Development and Application of Surface-Enhanced Raman Scattering (SERS)
Abstract
:1. Introduction of Raman Scattering Spectroscopy
2. Development of SERS Technology
3. Theoretical Mechanisms for the Emergence of the SERS Phenomenon
- (1)
- Relating to the material of the SERS substrate: the SERS substrates refer to those materials that could provide the plasma resonance to produce the SERS effect. These materials generally refer to metallic materials such as gold, silver, copper, and aluminum, as well as other materials that are being investigated, such as electrolytes and semiconductor materials.
- (2)
- Related to “hot spots”: the electromagnetic enhancement on the surface of the SERS substrate is usually not uniform. Typically, the enhancement mainly occurs in very small and narrow areas, i.e., “hot spots”. From the physical structure, the signal enhancement is more efficient at the sharp tips and the gaps between the nanoparticles.
- (3)
- Distance-dependent: as the distance between the nanoparticles and the surface increases, the Raman signal intensity decreases very rapidly since the probe molecules need to be kept within 10 nm of the substrate surface for better enhancement.
3.1. Electromagnetic Enhancement Mechanism
3.2. Chemical Enhancement Mechanism
- (1)
- Resonance enhancement of the probe molecule;
- (2)
- Charge transfer resonance enhancement between the probe molecule and the substrate;
- (3)
- Non-resonance alteration enhancement, which refers to the static chemical enhancement of the molecular polarization rate due to interaction.
3.3. Theoretical Modeling of SERS Enhancement Factors
3.4. Evaluation of SERS Enhancement Factor
4. Application of SERS Technology
4.1. Food Additives and Pesticide Residues
4.2. Biomedical Applications
4.3. Reaction Process Monitoring, Sensing, and Imaging
4.4. Archaeology and Art
5. Conclusions and Prospects
Funding
Conflicts of Interest
References
- Smekal, A. Zur quantentheorie der dispersion. Naturwissenschaften 1923, 11, 873–875. [Google Scholar] [CrossRef]
- Raman, C.V.; Krishnan, K.S. A new type of secondary radiation. Nature 1928, 121, 501–502. [Google Scholar] [CrossRef]
- Settle, F. Handbook of instrumental techniques for analytical chemistry. Electr. Insul. Mag. IEEE 1997, 14, 42. [Google Scholar]
- John, M.C.; Howell, G.M.E.; Michael, D.H. Infrared and Raman Spectroscopy in Forensic Science; John Wiley & Sons: Hoboken, NJ, USA, 2012. [Google Scholar]
- Willard, H.H.; Merritt, L.L., Jr.; Dean, J.A.; Settle, F.A., Jr. Instrumental Methods of Analysis; John Wiley & Sons: Hoboken, NJ, USA, 1988. [Google Scholar]
- Ewen, S.; Geoffrey, D. Modern Raman Spectroscopy: A Practical Approach; John Wiley & Sons: Hoboken, NJ, USA, 2004. [Google Scholar]
- Baena, J.R.; Lendl, B. Raman spectroscopy in chemical bioanalysis. Curr. Opin. Chem. Biol. 2004, 8, 534–539. [Google Scholar] [CrossRef] [PubMed]
- Albraheem, L.; Al-Khalifa, H.S. Raman Spectroscopy for Chemical Analysis; John Wiley & Sons: Hoboken, NJ, USA, 2000. [Google Scholar]
- Sloane, H.J. The technique of Raman spectroscopy: A state-of-the-art comparison to infrared. Appl. Spectrosc. 1971, 25, 430–439. [Google Scholar] [CrossRef]
- Fleischmann, M.; Hendra, P.J.; McQuillan, A.J. Raman spectra of pyridine adsorbed at a silver electrode. Chem. Phys. Lett. 1974, 26, 163–166. [Google Scholar] [CrossRef]
