Regeneration of Antifog Performance of Laser-Induced Copper-Based Micro-Nano Structured Surfaces by Rapid Thermal Treatment
Abstract
1. Introduction
2. Experiments
2.1. Materials and Methods
2.2. Characterization
3. Results and Discussion
4. Conclusions
Funding
Data Availability Statement
Conflicts of Interest
References
- Hashimoto, D.; Shouji, M. Development of a fogless scope and its analysis using infrared radiation pyrometer. Surg. Endosc. 1997, 11, 805–808. [Google Scholar] [CrossRef] [PubMed]
- Ohdaira, T.; Nagai, H.; Kayano, S.; Kazuhito, H. Antifogging effects of a socket-type device with the superhydrophilic, titanium dioxide–coated glass for the laparoscope. Surg. Endosc. 2007, 21, 333–338. [Google Scholar] [CrossRef] [PubMed]
- Zuo, Y.; Zheng, L.; Zhao, C.; Liu, H. Micro-/nanostructured interface for liquid manipulation and its applications. Small 2020, 16, 1903849. [Google Scholar] [CrossRef]
- You, I.; Yun, N.; Lee, H. Surface-Tension-Confined Microfluidics and Their Applications. ChemPhysChem 2013, 14, 471–481. [Google Scholar] [CrossRef]
- Feng, K.; Peng, L.; Yu, L.; Zheng, Y.; Chen, R.; Zhang, W.; Chen, G.J. Universal antifogging and antimicrobial thin coating based on dopamine-containing glycopolymers. ACS Appl. Mater. Interfaces 2020, 12, 27632–27639. [Google Scholar] [CrossRef] [PubMed]
- Duran, I.R.; Laroche, G. Current trends, challenges, and perspectives of anti-fogging technology: Surface and material design, fabrication strategies, and beyond. Prog. Mater. Sci. 2019, 99, 106–186. [Google Scholar] [CrossRef]
- Aroussi, A.; Hassan, A.; Morsi, Y. Numerical simulation of the airflow over and heat transfer through a vehicle windshield defrosting and demisting system. Appl. Therm. Eng. 2003, 39, 401–405. [Google Scholar] [CrossRef]
- Kim, H.-J.; Kim, Y.; Jeong, J.-H.; Choi, J.-H.; Lee, J.; Choi, D.-G. A cupronickel-based micromesh film for use as a high-performance and low-voltage transparent heater. J. Mater. Chem. A 2015, 3, 16621–16626. [Google Scholar] [CrossRef]
