Toxicological Effects of Leachates Extracted from Photocatalytic Concrete Blocks with Nano-TiO2 on Daphnia magna
Abstract
1. Introduction
2. Materials
2.1. Nanoparticles
2.2. Cement
2.3. Mineral Aggregates
3. Methods
3.1. Production of Concrete Blocks for Paving
3.2. Leaching Process of the Concrete
3.3. Characterization of the Leachate Extracts
3.4. Toxicological Assessment of the Leachate Extracts in D. magna
3.4.1. Acute Toxicity in D. magna
3.4.2. Chronic Toxicity in D. magna
4. Results and Discussion
4.1. Characterization of the Leachate Extracts
4.2. Acute Toxicity in D. magna
4.3. Chronic Toxicity in D. magna
5. Conclusions and Future Prospects
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Meng, C.; Dong, P.; Tian, H.; Cheng, T.; Li, J.; Liu, Y.; Yang, X.; Xie, M.; Chen, X.; Xi, X. Photocatalytic Concrete Paving Block Reinforced by TiO2 Nanotubes for NO Removal. J. Mater. Sci. 2020, 55, 14280–14291. [Google Scholar] [CrossRef]
- de Rosso, L.T.; de Melo, J.V.S. Translucent Photocatalytic Concrete Blocks for Paving: Impact of Service Life on the Textural Characteristics and Photocatalytic Efficiency. Mater. Struct. 2023, 56, 142. [Google Scholar] [CrossRef]
- Ansari, M.A.; Shariq, M.; Ansari, S.S.; Husain, A. Efficiency Assessment of TiO2-Based Photocatalytic Concrete for Clean and Sustainable Construction: A State-of-the-Art Review. Iran. J. Sci. Technol. Trans. Civ. Eng. 2024. [Google Scholar] [CrossRef]
- Guo, M.-Z.; Poon, C.-S. Photocatalytic NO Removal of Concrete Surface Layers Intermixed with TiO2. Build. Environ. 2013, 70, 102–109. [Google Scholar] [CrossRef]
- Talaiekhozani, A.; Rezania, S.; Kim, K.-H.; Sanaye, R.; Amani, A.M. Recent Advances in Photocatalytic Removal of Organic and Inorganic Pollutants in Air. J. Clean. Prod. 2021, 278, 123895. [Google Scholar] [CrossRef]
- Zouzelka, R.; Martiniakova, I.; Duchacek, T.; Muzikova, B.; Mikyskova, E.; Rathousky, J. Photocatalytic Abatement of Air Pollutants: Focus on Their Interference in Mixtures. J. Photochem. Photobiol. A Chem. 2023, 434, 114235. [Google Scholar] [CrossRef]
- Kim, Y.K.; Hong, S.J.; Kim, H.B.; Lee, S.W. Evaluation of In-Situ NOx Removal Efficiency of Photocatalytic Concrete in Expressways. KSCE J. Civ. Eng. 2017, 22, 2274–2280. [Google Scholar] [CrossRef]
- Xie, X.; Hao, C.; Huang, Y.; Huang, Z. Influence of TiO2-Based Photocatalytic Coating Road on Traffic-Related NOx Pollutants in Urban Street Canyon by CFD Modeling. Sci. Total Environ. 2020, 724, 138059. [Google Scholar] [CrossRef]
- Boonen, E.; Beeldens, A. Recent Photocatalytic Applications for Air Purification in Belgium. Coatings 2014, 4, 553–573. [Google Scholar] [CrossRef]
- Padmanabhan, N.T.; John, H. Titanium Dioxide Based Self-Cleaning Smart Surfaces: A Short Review. J. Environ. Chem. Eng. 2020, 8, 104211. [Google Scholar] [CrossRef]
- Chen, K.; Qu, F.; Huang, Y.; Cai, J.; Wu, F.; Li, W. Advancing Photocatalytic Concrete Technologies in Design, Performance and Application for a Sustainable Future. Adv. Nanocompos. 2024, 1, 180–200. [Google Scholar] [CrossRef]
- García, A.; Castro-Fresno, D.; Polanco, J.A.; Thomas, C. Abrasive Wear Evolution in Concrete Pavements. Road Mater. Pavement Des. 2012, 13, 534–548. [Google Scholar] [CrossRef]
