Size-Dependent Internalization of Microplastics and Nanoplastics Using In Vitro Model of the Human Intestine—Contribution of Each Cell in the Tri-Culture Models
Abstract
1. Introduction
2. Materials and Methods
2.1. Cell Culture
2.2. PS Particles
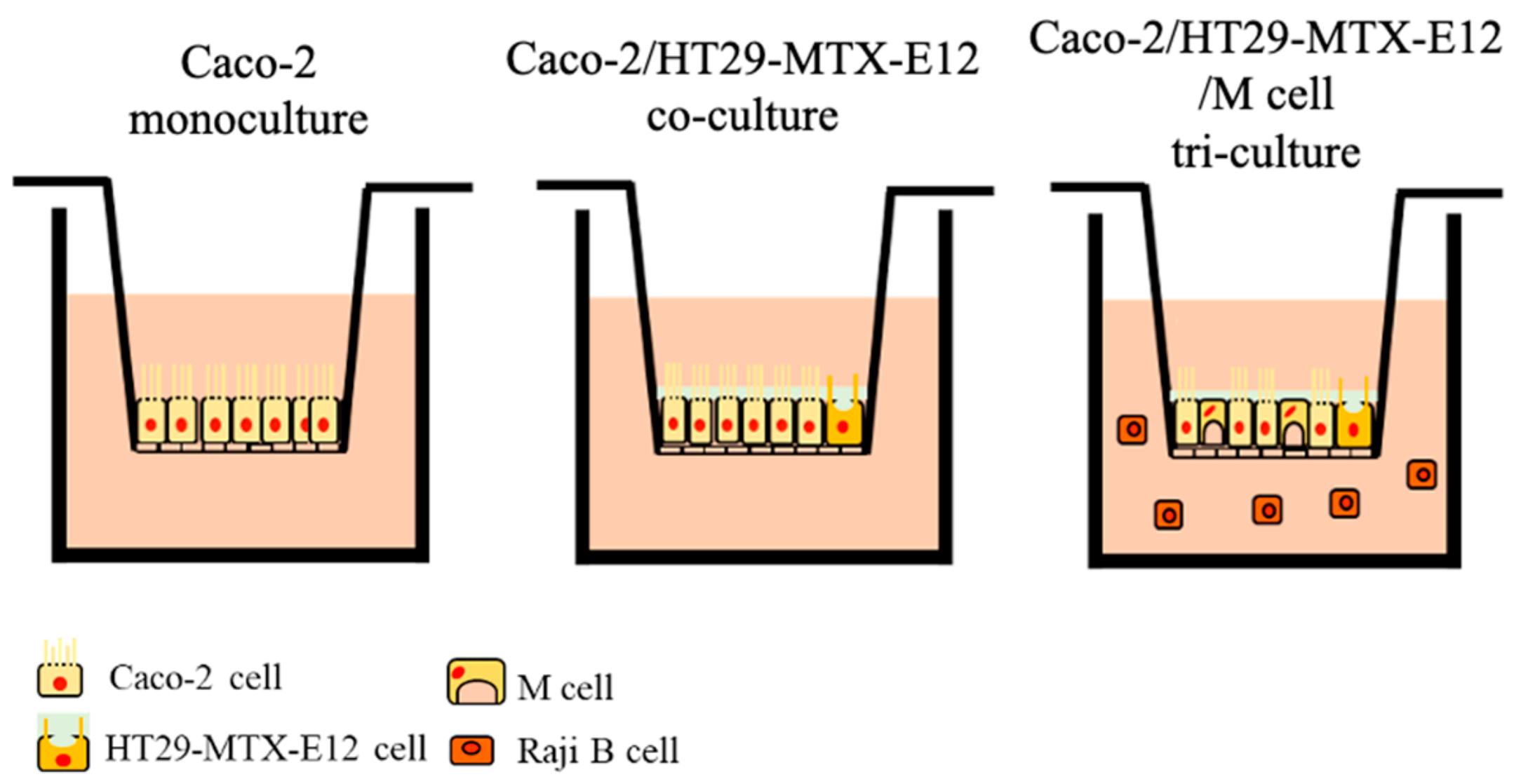
2.3. Culture Models for the MNP Exposure
