A Facile and Green Approach for the Preparation of Silver Nanoparticles on Graphene Oxide with Favorable Antibacterial Activity
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of GO
2.3. Preparation of the Seed Extract
2.4. Synthesis of Silver Nanoparticles: Approach 1
2.5. Synthesis of Silver Nanoparticles: Approach 2
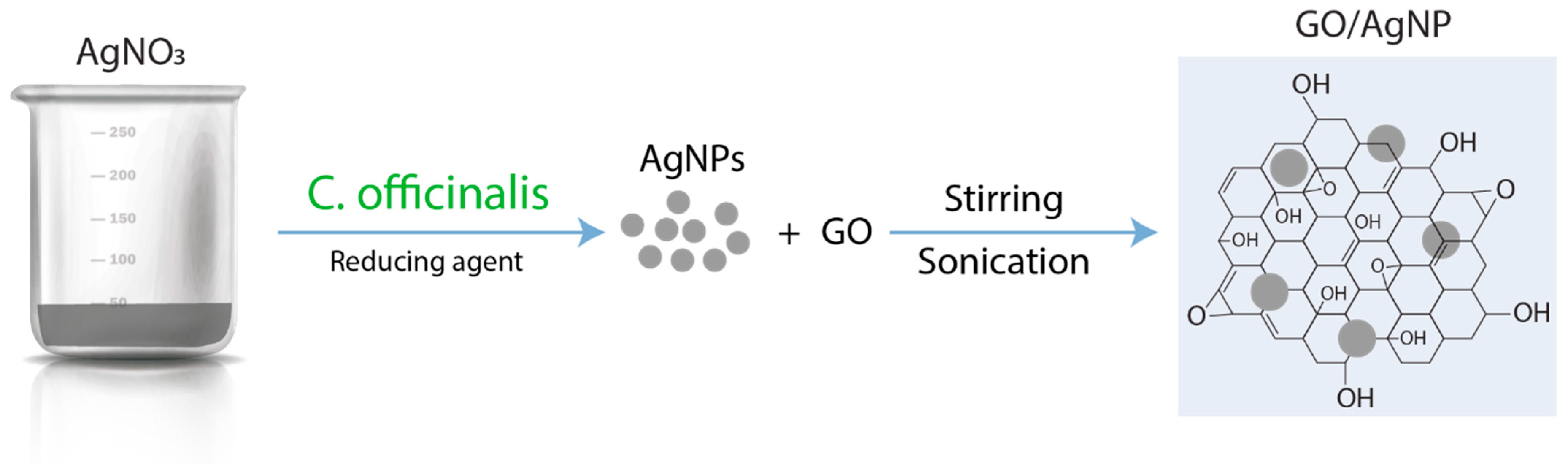
2.6. Preparation of GO/AgNP Composite
2.7. Culturing of Escherichia coli
2.8. Disk Diffusion Test
2.9. Minimum Bactericidal Concentration (MBC)
2.10. Colony-Forming Unit (CFU) Analysis
2.11. Characterization
3. Results and Discussion
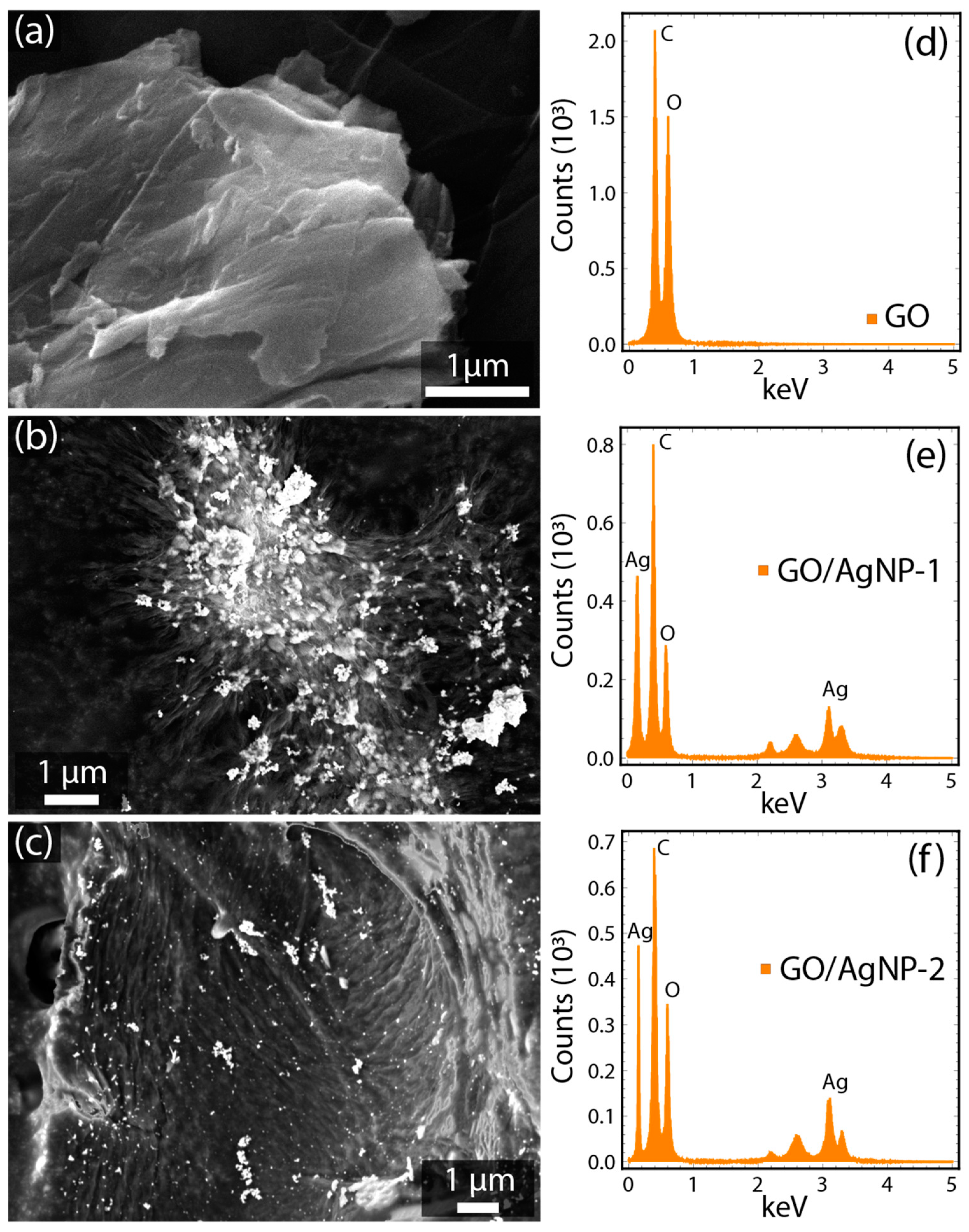
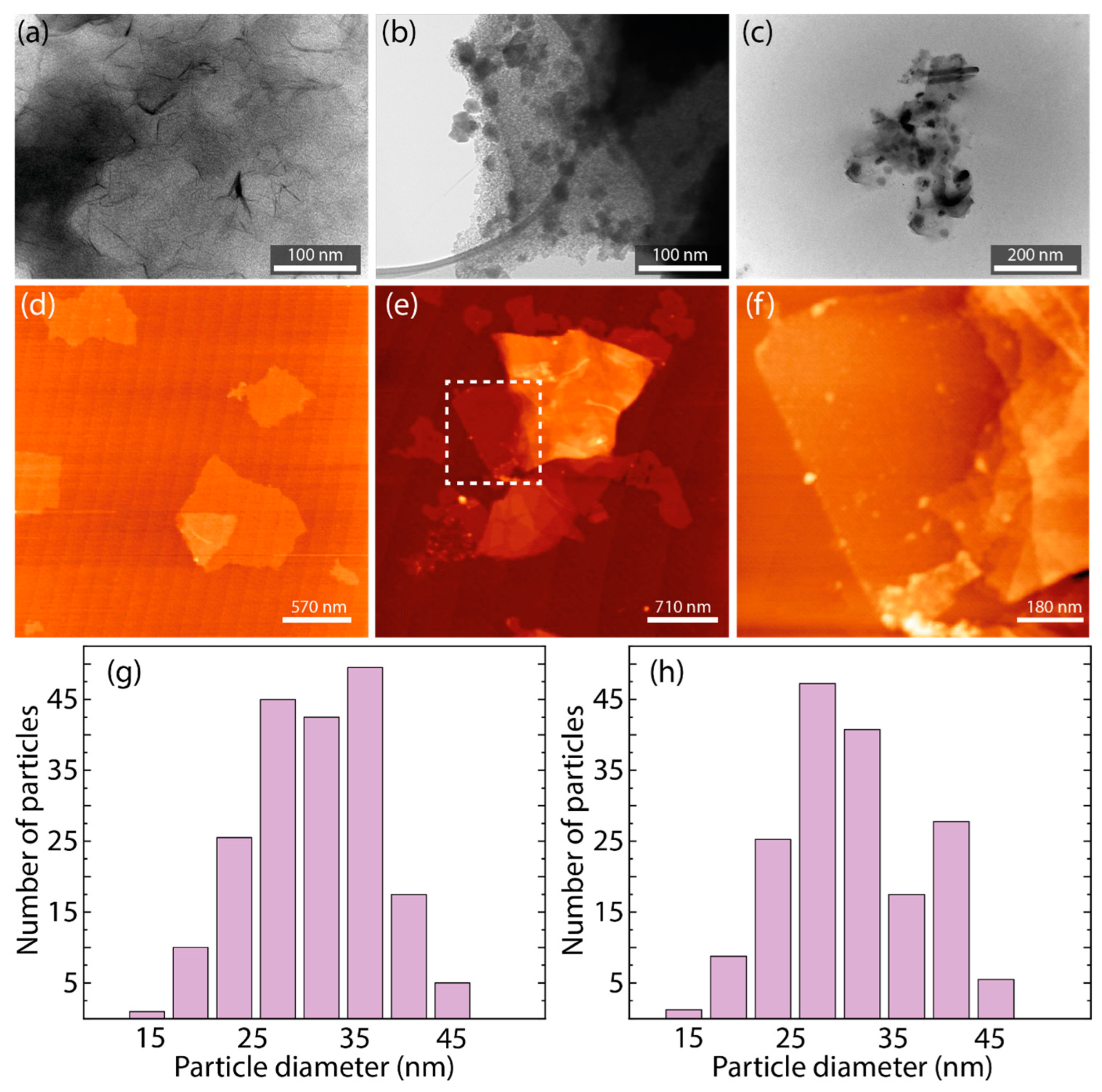
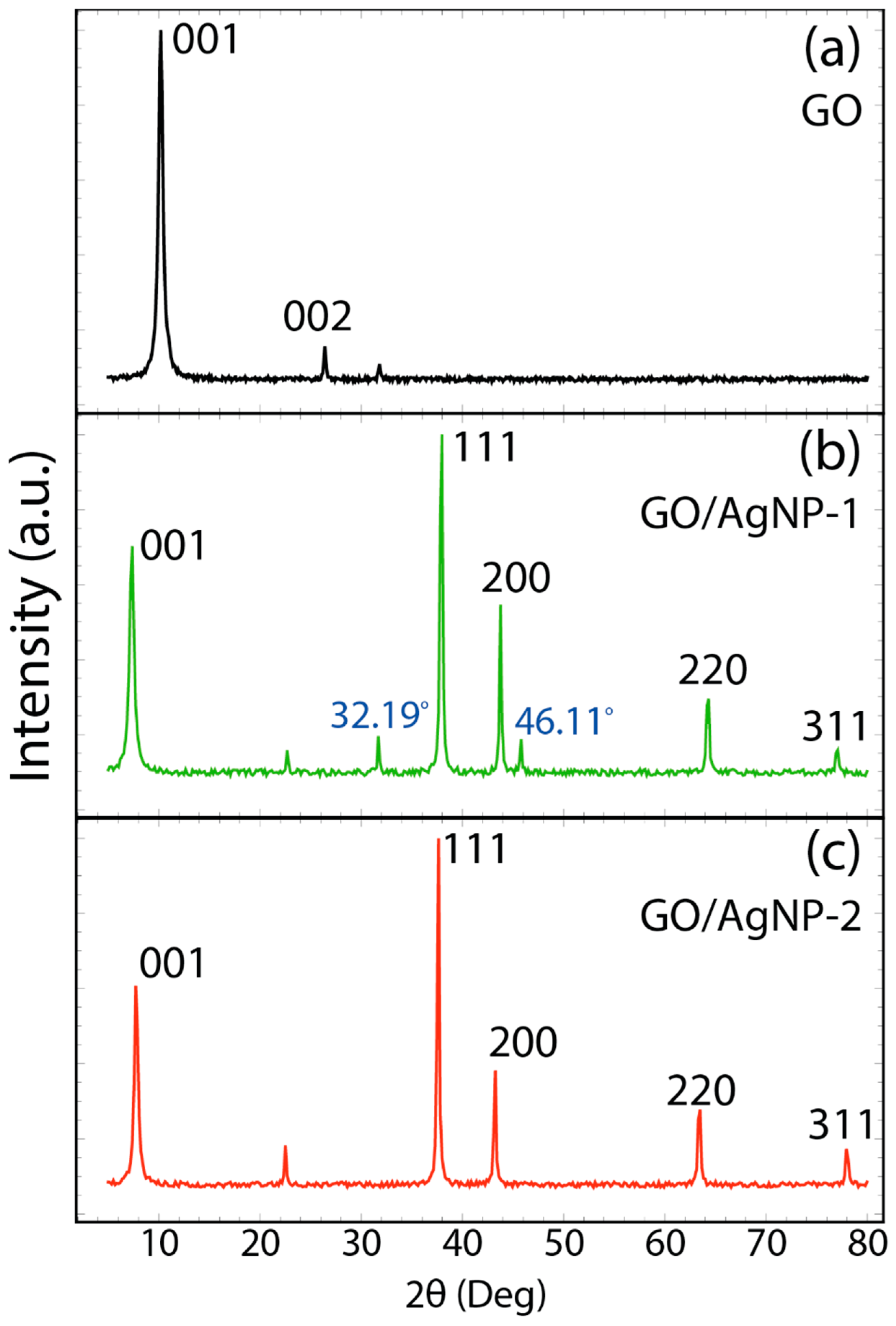
3.1. Morphological and Structural Characterization
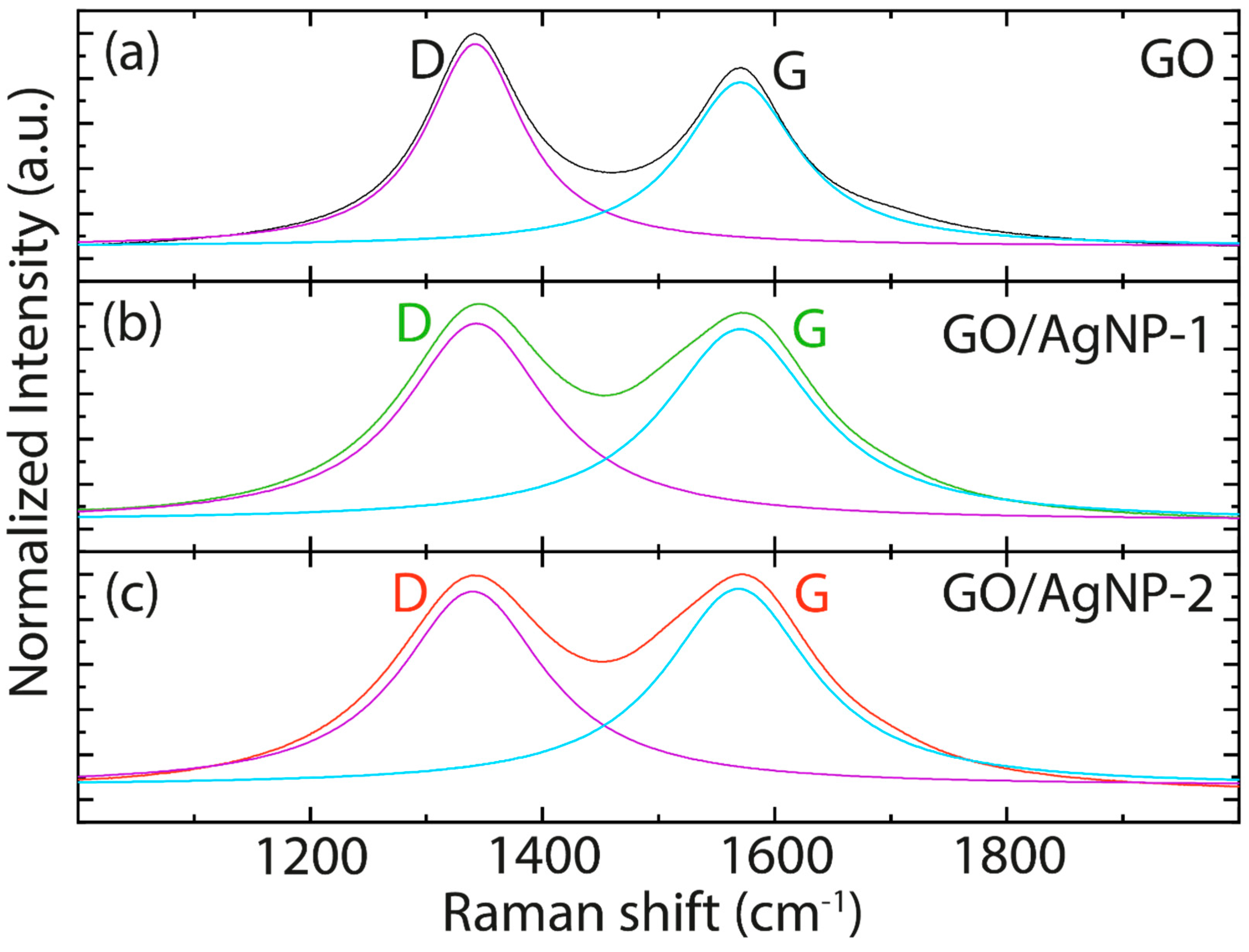
3.2. Spectroscopic Characterization
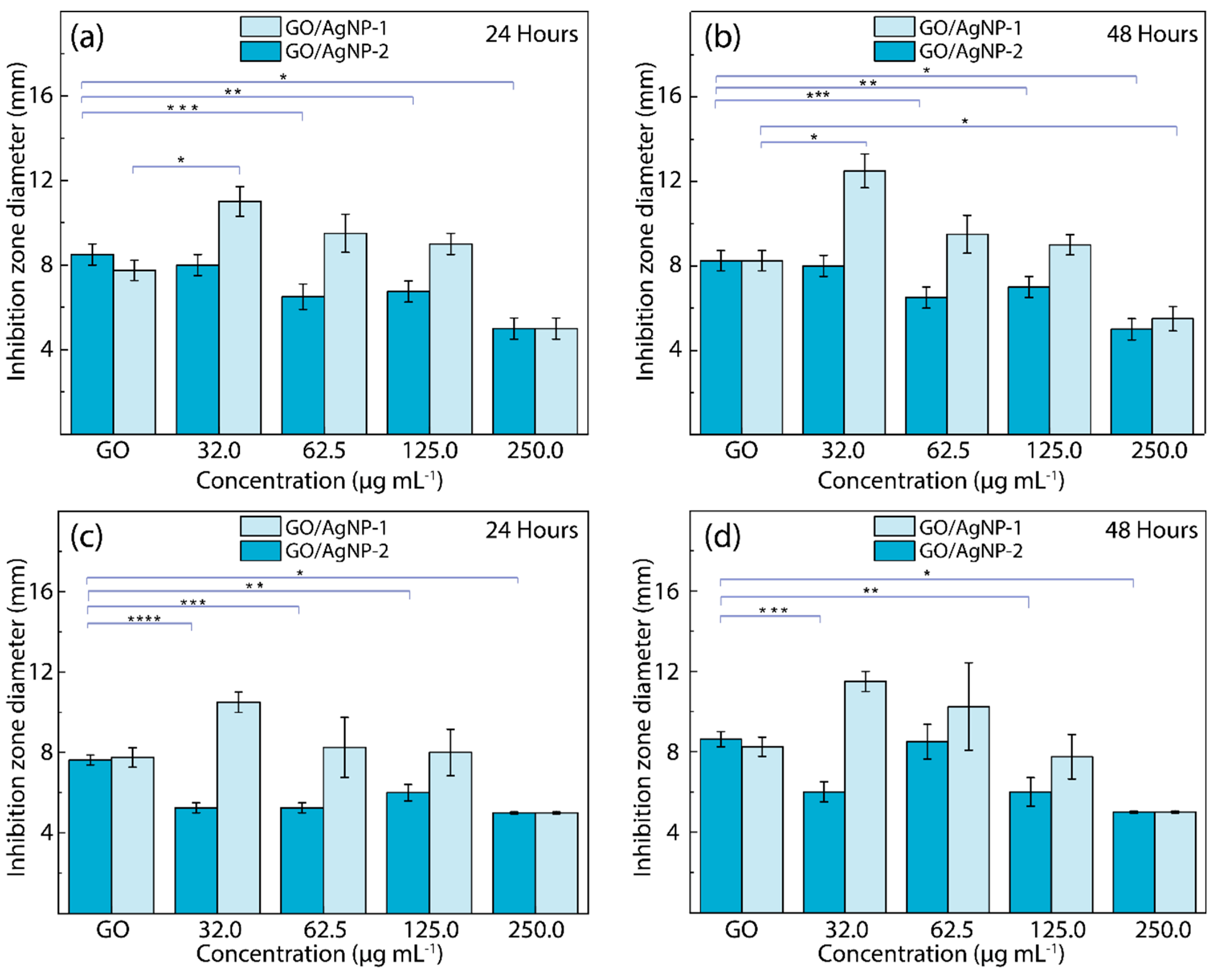
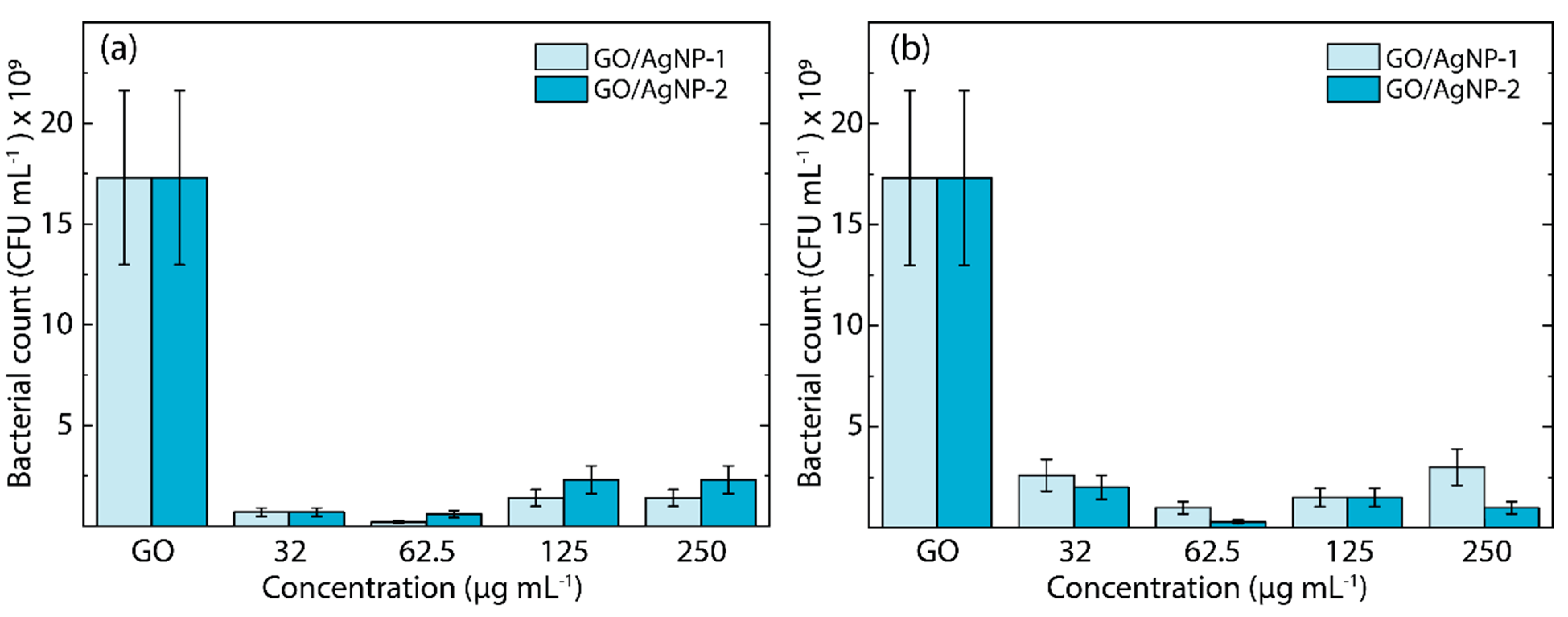
3.3. Antibacterial Feature
- At higher concentrations, AgNPs aggregate (see Figure 2), forming larger clusters that reduce the effective surface area available for interaction with bacterial cells, thereby diminishing antibacterial efficacy.
- The aggregation of AgNPs at higher concentrations leads to a decrease in the available surface area for generating ROS and interacting with bacterial membranes, resulting in lower antibacterial activity.
- Higher concentrations of AgNPs might saturate the bacterial environment, leading to less efficient interactions between nanoparticles and bacterial cells. This could reduce the ability of AgNPs to penetrate bacterial membranes effectively and exert their antibacterial effects.
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Jiang, A.; Liu, Z.; Lv, X.; Zhou, C.; Ran, T.; Tan, Z. Prospects and Challenges of Bacteriophage Substitution for Antibiotics in Livestock and Poultry Production. Biology 2024, 13, 28. [Google Scholar] [CrossRef]
- Fang, M.; Lin, L.; Zheng, M.; Liu, W.; Lin, R. Antibacterial functionalized carbon dots and their application in bacterial infections and inflammation. J. Mater. Chem. B 2023, 11, 9386–9403. [Google Scholar] [CrossRef] [PubMed]