- Folkeson, L.; Bækken, T.; Brenčič, M.; Dawson, A.; Frančois, D.; Kuřímská, P.; Leitão, T.; Ličbinský, R.; Vojtěšek, M. Sources and Fate of Water Contaminants in Roads. In Water in Road Structures; Dawson, A., Ed.; Springer: Dordrecht, The Netherlands, 2009; pp. 107–146. [Google Scholar] [CrossRef]
- Jimenez-Relinque, E.; Grande, M.; Duran, T.; Castillo, Á.; Castellote, M. Environmental Impact of Nano-Functionalized Construction Materials: Leaching of Titanium and Nitrates from Photocatalytic Pavements under Outdoor Conditions. Sci. Total Environ. 2020, 744, 140817. [Google Scholar] [CrossRef]
- Diamond, S.A.; Kennedy, A.J.; Melby, N.L.; Moser, R.D.; Poda, A.R.; Weiss, C.A., Jr.; Brame, J.A. Assessment of the Potential Hazard of Nano-Scale TiO2 in Photocatalytic Cement: Application of a Tiered Assessment Framework. NanoImpact 2017, 8, 11–19. [Google Scholar] [CrossRef]
- Turan, N.B.; Erkan, H.S.; Engin, G.O.; Bilgili, M.S. Nanoparticles in the Aquatic Environment: Usage, Properties, Transformation and Toxicity—A Review. Process Saf. Environ. Prot. 2019, 130, 238–249. [Google Scholar] [CrossRef]
- Zhang, W.; Xiao, B.; Fang, T. Chemical Transformation of Silver Nanoparticles in Aquatic Environments: Mechanism, Morphology and Toxicity. Chemosphere 2018, 191, 324–334. [Google Scholar] [CrossRef] [PubMed]
- Nanostructured & Amorphous Materials, Inc. Available online: https://www.nanoamor.com/home (accessed on 20 August 2019).
- Muller, R.H.; Shegokar, R.; Keck, C.M. 20 Years of Lipid Nanoparticles (SLN & NLC): Present State of Development & Industrial Applications. Curr. Drug Discov. Technol. 2011, 8, 207–227. [Google Scholar] [CrossRef] [PubMed]
- Riddick, T.M. Control of Colloid Stability through Zeta Potential: With a Closing Chapter on Its Relationship to Cardiovascular Disease; Livingston Publishing Company: Wynnewood, PA, USA, 1968. [Google Scholar]
- International Centre for Diffraction Data. Available online: https://www.icdd.com/ (accessed on 26 January 2020).
- ABNT NBR 16697; Cimento Portland—Requisitos. Associação Brasileira de Normas Técnicas: São Paulo, SP, Brazil, 2018.
- ASTM C150/C150M; Standard Specification for Portland Cement. ASTM International: West Conshohocken, PA, USA, 2020.
- ASTM C117; Standard Test Method for Materials Finer than 75-μm (No. 200) Sieve in Mineral Aggregates by Washing. ASTM International: West Conshohocken, PA, USA, 2017.
- ASTM C127; Standard Test Method for Relative Density (Specific Gravity) and Absorption of Coarse Aggregate. ASTM International: West Conshohocken, PA, USA, 2015.
- Talbot, A.N.; Richart, F.E. The Strength of Concrete, Its Relation to the Cement Aggregates and Water; Engineering Experiment Station Bulletin No. 137; University of Illinois: Urbana-Champagne, IL, USA, 1923; p. 122. [Google Scholar]
- ASTM C39/C39M; Standard Test Method for Compressive Strength of Cylindrical Concrete Specimens. ASTM International: West Conshohocken, PA, USA, 2018.
- ASTM C192/C192M; Standard Practice for Making and Curing Concrete Test Specimens in the Laboratory. ASTM International: West Conshohocken, PA, USA, 2018.
- ABNT NBR 10005; Procedimento para Obtenção de Extrato Lixiviado de Resíduos Sólidos. Associação Brasileira de Normas Técnicas: São Paulo, SP, Brazil, 2004.
