Self-Assembled Hybrid Halide Perovskite Quantum Wire Bundle/Dot for Multiband Applications
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
2.2.1. Precursor Synthesis
2.2.2. Synthesis of Perovskite Wire Crystal Structure
2.3. Characterization
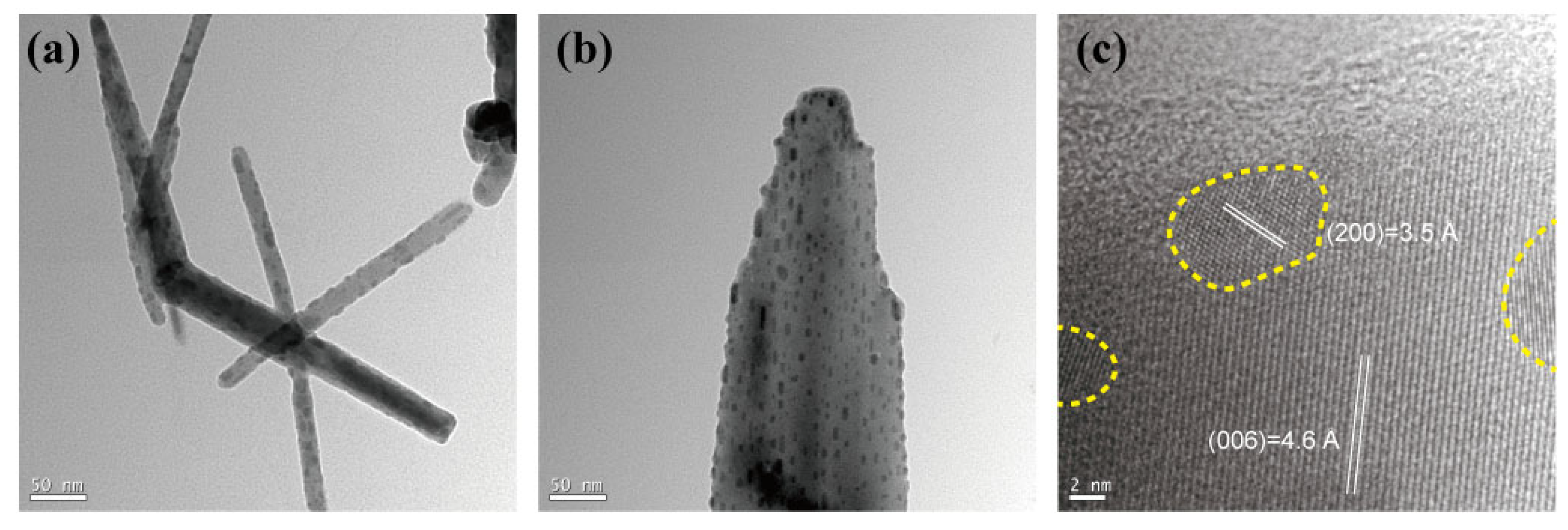
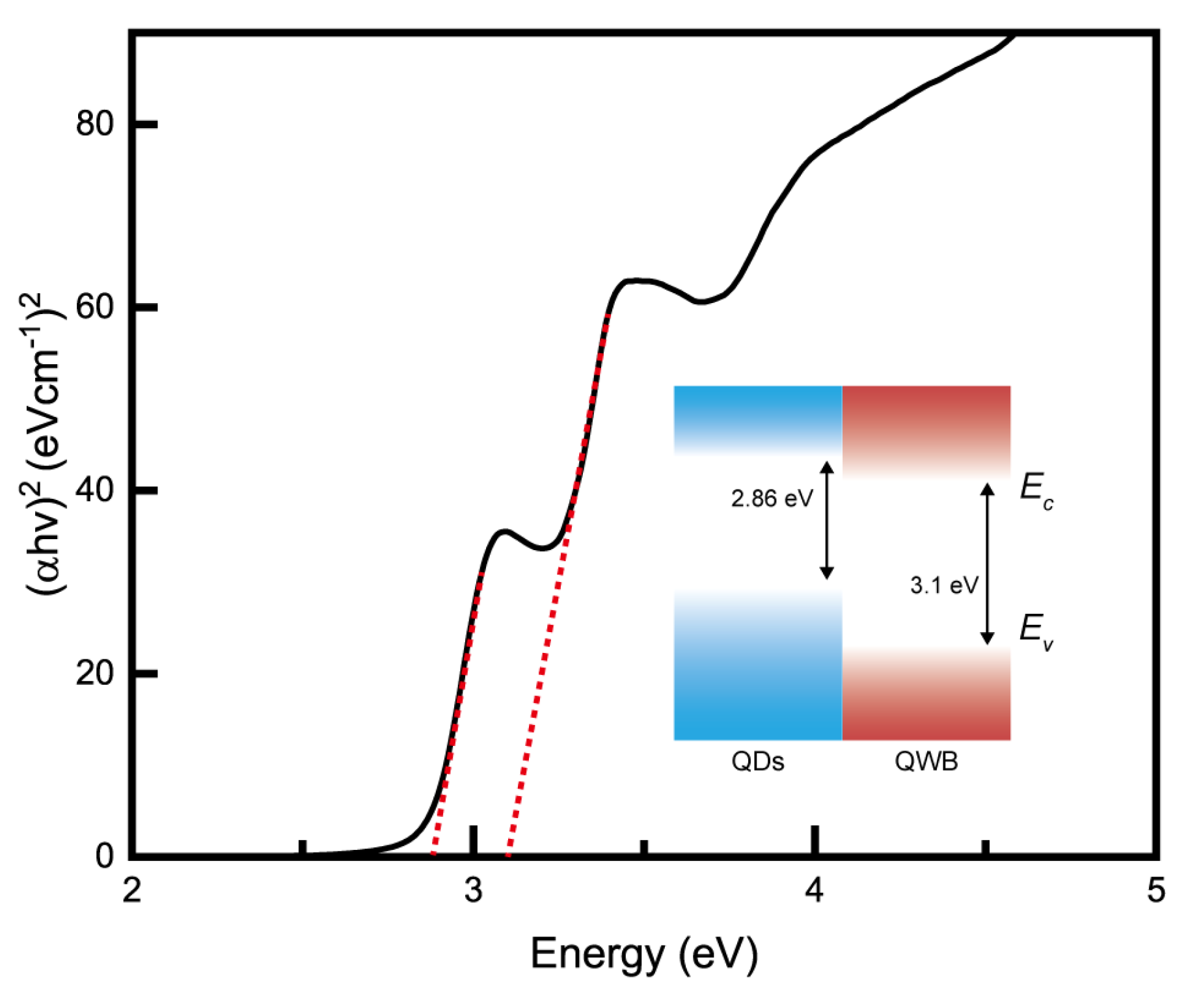
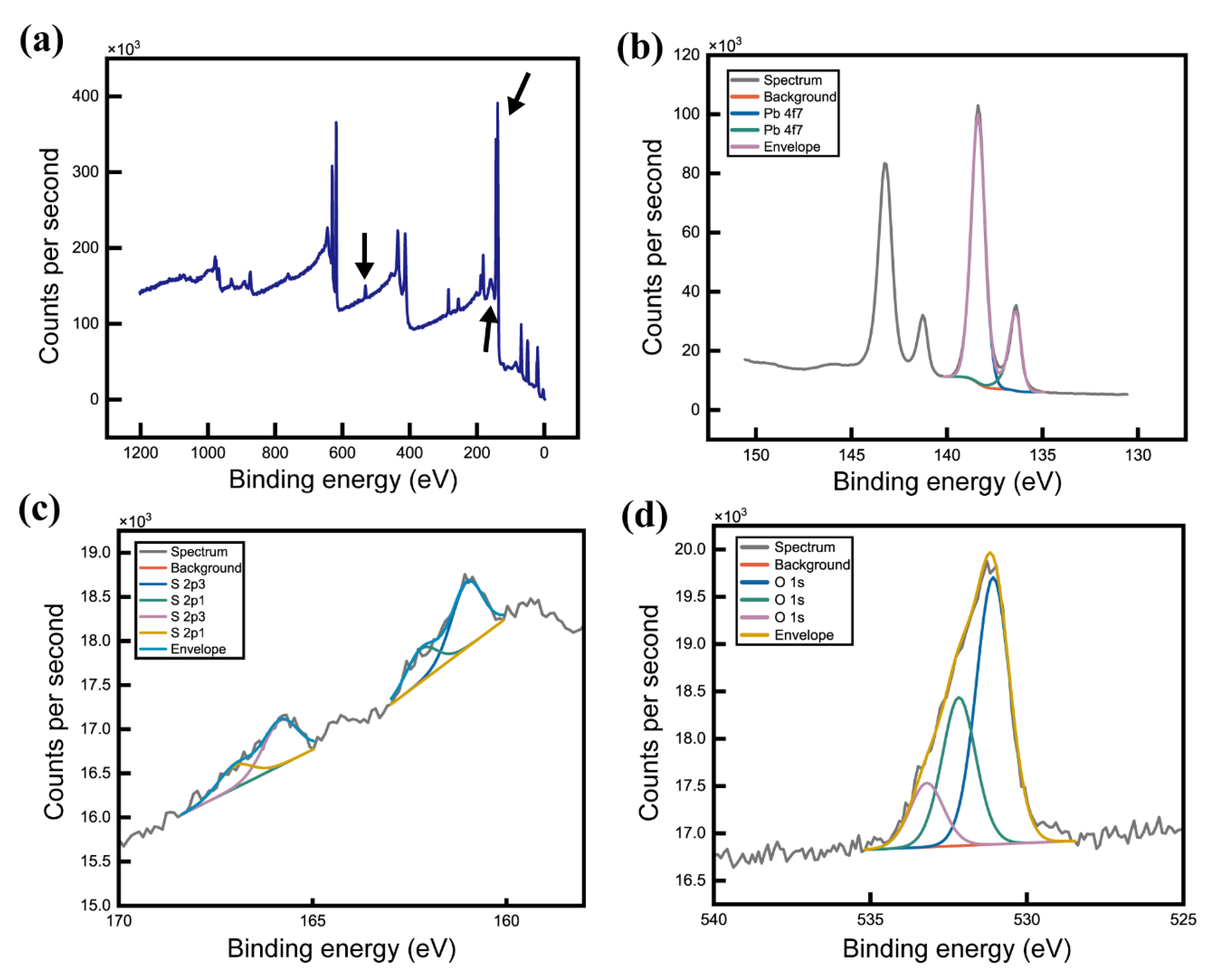
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Ravanat, J.L.; Douki, T.; Cadet, J. Direct and indirect effects of UV radiation on DNA and its components. J. Photochem. Photobiol. B Biol. 2001, 63, 88–102. [Google Scholar] [CrossRef] [PubMed]
- Lawrence, K.P.; Douki, T.; Sarkany, R.P.E.; Acker, S.; Herzog, B.; Young, A.R. The UV/visible radiation boundary region (385–405 nm) damages skin cells and induces “dark” cyclobutane pyrimidine dimers in human skin in vivo. Sci. Rep. 2018, 8, 12722. [Google Scholar] [CrossRef] [PubMed]
- Rastogi, R.P.; Richa; Kumar, A.; Tyagi, M.B.; Sinha, R.P. Molecular mechanisms of ultraviolet radiation-induced DNA damage and repair. J. Nucleic Acid. 2010, 2010, 592980. [Google Scholar] [CrossRef]
- Hockberger, P.E. The discovery of the damaging effect of sunlight on bacteria. J. Photochem. Photobiol. B Biol. 2000, 58, 185–191. [Google Scholar] [CrossRef]
- Richardson, T.B.; Porter, C.D. Inactivation of murine leukemia virus by exposure to visible light. Virology 2005, 341, 321–329. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Schuit, M.; Gardner, S.; Wood, S.; Bower, K. The influence of simulated sunlight on the inactivation of influenza virus in aerosols. J. Infect. Dis. 2020, 221, 372–378. [Google Scholar] [CrossRef]