- Hu, C.; Yang, Y.; Lin, Y.; Wang, L.; Ma, R.; Zhang, Y.; Feng, X.; Wu, J.; Chen, L.; Shao, L. GO-based antibacterial composites: Application and design strategies. Adv. Drug Deliv. Rev. 2021, 178, 113967. [Google Scholar] [CrossRef]
- Elumalai, K.; Srinivasan, S.; Shanmugam, A. Review of the efficacy of nanoparticle-based drug delivery systems for cancer treatment. Biomed. Technol. 2024, 5, 109–122. [Google Scholar] [CrossRef]
- Siddique, R.; Saleem, A.; Muhammad, F.; Akhtar, M.F.; Akhtar, B.; Sharif, A. Nanomedicines for the Treatment of Bacterial Diseases. In Nanomedicine in Treatment of Diseases; Springer Nature: Singapore, 2024; Volume 1, pp. 89–122. [Google Scholar] [CrossRef]
- Nandhini, J.; Karthikeyan, E.; Rajeshkumar, S. Nanomaterials for wound healing: Current status and futuristic frontier. Biomed. Technol. 2024, 6, 26–45. [Google Scholar] [CrossRef]
- Thakur, N.; Thakur, N.; Kumar, A.; Thakur, V.K.; Kalia, S.; Arya, V.; Kumar, A.; Kumar, S.; Kyzas, G.Z. A critical review on the recent trends of photocatalytic, antibacterial, antioxidant and nanohybrid applications of anatase and rutile TiO2 nanoparticles. Sci. Total Environ. 2024, 914, 169815. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Deng, B.; Lv, M.; Li, J.; Zhang, Y.; Jiang, H.; Peng, H.; Li, J.; Shi, J.; Huang, Q.; et al. Graphene oxide-based antibacterial cotton fabrics. Adv. Healthc. Mater. 2013, 2, 1259–1266. [Google Scholar] [CrossRef]
- Gao, Y.; Wu, J.; Ren, X.; Tan, X.; Hayat, T.; Alsaedi, A.; Cheng, C.; Chen, C. Impact of graphene oxide on the antibacterial activity of antibiotics against bacteria. Environ. Sci. Nano 2017, 4, 1016–1024. [Google Scholar] [CrossRef]
- Lanai, V.; Chen, Y.; Naumovska, E.; Pandit, S.; Schröder, E.; Mijakovic, I.; Rahimi, S. Differences in interaction of graphene/graphene oxide with bacterial and mammalian cell membranes. Nanoscale 2024, 16, 1156–1166. [Google Scholar] [CrossRef]
- Shabbirahmed, A.M.; Somu, P.; Kendrekar, P.; Mishra, A.K.; Mahanta, S.K. Recent Nanotechnological Advancement of Graphene-Based Nanomaterials in Gene Delivery and Protein Delivery. In Graphene-Based Nanomaterials; CRC Press: Boca Raton, FL, USA, 2024; Volume 1, pp. 185–195. [Google Scholar]