- Jeanmaire, D.L.; Van Duyne, R.P. Surface Raman spectroelectrochemistry: Part I. Heterocyclic, aromatic, and aliphatic amines adsorbed on the anodized silver electrode. J. Electroanal. Chem. 1977, 84, 1–20. [Google Scholar] [CrossRef]
- Albrecht, M.G.; Creighton, J.A. Anomalously intense Raman spectra of pyridine at a silver electrode. J. Am. Chem. Soc. 1977, 99, 5215–5217. [Google Scholar] [CrossRef]
- Stiles, P.L.; Dieringer, J.A.; Shah, N.C.; Van Duyne, R.P. Surface-enhanced Raman spectroscopy. Annu. Rev. Anal. Chem. 2008, 1, 601–626. [Google Scholar] [CrossRef]
- Camden, J.P.; Dieringer, J.A.; Wang, Y.; Masiello, D.J.; Marks, L.D.; Schatz, G.C.; Van Duyne, R.P. Probing the structure of single-molecule surface-enhanced Raman scattering hot spots. J. Am. Chem. Soc. 2008, 130, 12616–12617. [Google Scholar] [CrossRef]
- Tong, L.; Zhu, T.; Liu, Z. Approaching the electromagnetic mechanism of surface-enhanced Raman scattering: From self-assembled arrays to individual gold nanoparticles. Chem. Soc. Rev. 2011, 40, 1296–1304. [Google Scholar] [CrossRef]
- Knoll, W. Interfaces and thin films as seen by bound electromagnetic waves. Annu. Rev. Phys. Chem. 1998, 49, 569–638. [Google Scholar] [CrossRef] [PubMed]
- Moskovits, M. Surface-enhanced Raman spectroscopy: A brief retrospective. J. Raman Spectrosc. 2010, 36, 485–496. [Google Scholar] [CrossRef]
- Guillot, N.; Chapelle, M.L. The electromagnetic effect in surface enhanced Raman scattering: Enhancement optimization using precisely controlled nanostructures. J. Quant. Spectrosc. Radiat. Transf. 2012, 113, 51–63. [Google Scholar] [CrossRef]
- Moskovits, M.; DiLella, D.P.; Maynard, K.J. Surface Raman spectroscopy of a number of cyclic aromatic molecules adsorbed on silver: Selection rules and molecular reorientation. Langmuir 1988, 4, 67–76. [Google Scholar] [CrossRef]
- Nitzan, A.; Brus, L.E. Theoretical model for enhanced photochemistry on rough surfaces. J. Chem. Phys. 1981, 75, 2205–2214. [Google Scholar] [CrossRef]
- Ding, S.-Y.; Yi, J.; Li, J.-F.; Ren, B.; Wu, D.-Y.; Panneerselvam, R.; Tian, Z.-Q. Nanostructure-based plasmon-enhanced Raman spectroscopy for surface analysis of materials. Nat. Rev. Mater. 2016, 1, 16021. [Google Scholar] [CrossRef]
- Yu, H.; Peng, Y.; Yang, Y.; Li, Z.-Y. Plasmon-enhanced light-matter interactions and applications. NPJ Comput. Mater. 2019, 5, 45. [Google Scholar] [CrossRef]
- Kruszewskh, S.; Skonieczny, J. Roughness effects in surface enhanced Raman scattering-evidence for electromagnetic and charge transfer enhancement mechanism. Acta Phys. Pol. A 1991, 80, 611–620. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, J.; Jia, H.; Li, M.; Zeng, J.; Yang, B.; Zhao, B.; Xu, W.; Lombardi, J.R. Mercaptopyridine surface-functionalized CdTe quantum dots with enhanced Raman scattering properties. J. Phys. Chem. C 2008, 112, 996–1000. [Google Scholar] [CrossRef]
- Zeman, E.J.; Schatz, G.C. An accurate electromagnetic theory study of surface enhancement factors for silver, gold, copper, lithium, sodium, aluminum, gallium, indium, zinc, and cadmium. J. Phys. Chem. 1987, 91, 634–643. [Google Scholar] [CrossRef]
