Potential of Enzymatically Synthesized Hemozoin Analog as Th1 Cell Adjuvant
Abstract
:1. Introduction
2. Materials and Methods
2.1. Reagents
2.2. Expression and Purification of HDP
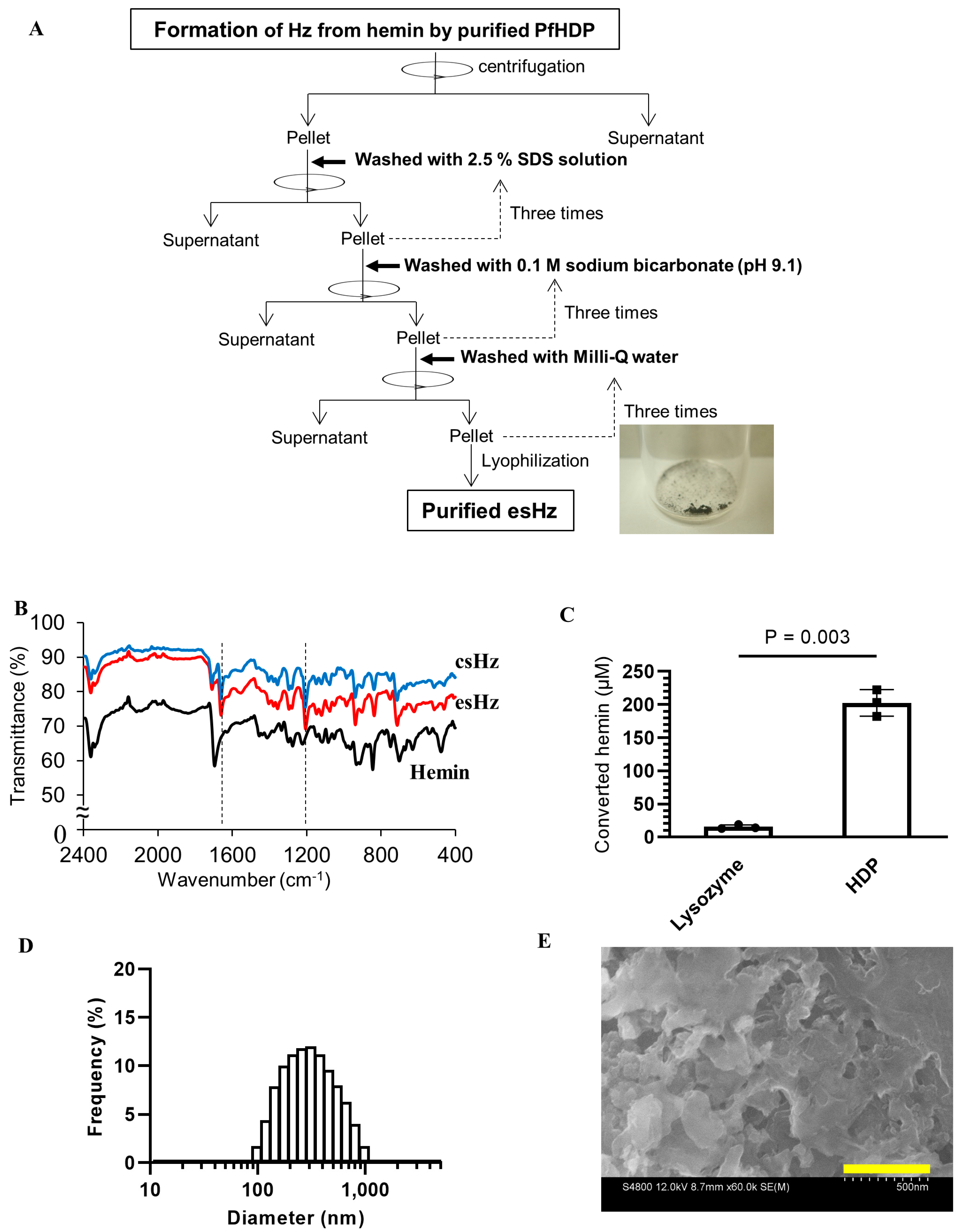
2.3. esHz Formation Assay
2.4. csHz Preparation
2.5. Generation of Infra-Red (IR) Spectra and Scanning Electron Microscope (SEM) Images
2.6. Mammalian Cell Culture and Immunostimulation
2.7. RT-qPCR and ELISA
2.8. Administration of OVA with esHz in Mice and Measurement of OVA-Specific IgG Level
2.9. Statistical Analysis
3. Results
3.1. Cold-Shock with Co-Expression of Chaperones Improves the Solubility of PfHDP
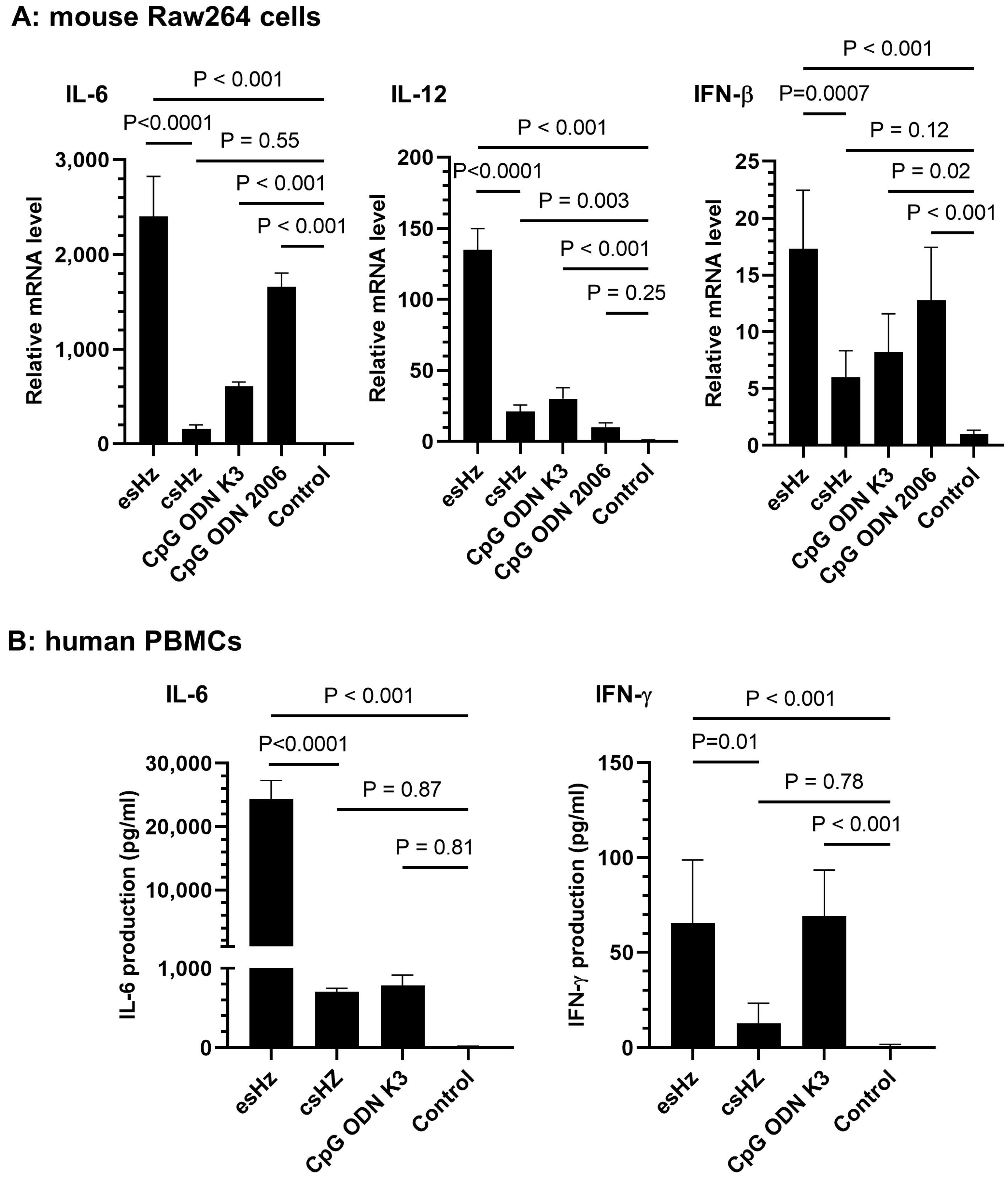
3.2. esHz Induces Immunostimulatory Cytokine Production in RAW264 Cells and PBMCs
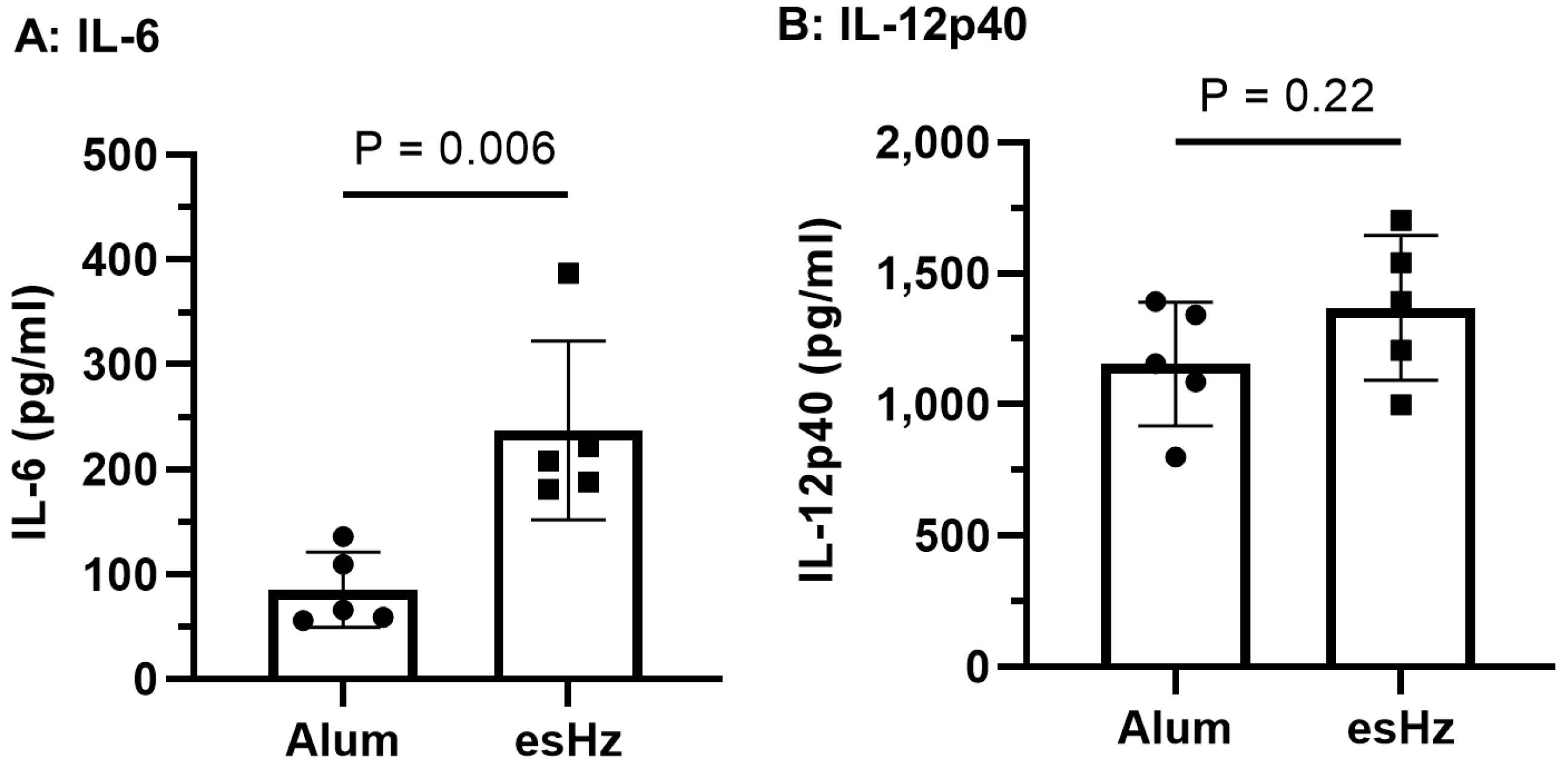
3.3. esHz Induces the Cytokines and OVA-Specific IgG Induction in Mice
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Higdon, A.N.; Benavides, G.A.; Chacko, B.K.; Ouyang, X.; Johnson, M.S.; Landar, A.; Zhang, J.; Darley-Usmar, V.M. Hemin causes mitochondrial dysfunction in endothelial cells through promoting lipid peroxidation: The protective role of autophagy. Am. J. Physiol. Heart Circ. Physiol. 2012, 302, H1394–H1409. [Google Scholar] [CrossRef] [PubMed]
- Jani, D.; Nagarkatti, R.; Beatty, W.; Angel, R.; Slebodnick, C.; Andersen, J.; Kumar, S.; Rathore, D. HDP—A novel heme detoxification protein from the malaria parasite. PLoS Pathog. 2008, 4, e1000053. [Google Scholar] [CrossRef]