- Coello-Fiallos, D.; Tene, T.; Guayllas, J.L.; Haro, D.; Haro, A.; Gomez, C.V. DFT comparison of structural and electronic properties of graphene and germanene: Monolayer and bilayer systems. Mater. Today Proc. 2017, 4, 6835–6841. [Google Scholar] [CrossRef]
- Gomez, C.V.; Robalino, E.; Haro, D.; Tene, T.; Escudero, P.; Haro, A.; Orbe, J. Structural and electronic properties of graphene oxide for different degree of oxidation. Mater. Today Proc. 2016, 3, 796–802. [Google Scholar] [CrossRef]
- Liao, C.; Li, Y.; Tjong, S.C. Graphene Nanomaterials: Synthesis, Biocompatibility, and Cytotoxicity. Int. J. Mol. Sci. 2018, 19, 3564. [Google Scholar] [CrossRef] [PubMed]
- Fouda, A.; Abdel-Maksoud, G.; Saad, H.A.; Gobouri, A.A.; Mohammedsaleh, Z.M.; Abdel-Haleem El-Sadany, M. The Efficacy of Silver Nitrate (AgNO3) as a Coating Agent to Protect Paper against High Deteriorating Microbes. Catalysts 2021, 11, 310. [Google Scholar] [CrossRef]
- Fareed, N.; Nisa, S.; Bibi, Y.; Fareed, A.; Ahmed, W.; Sabir, M.; Alma, S.; Sajjad, A.; Kumar, S.; Hussain, M.; et al. Green synthesized silver nanoparticles using carrot extract exhibited strong antibacterial activity against multidrug resistant bacteria. J. King Saud Univ.-Sci. 2023, 35, 102477. [Google Scholar] [CrossRef]
- Ameen, F.; Al-Homaidan, A.A.; Al-Sabri, A.; Almansob, A.; AlNAdhari, S. Anti-oxidant, anti-fungal and cytotoxic effects of silver nanoparticles synthesized using marine fungus Cladosporium halotolerans. Appl. Nanosci. 2023, 13, 623–631. [Google Scholar] [CrossRef]
- Lal, H.M.; Uthaman, A.; Thomas, S. Silver Nanoparticle as an Effective Antiviral Agent. In Polymer Nanocomposites Based on Silver Nanoparticles; Synthesis, Characterization and Applications; Springer: Cham, Switzerland, 2021; Volume 1, pp. 247–265. [Google Scholar] [CrossRef]
- Barbosa, A.I.; Rebelo, R.; Reis, R.L.; Bhattacharya, M.; Correlo, V.M. Current nanotechnology advances in diagnostic biosensors. Med. Devices Sens. 2021, 4, e10156. [Google Scholar] [CrossRef]
- Fujii, R.; Okubo, K.; Takashiba, S.; Bianco, A.; Nishina, Y. Tailoring the interaction between graphene oxide and antibacterial pyridinium salts by terminal functional groups. Carbon 2020, 160, 204–210. [Google Scholar] [CrossRef]