- Polavarapu, L.; Liz-Marzán, L.M. Growth and galvanic replacement of silver nanocubes in organic media. Nanoscale 2013, 5, 4355–4361. [Google Scholar] [CrossRef] [PubMed]
- Shiohara, A.; Wang, Y.S.; Liz-Marzan, L.M. Recent approaches toward creation of hot spots for SERS detection. J. Photochem. Photobiol. C Photochem. Rev. 2014, 21, 2–25. [Google Scholar] [CrossRef]
- Yang, Y.; Li, Z.-Y.; Yamaguchi, K.; Tanemura, M.; Huang, Z.; Jiang, D.; Chen, Y.; Zhou, F.; Nogami, M. Controlled fabrication of silver nanoneedles array for SERS and their application in rapid detection of narcotics. Nanoscale 2012, 4, 2663–2669. [Google Scholar] [CrossRef] [PubMed]
- Le Ru, E.C.; Grand, J.; Sow, I.; Somerville, W.R.; Etchegoin, P.G.; Treguer-Delapierre, M.; Charron, G.; Felidj, N.; Levi, G.; Aubard, J. A scheme for detecting every single target molecule with surface-enhanced Raman spectroscopy. Nano Lett. 2011, 11, 5013–5021. [Google Scholar] [CrossRef]
- Hakonen, A.; Svedendahl, M.; Ogier, R.; Yang, Z.-J. Dimer-on-mirror SERS substrates with attogram sensitivity fabricated by colloidal lithography. Nanoscale 2015, 7, 9405–9410. [Google Scholar] [CrossRef]
- Brus, L. Noble metal nanocrystals: Plasmon electron transfer photochemistry and singlemolecule Raman spectroscopy. Acc. Chem. Res. 2008, 41, 1742–1749. [Google Scholar] [CrossRef]
- Roy, S.; Muhammed, A.C.; Baik, S.; Kim, J. Silver nanoflowers for single-particle SERS with 10 pm sensitivity. Nanotechnology 2017, 28, 465705. [Google Scholar] [CrossRef]
- Shegai, T.; Vaskevich, A.; Rubinstein, I.; Haran, G. Raman spectroelectrochemistry of molecules within individual electromagnetic hot spots. J. Am. Chem. Soc. 2009, 131, 14390–14398. [Google Scholar] [CrossRef]
- Kleinman, S.L.; Frontiera, R.R.; Henry, A.I.; Dieringer, J.A.; Van Duyne, R.P. Creating, characterizing, and controlling chemistry with SERS hot spots. Phys. Chem. Chem. Phys. 2013, 15, 21–36. [Google Scholar] [CrossRef]
- Metiu, H. Surface enhanced spectroscopy. Prog. Surf. Sci. 1984, 17, 153–320. [Google Scholar] [CrossRef]
- Campion, A.; Kambhampati, P. Surface-enhanced Raman scattering. Chem. Soc. Rev. 1998, 27, 241–250. [Google Scholar] [CrossRef]
- Valley, N.; Greeneltch, N.; Van Duyne, R.P.; Schatz, G.C. A look at the origin and magnitude of the chemical contribution to the enhancement mechanism of surface-enhanced Raman spectroscopy (SERS): Theory and experiment. J. Phys. Chem. Lett. 2013, 4, 2599–2604. [Google Scholar] [CrossRef]
- Saikin, S.K.; Olivares-Amaya, R.; Rappoport, D.; Stopa, M.; Aspuru-Guzik, A. On the chemical bonding effects in the Raman response: Benzenethiol adsorbed on silver clusters. Phys. Chem. Chem. Phys. 2009, 11, 9401–9411. [Google Scholar] [CrossRef] [PubMed]
- Kneipp, K.; Kneipp, H.; Itzkan, I.; Dasari, R.R.; Feld, M.S. Ultrasensitive chemical analysis by Raman spectroscopy. Chem. Rev. 1999, 99, 2957–2976. [Google Scholar] [CrossRef]
- Dresselhaus, M.S.; Avouris, P. Introduction to carbon materials research. In Topics in Applied Physics; Springer: Berlin/Heidelberg, Germany, 2001; Volume 80, pp. 1–9. [Google Scholar]
- Cronin, S.B.; Swan, A.K.; Unlu, M.S.; Goldberg, B.B.; Dresselhaus, M.S.; Tinkham, M. Measuring the uniaxial strain of individual single-wall carbon nanotubes: Resonance Raman spectra of atomic-force-microscope modified single-wall nanotubes. Phys. Rev. Lett. 2004, 93, 167401. [Google Scholar] [CrossRef]