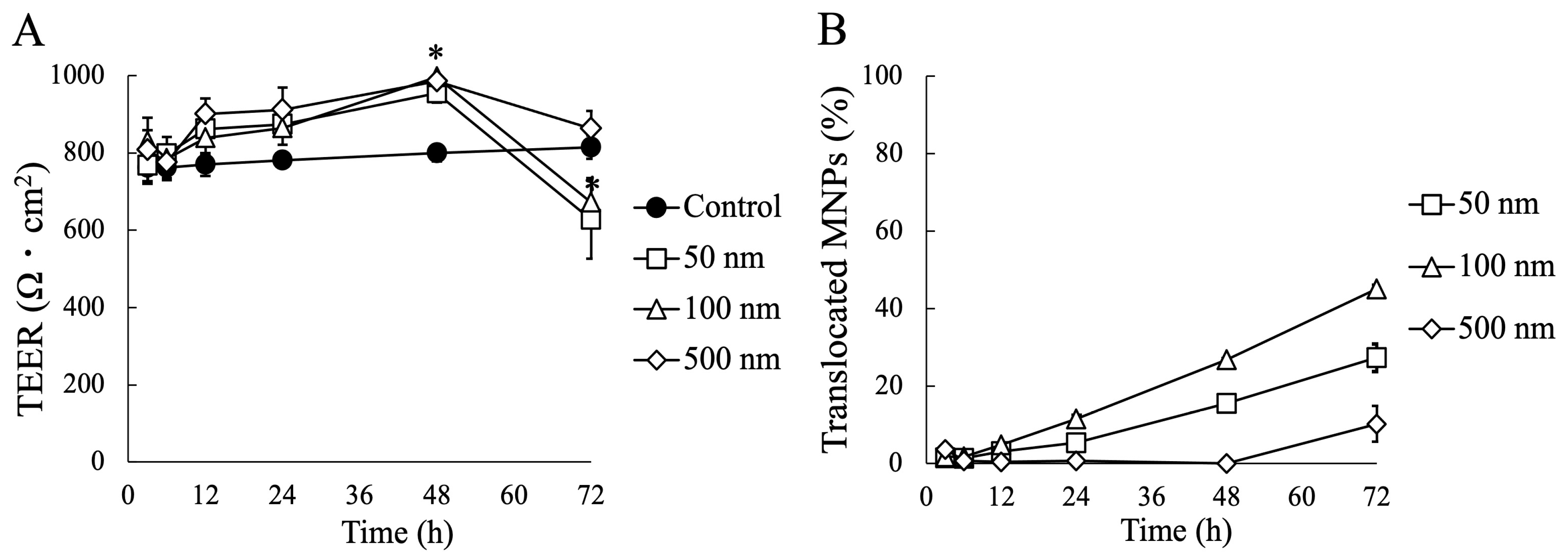
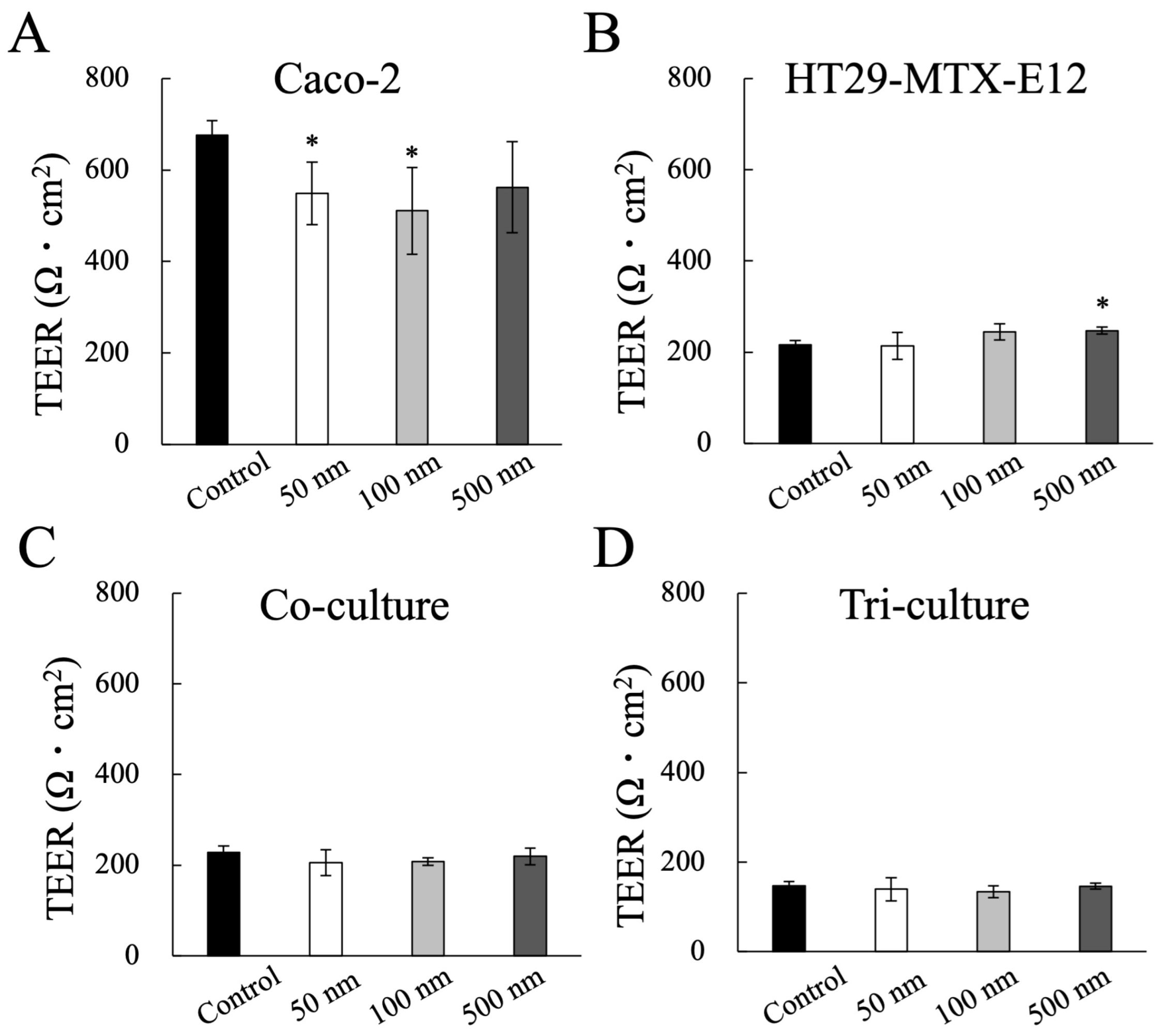
2.4. Evaluation of Epithelial Barrier Function: Transepithelial Electrical Resistance (TEER)
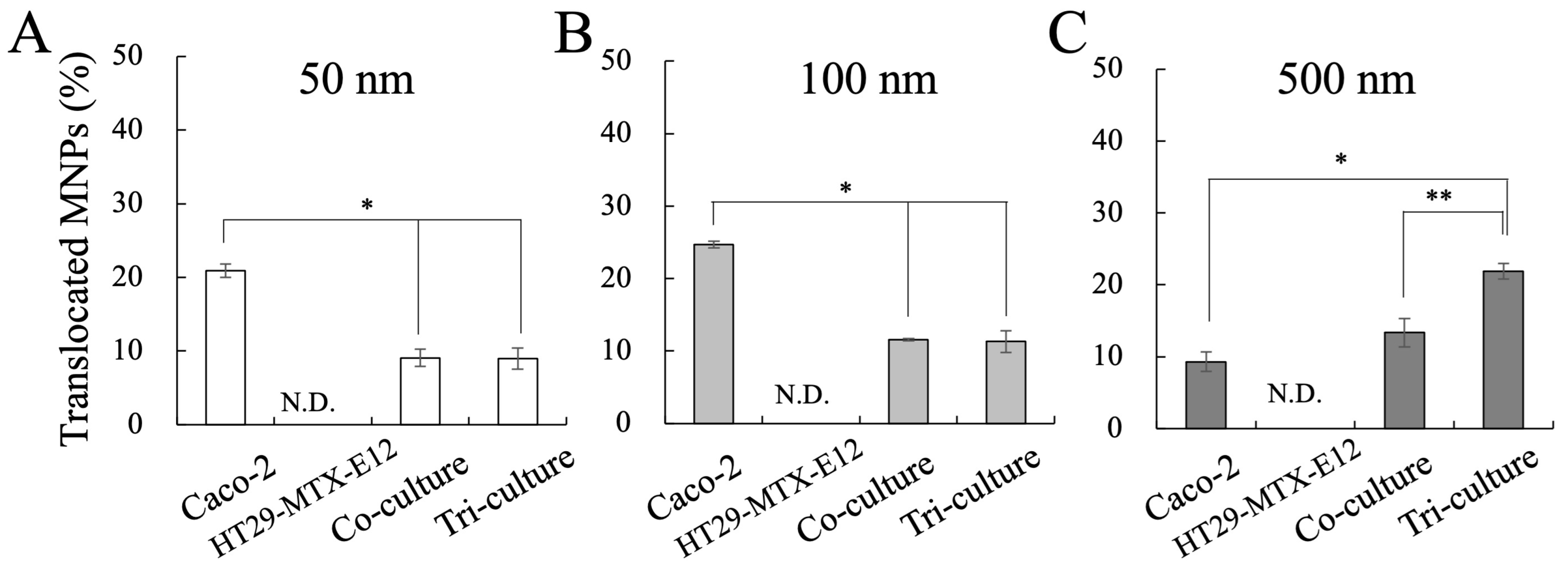
2.5. Measurement of MNP Translocation
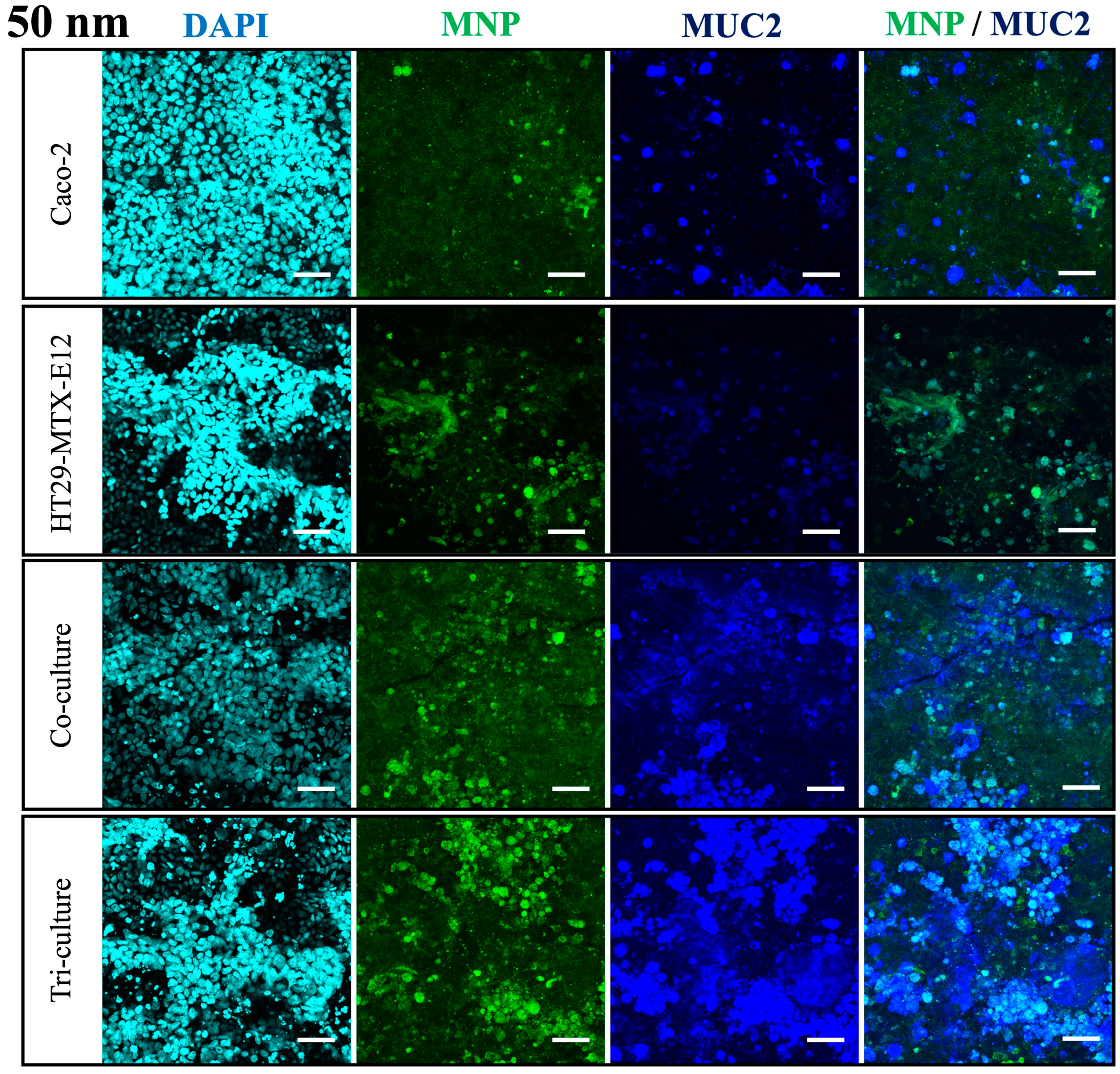
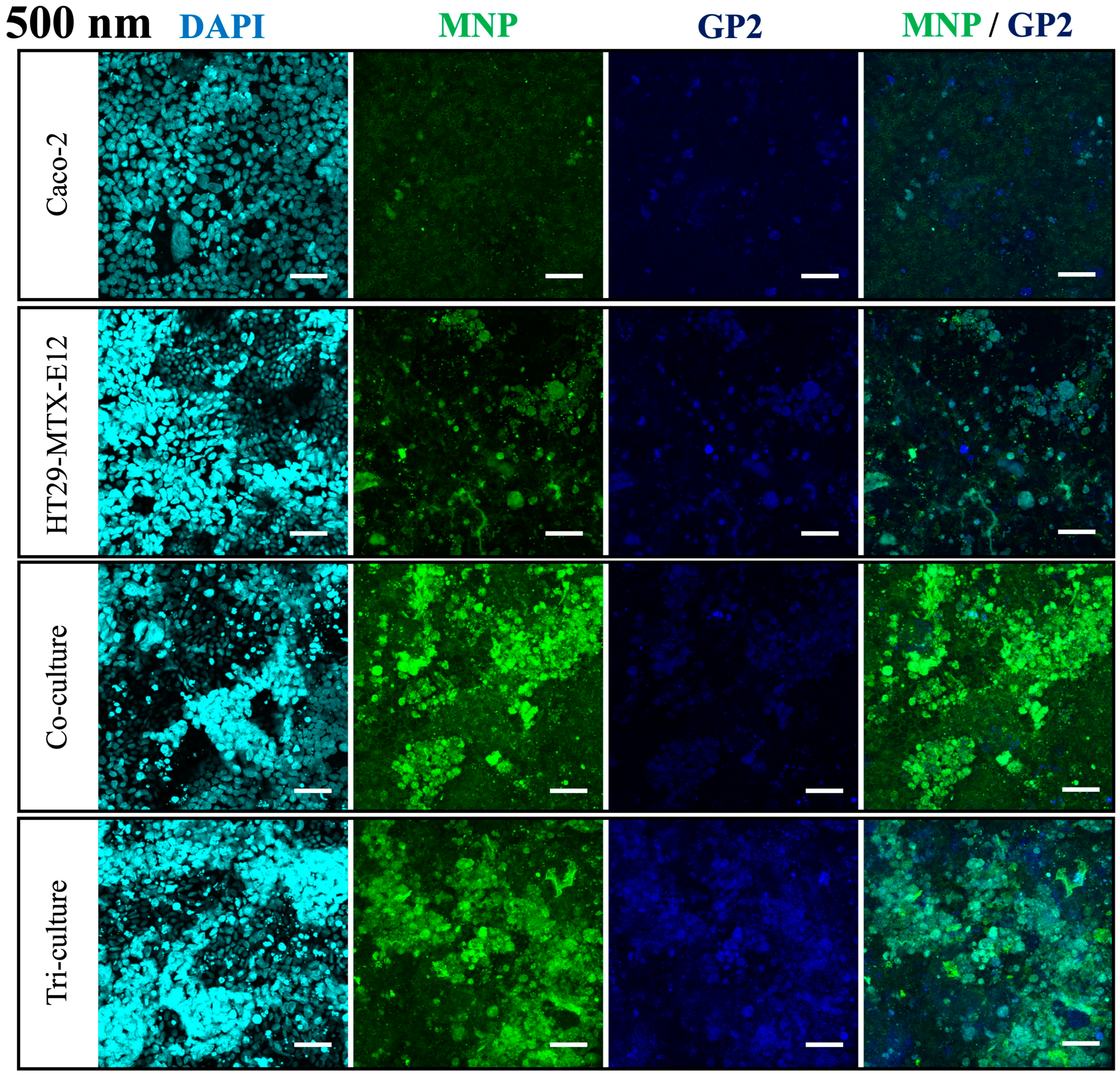