- Soni, A.; Goyal, M.; Prakash, K.; Bhardwaj, J.; Siddiqui, A.J.; Puri, S.K. Cloning, expression and functional characterization of heme detoxification protein (HDP) from the rodent malaria parasite Plasmodium vinckei. Gene 2015, 566, 109–119. [Google Scholar] [CrossRef] [PubMed]
- Nakatani, K.; Ishikawa, H.; Aono, S.; Mizutani, Y. Identification of essential histidine residues involved in heme binding and Hemozoin formation in heme detoxification protein from Plasmodium falciparum. Sci. Rep. 2014, 4, 6137. [Google Scholar] [CrossRef]
- Nakatani, K.; Ishikawa, H.; Aono, S.; Mizutani, Y. Heme-binding properties of heme detoxification protein from Plasmodium falciparum. Biochem. Biophys. Res. Commun. 2013, 439, 477–480. [Google Scholar] [CrossRef] [PubMed]
- Coban, C.; Igari, Y.; Yagi, M.; Reimer, T.; Koyama, S.; Aoshi, T.; Ohata, K.; Tsukui, T.; Takeshita, F.; Sakurai, K.; et al. Immunogenicity of whole-parasite vaccines against Plasmodium falciparum involves malarial hemozoin and host TLR9. Cell Host Microbe 2010, 7, 50–61. [Google Scholar] [CrossRef]
- Coban, C.; Yagi, M.; Ohata, K.; Igari, Y.; Tsukui, T.; Horii, T.; Ishii, K.J.; Akira, S. The malarial metabolite hemozoin and its potential use as a vaccine adjuvant. Allergol. Int. 2010, 59, 115–124. [Google Scholar] [CrossRef]
- Griffith, J.W.; Sun, T.; McIntosh, M.T.; Bucala, R. Pure Hemozoin is inflammatory in vivo and activates the NALP3 inflammasome via release of uric acid. J. Immunol. 2009, 183, 5208–5220. [Google Scholar] [CrossRef]