- Zhang, G.; Zou, H.; Qin, F.; Xue, Q.; Tian, C. Investigation on an improved heat pump AC system with the view of return air utilization and anti-fogging for electric vehicles. Appl. Therm. Eng. 2017, 115, 726–735. [Google Scholar] [CrossRef]
- Chen, Y.; Zhang, Y.; Shi, L.; Li, J.; Xin, Y.; Yang, T.; Guo, Z. Transparent superhydrophobic/superhydrophilic coatings for self-cleaning and anti-fogging. Appl. Phys. Lett. 2012, 101, 033701. [Google Scholar] [CrossRef]
- Long, J.; Zhou, P.; Huang, Y.; Xie, X. Enhancing the Long-Term Robustness of Dropwise Condensation on Nanostructured Superhydrophobic Surfaces by Introducing 3D Conical Microtextures Prepared by Femtosecond Laser. Adv. Mater. Interfaces 2020, 7, 2000997. [Google Scholar] [CrossRef]
- Mouterde, T.; Lehoucq, G.; Xavier, S.; Checco, A.; Black, C.T.; Rahman, A.; Midavaine, T.; Clanet, C.; Quéré, D. Antifogging abilities of model nanotextures. Nat. Mater. 2017, 16, 658–663. [Google Scholar] [CrossRef] [PubMed]
- Gao, X.; Yan, X.; Yao, X.; Xu, L.; Zhang, K.; Zhang, J.; Yang, B.; Jiang, L. The dry-style antifogging properties of mosquito compound eyes and artificial analogues prepared by soft lithography. Adv. Mater. 2007, 19, 2213–2217. [Google Scholar] [CrossRef]
- Kong, R.; Ren, J.; Mo, M.; Zhang, L.; Zhu, J. Multifunctional antifogging, self-cleaning, antibacterial, and self-healing coatings based on polyelectrolyte complexes. Colloid Surf. A 2023, 656, 130484. [Google Scholar] [CrossRef]
- Deng, W.; Su, Y.; Zhang, C.; Wang, W.; Xu, L.; Liu, P.; Wang, J.; Yu, X.; Zhang, Y. Transparent superhydrophilic composite coating with anti-fogging and self-cleaning properties. J. Colloid Interface Sci. 2023, 642, 255–263. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.-J.; Lee, Y.-T. Self-cleaning and antireflection properties of titanium oxide film by liquid phase deposition. Surf. Coat. Technol. 2013, 231, 257–260. [Google Scholar] [CrossRef]
- Huang, K.-T.; Yeh, S.-B.; Huang, C.-J. Surface modification for superhydrophilicity and underwater superoleophobicity: Applications in antifog, underwater self-cleaning, and oil–water separation. ACS Appl. Mater. Interfaces 2015, 7, 21021–21029. [Google Scholar] [CrossRef]