2.6. Immunofluorescent Staining
2.7. Statistical Analysis
3. Results
3.1. The Characterization of MNPs
3.2. Impact of MNP Exposure on Caco-2 Cell Monoculture: Barrier Integrity and Behavior of MNPs during Exposure
3.3. Impact of MNP Exposure on Co- and Tri-Culture of Intestinal Epithelium
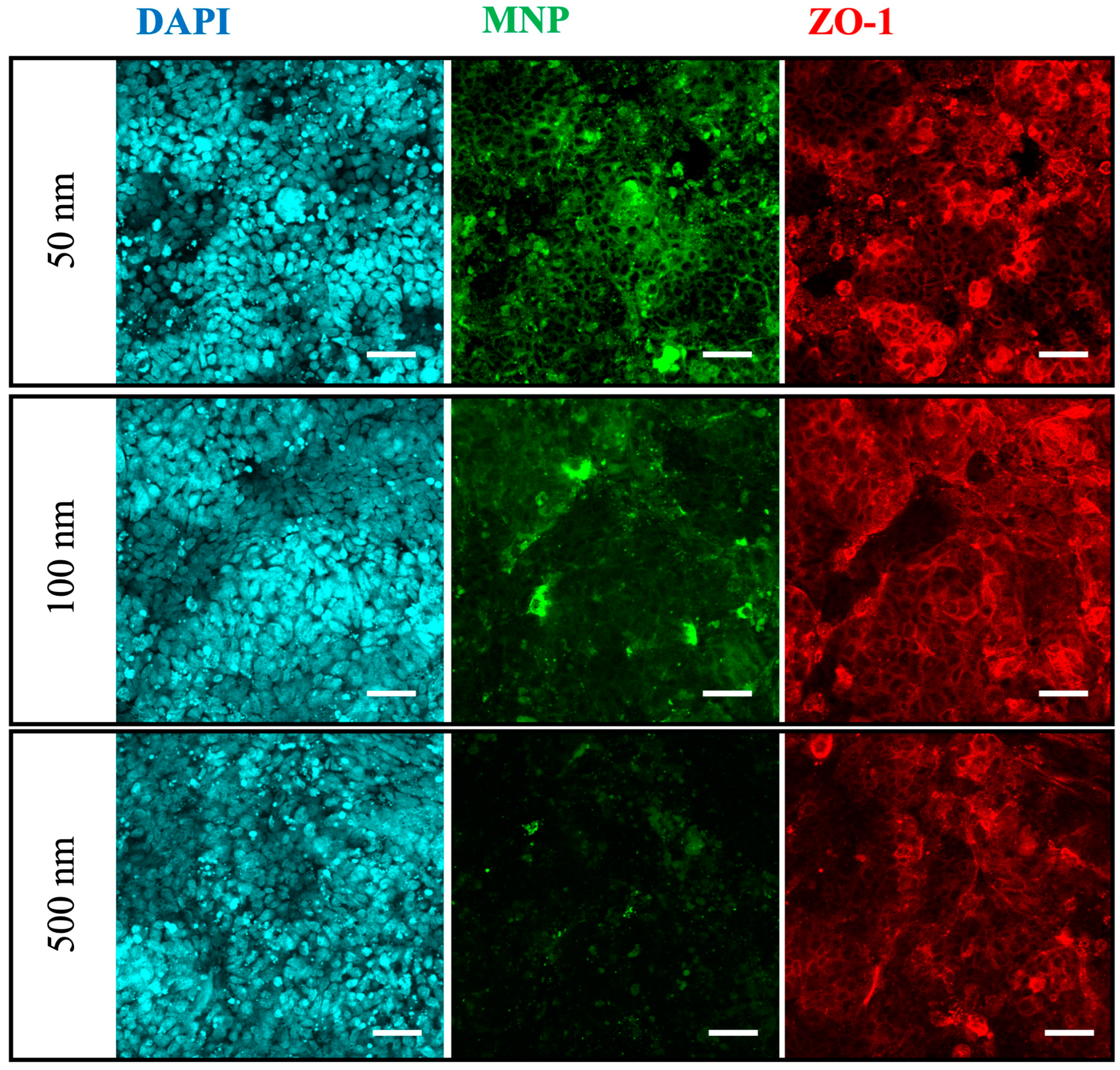
3.4. Translocation and Localization of MNPs in Co- and Tri-Culture of Intestinal Epithelium
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Plastics Europe Plastics—The Fast Facts 2023. Available online: https://plasticseurope.org/knowledge-hub/plastics-the-fast-facts-2023/ (accessed on 5 July 2024).