- Lytle, C.D.; Sagripanti, J.L. Predicted inactivation of viruses of relevance to biodefense by solar radiation. J. Virol. 2005, 79, 14244–14252. [Google Scholar] [CrossRef]
- Rywkin, S.; Ben-Hur, E.; Malik, Z.; Prince, A.M.; Li, Y.S.; Kenney, M.E.; Oleinick, N.L.; Horowitz, B. New phthalocyanines for photodynamic virus inactivation in red blood cell concentrates. Photochem. Photobiol. 1994, 60, 165–170. [Google Scholar] [CrossRef]
- Käsermann, F.; Kempf, C. Photodynamic inactivation of enveloped viruses by buckminsterfullerene. Antivir. Res. 1997, 34, 65–70. [Google Scholar] [CrossRef]
- Tryba, B.; Wozniak, M.; Zolnierkiewicz, G.; Guskos, N.; Morawski, A.; Colbeau-Justin, C.; Wrobel, R.; Nitta, A.; Ohtani, B. Influence of an electronic structure of N-TiO2 on its photocatalytic activity towards decomposition of acetaldehyde under UV and fluorescent lamps irradiation. Catalysts 2018, 8, 85. [Google Scholar] [CrossRef]
- Wang, L.; Jin, J.; Mi, C.; Hao, Z.; Luo, Y.; Sun, C.; Han, Y.; Xiong, B.; Wang, J.; Li, H. A review on experimental measurements for understanding efficiency droop in InGaN-based light-emitting diodes. Materials 2017, 10, 1233. [Google Scholar] [CrossRef]
- Chouhan, L.; Ghimire, S.; Challapalli, S.; Miyasaka, T. Synthesis, optoelectronic properties and applications of halide perovskites. Chem. Soc. Rev. 2020, 49, 2869–2885. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; Wang, Q.; Han, L.; Zhao, Y.; He, Z.; Song, W.; Zong, C.; Miao, Z. Spintronic phenomena and applications in hybrid organic–inorganic perovskites. Adv. Func. Mater. 2024, 34, 2314427. [Google Scholar] [CrossRef]