- Syafiq, A.; Balakrishnan, V.; Rahim, N.A. Durable self-cleaning nano-titanium dioxide superhydrophilic coating with anti-fog property. Pigment. Resin Technol. 2022, 53, 261–270. [Google Scholar] [CrossRef]
- Syafiq, A.; Rahim, N.A.; Balakrishnan, V.; Pandey, A.K. Development of self-cleaning polydimethylsiloxane/nano-calcium carbonate-titanium dioxide coating with fog-resistance response for building glass. Pigment. Resin Technol. 2024, 53, 249–260. [Google Scholar] [CrossRef]
- Tran, H.T.; Mai, D.H.; Thi, T.T.N.; Tran, T.M.; Nguyen, D.T.; Trinh, X.A.; Vu, D.H.; Dang, Q.K.; Nguyen, V.G.; Nguyen, D.C. Characterization of closed-surface antireflective TiO2–SiO2 films for application in solar-panel glass. Mater. Lett. 2022, 326, 132921. [Google Scholar] [CrossRef]
- Wang, S.; Meng, K.; Zhao, L.; Jiang, Q.; Lian, J.S. Superhydrophilic Cu-doped TiO2 thin film for solar-driven photocatalysis. Ceram. Int. 2014, 40, 5107–5110. [Google Scholar] [CrossRef]
- Yang, Y.; Sun, T.; Ma, F.; Huang, L.-F.; Zeng, Z. Superhydrophilic Fe3+ doped TiO2 films with long-lasting antifogging performance. ACS Appl. Mater. Interfaces 2021, 13, 3377–3386. [Google Scholar] [CrossRef] [PubMed]
- Yu, X.; Zhao, J.; Wu, C.; Li, B.; Sun, C.; Huang, S.; Tian, X. Highly durable antifogging coatings resistant to long-term airborne pollution and intensive UV irradiation. Mater. Des. 2020, 194, 108956. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, S.; Wu, S. Room-temperature fabrication of TiO2-PHEA nanocomposite coating with high transmittance and durable superhydrophilicity. Chem. Eng. J. 2019, 371, 609–617. [Google Scholar] [CrossRef]
- Huang, J.; Liu, Y.; Jin, S.; Wang, Z.; Qi, Y.; Zhang, J.; Wang, K.; Qiu, R. Uniformity control of laser-induced periodic surface structures. Front. Phys. 2022, 10, 932284. [Google Scholar] [CrossRef]