- Hirt, N.; Body-Malapel, M. Immunotoxicity and Intestinal Effects of Nano- and Microplastics: A Review of the Literature. Part. Fibre Toxicol. 2020, 17, 57. [Google Scholar] [CrossRef]
- Alimba, C.G.; Faggio, C. Microplastics in the Marine Environment: Current Trends in Environmental Pollution and Mechanisms of Toxicological Profile. Environ. Toxicol. Pharmacol. 2019, 68, 61–74. [Google Scholar] [CrossRef] [PubMed]
- Kannan, K.; Vimalkumar, K. A Review of Human Exposure to Microplastics and Insights Into Microplastics as Obesogens. Front. Endocrinol. 2021, 12, 1–19. [Google Scholar] [CrossRef]
- Andrady, A.L. Microplastics in the Marine Environment. Mar. Pollut. Bull. 2011, 62, 1596–1605. [Google Scholar] [CrossRef] [PubMed]
- Law, K.L. Plastics in the Marine Environment. Ann. Rev. Mar. Sci. 2017, 9, 205–229. [Google Scholar] [CrossRef]
- Rochman, C.M.; Brookson, C.; Bikker, J.; Djuric, N.; Earn, A.; Bucci, K.; Athey, S.; Huntington, A.; McIlwraith, H.; Munno, K.; et al. Rethinking Microplastics as a Diverse Contaminant Suite. Environ. Toxicol. Chem. 2019, 38, 703–711. [Google Scholar] [CrossRef]
- Gewert, B.; Plassmann, M.M.; Macleod, M. Pathways for Degradation of Plastic Polymers Floating in the Marine Environment. Environ. Sci. Process. Impacts 2015, 17, 1513–1521. [Google Scholar] [CrossRef]
- Li, W.C.; Tse, H.F.; Fok, L. Plastic Waste in the Marine Environment: A Review of Sources, Occurrence and Effects. Sci. Total Environ. 2016, 566–567, 333–349. [Google Scholar] [CrossRef]
- Stock, V.; Böhmert, L.; Lisicki, E.; Block, R.; Cara-Carmona, J.; Pack, L.K.; Selb, R.; Lichtenstein, D.; Voss, L.; Henderson, C.J.; et al. Uptake and Effects of Orally Ingested Polystyrene Microplastic Particles In Vitro and In Vivo. Arch. Toxicol. 2019, 93, 1817–1833. [Google Scholar] [CrossRef]
- Frias, J.P.G.L.; Nash, R. Microplastics: Finding a Consensus on the Definition. Mar. Pollut. Bull. 2019, 138, 145–147. [Google Scholar] [CrossRef] [PubMed]
- Galloway, T.S.; Cole, M.; Lewis, C. Interactions of Microplastic Debris throughout the Marine Ecosystem. Nat. Ecol. Evol. 2017, 1, 116. [Google Scholar] [CrossRef] [PubMed]
- Ragusa, A.; Svelato, A.; Santacroce, C.; Catalano, P.; Notarstefano, V.; Carnevali, O.; Papa, F.; Rongioletti, M.C.A.; Baiocco, F.; Draghi, S.; et al. Plasticenta: First Evidence of Microplastics in Human Placenta. Environ. Int. 2021, 146, 106274. [Google Scholar] [CrossRef] [PubMed]
- Mintenig, S.M.; Löder, M.G.J.; Primpke, S.; Gerdts, G. Low Numbers of Microplastics Detected in Drinking Water from Ground Water Sources. Sci. Total Environ. 2019, 648, 631–635. [Google Scholar] [CrossRef] [PubMed]
- Galgani, F.; Leaute, J.P.; Moguedet, P.; Souplet, A.; Verin, Y.; Carpentier, A.; Goraguer, H.; Latrouite, D.; Andral, B.; Cadiou, Y.; et al. Litter on the Sea Floor along European Coasts. Mar. Pollut. Bull. 2000, 40, 516–527. [Google Scholar] [CrossRef]
- Meaza, I.; Toyoda, J.H.; Wise, J.P. Microplastics in Sea Turtles, Marine Mammals and Humans: A One Environmental Health Perspective. Front. Environ. Sci. 2021, 8, 575614. [Google Scholar] [CrossRef]
- Smith, M.; Love, D.C.; Rochman, C.M.; Neff, R.A. Microplastics in Seafood and the Implications for Human Health. Curr. Environ. Health Rep. 2018, 5, 375–386. [Google Scholar] [CrossRef]
- Panel, E.; Chain, F. Presence of Microplastics and Nanoplastics in Food, with Particular Focus on Seafood. EFSA J. 2016, 14, e04501. [Google Scholar] [CrossRef]
- Liang, B.; Zhong, Y.; Huang, Y.; Lin, X.; Liu, J.; Lin, L.; Hu, M.; Jiang, J.; Dai, M.; Wang, B.; et al. Underestimated Health Risks: Polystyrene Micro- and Nanoplastics Jointly Induce Intestinal Barrier Dysfunction by ROS-Mediated Epithelial Cell Apoptosis. Part. Fibre Toxicol. 2021, 18, 20. [Google Scholar] [CrossRef]
