun, Y. Sulphur-doped Carbon Nanosheets Derived from Biomass as High-performance Anode Materials for Sodium-ion Batteries. Nano Energy 2020, 67, 104219. [Google Scholar] [CrossRef]
- Li, W.; Zhou, M.; LI, H.M.; Wang, K.L.; Cheng, S.J.; Jiang, K. A high Performance Sulfur-doped Disordered Carbon Anode for Sodium ion Batteries. Energy Environ. Sci. 2015, 8, 2916–2921. [Google Scholar] [CrossRef]
- Qian, J.; Wu, F.; Ye, Y.S.; Zhang, M.L.; Huang, Y.X.; Xing, Y.; Qu, W.; Li, L.; Chen, R.J. Boosting Fast Sodium Storage of a Large-Scalable Carbon Anode with an Ultralong Cycle Life. Adv. Energy Mater. 2018, 8, 1703159. [Google Scholar] [CrossRef]
- Liu, L.T.; Li, Y.M.; Wang, S.Z.; Lu, Y.P.; Zhang, J.P.; Wang, D.K.; Ding, Y.H.; Qiu, D.P.; Niu, J.; Yu, Y.C.; et al. High Sulfur-doped hollow carbon sphere with multicavity for high-performance Potassium-ion hybrid capacitors. J. Colloid Interface Sci. 2022, 628, 975–983. [Google Scholar] [CrossRef]
- Liu, Y.X.; Guo, X.; Tian, X.D.; Liu, Z.J. Coal-Based Semicoke-Derived Carbon Anode Materials with Tunable Microcrystalline Structure for Fast Lithium-Ion Storage. Nanomaterials 2022, 12, 4067. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.X.; Shi, L.L.; Li, A.; Zhang, S.; Guo, M.Y.; Chen, X.H.; Zhou, J.S.; Song, H.H. Capacity Enhancement of Porous Carbon Electrodes during Long-Term Cycling in Lithium-Ion Batteries. J. Electrochem. Soc. 2017, 164, A2000–A2006. [Google Scholar] [CrossRef]
- Zhang, S.; Liu, Q.F.; Zhang, H.; Ma, R.J.; Li, K.; Wu, Y.K.; Teppen, B.J. Structural Order Evaluation and Structural Evolution of Coal Derived Natural Graphite During Graphitization. Carbon 2020, 157, 714–723. [Google Scholar] [CrossRef]
- Sadezky, A.; Muckenhuber, H.; Grothe, H.; Niessner, R.; Pöschl, U. Raman Micro Spectroscopy of Soot and Related Carbonaceous Materials: Spectral Analysis and Structural Information. Carbon 2005, 43, 1731–1742. [Google Scholar] [CrossRef]
- Feng, Q.X.; Li, H.X.; Tan, Z.; Huang, Z.Y.; Jiang, L.L.; Zhou, H.H.; Pan, H.Y.; Zhou, Q.; Ma, S.; Kuang, Y.F. Design and Preparation of Three-dimensional MnO/N-doped Carbon Nanocomposites Based on Waste Biomass for High Storage and Ultra-fast Transfer of Lithium ions. J. Mater. Chem. A 2018, 6, 19479–19487. [Google Scholar] [CrossRef]
- Qiu, D.P.; Guan, J.Y.; Li, M.; Kang, C.H.; Wei, J.Y.; Li, Y.; Xie, Z.Y.; Wang, F.; Yang, R. Kinetics Enhanced Nitrogen-Doped Hierarchical Porous Hollow Carbon Spheres Boosting Advanced Potassium-Ion Hybrid Capacitors. Adv. Funct. Mater. 2019, 29, 1903496. [Google Scholar] [CrossRef]
- Jiang, F.; Zhang, L.M.; Zhao, W.Q.; Zhou, J.H.; Ge, P.; Wang, L.; Yang, Y.; Sun, W.; Chang, X.H.; Ji, X.B. Microstructured Sulfur-Doped Carbon-Coated Fe7S8 Composite for High-Performance Lithium and Sodium Storage. Acs Sustain. Chem. Eng. 2020, 8, 11783–11794. [Google Scholar] [CrossRef]