- Bondareva, N.E.; Sheremet, A.B.; Morgunova, E.Y.; Khisaeva, I.R.; Parfenova, A.S.; Chernukha, M.Y.; Omran, F.S.; Emelyanenko, A.M.; Boinovich, L.B. Study of the antibacterial activity of superhydrophilic and superhydrophobic copper substrates against multi-drug-resistant hospital-acquired Pseudomonas aeruginosa isolates. Int. J. Mol. Sci. 2024, 25, 779. [Google Scholar] [CrossRef]
- Chen, Z.; Wu, Z.; Zhang, G.; Li, D.; Han, F. Wettability regulation from superhydrophilic to superhydrophobic via nanosecond laser ablation. Sci. China Technol. Sci. 2024, 67, 1829–1841. [Google Scholar] [CrossRef]
- Gao, H.; Zhao, H.; Chang, S.; Meng, Z.; Han, Z.; Liu, Y. Multi-biomimetic Double Interlaced Wetting Janus Surface for Efficient Fog Collection. Nano Lett. 2024, 24, 7774–7782. [Google Scholar] [CrossRef]
- He, Y.; Yin, K.; Wang, L.; Wu, T.; Chen, Y.; Arnusch, C.J. Femtosecond laser structured black superhydrophobic cork for efficient solar-driven cleanup of crude oil. Appl. Phys. Lett. 2024, 124, 171601. [Google Scholar] [CrossRef]
- Ngo, C.-V.; Chun, D.-M. Fabrication of un-coated transparent superhydrophobic sapphire surface using laser surface ablation and heat treatment. CIRP Ann. 2018, 67, 571–574. [Google Scholar] [CrossRef]
- Vasiliev, M.M.; Shukhov, Y.G.; Rodionov, A.A.; Sulyaeva, V.S.; Markovich, D.M.; Starinskiy, S.V. Why do metals become superhydrophilic during nanosecond laser processing? Design of superhydrophilic, anisotropic and biphilic surfaces. Appl. Surf. Sci. 2024, 653, 159392. [Google Scholar] [CrossRef]
- Wang, R.; Ma, W.; Zhao, J.; Guo, Z.; Cui, Y.; Zhu, Y.; Chen, H. Nanosecond laser etching for enhanced multi-scale layered micro-nano superhydrophilic 304 stainless steel surface. Opt. Laser Technol. 2024, 174, 110645. [Google Scholar] [CrossRef]
- Weng, W.-X.; Deng, Q.-W.; Yang, P.-Y.; Yin, K. Femtosecond laser-chemical hybrid processing for achieving substrate-independent superhydrophobic surfaces. J. Cent. South Univ. 2024, 31, 1–10. [Google Scholar] [CrossRef]
- Wu, T.; Yin, K.; Pei, J.; He, Y.; Duan, J.-A.; Arnusch, C.J. Femtosecond laser-textured superhydrophilic coral-like structures spread AgNWs enable strong thermal camouflage and anti-counterfeiting. Appl. Phys. Lett. 2024, 124, 161602. [Google Scholar] [CrossRef]