- Deng, Y.; Zhang, Y.; Lemos, B.; Ren, H. Tissue Accumulation of Microplastics in Mice and Biomarker Responses Suggest Widespread Health Risks of Exposure. Sci. Rep. 2017, 7, srep46687. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; Zhang, Y.; Deng, Y.; Jiang, W.; Zhao, Y.; Geng, J.; Ding, L.; Ren, H. Uptake and Accumulation of Polystyrene Microplastics in Zebrafish (Danio Rerio) and Toxic Effects in Liver. Environ. Sci. Technol. 2016, 50, 4054–4060. [Google Scholar] [CrossRef]
- Jenner, L.C.; Rotchell, J.M.; Bennett, R.T.; Cowen, M.; Tentzeris, V.; Sadofsky, L.R. Detection of Microplastics in Human Lung Tissue Using ΜFTIR Spectroscopy. Sci. Total Environ. 2022, 831, 154907. [Google Scholar] [CrossRef] [PubMed]
- Horvatits, T.; Tamminga, M.; Liu, B.; Sebode, M.; Carambia, A.; Fischer, L.; Püschel, K.; Huber, S.; Fischer, E.K. Microplastics Detected in Cirrhotic Liver Tissue. EBioMedicine 2022, 82, 104147. [Google Scholar] [CrossRef]
- Leslie, H.A.; van Velzen, M.J.M.; Brandsma, S.H.; Vethaak, A.D.; Garcia-Vallejo, J.J.; Lamoree, M.H. Discovery and Quantification of Plastic Particle Pollution in Human Blood. Environ. Int. 2022, 163, 107199. [Google Scholar] [CrossRef]
- Schwabl, P.; Koppel, S.; Konigshofer, P.; Bucsics, T.; Trauner, M.; Reiberger, T.; Liebmann, B. Detection of Various Microplastics in Human Stool: A Prospective Case Series. Ann. Intern. Med. 2019, 171, 453–457. [Google Scholar] [CrossRef]
- Hou, J.; Lei, Z.; Cui, L.; Hou, Y.; Yang, L.; An, R.; Wang, Q.; Li, S.; Zhang, H.; Zhang, L. Polystyrene Microplastics Lead to Pyroptosis and Apoptosis of Ovarian Granulosa Cells via NLRP3/Caspase-1 Signaling Pathway in Rats. Ecotoxicol. Environ. Saf. 2021, 212, 453–457. [Google Scholar] [CrossRef] [PubMed]
- DeLoid, G.M.; Cao, X.; Bitounis, D.; Singh, D.; Llopis, P.M.; Buckley, B.; Demokritou, P. Toxicity, Uptake, and Nuclear Translocation of Ingested Micro-Nanoplastics in an in Vitro Model of the Small Intestinal Epithelium. Food Chem. Toxicol. 2021, 158, 112609. [Google Scholar] [CrossRef] [PubMed]
- Yong, C.Q.Y.; Valiyaveettil, S.; Tang, B.L. Toxicity of Microplastics and Nanoplastics in Mammalian Systems. Int. J. Environ. Res. Public. Health 2020, 17, 1509. [Google Scholar] [CrossRef]
- Wu, B.; Wu, X.; Liu, S.; Wang, Z.; Chen, L. Size-Dependent Effects of Polystyrene Microplastics on Cytotoxicity and Efflux Pump Inhibition in Human Caco-2 cells. Chemosphere 2019, 221, 333–341. [Google Scholar] [CrossRef]
- Wright, S.L.; Kelly, F.J. Plastic and Human Health: A Micro Issue? Environ. Sci. Technol. 2017, 51, 6634–6647. [Google Scholar] [CrossRef]
- Stock, V.; Laurisch, C.; Franke, J.; Dönmez, M.H.; Voss, L.; Böhmert, L.; Braeuning, A.; Sieg, H. Uptake and Cellular Effects of PE, PP, PET and PVC Microplastic Particles. Toxicol. Vitr. 2021, 70, 105021. [Google Scholar] [CrossRef] [PubMed]
- Hesler, M.; Aengenheister, L.; Ellinger, B.; Drexel, R.; Straskraba, S.; Jost, C.; Wagner, S.; Meier, F.; von Briesen, H.; Büchel, C.; et al. Multi-Endpoint Toxicological Assessment of Polystyrene Nano- and Microparticles in Different Biological Models In Vitro. Toxicol. Vitr. 2019, 61, 104610. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Williams, A.M.; Gordon, E.B.; Rudolph, S.E.; Longo, B.N.; Li, G.; Kaplan, D.L. Biological Effects of Polystyrene Micro- and Nano-Plastics on Human Intestinal Organoid-Derived Epithelial Tissue Models without and with M Cells. Nanomedicine 2023, 50, 102680. [Google Scholar] [CrossRef]
- Donkers, J.M.; Höppener, E.M.; Grigoriev, I.; Will, L.; Melgert, B.N.; van der Zaan, B.; van de Steeg, E.; Kooter, I.M. Advanced Epithelial Lung and Gut Barrier Models Demonstrate Passage of Microplastic Particles. Microplastics Nanoplastics 2022, 2, 6. [Google Scholar] [CrossRef]