- Otto, A. The ‘chemical’ (electronic) contribution to surface-enhanced Raman scattering. J. Raman Spectrosc. 2005, 36, 497–509. [Google Scholar] [CrossRef]
- Campion, A.; Ivanecky, J.E., III; Child, C.M.; Foster, M. On the mechanism of chemical enhancement in surface-enhanced Raman scattering. J. Am. Chem. Soc. 1995, 117, 11807–11808. [Google Scholar] [CrossRef]
- Zhao, L.L.; Jensen, L.; Schatz George, C. Surface-enhanced Raman scattering of pyrazine at the junction between two Ag20 nanoclusters. Nano Lett. 2006, 6, 1229–1234. [Google Scholar] [CrossRef]
- Yee, K. Numerical solution of initial boundary value problems involving maxwell’s equations in isotropic media. IEEE Trans. Antennas 1966, 14, 302–307. [Google Scholar]
- Kunz, K.S.; Luebbers, R.J. The Finite Difference Time Domain Method for Electromagnetics; CRC Press: Boca Raton, FL, USA, 1993. [Google Scholar]
- Krug, J.T.; Sanchez, E.J.; Xie, X.S. Design of near-field optical probes with optimal field enhancement by finite difference time domain electromagnetic simulation. J. Chem. Phys. 2002, 116, 10895–10901. [Google Scholar] [CrossRef]
- Sullivan, D.M. Electromagnetic Simulation Using the FDTD Method; John Wiley & Sons: Hoboken, NJ, USA, 2013. [Google Scholar]
- Futamata, M.; Maruyama, Y.; Ishikawa, M. Local electric field and scattering cross section of ag nanoparticles under surface plasmon resonance by finite difference time domain method. J. Phys. Chem. B 2003, 107, 7607–7617. [Google Scholar] [CrossRef]
- Futamata, M.; Maruyama, Y.; Ishikawa, M. Microscopic morphology and SERS activity of ag colloidal particles. Vib. Spectrosc. 2002, 30, 17–23. [Google Scholar] [CrossRef]
- Liu, Q.H. The PSTD algorithm: A time-domain method requiring only two cells per wavelength. Microw. Opt. Technol. Lett. 1997, 15, 158–165. [Google Scholar] [CrossRef]
- Liu, Q.H. PML and PSTD algorithm for arbitrary lossy anisotropic media. IEEE Microw. Guid. Wave Lett. 1999, 9, 48–50. [Google Scholar]
- Mishchenko, M. Light scattering by randomly oriented axially symmetric particles. J. Opt. Soc. Am. A 1991, 8, 871–882. [Google Scholar] [CrossRef]
- Mishchenko, M.I.; Travis, L.D.; Mackowski, D.W. T-matrix computations of light scattering by nonspherical particles: A review. J. Quant. Spectrosc. Radiat. Transf. 1996, 55, 535–575. [Google Scholar] [CrossRef]
- Kottmann, J.P.; Martin, O.J.; Smith, D.R.; Schultz, S. Plasmon resonances of silver nanowires with a nonregular cross section. Phys. Rev. B 2001, 64, 235402. [Google Scholar] [CrossRef]
- Micic, M.; Klymyshyn, N.; Suh, Y.D.; Lu, H.P. Finite element method simulation of the field distribution for AFM tip-enhanced surface-enhanced Raman scanning microscopy. J. Phys. Chem. B 2003, 107, 1574–1584. [Google Scholar] [CrossRef]
- Kottmann, J.P.; Martin, O.J.F.; Smith, D.R.; Schultz, S. Dramatic localized electromagnetic enhancement in plasmon resonant nanowires. Chem. Phys. Lett. 2001, 341, 1–6. [Google Scholar] [CrossRef]
- Klein, S.; Geshev, P.; Witting, T.; Dickmann, K.; Hietschold, M. Enhanced Raman scattering in the near field of a scanning tunneling tip—An approach to single molecule Raman spectroscopy. Electrochemistry 2003, 71, 114–116. [Google Scholar] [CrossRef]