- Hoang, A.N.; Sandlin, R.D.; Omar, A.; Egan, T.J.; Wright, D.W. The neutral lipid composition present in the digestive vacuole of Plasmodium falciparum concentrates heme and mediates β-hematin formation with an unusually low activation energy. Biochemistry 2010, 49, 10107–10116. [Google Scholar] [CrossRef]
- Jaramillo, M.; Godbout, M.; Olivier, M. Hemozoin induces macrophage chemokine expression through oxidative stress-dependent and -independent mechanisms. J. Immunol. 2005, 174, 475–484. [Google Scholar] [CrossRef]
- Jaramillo, M.; Gowda, D.C.; Radzioch, D.; Olivier, M. Hemozoin increases IFN-γ-inducible macrophage nitric oxide generation through extracellular signal-regulated kinase- and NF-κ B-dependent pathways. J. Immunol. 2003, 171, 4243–4253. [Google Scholar] [CrossRef] [PubMed]
- Jaramillo, M.; Plante, I.; Ouellet, N.; Vandal, K.; Tessier, P.A.; Olivier, M. Hemozoin-inducible proinflammatory events in vivo: Potential role in malaria infection. J. Immunol. 2004, 172, 3101–3110. [Google Scholar] [CrossRef] [PubMed]
- Coban, C.; Ishii, K.J.; Horii, T.; Akira, S. Manipulation of host innate immune responses by the malaria parasite. Trends Microbiol. 2007, 15, 271–278. [Google Scholar] [CrossRef]