- Yang, Z.; Hao, X.; Chen, S.; Ma, Z.; Wang, W.; Wang, C.; Wang, C.; Yue, L.; Sun, H.; Shao, Q.; et al. Long-term antibacterial stable reduced graphene oxide nanocomposites loaded with cuprous oxide nanoparticles. J. Colloid Interface Sci. 2019, 533, 13–23. [Google Scholar] [CrossRef]
- Zhao, J.; Wang, X.; Hoang, S.A.; Bolan, N.S.; Kirkham, M.B.; Liu, J.; Xia, X.; Li, Y. Silver nanoparticles in aquatic sediments: Occurrence, chemical transformations, toxicity, and analytical methods. J. Hazard. Mater. 2021, 418, 126368. [Google Scholar] [CrossRef]
- Cobos, M.; De-La-Pinta, I.; Quindós, G.; Fernández, M.J.; Fernández, M.D. Graphene Oxide–Silver Nanoparticle Nanohybrids: Synthesis, Characterization, and Antimicrobial Properties. Nanomaterials 2020, 10, 376. [Google Scholar] [CrossRef]
- Badoni, A.; Prakash, J. Noble metal nanoparticles and graphene oxide based hybrid nanostructures for antibacterial applications: Recent advances, synergistic antibacterial activities, and mechanistic approaches. Micro Nano Eng. 2024, 22, 100239. [Google Scholar] [CrossRef]
- Marcano, D.C.; Kosynkin, D.V.; Berlin, J.M.; Sinitskii, A.; Sun, Z.; Slesarev, A.; Alemany, L.B.; Lu, W.; Tour, J.M. Improved synthesis of graphene oxide. ACS Nano 2010, 4, 4806–4814. [Google Scholar] [CrossRef]
- Chen, J.; Yao, B.; Li, C.; Shi, G. An improved Hummers method for eco-friendly synthesis of graphene oxide. Carbon 2013, 64, 225–229. [Google Scholar] [CrossRef]
- Kaur, R.; Singh, K.; Agarwal, S.; Masih, M.; Chauhan, A.; Gautam, P.K. Silver nanoparticles induces apoptosis of cancer stem cells in head and neck cancer. Toxicol. Rep. 2023, 12, 10–17. [Google Scholar] [CrossRef] [PubMed]
- Ranoszek-Soliwoda, K.; Tomaszewska, E.; Socha, E.; Krzyczmonik, P.; Ignaczak, A.; Orlowski, P.; Krzyzowska, M.; Celichowski, G.; Grobelny, J. The role of tannic acid and sodium citrate in the synthesis of silver nanoparticles. J. Nanoparticle Res. 2017, 19, 1–15. [Google Scholar] [CrossRef]