- Kim, Y.S.; Jin, Z.; Park, M.W.; Jeon, H.C.; Lim, J.Y. Multiferroic-field coupling in ultrathin nanofilm halide perovskite at room temperature, Mater. Today Phys. 2023, 35, 101109. [Google Scholar]
- Tan, Z.K.; Moghaddam, R.S.; Lai, M.L.; Docampo, P.; Higler, R.; Deschler, F.; Price, M.; Sadhanala, A.; Pazos, L.M.; Credgington, D.; et al. Bright light-emitting diodes based on organometal halide perovskite. Nat. Nanotechnol. 2014, 9, 687–692. [Google Scholar] [CrossRef] [PubMed]
- Dou, L.; Wong, A.B.; Yu, Y.; Lai, M.; Kornienko, N.; Eaton, S.W.; Fu, A.; Bischak, C.G.; Ma, J.; Ding, T.; et al. Atomically thin two-dimensional organic-inorganic hybrid perovskites. Science 2015, 349, 1518–1521. [Google Scholar] [CrossRef]
- Fang, X.; Zhang, K.; Li, Y.; Yao, L.; Zhang, Y.; Wang, Y.; Zhai, W.; Tao, L.; Du, H.; Ran, G. Effect of excess PbBr2 on photoluminescence spectra of CH3NH3PbBr3 perovskite particles at room temperature. Appl. Phys. Lett. 2016, 108, 7. [Google Scholar] [CrossRef]
- Dong, J.; Zhao, Y.; Ouyang, G.; Yang, G. A perspective on optimizing photoelectric conversion process in 2D transition-metal dichalcogenides and related heterostructures. Appl. Phy. Lett. 2022, 120, 8. [Google Scholar] [CrossRef]
- Cai, B.; Zhao, Y.; Xu, D.; Ouyang, G. Optimized photoelectric conversion properties of PbSxSe1−x−QD/MoS2-NT 0D–1D mixed-dimensional van der Waals heterostructures. New J. Phys. 2022, 24, 063012. [Google Scholar] [CrossRef]