- ABNT NBR 12713; Ecotoxicologia Aquática—Toxicidade Aguda—Método de Ensaio com Daphnia spp (Crustacea, Cladocera). Associação Brasileira de Normas Técnicas: São Paulo, SP, Brazil, 2016.
- ISO 6341; Water Quality—Determination of the Inhibition of the Mobility of Daphnia magna Straus (Cladocera, Crustacea)—Acute Toxicity Test. International Organization for Standardization: Geneva, Switzerland, 2012.
- Nogueira, D.J.; Vaz, V.P.; Neto, O.S.; Silva, M.L.N.; Simioni, C.; Ouriques, L.C.; Vicentini, D.S.; Matias, W.G. Crystalline Phase-Dependent Toxicity of Aluminum Oxide Nanoparticles toward Daphnia magna and Ecological Risk Assessment. Environ. Res. 2020, 182, 108987. [Google Scholar] [CrossRef]
- Costa Puerari, R.; Gonçalves, R.A.; Mottim Justino, N.; Schulz Vicentini, D.; Gerson Matias, W. The Influence of Amine-Functionalized SiO2 Nanostructures upon Nanofiltration Membranes. Environ. Nanotechnol. Monit. Manag. 2020, 13, 100287. [Google Scholar] [CrossRef]
- Totoni Mofrad, F.; Jonidi Jafari, A.; Kermani, M.; Farzadkia, M.; Esrafili, A. 4-Chloro-2-Methylphenoxyacetic Acid Photo-Reduction with Sulfite Excitation under UV Irradiation: Kinetics, Energy Consumption, Operational Variables and Toxicology with Daphnia magna. Desalination Water Treat. 2024, 317, 100276. [Google Scholar] [CrossRef]
- Ghosh, M.; Dey, P.; Das, A.; Giri, A.; Nath, S.; Giri, S. Evaluation of Arsenic Induced Genotoxicity and Its Impact on Life Processes of Daphnia magna. Mutat. Res. Genet. Toxicol. Environ. Mutagen. 2024, 899, 503804. [Google Scholar] [CrossRef]