- Yang, C.; Peng, Y.; Lv, J.; Guan, X.; You, H. Water collection and transportation on superhydrophilic/superhydrophobic bioinspired heterogeneous wettability surface. Colloids Surf. A Physicochem. Eng. Asp. 2024, 687, 133472. [Google Scholar] [CrossRef]
- Yang, P.; Yin, K.; Song, X.; Wang, L.; Deng, Q.; Pei, J.; He, Y.; Arnusch, C.J. Airflow triggered water film self-sculpturing on femtosecond laser-induced heterogeneously wetted micro/nanostructured surfaces. Nano Lett. 2024, 24, 3133–3141. [Google Scholar] [CrossRef]
- Vorobyev, A.; Guo, C. Water sprints uphill on glass. J. Appl. Phys. 2010, 108, 123512. [Google Scholar] [CrossRef]
- Wang, B.; Hua, Y.; Ye, Y.; Chen, R.; Li, Z. Transparent superhydrophobic solar glass prepared by fabricating groove-shaped arrays on the surface. Appl. Surf. Sci. 2017, 426, 957–964. [Google Scholar] [CrossRef]
- Yang, L.; Luo, X.; Chang, W.; Tian, Y.; Wang, Z.; Gao, J.; Cai, Y.; Qin, Y.; Duxbury, M. Manufacturing of anti-fogging super-hydrophilic microstructures on glass by nanosecond laser. J. Manuf. Process. 2020, 59, 557–565. [Google Scholar] [CrossRef]
- Skruibis, J.; Balachninaite, O.; Butkus, S.; Vaicaitis, V.; Sirutkaitis, V. Multiple-pulse Laser-induced breakdown spectroscopy for monitoring the femtosecond laser micromachining process of glass. Opt. Laser Technol. 2019, 111, 295–302. [Google Scholar] [CrossRef]
- Verma, N.; Anoop, K.; Dominic, P.; Philip, R. Fabrication of durable superhydrophilic silicon surfaces using nanosecond laser pulses. J. Appl. Phys. 2020, 128, 135304. [Google Scholar] [CrossRef]
- Yang, Z.; Liu, X.; Tian, Y. Insights into the wettability transition of nanosecond laser ablated surface under ambient air exposure. J. Colloid Interface Sci. 2019, 533, 268–277. [Google Scholar] [CrossRef]
- Chen, X.; Liu, C.; Mi, L.; Cui, H. Self-cleaning anti-fog micro-nanostructures by laser marker patterning nickel-coated glass. Surf. Eng. 2024, 40, 216–225. [Google Scholar] [CrossRef]