- Coban, C.; Ishii, K.J.; Sullivan, D.J.; Kumar, N. Purified malaria pigment (hemozoin) enhances dendritic cell maturation and modulates the isotype of antibodies induced by a DNA vaccine. Infect. Immun. 2002, 70, 3939–3943. [Google Scholar] [CrossRef]
- Coban, C.; Ishii, K.J.; Kawai, T.; Hemmi, H.; Sato, S.; Uematsu, S.; Yamamoto, M.; Takeuchi, O.; Itagaki, S.; Kumar, N.; et al. Toll-like receptor 9 mediates innate immune activation by the malaria pigment hemozoin. J. Exp. Med. 2005, 201, 19–25. [Google Scholar] [CrossRef] [PubMed]
- Deroost, K.; Lays, N.; Pham, T.T.; Baci, D.; Van den Eynde, K.; Komuta, M.; Prato, M.; Roskams, T.; Schwarzer, E.; Opdenakker, G.; et al. Hemozoin Induces Hepatic Inflammation in Mice and Is Differentially Associated with Liver Pathology Depending on the Plasmodium Strain. PLoS ONE 2014, 9, e113519. [Google Scholar] [CrossRef]
- Deroost, K.; Tyberghein, A.; Lays, N.; Noppen, S.; Schwarzer, E.; Vanstreels, E.; Komuta, M.; Prato, M.; Lin, J.W.; Pamplona, A.; et al. Hemozoin Induces Lung Inflammation and Correlates with Malaria-Associated Acute Respiratory Distress Syndrome. Am. J. Respir. Cell Mol. 2013, 48, 589–600. [Google Scholar] [CrossRef]
- Raulf, M.K.; Johannssen, T.; Matthiesen, S.; Neumann, K.; Hachenberg, S.; Mayer-Lambertz, S.; Steinbeis, F.; Hegermann, J.; Seeberger, P.H.; Baumgartner, W.; et al. The C-type Lectin Receptor CLEC12A Recognizes Plasmodial Hemozoin and Contributes to Cerebral Malaria Development. Cell Rep. 2019, 28, 30–38.e5. [Google Scholar] [CrossRef]