- Yang, K.; Huang, L.-j.; Wang, Y.-x.; Du, Y.-c.; Zhang, Z.-j.; Wang, Y.; Kipper, M.J.; Belfiore, L.A.; Tang, J.-g. Graphene Oxide Nanofiltration Membranes Containing Silver Nanoparticles: Tuning Separation Efficiency via Nanoparticle Size. Nanomaterials 2020, 10, 454. [Google Scholar] [CrossRef]
- de Luna, L.A.V.; de Moraes, A.C.M.; Consonni, S.R.; Pereira, C.D.; Cadore, S.; Giorgio, S.; Alves, O.L. Comparative in vitro toxicity of a graphene oxide-silver nanocomposite and the pristine counterparts toward macrophages. J. Nanobiotechnol. 2016, 14, 1–17. [Google Scholar] [CrossRef]
- Tene, T.; Tubon Usca, G.; Guevara, M.; Molina, R.; Veltri, F.; Arias, M.; Caputi, L.S.; Vacacela Gomez, C. Toward Large-Scale Production of Oxidized Graphene. Nanomaterials 2020, 10, 279. [Google Scholar] [CrossRef]
- Salazar, Y.C.; Bellucci, S.; Guevara, M.; Gomez, C.V.; Caicedo, I.; Buñay, J.; Lopez, S.; Mayorga, D.; Scarcello, A.; Arias Polanco, M.; et al. An eco-friendly bandgap engineering of semiconductor graphene oxide. Solid-State Electron. 2023, 207, 108705. [Google Scholar] [CrossRef]
- Tene, T.; Arias Arias, F.; Guevara, M.; Nuñez, A.; Villamagua, L.; Tapia, C.; Pisarra, M.; Torres, F.J.; Caputi, L.S.; Vacacela Gomez, C. Removal of mercury (II) from aqueous solution by partially reduced graphene oxide. Sci. Rep. 2022, 12, 6326. [Google Scholar] [CrossRef]
- Baghizadeh, A.; Ranjbar, S.; Gupta, V.K.; Asif, M.; Pourseyedi, S.; Karimi, M.J.; Mohammadinejad, R. Green synthesis of silver nanoparticles using seed extract of Calendula officinalis in liquid phase. J. Mol. Liq. 2015, 207, 159–163. [Google Scholar] [CrossRef]
- John, T.; Stevenson, P.C.; Hillier, J. A study of the nucleation and growth processes in the synthesis of colloidal gold. Discuss. Faraday Soc. 1951, 11, 55–75. [Google Scholar]
- Zhou, X.; Huang, X.; Qi, X.; Wu, S.; Xue, C.; Boey, F.Y.; Yan, G.; Chen, P.; Zhang, H. In situ synthesis of metal nanoparticles on single-layer graphene oxide and reduced graphene oxide surfaces. J. Phys. Chem. C 2009, 113, 10842–10846. [Google Scholar] [CrossRef]
- Manimegalai, G.; Shanthakumar, S.; Sharma, C. Silver nanoparticles: Synthesis and application in mineralization of pesticides using membrane support. Int. Nano Lett. 2014, 4, 105. [Google Scholar] [CrossRef]