- Demming, F.; Jersch, J.; Dickmann, K.; Geshev, P.I. Calculation of the field enhancement on laser-illuminated scanning probe tips by the boundary element method. Appl. Phys. B Lasers Opt. 1998, 66, 593–598. [Google Scholar] [CrossRef]
- Hao, E.; Schatz, G.C. Electromagnetic fields around silver nanoparticles and dimers. J. Chem. Phys. 2004, 120, 357–366. [Google Scholar] [CrossRef]
- Yang, W.H.; Schatz, G.C.; Van Duyne, R.P. Discrete dipole approximation for calculating extinction and Raman intensities for small particles with arbitrary shapes. J. Chem. Phys. 1995, 103, 869–875. [Google Scholar] [CrossRef]
- Futamata, M.; Maruyama, Y.; Ishikawa, M. Critical importance of the junction in touching ag particles for single molecule sensitivity in SERS. J. Mol. Struct. 2005, 735, 75–84. [Google Scholar] [CrossRef]
- Oubre, C.; Nordlander, P. Finite-difference time-domain studies of the optical properties of nanoshell dimers. J. Phys. Chem. B 2005, 109, 10042–10051. [Google Scholar] [CrossRef]
- Futamata, M.; Maruyama, Y.; Ishikawa, M. Metal nanostructures with single molecule sensitivity in surface enhanced Raman scattering. Vib. Spectrosc. 2004, 35, 121–129. [Google Scholar] [CrossRef]
- Lin, X.M.; Cui, Y.; Xu, Y.H.; Ren, B.; Tian, Z.Q. Surface-enhanced Raman spectroscopy: Substrate-related issues. Anal. Bioanal. Chem. 2009, 394, 1729–1745. [Google Scholar] [CrossRef]
- Leru, E.C.; Blackie, E.; Meyer, M.; Etchegoin, P.G. Surface enhanced Raman scattering enhancement factors: A comprehensive study. J. Phys. Chem. C 2007, 111, 13794–13803. [Google Scholar] [CrossRef]
- Li, W.; Guo, L.; Ding, X.L.; Ding, Y.; Ji, L.N.; Xia, X.H.; Wang, K. High-Throughput Single-Molecule Surface-Enhanced Raman Spectroscopic Profiling of Single-Amino Acid Substitutions in Peptides by a Gold Plasmonic Nanopore. ACS Nano 2024, 18, 19200–19207. [Google Scholar] [CrossRef]
- Li, X.; Ye, S.; Luo, X. Sensitive SERS detection of miRNA via enzyme-free DNA machine signal amplification. Chem. Commun. 2016, 52, 10269–10272. [Google Scholar] [CrossRef] [PubMed]
- Schlacker, P. Surface-enhanced Raman spectroscopy: Concepts and chemical applications. Angew. Chem. Int. Ed. 2014, 53, 4756–4795. [Google Scholar] [CrossRef]
- Huang, Z.C.; Zhang, A.M.; Zhang, Q.; Cui, D.X. Nanomaterial-based SERS sensing technology for biomedical application. J. Mater. Chem. B 2019, 7, 3755–3774. [Google Scholar] [CrossRef]
- Li, Z.; Jiang, S.; Huo, Y.; Ning, T.; Liu, A.; Zhang, C.; He, Y.; Wang, M.; Li, C.; Man, B. 3D silver nanoparticles with multilayer graphene oxide as a spacer for surface enhanced Raman spectroscopy analysis. Nanoscale 2018, 10, 5897–5905. [Google Scholar] [CrossRef] [PubMed]
- Liang, X.; Wang, Y.S.; You, T.T.; Zhang, X.-J.; Yang, N.; Wang, G.-S.; Yin, P.-G. Interfacial synthesis of a three-dimensional hierarchical MoS2-NS@Ag-NP nanocomposite as a SERS nanosensor for ultrasensitive thiram detection. Nanoscale 2017, 9, 8879–8888. [Google Scholar] [CrossRef]
- Chen, S.; Liu, D.; Wang, Z.; Sun, X.; Cui, D.; Chen, X. Picomolar detection of mercuric ions by means of gold-silver core-shell nanorods. Nanoscale 2013, 5, 6731–6735. [Google Scholar] [CrossRef]
- Zhong, L.B.; Yin, J.; Zheng, Y.M.; Liu, Q.; Cheng, X.X.; Luo, F.H. Self-assembly of au nanoparticles on PMMA template as flexible, transparent, and highly active SERS substrates. Anal. Chem. 2014, 86, 6262–6267. [Google Scholar] [CrossRef]