- Strugari, A.F.G.; Stan, M.S.; Gharbia, S.; Hermenean, A.; Dinischiotu, A. Characterization of Nanoparticle Intestinal Transport Using an in Vitro Co-Culture Model. Nanomaterials 2019, 9, 5. [Google Scholar] [CrossRef] [PubMed]
- Ejazi, S.A.; Louisthelmy, R.; Maisel, K. Mechanisms of Nanoparticle Transport across Intestinal Tissue: An Oral Delivery Perspective. ACS Nano 2023, 17, 13044–13061. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Lu, Y.; Qi, J.; Wu, W. An Update on Oral Drug Delivery via Intestinal Lymphatic Transport. Acta Pharm. Sin. B 2021, 11, 2449–2468. [Google Scholar] [CrossRef]
- Yu, M.; Yang, Y.; Zhu, C.; Guo, S.; Gan, Y. Advances in the Transepithelial Transport of Nanoparticles. Drug Discov. Today 2016, 21, 1155–1161. [Google Scholar] [CrossRef] [PubMed]
- Natoli, M.; Leoni, B.D.; D’Agnano, I.; Zucco, F.; Felsani, A. Good Caco-2 Cell Culture Practices. Toxicology In Vitro 2012, 26, 1243–1246. [Google Scholar] [CrossRef] [PubMed]
- Bandi, S.P.; Kumbhar, Y.S.; Venuganti, V.V.K. Effect of Particle Size and Surface Charge of Nanoparticles in Penetration through Intestinal Mucus Barrier. J. Nanoparticle Res. 2020, 22, 62. [Google Scholar] [CrossRef]
- Lai, S.K.; Elizabeth O’hanlon, D.; Harrold, S.; Man, S.T.; Wang, Y.-Y.; Cone, R.; Hanes, J. Rapid Transport of Large Polymeric Nanoparticles in Fresh Undiluted Human Mucus. Proc. Natl. Acad. Sci. USA 2007, 104, 1482–1487. [Google Scholar] [CrossRef]
- Liu, S.; Wu, X.; Gu, W.; Yu, J.; Wu, B. Influence of the Digestive Process on Intestinal Toxicity of Polystyrene Microplastics as Determined by in Vitro Caco-2 Models. Chemosphere 2020, 256, 127204. [Google Scholar] [CrossRef] [PubMed]
- Banerjee, A.; Qi, J.; Gogoi, R.; Wong, J.; Mitragotri, S. Role of Nanoparticle Size, Shape and Surface Chemistry in Oral Drug Delivery. J. Control. Release 2016, 238, 176–185. [Google Scholar] [CrossRef]
- Dawson, A.L.; Bose, U.; Ni, D.; Nelis, J.L.D. Unravelling Protein Corona Formation on Pristine and Leached Microplastics. Microplastics Nanoplastics 2024, 4, 9. [Google Scholar] [CrossRef]
- Araújo, F.; Sarmento, B. Towards the Characterization of an in Vitro Triple Co-Culture Intestine Cell Model for Permeability Studies. Int. J. Pharm. 2013, 458, 128–134. [Google Scholar] [CrossRef] [PubMed]
- Xavier, M.; García-Hevia, L.; Amado, I.R.; Pastrana, L.; Gonçalves, C. In Vitro Intestinal Uptake and Permeability of Fluorescently-Labelled Hyaluronic Acid Nanogels. Int. J. Nanomed. 2019, 14, 9077–9088. [Google Scholar] [CrossRef] [PubMed]
- Lehner, R.; Wohlleben, W.; Septiadi, D.; Landsiedel, R.; Petri-Fink, A.; Rothen-Rutishauser, B. A Novel 3D Intestine Barrier Model to Study the Immune Response upon Exposure to Microplastics. Arch. Toxicol. 2020, 94, 2463–2479. [Google Scholar] [CrossRef]
- He, C.; Yin, L.; Tang, C.; Yin, C. Size-Dependent Absorption Mechanism of Polymeric Nanoparticles for Oral Delivery of Protein Drugs. Biomaterials 2012, 33, 8569–8578. [Google Scholar] [CrossRef] [PubMed]
- Lock, J.Y.; Carlson, T.L.; Carrier, R.L. Mucus Models to Evaluate the Diffusion of Drugs and Particles. Adv. Drug Deliv. Rev. 2018, 124, 34–49. [Google Scholar] [CrossRef]
- Merkley, S.D.; Moss, H.C.; Goodfellow, S.M.; Ling, C.L.; Meyer-Hagen, J.L.; Weaver, J.; Campen, M.J.; Castillo, E.F. Polystyrene Microplastics Induce an Immunometabolic Active State in Macrophages. Cell Biol. Toxicol. 2022, 38, 31–41. [Google Scholar] [CrossRef] [PubMed]
- Tavakolpournegari, A.; Annangi, B.; Villacorta, A.; Banaei, G.; Martin, J.; Pastor, S.; Marcos, R.; Hernández, A. Hazard Assessment of Different-Sized Polystyrene Nanoplastics in Hematopoietic Human Cell Lines. Chemosphere 2023, 325, 138360. [Google Scholar] [CrossRef]