- Van Leeuwen, K.; Van Leeuwen, C.J. Technical Guidance Document on Risk Assessment in Support of Commission Directive 93/67/EEC on Risk Assessment for New Notified Substances and Commission Regulation (EC) No 1488/94 on Risk Assessment for Existing Substances Part II; European Commission: Brussels, Belgium, 1996; pp. 1–337.
- ISO 10706; Water Quality—Determination of Long Term Toxicity of Substances to Daphnia magna Straus (Cladocera, Crustacea). International Organization for Standardization: Geneva, Switzerland, 2000.
- OCED. Test No. 211: Daphnia magna Reproduction Test. In OECD Guidelines for the Testing of Chemicals, Section 2; OCED: Paris, France, 2012. [Google Scholar] [CrossRef]
- Kumar, P.M.; Badrinarayanan, S.; Sastry, M. Nanocrystalline TiO2 Studied by Optical, FTIR and X-ray Photoelectron Spectroscopy: Correlation to Presence of Surface States. Thin Solid Films 2000, 358, 122–130. [Google Scholar] [CrossRef]
- Chougala, L.S.; Yatnatti, M.S.; Linganagoudar, R.K.; Kamble, R.R.; Kadadevarmath, J.S. A Simple Approach on Synthesis of TiO2 Nanoparticles and Its Application in Dye Sensitized Solar Cells. J. Nano Electron. Phys. 2017, 9, 04005-1–04005-6. [Google Scholar] [CrossRef]
- Cheng, X.; Shang, Y.; Cui, Y.; Shi, R.; Zhu, Y.; Yang, P. Enhanced Photoelectrochemical and Photocatalytic Properties of Anatase-TiO2(B) Nanobelts Decorated with CdS Nanoparticles. Solid State Sci. 2020, 99, 106075. [Google Scholar] [CrossRef]