- Chen, L.M.; Liu, Y.N. Surface-enhanced Raman detection of melamine on silver-nanoparticle-decorated silver/carbon nanospheres: Effect of metal ions. ACS Appl. Mater. Interfaces 2011, 3, 3091–3096. [Google Scholar] [CrossRef]
- Ma, W.; Sun, M.; Xu, L.; Wang, L.; Kuang, H.; Xu, C. A SERS active gold nanostar dimer for mercury ion detection. Chem. Commun. 2013, 49, 4989–4991. [Google Scholar] [CrossRef]
- Wei, J.; Yue, W.; Tanabe, I.; Han, X.; Zhao, B.; Ozaki, Y. Semiconductor-driven “turn-off” surface-enhanced Raman scattering spectroscopy: Application in selective determination of chromium (vi) in water. Chem. Sci. 2015, 6, 342–348. [Google Scholar]
- Yu, S.; Chen, N.; Su, Y.Y. Silicon nanohybrid-based SERS chips armed with an internal standard for broad-range, sensitive and reproducible simultaneous quantification of lead(ii) and mercury(ii) in real systems. Nanoscale 2018, 10, 4010–4018. [Google Scholar]
- Jones, C.L.; Bantz, K.C.; Haynes, C.L. Partition layer-modified substrates for reversible surface-enhanced Raman scattering detection of polycyclic aromatic hydrocarbons. Anal. Bioanal. Chem. 2009, 394, 303–311. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.; Lee, S.; Seo, H.W.; Kang, B.; Moon, J.; Lee, K.G.; Yong, D.; Kang, H.; Jung, J.; Lim, E.K.; et al. Clustered Regularly Interspaced Short Palindromic Repeats-Mediated Surface-Enhanced Raman Scattering Assay for Multidrug-Resistant Bacteria. ACS Nano 2020, 14, 17241–17253. [Google Scholar] [CrossRef]
- Choi, J.H.; Shin, M.; Yang, L.; Conley, B.; Yoon, J.; Lee, S.N.; Lee, K.B.; Choi, J.W. Clustered Regularly Interspaced Short Palindromic Repeats-Mediated Amplification-Free Detection of Viral DNAs Using Surface-Enhanced Raman Spectroscopy-Active Nanoarray. ACS Nano 2021, 15, 13475–13485. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Parchur, A.K.; Zhou, A. In vitro biomechanical properties, fluorescence imaging, surface-enhanced Raman spectroscopy, and photothermal therapy evaluation of luminescent functionalized CaMoO4: Eu@Au hybrid nanorods on human lung adenocarcinoma epithelial cells. Sci. Technol. Adv. Mater. 2016, 17, 346–360. [Google Scholar] [CrossRef] [PubMed]
- Moore, T.J.; Moody, A.S.; Payne, T.D.; Sarabia, G.M.; Daniel, A.R.; Sharma, B. In vitro and in vivo SERS biosensing for disease diagnosis. Biosensors 2018, 8, 46. [Google Scholar] [CrossRef]
- Haldavnekar, R.; Venkatakrishnan, K.; Tan, B. Non plasmonic semiconductor quantum SERS probe as a pathway for in vitro cancer detection. Nat. Commun. 2018, 9, 3065. [Google Scholar] [CrossRef]
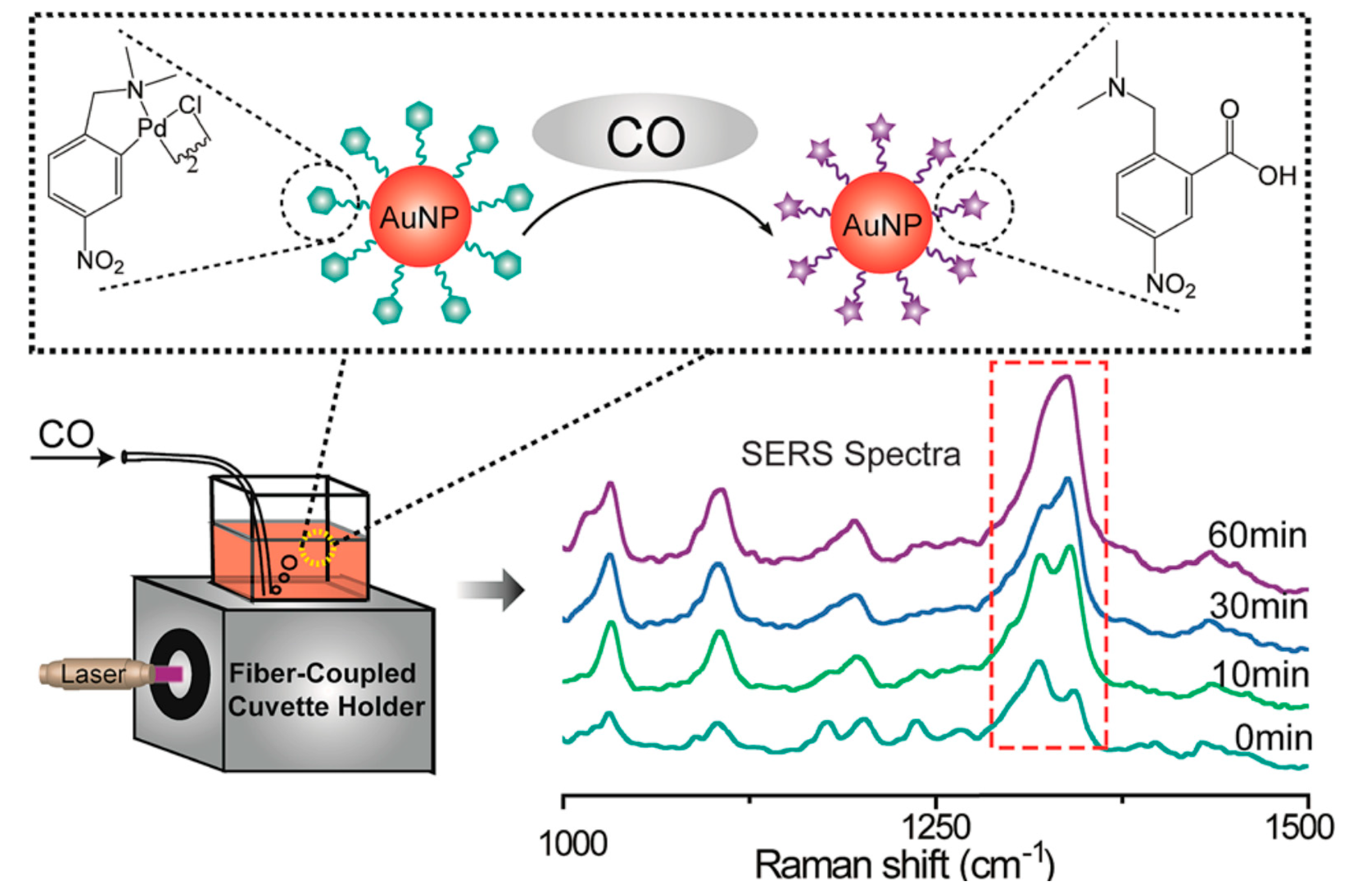
- Hu, K.; Li, D.W.; Cui, J.; Cao, Y.; Long, Y.-T. In situ monitoring of palladacycle-mediated carbonylation by surface enhanced Raman spectroscopy. RSC Adv. 2015, 5, 97734–97737. [Google Scholar] [CrossRef]
- Zheng, X.; Zong, C.; Xu, M.; Wang, X.; Ren, B. Raman imaging from microscopy to nanoscopy, and to macroscopy. Small 2015, 11, 3395–3406. [Google Scholar] [CrossRef]
- Zengin, A.; Tamer, U.; Caykara, T. Sers detection of hepatitis b virus DNA in a temperature-responsive sandwich-hybridization assay. J. Raman Spectrosc. 2017, 48, 668–672. [Google Scholar] [CrossRef]