- Zhang, J.; Yang, H.; Shen, G.; Cheng, P.; Zhang, J.; Guo, S. Reduction of graphene oxide via L-ascorbic acid. Chem. Commun. 2010, 46, 1112–1114. [Google Scholar] [CrossRef]
- Zhao, J.; Zhang, Z.; Yang, S.; Zheng, H.; Li, Y. Facile synthesis of MoS2 nanosheet-silver nanoparticles composite for surface enhanced Raman scattering and electrochemical activity. J. Alloys Compd. 2013, 559, 87–91. [Google Scholar] [CrossRef]
- Anandalakshmi, K.; Venugobal, J.; Ramasamy, V.J.A.N. Characterization of silver nanoparticles by green synthesis method using Pedalium murex leaf extract and their antibacterial activity. Appl. Nanosci. 2016, 6, 399–408. [Google Scholar] [CrossRef]
- Suvith, V.S.; Philip, D. Catalytic degradation of methylene blue using biosynthesized gold and silver nanoparticles. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2014, 118, 526–532. [Google Scholar] [CrossRef]
- Jeeva, K.; Thiyagarajan, M.; Elangovan, V.; Geetha, N.; Venkatachalam, P. Caesalpinia coriaria leaf extracts mediated biosynthesis of metallic silver nanoparticles and their antibacterial activity against clinically isolated pathogens. Ind. Crops Prod. 2014, 52, 714–720. [Google Scholar] [CrossRef]
- Ajitha, B.; Reddy, Y.A.K.; Reddy, P.S. Biogenic nano-scale silver particles by Tephrosia purpurea leaf extract and their inborn antimicrobial activity. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2014, 121, 164–172. [Google Scholar] [CrossRef]
- Kumar, R.; Singh, R.; Gurjar, A.; Kashyap, R.; Kumar, M.; Kumar, D. Study the thermal stability of functionalized graphene oxide. AIP Conf. Proc. 2019, 2142, 040015. [Google Scholar] [CrossRef]
- Menazea, A.A.; Ahmed, M.K. Silver and copper oxide nanoparticles-decorated graphene oxide via pulsed laser ablation technique: Preparation, characterization, and photoactivated antibacterial activity. Nano-Struct. Nano-Objects 2020, 22, 100464. [Google Scholar] [CrossRef]
- He, D.; Peng, Z.; Gong, W.; Luo, Y.; Zhao, P.; Kong, L. Mechanism of a green graphene oxide reduction with reusable potassium carbonate. RSC Adv. 2015, 5, 11966–11972. [Google Scholar] [CrossRef]