- Cui, H.; Teng, C.; Xie, X.; Qi, X. Durable anti-fog micro-nano structures fabricated by laser ablation of aluminum film on resin/glass. Discov. Nano 2024, 19, 58. [Google Scholar] [CrossRef] [PubMed]
- Cui, H.; Teng, C.; Zhao, Y.; Wang, P.; Guo, Y.; Qi, X. Durable self-cleaning antireflective and antifog Al micro-nano structure on glass by laser marker ablation. Opt. Mater. 2024, 147, 114675. [Google Scholar] [CrossRef]
- Guo, Y.; Qi, X.; Wang, P.; Teng, C.; Li, Y.; Mi, L.; Chen, X.; Cui, H. Durable self-cleaning anti-reflective and antifog micro-nanostructures fabricated by laser ablation of vanadium-coated glass surfaces. J. Appl. Phys. 2024, 135, 105301. [Google Scholar] [CrossRef]
- Okoye, P.; Azi, S.; Qahtan, T.; Owolabi, T.; Saleh, T.A. Synthesis, properties, and applications of doped and undoped CuO and Cu2O nanomaterials. Mater. Today Chem. 2023, 30, 101513. [Google Scholar] [CrossRef]
- Tsvetkov, P.; Coy, S.; Petrova, B.; Dreishpoon, M.; Verma, A.; Abdusamad, M.; Rossen, J.; Joesch-Cohen, L.; Humeidi, R.; Spangler, R.D.; et al. Copper induces cell death by targeting lipoylated TCA cycle proteins. Science 2022, 375, 1254–1261. [Google Scholar] [CrossRef]
- Mehmood, A.; Bibi, K.; Ali, F.; Nazir, A.; Sandhu, Z.A.; Raza, M.A.; Bhalli, A.H.; Ashraf, A.; Aslam, M.; Al-Sehemi, A.G. Innovative approach to synthesize Mo-Doped CuO Nanostructures: Uncovering structural and photocatalytic insights. J. Mol. Liq. 2024, 395, 123768. [Google Scholar] [CrossRef]
- Shin, J.H.; Park, J.H.; Seo, J.; Im, T.H.; Kim, J.C.; Lee, H.E.; Kim, D.H.; Woo, K.Y.; Jeong, H.Y.; Cho, Y.H.; et al. A Flash-Induced Robust Cu Electrode on Glass Substrates and Its Application for Thin-Film Mleds. Adv. Mater. 2021, 33, 2007186. [Google Scholar] [CrossRef]
- Park, J.H.; Seo, J.; Kim, C.; Joe, D.J.; Lee, H.E.; Im, T.H.; Seok, J.Y.; Jeong, C.K.; Ma, B.S.; Park, H.K.; et al. Flash-Induced Stretchable Cu Conductor Via Multiscale-Interfacial Couplings. Adv. Sci. 2018, 5, 1801146. [Google Scholar] [CrossRef] [PubMed]