- Hubarevich, A.; Huang, J.A.; Giovannini, G.; Schirato, A.; Zhao, Y.; Maccaferri, N.; Angelis, F.D.; Alabastri, A.; Garoli, D. Λ-DNA through porous materials-surface-enhanced Raman scattering in a simple plasmonic nanopore. J. Phys. Chem. C 2020, 124, 22663–22670. [Google Scholar] [CrossRef]
- Chourpa, I.; Morjani, H.; Riou, J.F.; Manfait, M. Intracellular molecular interactions of antitumor drug amsacrine (m-AMSA) as revealed by surface-enhanced Raman spectroscopy. FEBS Lett. 1996, 397, 61–64. [Google Scholar] [CrossRef]
- Cao, Y.W.; Jin, R.; Mirkin, C.; Northwestern, U. Nanoparticles with Raman spectroscopic fingerprints for DNA and RNA detection. Science 2002, 297, 1536–1540. [Google Scholar] [CrossRef] [PubMed]
- Zheng, J.W.; Li, X.W.; Xu, H.Y.; Zhou, Y.G.; Gu, R.A. A non-resonance surface-enhanced Raman spectroscopic study of hemin on a roughened silver electrode. Guang Pu Xue Yu Guang Pu Fen Xi 2003, 23, 294–296. [Google Scholar] [PubMed]
- Sun, F.; Hung, H.C.; Sinclair, A.; Zhang, P.; Bai, T.; Galvan, D.D.; Jain, P.; Li, B.; Jiang, S.; Yu, Q. Hierarchical zwitterionic modification of a SERS substrate enables real-time drug monitoring in blood plasma. Nat. Commun. 2016, 7, 13437. [Google Scholar] [CrossRef]
- Zheng, X.S.; Hu, P.; Cui, Y.; Zong, C.; Feng, J.-M.; Wang, X.; Ren, B. BSA-coated nanoparticles for improved SERS-based intracellular pH sensing. Anal. Chem. 2014, 86, 12250–12257. [Google Scholar] [CrossRef]
- Torre, L.A.; Bray, F.; Siegel, R.L.; Ferlay, J.; Lortet-Tieulent, J.; Jemal, A. Global cancer statistics. CA Cancer J. Clin. 2015, 65, 87–108. [Google Scholar] [CrossRef]
- Yazici, O.; Sendur, M.A.; Ozdemir, N.; Aksoy, S. Targeted therapies in gastric cancer and future perspectives. World J. Gastroenterol. 2016, 22, 471–489. [Google Scholar] [CrossRef]
- Wang, L.; Guo, T.; Lu, Q.; Yan, X.; Zhong, D.; Zhang, Z.; Ni, Y.; Han, Y.; Cui, D.; Li, X.; et al. Sea-urchin-like au nanocluster with surface-enhanced Raman scattering in detecting epidermal growth factor receptor (EGFR) mutation status of malignant pleural effusion. ACS Appl. Mater. Interfaces 2015, 7, 359–369. [Google Scholar] [CrossRef]
- Hakim, M.; Broza, Y.Y.; Barash, O.; Peled, N.; Phillips, M.; Amann, A.; Haick, H. Volatile organic compounds of lung cancer and possible biochemical pathways. Chem. Rev. 2012, 112, 5949–5966. [Google Scholar] [CrossRef]
- Chen, Y.S.; Zhang, Y.X.; Pan, F.; Liu, J. Breath analysis based on surface-enhanced Raman scattering sensors distinguishes early and advanced gastric cancer patients from healthy persons. ACS Nano 2016, 10, 8169–8179. [Google Scholar] [CrossRef]
- Qian, X.M.; Nie, S.M. Surface-enhanced Raman nanoparticles for in-vivo tumor targeting and spectroscopic detection. AIP Conf. Proc. 2010, 1267, 81. [Google Scholar]
- Lee, S.; Chon, H.; Lee, J.Y. Rapid and sensitive phenotypic marker detection on breast cancer cells using surface-enhanced Raman scattering (SERS) imaging. Biosensors 2014, 51, 238–243. [Google Scholar] [CrossRef] [PubMed]
- Ding, Q.; Zhou, H.; Zhang, H.; Zhang, Y.; Wang, G.; Zhao, H. 3D Fe3O4@Au@Ag nanoflowers assembled magnetoplasmonic chains for in situ SERS monitoring of plasmon-assisted catalytic reactions. J. Mater. Chem. A 2016, 4, 8866–8874. [Google Scholar] [CrossRef]