- Cai, B.; Tan, J.; Zhang, L.; Xu, D.; Dong, J.; Ouyang, G. Ultrafast interfacial charge transfer and superior photoelectric conversion properties in one-dimensional Janus-MoSSe/WSe2 van der Waals heterostructures. Phys. Rev. B 2023, 108, 045416. [Google Scholar] [CrossRef]
- Yuan, Z.; Zhou, C.; Tian, Y.; Shu, Y.; Messier, J.; Wang, J.C.; van de Burgt, L.J.; Kountouriotis, K.; Xin, Y.; Holt, E.; et al. One-dimensional organic lead halide perovskites with efficient bluish white-light emission. Nat. Commun. 2017, 8, 14051. [Google Scholar] [CrossRef] [PubMed]
- Petrov, A.A.; Graetzel, M.; Pellet, N.; Seo, J.Y.; Belich, N.A.; Kovalev, D.Y.; Shevelkov, A.V.; Goodilin, E.A.; Zakeeruddin, S.M.; Tarasov, A.B.; et al. New insight into the formation of hybrid perovskite nanowires via structure directing adducts. Chem. Mater. 2017, 29, 587–594. [Google Scholar] [CrossRef]
- Rashad, M.M.; Elseman, A.M.; Hassan, A.M. Facile synthesis, characterization and structural evolution of nanorods single-crystalline (C4H9NH3)2PbI2X2 mixed halide organometal perovskite for solar cell application. Optik 2016, 127, 9775–9787. [Google Scholar] [CrossRef]
- Berry, J.; Buonassisi, T.; Egger, D.A.; Hodes, G.; Kronik, L.; Loo, Y.-L.; Lubomirsky, I.; Marder, S.R.; Mastai, Y.; Miller, J.S.; et al. Hybrid organic–inorganic perovskites (HOIPs): Opportunities and challenges. Adv. Mat. 2015, 27, 5102–5112. [Google Scholar] [CrossRef]
- Ahmadian-Yazdi, M.R.; Zabihi, F.; Habibi, M.; Eslamian, M. Effects of process parameters on the characteristics of mixed-halide perovskite solar cells fabricated by one-step and two-step sequential coating. Nanoscale Res. Lett. 2016, 11, 408. [Google Scholar] [CrossRef]