- El-Hashemy, M.A.; Sallam, A. The inhibitive action of Calendula officinalis flower heads extract for mild steel corrosion in 1 M HCl solution. J. Mater. Res. Technol. 2020, 9, 13509–13523. [Google Scholar] [CrossRef]
- Kumari, S.; Sharma, P.; Yadav, S.; Kumar, J.; Vij, A.; Rawat, P.; Kumar, S.; Sinha, C.; Bhattacharya, J.; Srivastava, C.M.; et al. A novel synthesis of the graphene oxide-silver (GO-Ag) nanocomposite for unique physiochemical applications. ACS Omega 2020, 5, 5041–5047. [Google Scholar] [CrossRef] [PubMed]
- Panicker, S.; Ahmady, I.M.; Han, C.; Chehimi, M.; Mohamed, A.A. On demand release of ionic silver from gold-silver alloy nanoparticles: Fundamental antibacterial mechanisms study. Mater. Today Chem. 2020, 16, 100237. [Google Scholar] [CrossRef]
- Hajipour, P.; Bahrami, A.; Eslami, A.; Hosseini-Abari, A. Chemical bath synthesis of CuO-GO-Ag nanocomposites with enhanced antibacterial properties. J. Alloys Compd. 2020, 821, 153456. [Google Scholar] [CrossRef]
- Raffi, M.; Hussain, F.; Bhatti, T.M.; Akhter, J.I.; Hameed, A.; Hasan, M.M. Antibacterial characterization of silver nanoparticles against E. coli ATCC-15224. J. Mater. Sci. Technol. 2008, 24, 192–196. [Google Scholar]


| C (%) | O (%) | Ag (%) | |
|---|---|---|---|
| GO | 58.12 ± 0.25 | 41.88 ± 0.36 | --- |
| GO/AgNPs-1 | 53.23 ± 0.29 | 44.17 ± 0.29 | 2.60 ± 1.11 |
| GO/AgNPs-2 | 57.11 ± 0.25 | 40.82 ± 0.35 | 2.07 ± 1.03 |
| Agpeak | R2 | |||
|---|---|---|---|---|
| GO | 227.30 ± 0.16 | 321.22 ± 1.26 | --- | 0.999 |
| GO/AgNPs-1 | 222.12 ± 9.72 | --- | 411.10 ± 0.74 | 0.992 |
| GO/AgNPs-2 | 221.22 ± 0.40 | --- | 410.05 ± 0.47 | 0.997 |
| AgNPs-1 | --- | --- | 421.36 ± 1.30 | 0.942 |
| AgNPs-2 | --- | --- | 433.60 ± 0.51 | 0.985 |
| D | G | R2 | |
|---|---|---|---|
| GO | 1342.20 ± 0.25 | 1570.51 ± 0.33 | 0.982 |
| GO/AgNPs-1 | 1340.05 ± 0.54 | 1568.87 ± 0.53 | 0.973 |
| GO/AgNPs-2 | 1343.30 ± 0.30 | 1570.49 ± 0.32 | 0.992 |
| GO | GO/AgNPs-1 | GO/AgNPs-2 | Assignment | Ref. |
|---|---|---|---|---|
| 840.47 | N–H deformation of amines | [45] | ||
| 968.38 | 949.71 | epoxy group | [46] | |
| 1016.93 | C–O–C vibrations | [16,26,47] | ||
| 1150.45 | 1153.25 | C–H in-plane bending | [39] | |