- Li, H.; Du, T.; Xiao, H.; Zhang, Q. Crystallization of Calcium Silicate Hydrates on the Surface of Nanomaterials. J. Am. Ceram. Soc. 2017, 100, 3227–3238. [Google Scholar] [CrossRef]
- Avramescu, M.-L.; Chénier, M.; Palaniyandi, S.; Rasmussen, P.E. Dissolution Behavior of Metal Oxide Nanomaterials in Cell Culture Medium Versus Distilled Water. J. Nanopart. Res. 2020, 22, 222. [Google Scholar] [CrossRef]
- Novak, S.; Jemec Kokalj, A.; Hočevar, M.; Godec, M.; Drobne, D. The Significance of Nanomaterial Post-Exposure Responses in Daphnia Magna Standard Acute Immobilisation Assay: Example with Testing TiO2 Nanoparticles. Ecotoxicol. Environ. Saf. 2018, 152, 61–66. [Google Scholar] [CrossRef] [PubMed]
- Zhu, X.; Chang, Y.; Chen, Y. Toxicity and Bioaccumulation of TiO2 Nanoparticle Aggregates in Daphnia Magna. Chemosphere 2010, 78, 209–215. [Google Scholar] [CrossRef]
- Heinlaan, M.; Ivask, A.; Blinova, I.; Dubourguier, H.-C.; Kahru, A. Toxicity of Nanosized and Bulk ZnO, CuO and TiO2 to Bacteria Vibrio fischeri and Crustaceans Daphnia magna and Thamnocephalus platyurus. Chemosphere 2008, 71, 1308–1316. [Google Scholar] [CrossRef]
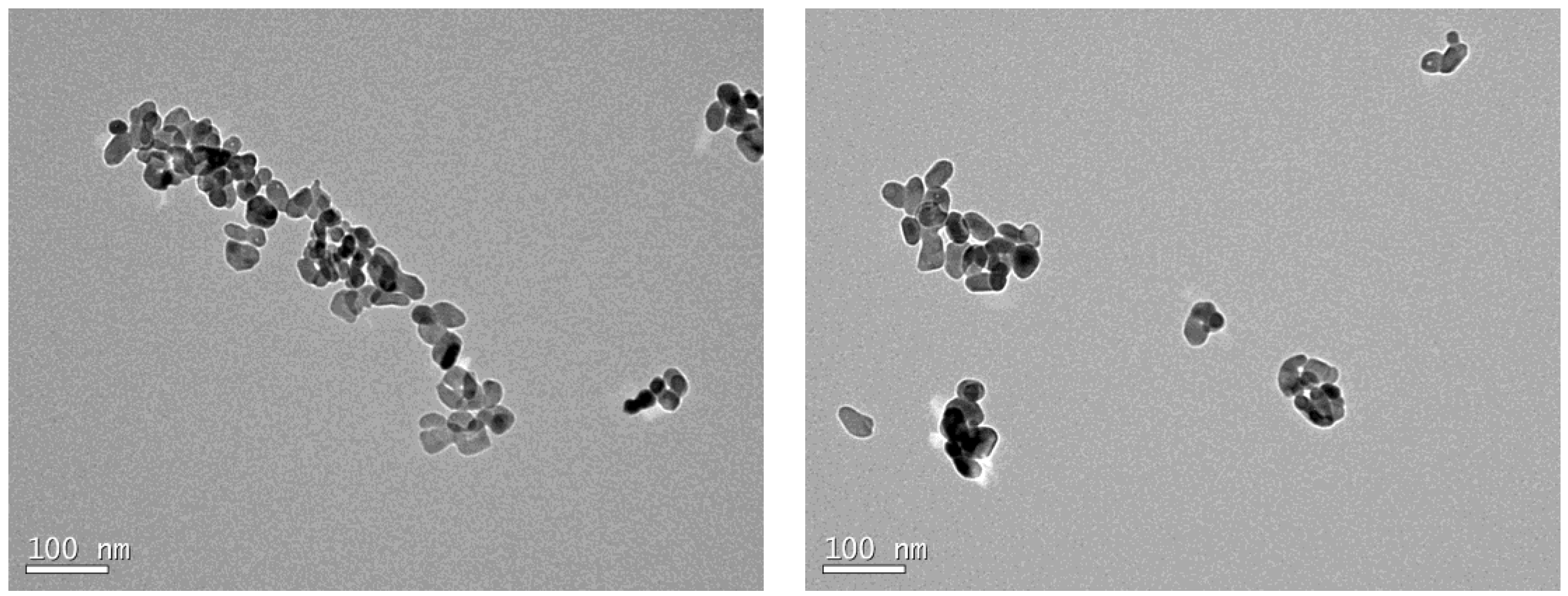
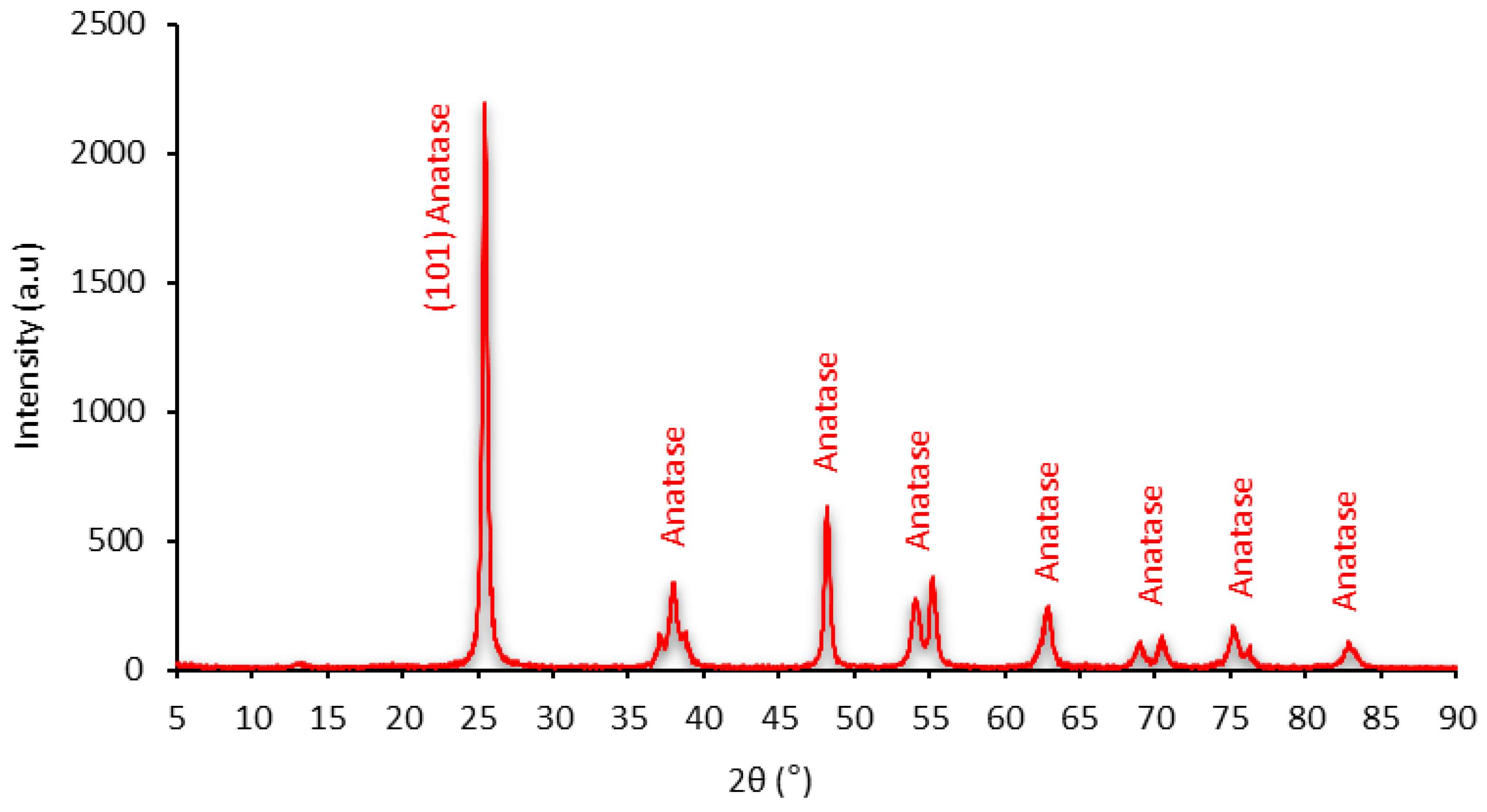


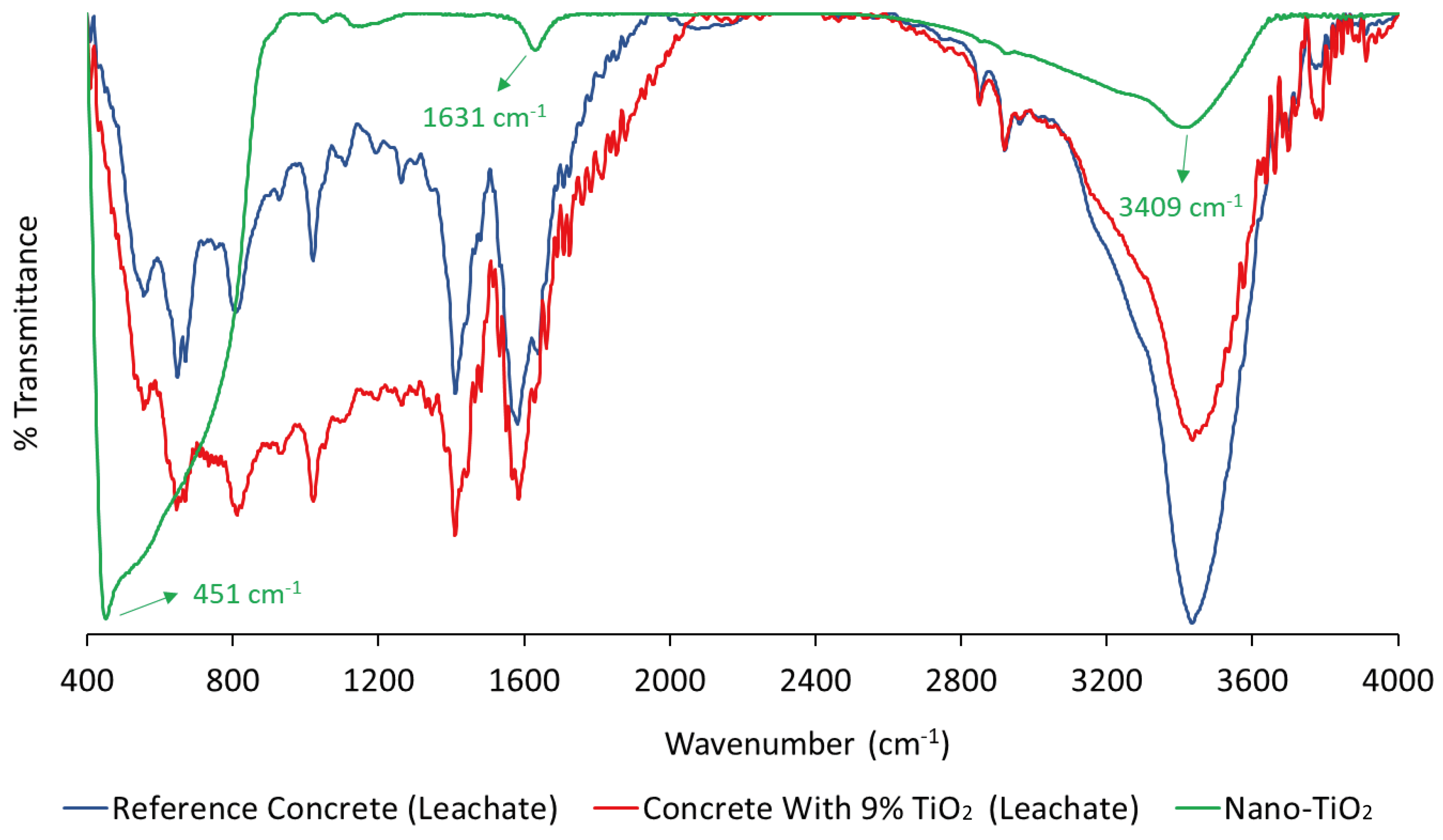
| Characteristics | Nano-TiO2 |
|---|---|
| Purity | >99% |
| Trace elements | Aluminum (Al) ≤ 17 ppm Magnesium (Mg) ≤ 65 ppm Silicon (Si) ≤ 120 ppm Calcium (Ca) ≤ 75 ppm Sulfur (S) ≤ 130 ppm Niobium (Nb) ≤ 80 ppm |
| Average particle size | 10 nm |
| Morphology of particles | Ellipsoidal and spherical |
| Specific surface area | ≥60 m2/g |