- Lassnig, A.; Terziyska, V.L.; Zalesak, J.; Jörg, T.; Toebbens, D.M.; Griesser, T.; Mitterer, C.; Pippan, R.; Cordill, M.J. Microstructural Effects on the Interfacial Adhesion of Nanometer-Thick Cu Films on Glass Substrates: Implications for Microelectronic Devices. ACS Appl. Nano Mater. 2020, 4, 61–70. [Google Scholar] [CrossRef] [PubMed]
- Long, J.; Xi, M.; Yang, P.; Huang, Z. Mechanisms of metal wettability transition and fabrication of durable superwetting/superhydrophilic metal surfaces. Appl. Surf. Sci. 2024, 654, 159497. [Google Scholar] [CrossRef]
- Long, J.; Zhong, M.; Fan, P.; Gong, D.; Zhang, H. Wettability conversion of ultrafast laser structured copper surface. J. Laser Appl. 2015, 27, S29107. [Google Scholar] [CrossRef]
- Long, J.; Zhong, M.; Zhang, H.; Fan, P. Superhydrophilicity to superhydrophobicity transition of picosecond laser microstructured aluminum in ambient air. J. Colloid Interface Sci. 2015, 441, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Jiang, Y.; Jiang, Z.; Li, Y.; Wen, C.; Lian, J. Reversible wettability transition between superhydrophilicity and superhydrophobicity through alternate heating-reheating cycle on laser-ablated brass surface. Appl. Surf. Sci. 2019, 492, 349–361. [Google Scholar] [CrossRef]
- Young, T. An Essay on the Cohesion of Fluids. Proc. R. Soc. Lond. 1832, 1, 171–172. [Google Scholar]
- Cui, M.; Huang, H.; Wu, H.; Zhang, L.; Yan, J. Achieving superhydrophobicity of Zr-based metallic glass surface with anti-corrosion and anti-icing properties by nanosecond laser ablation and subsequent heat treatment. Surf. Coat. Technol. 2023, 475, 130159. [Google Scholar] [CrossRef]
- Sharma, A.; Song, J.; Furfari, D.; Mannava, S.R.; Vasudevan, V.K. Remarkable near-surface microstructure of nanoparticles and oxide film in laser shock peened Al-Zn-Mg-Cu alloy. Scr. Mater. 2021, 202, 114012. [Google Scholar] [CrossRef]
- Wenzel, R.N. Resistance of solid surfaces to wetting by water. Ind. Eng. Chem. 1936, 28, 988–994. [Google Scholar] [CrossRef]