- Gustafsson, J.K.; Ermund, A.; Johansson, M.E.V.; Schütte, A.; Hansson, G.C.; Sjövall, H. An Ex Vivo Method for Studying Mucus Formation, Properties, and Thickness in Human Colonic Biopsies and Mouse Small and Large Intestinal Explants. Am. J. Physiol. Gastrointest. Liver Physiol. 2012, 302, 430–438. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, T. Recent Progress in Organoid Culture to Model Intestinal Epithelial Barrier Functions. Int. Immunol. 2018, 31, 13–21. [Google Scholar] [CrossRef] [PubMed]
- Min, S.; Kim, S.; Cho, S.W. Gastrointestinal Tract Modeling Using Organoids Engineered with Cellular and Microbiota Niches. Exp. Mol. Med. 2020, 52, 227–237. [Google Scholar] [CrossRef]
- Cornu, R.; Béduneau, A.; Martin, H. Ingestion of Titanium Dioxide Nanoparticles: A Definite Health Risk for Consumers and Their Progeny. Arch. Toxicol. 2022, 96, 2655–2686. [Google Scholar] [CrossRef] [PubMed]
- Lin, S.; Wu, F.; Cao, Z.; Liu, J. Advances in Nanomedicines for Interaction with the Intestinal Barrier. Adv. Nanobiomed Res. 2022, 2, 2100147. [Google Scholar] [CrossRef]
- Lopez-Escalera, S.; Wellejus, A. Evaluation of Caco-2 and Human Intestinal Epithelial Cells as in Vitro Models of Colonic and Small Intestinal Integrity. Biochem. Biophys. Rep. 2022, 31, 101314. [Google Scholar] [CrossRef] [PubMed]
- Busch, M.; Bredeck, G.; Kämpfer, A.A.M.; Schins, R.P.F. Investigations of Acute Effects of Polystyrene and Polyvinyl Chloride Micro- and Nanoplastics in an Advanced in Vitro Triple Culture Model of the Healthy and Inflamed Intestine. Environ. Res. 2021, 193, 110536. [Google Scholar] [CrossRef]
- Domenech, J.; de Britto, M.; Velázquez, A.; Pastor, S.; Hernández, A.; Marcos, R.; Cortés, C. Long-Term Effects of Polystyrene Nanoplastics in Human Intestinal Caco-2 Cells. Biomolecules 2021, 11, 1442. [Google Scholar] [CrossRef]
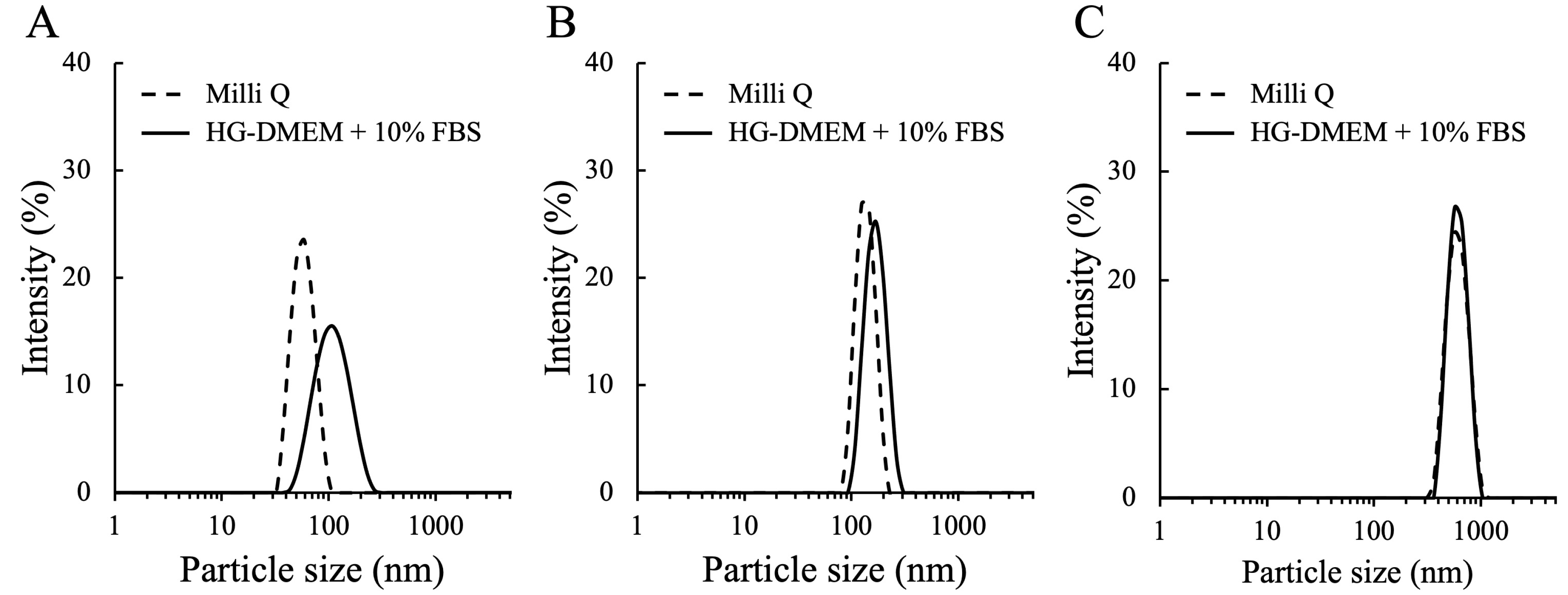
| MNP | Dispersant | Size (nm) | Zeta Potential (mV) | PDI |
|---|---|---|---|---|
| 50 nm | Milli Q | 58.76 ± 1.37 | −34.65 ± 1.20 | 0.04 ± 0.01 |
| HG-DMEM + 10% FBS | 113.40 ± 2.80 | −8.32 ± 1.47 | 0.17 ± 0.01 | |
| 100 nm | Milli Q | 139.00 ± 2.64 | −24.72 ± 2.74 | 0.02 ± 0.03 |
| HG-DMEM + 10% FBS | 171.40 ± 0.48 | −9.32 ± 0.74 | 0.02 ± 0.01 | |
| 500 nm | Milli Q | 603.50 ± 9.57 | −6.87 ± 0.09 | 0.05 ± 0.03 |
| HG-DMEM + 10% FBS | 625.10 ± 10.79 | −10.23 ± 1.25 | 0.04 ± 0.03 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Choi, H.; Kaneko, S.; Suzuki, Y.; Inamura, K.; Nishikawa, M.; Sakai, Y. Size-Dependent Internalization of Microplastics and Nanoplastics Using In Vitro Model of the Human Intestine—Contribution of Each Cell in the Tri-Culture Models. Nanomaterials 2024, 14, 1435. https://doi.org/10.3390/nano14171435
Choi H, Kaneko S, Suzuki Y, Inamura K, Nishikawa M, Sakai Y. Size-Dependent Internalization of Microplastics and Nanoplastics Using In Vitro Model of the Human Intestine—Contribution of Each Cell in the Tri-Culture Models. Nanomaterials. 2024; 14(17):1435. https://doi.org/10.3390/nano14171435
Chicago/Turabian StyleChoi, Hyunjin, Shohei Kaneko, Yusei Suzuki, Kosuke Inamura, Masaki Nishikawa, and Yasuyuki Sakai. 2024. "Size-Dependent Internalization of Microplastics and Nanoplastics Using In Vitro Model of the Human Intestine—Contribution of Each Cell in the Tri-Culture Models" Nanomaterials 14, no. 17: 1435. https://doi.org/10.3390/nano14171435
APA StyleChoi, H., Kaneko, S., Suzuki, Y., Inamura, K., Nishikawa, M., & Sakai, Y. (2024). Size-Dependent Internalization of Microplastics and Nanoplastics Using In Vitro Model of the Human Intestine—Contribution of Each Cell in the Tri-Culture Models. Nanomaterials, 14(17), 1435. https://doi.org/10.3390/nano14171435