- Ouyang, L.; Li, D.; Zhu, L.; Yang, W. A new plasmonic pickering emulsion based SERS sensor for in situ reaction monitoring and kinetic study. J. Mater. Chem. C 2016, 4, 736–744. [Google Scholar] [CrossRef]
- Leyi, F.; Xiao-Tong, P.; Kang, L.; Dechen, J.; Deju, Y.; Li-Na, J.; Kang, W.; Xing-Hua, X. Surface-Roughened SERS-Active Single Silver Nanowire for Simultaneous Detection of Intracellular and Extracellular pHs. ACS Appl. Mater. Interfaces 2023, 15, 20677–20685. [Google Scholar]
- García-Astrain, C.; Henriksen-Lacey, M.; Lenzi, E.; Renero-Lecuna, C.; Langer, J.; Piñeiro, P.; Molina-Martínez, B.; Plou, J.; Jimenez de Aberasturi, D.; Liz-Marzán, L.M. A Scaffold-Assisted 3D Cancer Cell Model for Surface-Enhanced Raman Scattering-Based Real-Time Sensing and Imaging. ACS Nano 2024, 18, 11257–11269. [Google Scholar] [CrossRef] [PubMed]
- Troncoso-Afonso, L.; Vinnacombe-Willson, G.A.; García-Astrain, C.; Liz-Márzan, L.M. SERS in 3D cell models: A powerful tool in cancer research. Chem. Soc. Rev. 2024, 53, 5118–5148. [Google Scholar] [CrossRef] [PubMed]
- Liz-Marzán, L.M.; Willets, K.A.; Chen, X. Fifty Years of Surface-Enhanced Spectroscopy. ACS Nano 2024, 18, 5995–5997. [Google Scholar] [CrossRef]
- Ardini, M.; Huang, J.A.; Sánchez, C.S.; Mousavi, M.Z.; Caprettini, V.; Maccaferri, N.; Melle, G.; Bruno, G.; Pasquale, L.; Garoli, D.; et al. Live Intracellular Biorthogonal Imaging by Surface Enhanced Raman Spectroscopy using Alkyne-Silver Nanoparticles Clusters. Sci. Rep. 2018, 8, 12652. [Google Scholar]
- Collins, D.; Groom, G.; Ireson, N.; Keegan, K.; Shaw, J.; Nichols, K. Renoir Paintings and Drawings at the Art Institute of Chicago; Art Institute of Chicago: Chicago, IL, USA, 2014. [Google Scholar]
- Canamares, M.V.; Leona, M. Surface-enhanced Raman scattering study of the red dye laccaic acid. J. Raman Spectrosc. 2007, 38, 1259–1266. [Google Scholar] [CrossRef]
- Jurasekova, Z.; Domingo, C.; Garcia-Ramos, J.V.; Sanchez-Cortes, S. Effect of pH on the chemical modification of quercetin and structurally related flavonoids characterized by optical (UV-visible and Raman) spectroscopy. Phys. Chem. Chem. Phys. 2014, 16, 12802–12811. [Google Scholar] [CrossRef] [PubMed]
- Jurasekova, Z.; del Puerto, E.; Bruno, G.; García-Ramos, J.V.; Sanchez-Cortes, S.; Domingo, C. Extractionless non-hydrolysis surface-enhanced Raman spectroscopic detection of historical mordant dyes on textile fibers. J. Raman Spectrosc. 2010, 41, 1455–1461. [Google Scholar] [CrossRef]







Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huang, Z.; Peng, J.; Xu, L.; Liu, P. Development and Application of Surface-Enhanced Raman Scattering (SERS). Nanomaterials 2024, 14, 1417. https://doi.org/10.3390/nano14171417
Huang Z, Peng J, Xu L, Liu P. Development and Application of Surface-Enhanced Raman Scattering (SERS). Nanomaterials. 2024; 14(17):1417. https://doi.org/10.3390/nano14171417
Chicago/Turabian StyleHuang, Zhenkai, Jianping Peng, Liguo Xu, and Peijiang Liu. 2024. "Development and Application of Surface-Enhanced Raman Scattering (SERS)" Nanomaterials 14, no. 17: 1417. https://doi.org/10.3390/nano14171417
APA StyleHuang, Z., Peng, J., Xu, L., & Liu, P. (2024). Development and Application of Surface-Enhanced Raman Scattering (SERS). Nanomaterials, 14(17), 1417. https://doi.org/10.3390/nano14171417