- Bohle, D.S.; Helms, J.B. Synthesis of β-hematin by dehydrohalogenation of hemin. Biochem. Biophys. Res. Commun. 1993, 193, 504–508. [Google Scholar] [CrossRef]
- Zhao, D.; Tu, A.T.T.; Shobo, M.; Le, N.B.T.; Yoshikawa, C.; Sugai, K.; Hakamata, Y.; Yamazaki, T. Non-Modified CpG Oligodeoxynucleotide Forming Guanine-Quadruplex Structure Complexes with ε-Poly-(L)-Lysine Induce Antibody Production as Vaccine Adjuvants. Biomolecules 2022, 12, 1868. [Google Scholar] [CrossRef]
- Takahashi, Y.; Maezawa, T.; Araie, Y.; Takahashi, Y.; Takakura, Y.; Nishikawa, M. In Vitro and In Vivo Stimulation of Toll-Like Receptor 9 by CpG Oligodeoxynucleotides Incorporated Into Polypod-Like DNA Nanostructures. J. Pharm. Sci. 2017, 106, 2457–2462. [Google Scholar] [CrossRef]
- Hyeon, J.E.; Jeong, D.W.; Ko, Y.J.; Kim, S.W.; Park, C.; Han, S.O. Biomimetic magnetoelectric nanocrystals synthesized by polymerization of heme as advanced nanomaterials for biosensing application. Biosens. Bioelectron. 2018, 114, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Noland, G.S.; Briones, N.; Sullivan, D.J., Jr. The shape and size of hemozoin crystals distinguishes diverse Plasmodium species. Mol. Biochem. Parasitol. 2003, 130, 91–99. [Google Scholar] [CrossRef] [PubMed]
- Belcher, J.D.; Chen, C.S.; Nguyen, J.; Milbauer, L.M.; Abdulla, F.; Alayash, A.I.; Smith, A.; Nath, K.A.; Hebbel, R.P.; Vercellotti, G.M. Heme triggers TLR4 signaling leading to endothelial cell activation and vaso-occlusion in murine sickle cell disease. Blood 2014, 123, 377–390. [Google Scholar] [CrossRef]