- Wan, H.; Ju, X.; He, T.; Chen, T.; Zhou, Y.M.; Zhang, C.; Wang, J.; Xu, Y.; Yao, B.; Zhuang, W.; et al. Sulfur-doped porous carbon as high-capacity anodes for lithium and sodium ions batteries. J. Alloys Compd. 2021, 863, 158078. [Google Scholar] [CrossRef]
- Ma, Z.; Zhuang, Y.C.; Deng, Y.M.; Song, X.N.; Zuo, X.X.; Xiao, X.; Nan, J.M. From Spent Graphite to Amorphous sp2 + sp3 Carbon-coated sp2 Graphite for High-performance Lithium-ion Batteries. J. Power Sources 2018, 376, 91–99. [Google Scholar] [CrossRef]
- Ma, C.L.; Zhao, Y.; Li, J.; Song, Y.; Shi, J.L.; Guo, Q.G.; Liu, L. Synthesis and Electrochemical Properties of Artificial Graphite as an Anode for High-performance Lithium-ion Batteries. Carbon 2013, 64, 553–556. [Google Scholar] [CrossRef]
- Xing, B.; Zhang, C.; Cao, Y.; Huang, G.; Liu, Q.; Zhang, C.; Chen, Z.; Yi, G.; Chen, L.; Yu, J. Preparation of Synthetic Graphite from Bituminous Coal as Anode Materials for High Performance Lithium-ion Batteries. Fuel Process. Technol. 2018, 172, 162–171. [Google Scholar] [CrossRef]
- Cuesta, N.; Cameán, I.; Ramos, A.; García, A.B. Graphitized biogas-derived carbon nanofibers as anodes for lithium-ion batteries. Electrochim. Acta 2016, 222, 264–270. [Google Scholar] [CrossRef]
- Chen, C.; Li, N.; Zhang, X.Y.; Zhang, C.H.; Qiu, J.S.; Yu, L. Interlayer-Expanded Titanate Hierarchical Hollow Spheres Embedded in Carbon Nanofibers for Enhanced Na Storage. Small 2022, 18, 2107890. [Google Scholar] [CrossRef] [PubMed]
- Yang, W.; Yang, W.; Zhang, F.; Wang, G.X.; Shao, G.J. Hierarchical Interconnected Expanded Graphitic Ribbons Embedded with Amorphous Carbon: An Advanced Carbon Nanostructure for Superior Lithium and Sodium Storage. Small 2018, 14, 1802221. [Google Scholar] [CrossRef]
- Zhang, X.S.; Tian, X.D.; Song, Y.; Wu, J.R.; Yang, T.; Liu, Z.J. High-performance activated carbon cathodes from green cokes for Zn-ion hybrid supercapacitors. Fuel 2022, 310, 122485. [Google Scholar] [CrossRef]
- Zhang, X.S.; Tian, X.D.; Song, Y.; Wu, J.R.; Yang, T.; Liu, Z.J. Boosting Zn-ion storage capacity of pitch coke-based activated carbon via pre-oxidation assisted KOH activation strategy. Microporous Mesoporous Mater. 2022, 333, 111721. [Google Scholar] [CrossRef]
- Yang, T.; Song, Y.; Tian, X.D.; Song, H.H.; Liu, Z.J. Pitch-Based Laminated Carbon Formed by Pressure Driving at Low Temperature as High-Capacity Anodes for Lithium Energy Storage Systems. Chem.-A Eur. J. 2020, 26, 16514–16520. [Google Scholar] [CrossRef]
- Zheng, J.; Yu, K.F.; Wang, X.F.; Liang, J.C.; Liang, C. Nitrogen self-doped porous carbon based on sunflower seed hulls as excellent double anodes for potassium/sodium ion batteries. Diam. Relat. Mater. 2023, 131, 109593. [Google Scholar] [CrossRef]