| Crystallographic structure | Anatase |
| Bulk density | 0.2–0.3 g/cm3 |
| Density (at 20 °C) | 3.9 |
| Nano | Test | Medium | ||
|---|---|---|---|---|
| Ultrapure Water | ISO | M4 | ||
| TiO2 | Zeta potential (mV) | 12.89 ± 0.38 | −2.85 ± 0.09 | 3.84 ± 0.35 |
| Effective diameter (nm) | 580.00 ± 1.39 | 1708.58 ± 6.96 | 1539.34 ± 8.12 | |
| Polydispersity index | 0.16 ± 0.03 | 0.21 ± 0.03 | 0.16 ± 0.01 | |
| pH | 7.21 | 7.90 | 7.62 | |
| Chemical Characteristics | |
| Loss on ignition | 5.43% |
| Magnesium oxide | 5.76% |
| Sulfur trioxide | 2.79% |
| Sodium oxide | 0.23% |
| Potassium oxide | 1.09% |
| Insoluble residue | 11.2% |
| Physical Characteristics | |
| Fineness—residue on the sieve—0.075 mm | 2.3% |
| Fineness—residue on the sieve—0.044 mm | 11.3% |
| Specific surface—Blaine | 372 m2/kg |
| Specific mass | 2.970 g/cm3 |
| Autoclave expansion | 0.73 mm |
| Water from the paste with normal consistency | 27.9% |
| Time of setting, not less than | 257 min |
| Time of setting, not more than | 335 min |
| Compressive strength (3 days) | 33.9 MPa |
| Compressive strength (7 days) | 39.2 MPa |
| Compressive strength (28 days) | 47.4 MPa |
| Concentrations | Nano-TiO2 (mg/L) | Reference Concrete (mL/L) | Concrete with 9% TiO2 (mL/L) |
|---|---|---|---|
| C0 (control) | 0 | 0 | 0 |
| C1 | 0.63 | 0.25 | 0.5 |
| C2 | 1.25 | 0.5 | 2.0 |
| C3 | 2.5 | 1.0 | 3.5 |
| C4 | 5.0 * | 2.0 | 5.0 ** |
| C5 | 10 | 4.0 | 6.5 |
| Pure Powders of Nanoparticles: Nano-TiO2 | ||||
|---|---|---|---|---|