- Hass, K.C.; Schneider, W.F.; Curioni, A.; Andreoni, W. The chemistry of water on alumina surfaces: Reaction dynamics from first principles. Science 1998, 282, 265–268. [Google Scholar] [CrossRef] [PubMed]
- Siddique, R.H.; Gomard, G.; Hölscher, H. The role of random nanostructures for the omnidirectional anti-reflection properties of the glasswing butterfly. Nat. Commun. 2015, 6, 6909. [Google Scholar] [CrossRef] [PubMed]
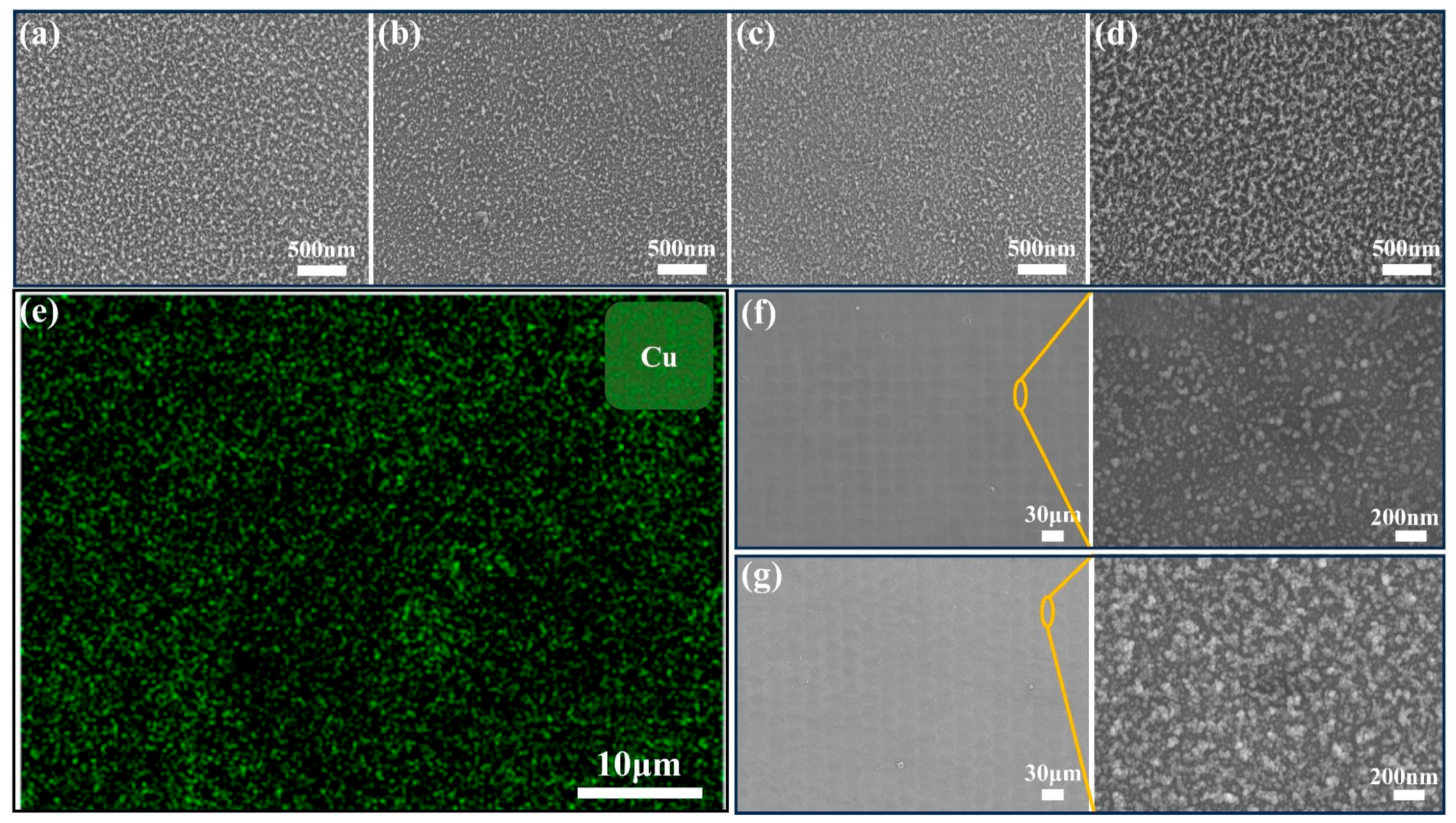
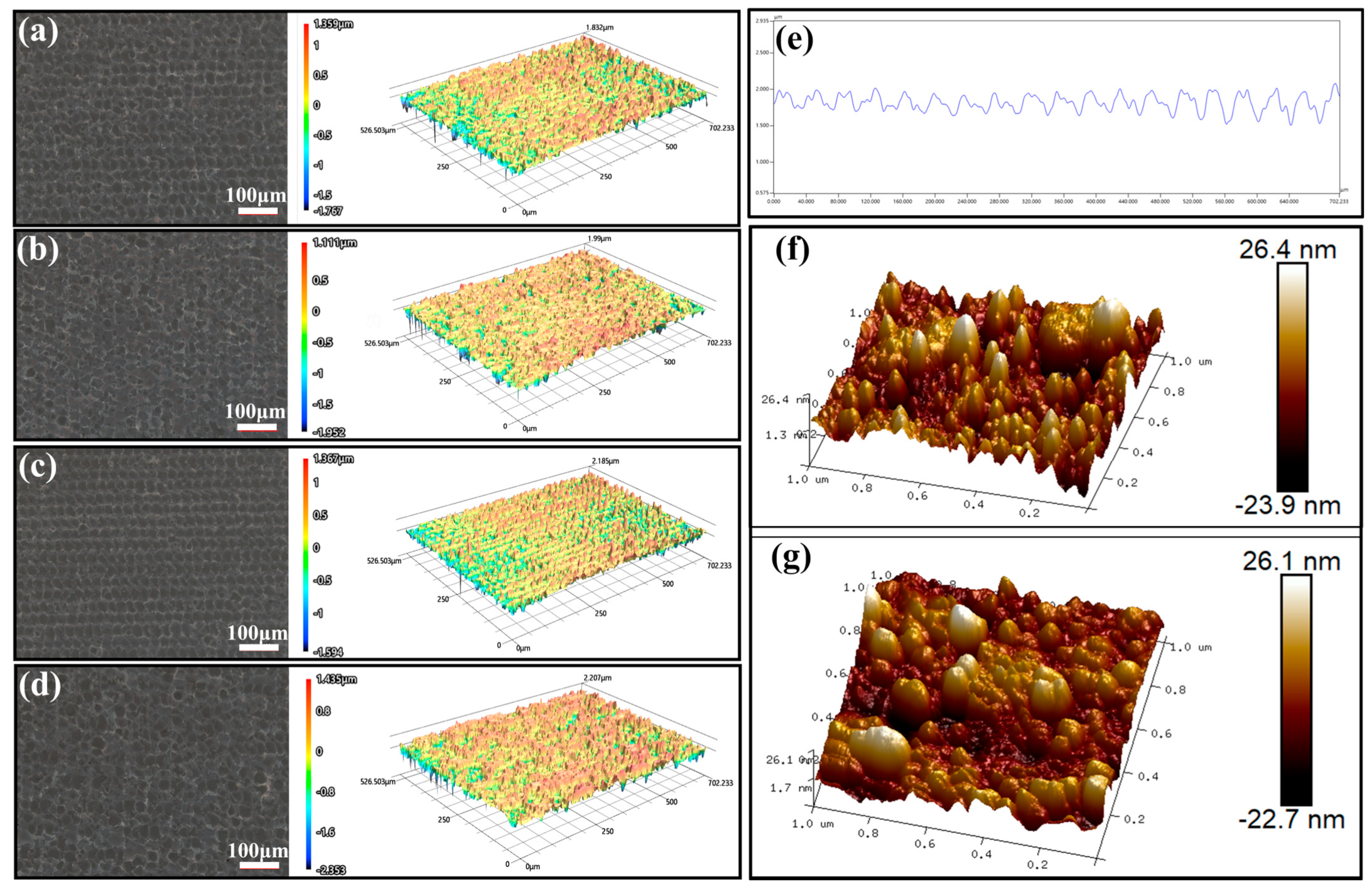
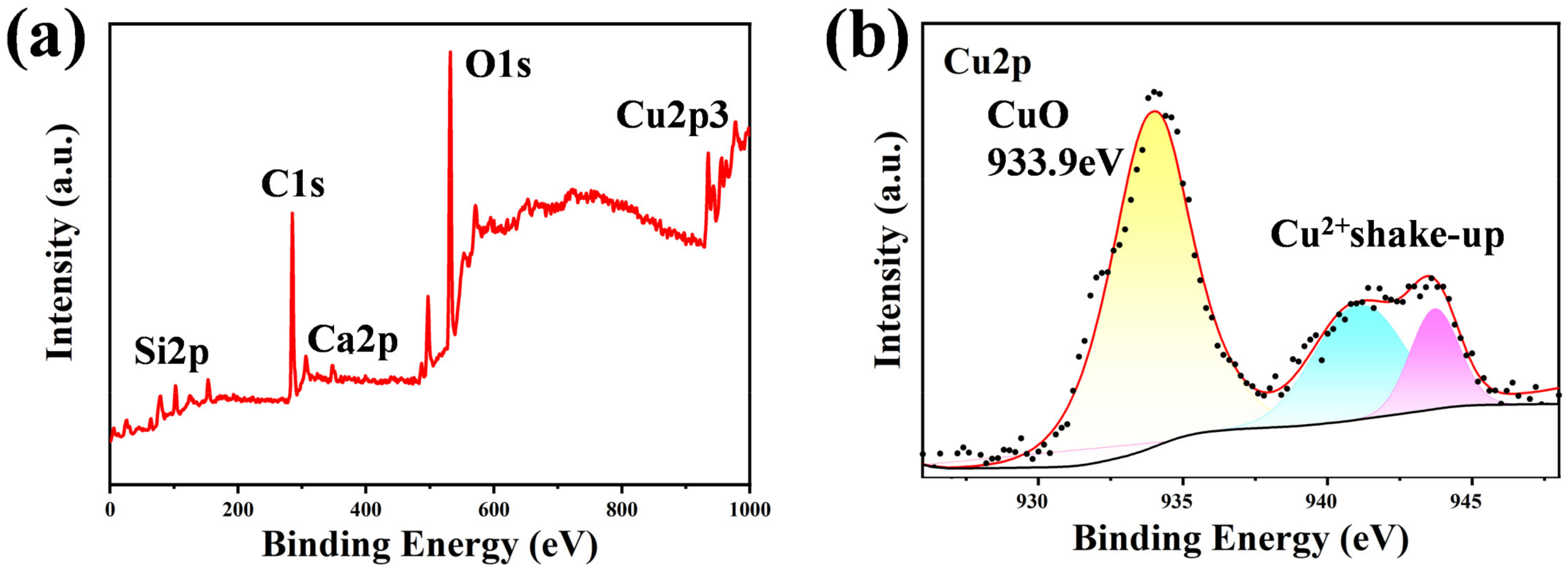

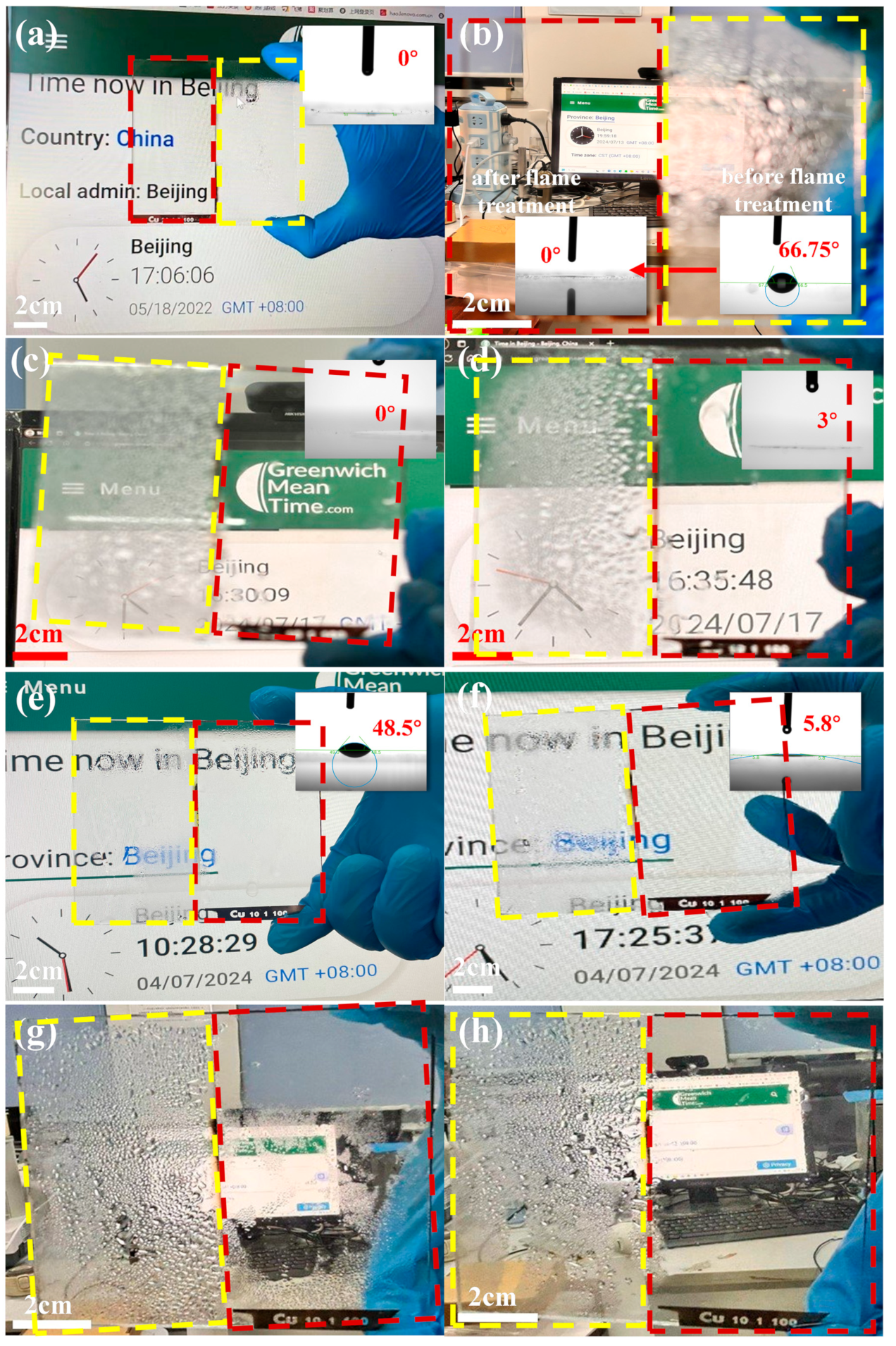
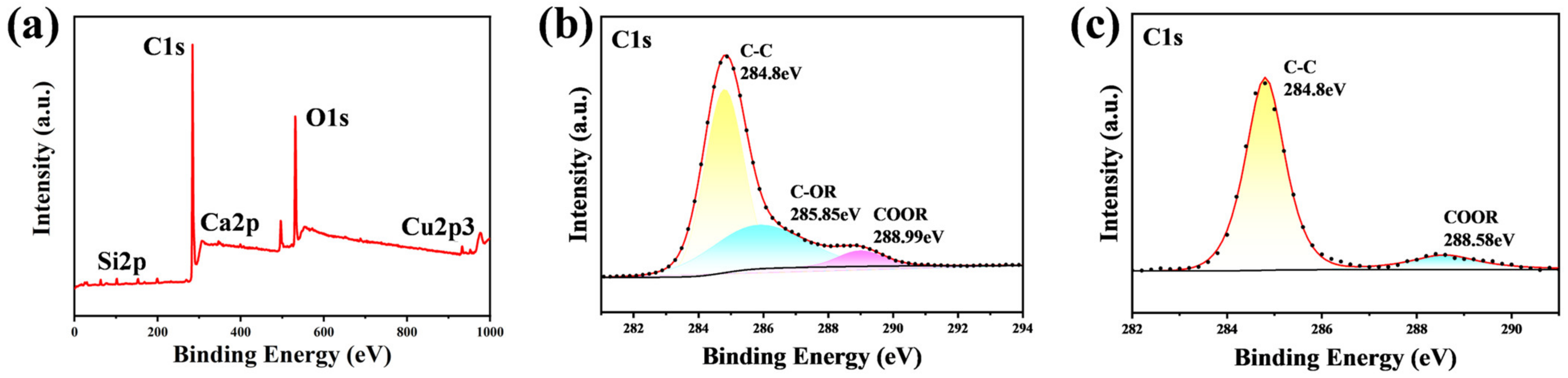
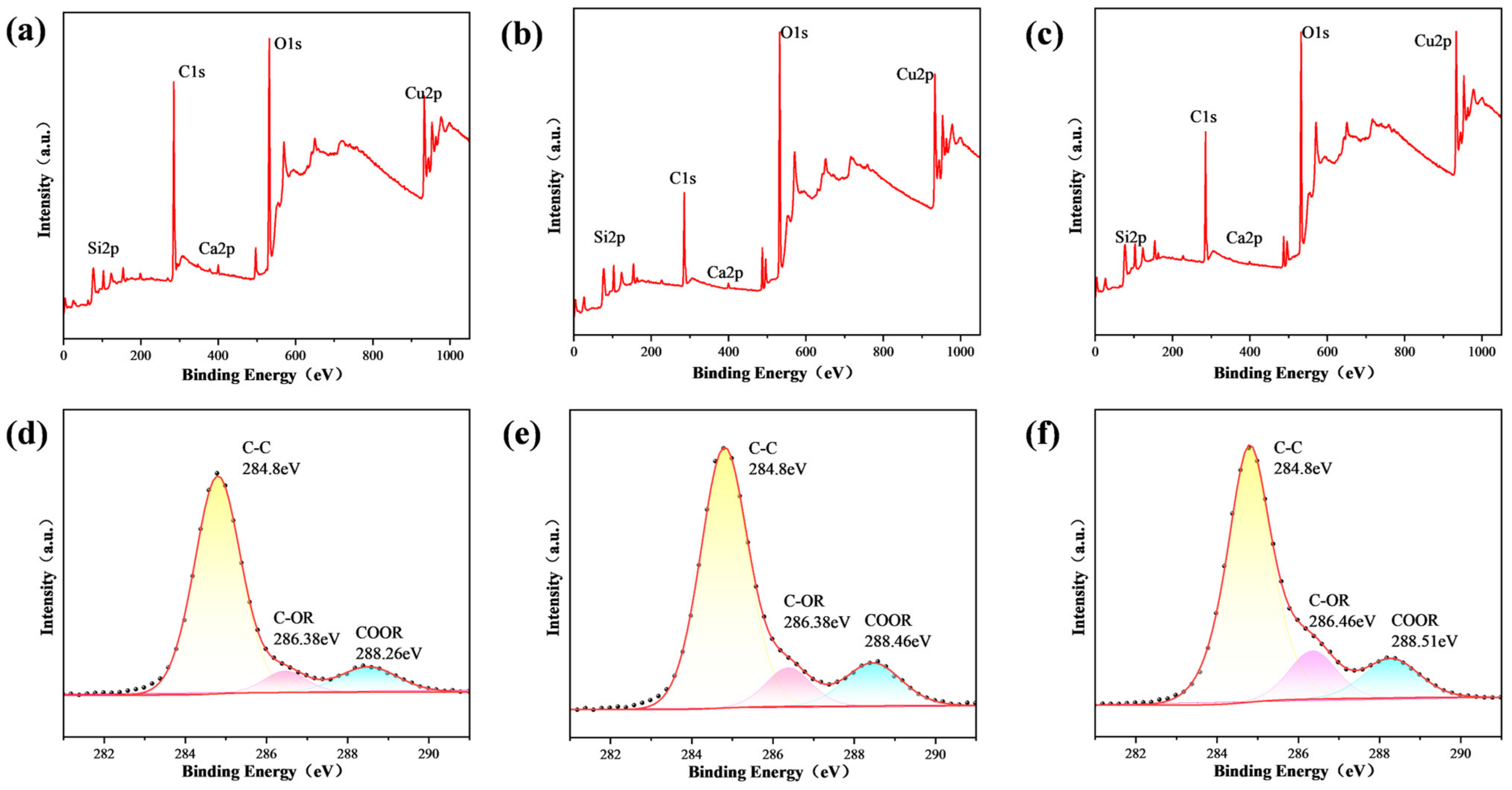
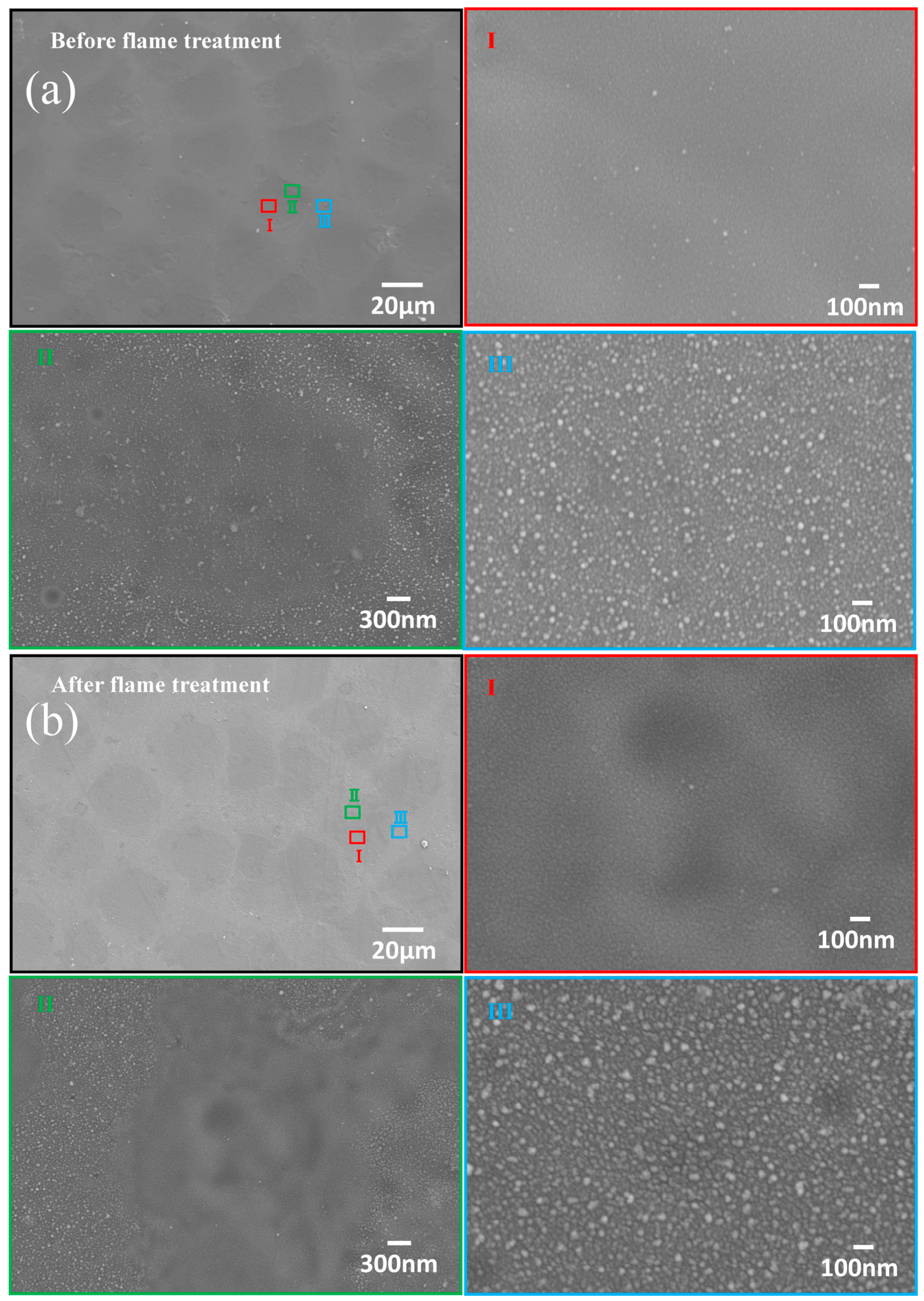
| Sample Name | 100 nm-Cu | 200 nm-Cu | 400 nm-Cu | 600 nm-Cu |
|---|---|---|---|---|
| Surface roughness (μm) | 0.472 | 0.447 | 0.437 | 0.604 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, H.; Xie, X.; Qi, X.; Liu, C.; Wang, C.; Fang, X.; Wang, Y.; Cui, H.; Dong, J. Regeneration of Antifog Performance of Laser-Induced Copper-Based Micro-Nano Structured Surfaces by Rapid Thermal Treatment. Nanomaterials 2024, 14, 1415. https://doi.org/10.3390/nano14171415
Zhang H, Xie X, Qi X, Liu C, Wang C, Fang X, Wang Y, Cui H, Dong J. Regeneration of Antifog Performance of Laser-Induced Copper-Based Micro-Nano Structured Surfaces by Rapid Thermal Treatment. Nanomaterials. 2024; 14(17):1415. https://doi.org/10.3390/nano14171415
Chicago/Turabian StyleZhang, Huixing, Xinyi Xie, Xiaowen Qi, Chengling Liu, Chenrui Wang, Xiaolong Fang, Youfu Wang, Hongtao Cui, and Ji Dong. 2024. "Regeneration of Antifog Performance of Laser-Induced Copper-Based Micro-Nano Structured Surfaces by Rapid Thermal Treatment" Nanomaterials 14, no. 17: 1415. https://doi.org/10.3390/nano14171415
APA StyleZhang, H., Xie, X., Qi, X., Liu, C., Wang, C., Fang, X., Wang, Y., Cui, H., & Dong, J. (2024). Regeneration of Antifog Performance of Laser-Induced Copper-Based Micro-Nano Structured Surfaces by Rapid Thermal Treatment. Nanomaterials, 14(17), 1415. https://doi.org/10.3390/nano14171415