- Rath, P.C.; Patra, J.; Saikia, D.; Mishra, M.; Tseng, C.M.; Chang, J.K.; Kao, H.M. Comparative Study on the Morphology-Dependent Performance of Various CuO Nanostructures as Anode Materials for Sodium-Ion Batteries. ACS Sustain. Chem. Eng. 2018, 6, 10876–10885. [Google Scholar] [CrossRef]
- Zhang, Q.T.; Dai, Q.Q.; Yan, C.; Su, C.; Li, A. Nitrogen-doped porous carbon nanoparticle derived from nitrogen containing conjugated microporous polymer as high performance lithium battery anode. J. Alloys Compd. 2017, 714, 204–212. [Google Scholar] [CrossRef]
- Fu, S.W.; Yang, T.; Song, Y.; Tian, X.D.; Wang, C.; Ma, Z.H.; Wu, J.R.; Liu, Z.J. Effect of iodine treatment on structural and electrochemical performance of coal tar pitch based carbon materials for sodium ion batteries. Appl. Surf. Sci. 2024, 657, 159731. [Google Scholar] [CrossRef]



| C/% | H/% | O/% | N/% | S/% | SP/°C |
|---|---|---|---|---|---|
| 93.17 | 4.38 | 0.63 | 0.95 | 0.64 | 110.5 |
| Samples | Elemental Composition (%) | C/H [b] | ||||
|---|---|---|---|---|---|---|
| N | C | H | S | O [a] | ||
| PC | 1.00 | 93.48 | 4.12 | 0.58 | 0.82 | 1.89 |
| SPC | 0.89 | 80.59 | 2.61 | 15.30 | 0.61 | 2.57 |
| Samples | Capacity (mAh g−1) | Capacity Retention | Rate Capacity (mAh g−1) | Ref. |
|---|---|---|---|---|
| SPC-800 | 478.1 (0.1 A g−1) | 100% (100 cycles, 5 A g−1) | 150.5 (5 A g−1) | This work |
| CSC-0 | 311 (0.1 A g−1) | - | 146 (5 A g−1) | [31] |
| AC@G | 434.1 (0.1 C) | 96.8% (100 cycles, 6 C) | 115.3 (5 C) | [32] |
| NiP + G | 460 (20 mA g−1) | - | 220 (1 A g−1) | [33] |
| BCG | 310 (0.1 A g−1) | 95.3% (100 cycles, 0.2 C) | 153.5 (5 A g−1) | [34] |
| BCNF | 321 (0.1 C) | 77% (50 cycles, 0.1 C) | 50 (2 C) | [35] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ma, Z.; Wen, Z.; Song, Y.; Yang, T.; Tian, X.; Wu, J.; Liu, Y.; Liu, Z.; Wang, H. Effect of Sulfur Modification on Structural and Electrochemical Performance of Pitch-Based Carbon Materials for Lithium/Sodium Ion Batteries. Nanomaterials 2024, 14, 1410. https://doi.org/10.3390/nano14171410
Ma Z, Wen Z, Song Y, Yang T, Tian X, Wu J, Liu Y, Liu Z, Wang H. Effect of Sulfur Modification on Structural and Electrochemical Performance of Pitch-Based Carbon Materials for Lithium/Sodium Ion Batteries. Nanomaterials. 2024; 14(17):1410. https://doi.org/10.3390/nano14171410
Chicago/Turabian StyleMa, Zihui, Zhe Wen, Yan Song, Tao Yang, Xiaodong Tian, Jinru Wu, Yaxiong Liu, Zhanjun Liu, and Huiqi Wang. 2024. "Effect of Sulfur Modification on Structural and Electrochemical Performance of Pitch-Based Carbon Materials for Lithium/Sodium Ion Batteries" Nanomaterials 14, no. 17: 1410. https://doi.org/10.3390/nano14171410
APA StyleMa, Z., Wen, Z., Song, Y., Yang, T., Tian, X., Wu, J., Liu, Y., Liu, Z., & Wang, H. (2024). Effect of Sulfur Modification on Structural and Electrochemical Performance of Pitch-Based Carbon Materials for Lithium/Sodium Ion Batteries. Nanomaterials, 14(17), 1410. https://doi.org/10.3390/nano14171410