- Lee, M.S.; Igari, Y.; Tsukui, T.; Ishii, K.J.; Coban, C. Current status of synthetic hemozoin adjuvant: A preliminary safety evaluation. Vaccine 2016, 34, 2055–2061. [Google Scholar] [CrossRef]


| Gene | Sequence (5′-3′) |
|---|---|
| GAPDH | FW: GTGGACCTCATGGCCTACAT RV: TGTGAGGGAGATGCTCAGTG |
| IL-6 | FW: TCCTTCCTACCCCAATTTCC RV: CGCACTAGGTTTGCCGAGTA |
| IL-12 | FW: GAAAGGCTGGGTATCGG RV: GGCTGTCCTCAAACTCAC |
| IFN-β | FW: GGTCCGAGCAGAGATCTTCA RV: TCACTACCAGTCCCAGAGTCC |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hoshi, K.; Tu, A.T.T.; Shobo, M.; Kettisen, K.; Ye, L.; Bülow, L.; Hakamata, Y.; Furuya, T.; Asano, R.; Tsugawa, W.; et al. Potential of Enzymatically Synthesized Hemozoin Analog as Th1 Cell Adjuvant. Nanomaterials 2024, 14, 1440. https://doi.org/10.3390/nano14171440
Hoshi K, Tu ATT, Shobo M, Kettisen K, Ye L, Bülow L, Hakamata Y, Furuya T, Asano R, Tsugawa W, et al. Potential of Enzymatically Synthesized Hemozoin Analog as Th1 Cell Adjuvant. Nanomaterials. 2024; 14(17):1440. https://doi.org/10.3390/nano14171440
Chicago/Turabian StyleHoshi, Kazuaki, Anh Thi Tram Tu, Miwako Shobo, Karin Kettisen, Lei Ye, Leif Bülow, Yoji Hakamata, Tetsuya Furuya, Ryutaro Asano, Wakako Tsugawa, and et al. 2024. "Potential of Enzymatically Synthesized Hemozoin Analog as Th1 Cell Adjuvant" Nanomaterials 14, no. 17: 1440. https://doi.org/10.3390/nano14171440