| 1250.13 | 1293.30 | C–O vibrations | [25,26] | |
| 1341.85 | NO2 (N from agNO3) | [16] | ||
| 1353.99 | C=O stretching | [48] | ||
| 1427.75 | vibration of carboxyl groups | [45] | ||
| 1572.46 | 1591.14 | 1596.74 | C=C stretching | [25] |
| 1727.45 | 1721.85 | 1731.19 | C=O stretching vibrations | [25,47] |
| 2788.10 | [47] | |||
| 2824.51 | C–H asymmetric and symmetric stretching vibration | |||
| 2940.29 | 2943.09 | 2943.09 | ||
| 3020–3600 | 3020–3600 | 3020–3600 | ||
| 3635.87 | 3633.07 | [25,26] | ||
| 3705.90 | 3699.36 | 3705.90 | O–H absorbed water | |
| 3778.72 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tene, T.; Bellucci, S.; Pachacama, J.; Cuenca-Lozano, M.F.; Tubon-Usca, G.; Guevara, M.; La Pietra, M.; Cruz Salazar, Y.; Scarcello, A.; Arias Polanco, M.; et al. A Facile and Green Approach for the Preparation of Silver Nanoparticles on Graphene Oxide with Favorable Antibacterial Activity. Nanomaterials 2024, 14, 1455. https://doi.org/10.3390/nano14171455
Tene T, Bellucci S, Pachacama J, Cuenca-Lozano MF, Tubon-Usca G, Guevara M, La Pietra M, Cruz Salazar Y, Scarcello A, Arias Polanco M, et al. A Facile and Green Approach for the Preparation of Silver Nanoparticles on Graphene Oxide with Favorable Antibacterial Activity. Nanomaterials. 2024; 14(17):1455. https://doi.org/10.3390/nano14171455
Chicago/Turabian StyleTene, Talia, Stefano Bellucci, Joseth Pachacama, María F. Cuenca-Lozano, Gabriela Tubon-Usca, Marco Guevara, Matteo La Pietra, Yolenny Cruz Salazar, Andrea Scarcello, Melvin Arias Polanco, and et al. 2024. "A Facile and Green Approach for the Preparation of Silver Nanoparticles on Graphene Oxide with Favorable Antibacterial Activity" Nanomaterials 14, no. 17: 1455. https://doi.org/10.3390/nano14171455
APA StyleTene, T., Bellucci, S., Pachacama, J., Cuenca-Lozano, M. F., Tubon-Usca, G., Guevara, M., La Pietra, M., Cruz Salazar, Y., Scarcello, A., Arias Polanco, M., Gahramanli, L. R., Vacacela Gomez, C., & Caputi, L. S. (2024). A Facile and Green Approach for the Preparation of Silver Nanoparticles on Graphene Oxide with Favorable Antibacterial Activity. Nanomaterials, 14(17), 1455. https://doi.org/10.3390/nano14171455