| Concentrations (mg/L) | Longevity (%) | Size (Length) ± SD (mm) | Reproduction ± SD (Number of Neonates) | Age of First Posture ± SD (Days) |
| 0 (Control) | 100 | 4.125 ± 0.34 | 3.392 ± 1.56 | 11.44 ± 1.01 |
| 0.63 | 70 | 4.385 ± 0.12 | 2.408 ± 1.84 | 12.00 ± 1.76 |
| 1.25 | 90 | 4.176 ± 0.12 | 2.919 ± 1.26 | 12.20 ± 1.98 |
| 2.5 | 90 | 4.371 ± 0.09 | 2.704 ± 1.16 | 11.67 ± 1.12 |
| 5.0 | 100 | 3.713 ± 0.24 * | 2.575 ± 0.63 | 12.70 ± 1.83 |
| 10 | 40 * | 3.624 ± 0.25 * | 0.417 ± 0.92 * | 14.00 ± 0.00 |
| LOEC (mg/L) | 10.0 | 5.0 | 10.0 | >10.0 (NF) |
| NOEC (mg/L) | 5.0 | 2.5 | 5.0 | >10.0 (NF) |
| Leachate: Reference Concrete | ||||
|---|---|---|---|---|
| Concentrations (mL/L) | Longevity (%) | Size (Length) ± SD (mm) | Reproduction ± SD (Number of Neonates) | Age of First Posture ± SD (Days) |
| 0 (Control) | 90 | 2.860 ± 0.13 | 3.533 ± 2.06 | 12.88 ± 1.13 |
| 0.25 | 100 | 2.766 ± 0.11 | 4.090 ± 1.97 | 12.70 ± 0.83 |
| 0.5 | 80 | 2.777 ± 0.13 | 4.550 ± 1.17 | 11.38 ± 1.30 |
| 1.0 | 90 | 2.740 ± 0.13 | 4.511 ± 1.09 | 13.00 ± 1.22 |
| 2.0 | 100 | 2.780 ± 0.09 | 4.510 ± 0.54 | 12.70 ± 2.40 |
| 4.0 | 100 | 2.714 ± 0.11 * | 3.560 ± 1.55 | 12.89 ± 1.16 |
| LOEC (mL/L) | >4.0 (NF) | 4.0 | >4.0 (NF) | >4.0 (NF) |
| NOEC (mL/L) | >4.0 (NF) | 2.0 | >4.0 (NF) | >4.0 (NF) |
| Leachate: Concrete with 9% TiO2 | ||||
|---|---|---|---|---|
| Concentrations (mL/L) | Longevity (%) | Size (Length) ± SD (mm) | Reproduction ± SD (Number of Neonates) | Age of First Posture ± SD (Days) |
| 0 (Control) | 100 | 2.854 ± 0.07 | 6.830 ± 0.99 | 10.40 ± 1.26 |