- Chang, Y.H.; Lin, J.C.; Chen, Y.C.; Kuo, T.R.; Wand, D.Y. Facile synthesis of two-dimensional Ruddlesden–Popper perovskite quantum dots with fine-tunable optical properties. Nanoscale Res. Lett. 2018, 13, 1–7. [Google Scholar] [CrossRef]
- Horváth, E.; Spina, M.; Szekrényes, Z.; Kamarás, K.; Gaal, R.; Gachet, D.; Forró, L. Nanowires of methylammonium lead iodide (CH3NH3PbI3) prepared by low temperature solution-mediated crystallization. Nano Lett. 2014, 14, 6761–6766. [Google Scholar] [CrossRef]
- Im, J.H.; Luo, J.; Franckevičius, M.; Pellet, N.; Gao, P.; Moehl, T.; Zakeeruddin, S.M.; Nazeeruddin, M.K.; Grätzel, M.; Park, N.G. Nanowire perovskite solar cell. Nano Lett. 2015, 15, 2120–2126. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jeon, H.C.; Kim, S.; Kim, Y.-S. Self-Assembled Hybrid Halide Perovskite Quantum Wire Bundle/Dot for Multiband Applications. Nanomaterials 2024, 14, 1443. https://doi.org/10.3390/nano14171443
Jeon HC, Kim S, Kim Y-S. Self-Assembled Hybrid Halide Perovskite Quantum Wire Bundle/Dot for Multiband Applications. Nanomaterials. 2024; 14(17):1443. https://doi.org/10.3390/nano14171443
Chicago/Turabian StyleJeon, Hee Chang, Seonghwan Kim, and Young-Seong Kim. 2024. "Self-Assembled Hybrid Halide Perovskite Quantum Wire Bundle/Dot for Multiband Applications" Nanomaterials 14, no. 17: 1443. https://doi.org/10.3390/nano14171443
APA StyleJeon, H. C., Kim, S., & Kim, Y.-S. (2024). Self-Assembled Hybrid Halide Perovskite Quantum Wire Bundle/Dot for Multiband Applications. Nanomaterials, 14(17), 1443. https://doi.org/10.3390/nano14171443