| 0.5 | 100 | 2.804 ± 0.08 | 5.670 ± 2.43 | 11.00 ± 1.58 |
| 2.0 | 100 | 2.843 ± 0.11 | 5.140 ± 0.89 | 11.00 ± 1.88 |
| 3.5 | 100 | 2.834 ± 0.06 | 5.850 ± 1.93 | 10.70 ± 1.63 |
| 5.0 | 100 | 2.858 ± 0.09 | 5.590 ± 0.58 | 10.30 ± 0.67 |
| 6.5 | 100 | 2.850 ± 0.05 | 5.480 ± 1.53 | 10.50 ± 1.58 |
| LOEC (mL/L) | >6.5 (NF) | >6.5 (NF) | >6.5 (NF) | >6.5 (NF) |
| NOEC (mL/L) | >6.5 (NF) | >6.5 (NF) | >6.5 (NF) | >6.5 (NF) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Facin, F.; Staub de Melo, J.V.; Costa Puerari, R.; Matias, W.G. Toxicological Effects of Leachates Extracted from Photocatalytic Concrete Blocks with Nano-TiO2 on Daphnia magna. Nanomaterials 2024, 14, 1447. https://doi.org/10.3390/nano14171447
Facin F, Staub de Melo JV, Costa Puerari R, Matias WG. Toxicological Effects of Leachates Extracted from Photocatalytic Concrete Blocks with Nano-TiO2 on Daphnia magna. Nanomaterials. 2024; 14(17):1447. https://doi.org/10.3390/nano14171447
Chicago/Turabian StyleFacin, Fernanda, João Victor Staub de Melo, Rodrigo Costa Puerari, and William Gerson Matias. 2024. "Toxicological Effects of Leachates Extracted from Photocatalytic Concrete Blocks with Nano-TiO2 on Daphnia magna" Nanomaterials 14, no. 17: 1447. https://doi.org/10.3390/nano14171447
APA StyleFacin, F., Staub de Melo, J. V., Costa Puerari, R., & Matias, W. G. (2024). Toxicological Effects of Leachates Extracted from Photocatalytic Concrete Blocks with Nano-TiO2 on Daphnia magna. Nanomaterials, 14(17), 1447. https://doi.org/10.3390/nano14171447






