Physical Properties of an Efficient MAPbBr3/GaAs Hybrid Heterostructure for Visible/Near-Infrared Detectors
Abstract
:1. Introduction
2. Experimental Details
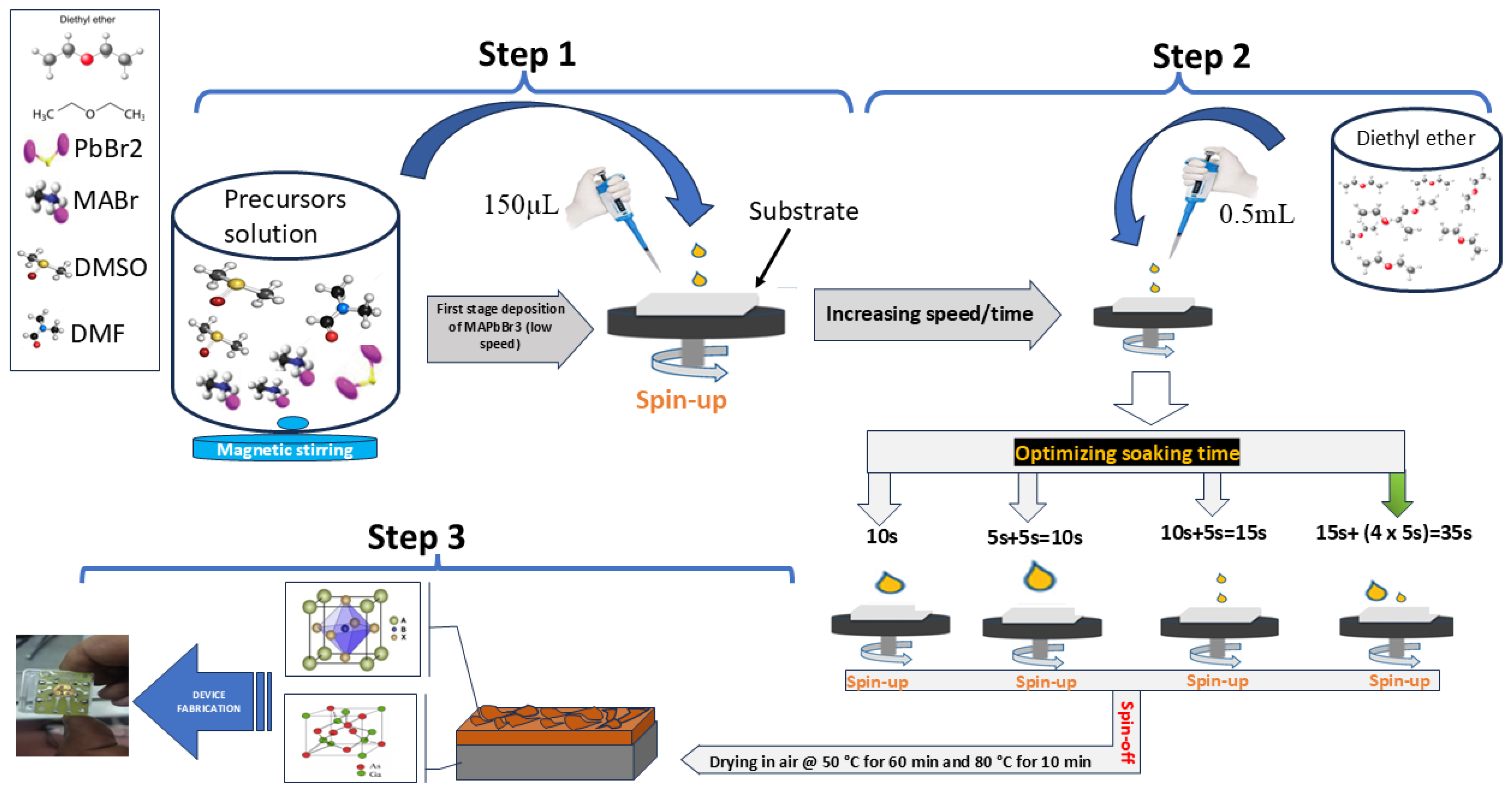
2.1. Preparation of MAPbBr3/GaAs Heterojunction
2.2. Characterization
3. Results and Discussion
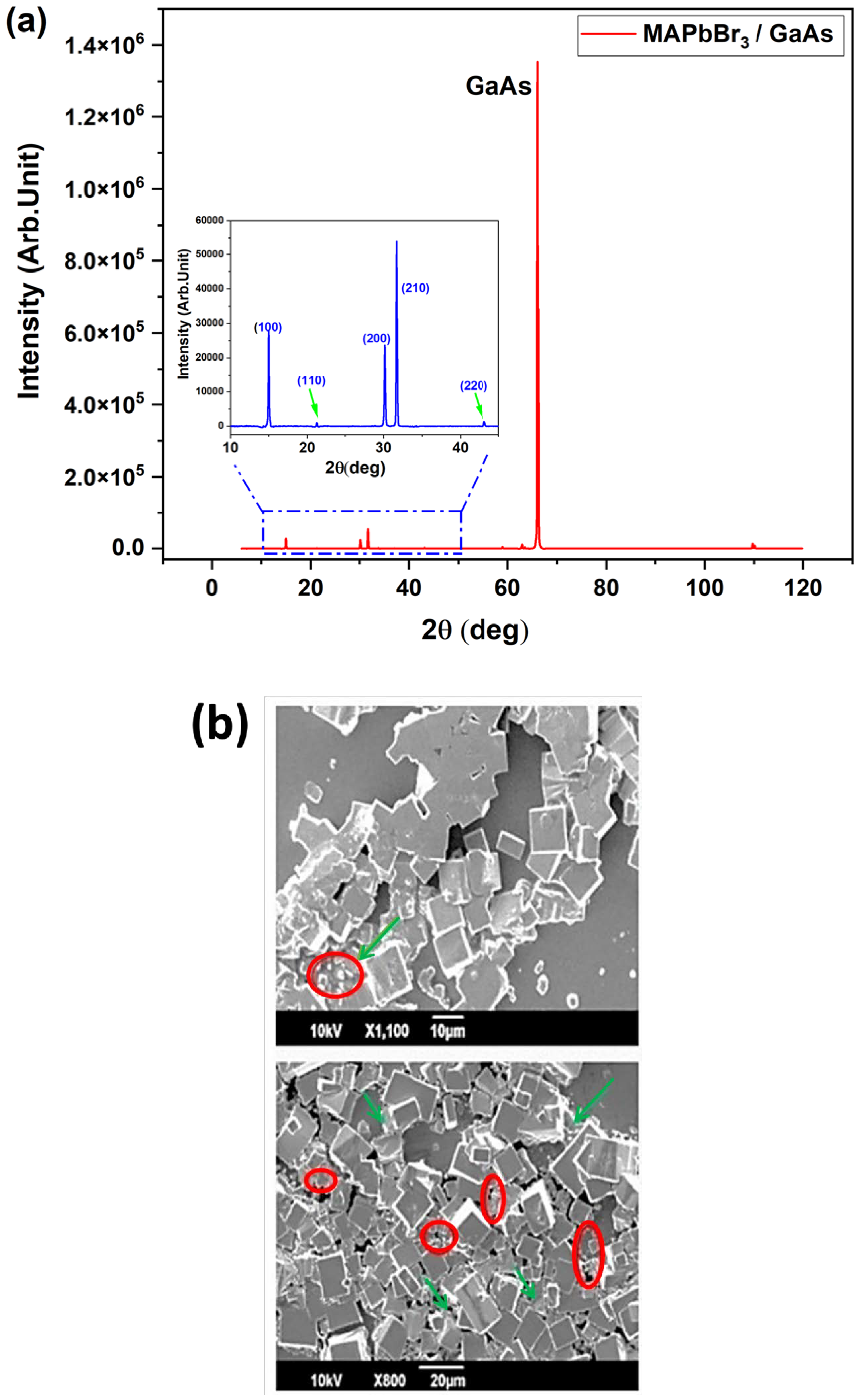
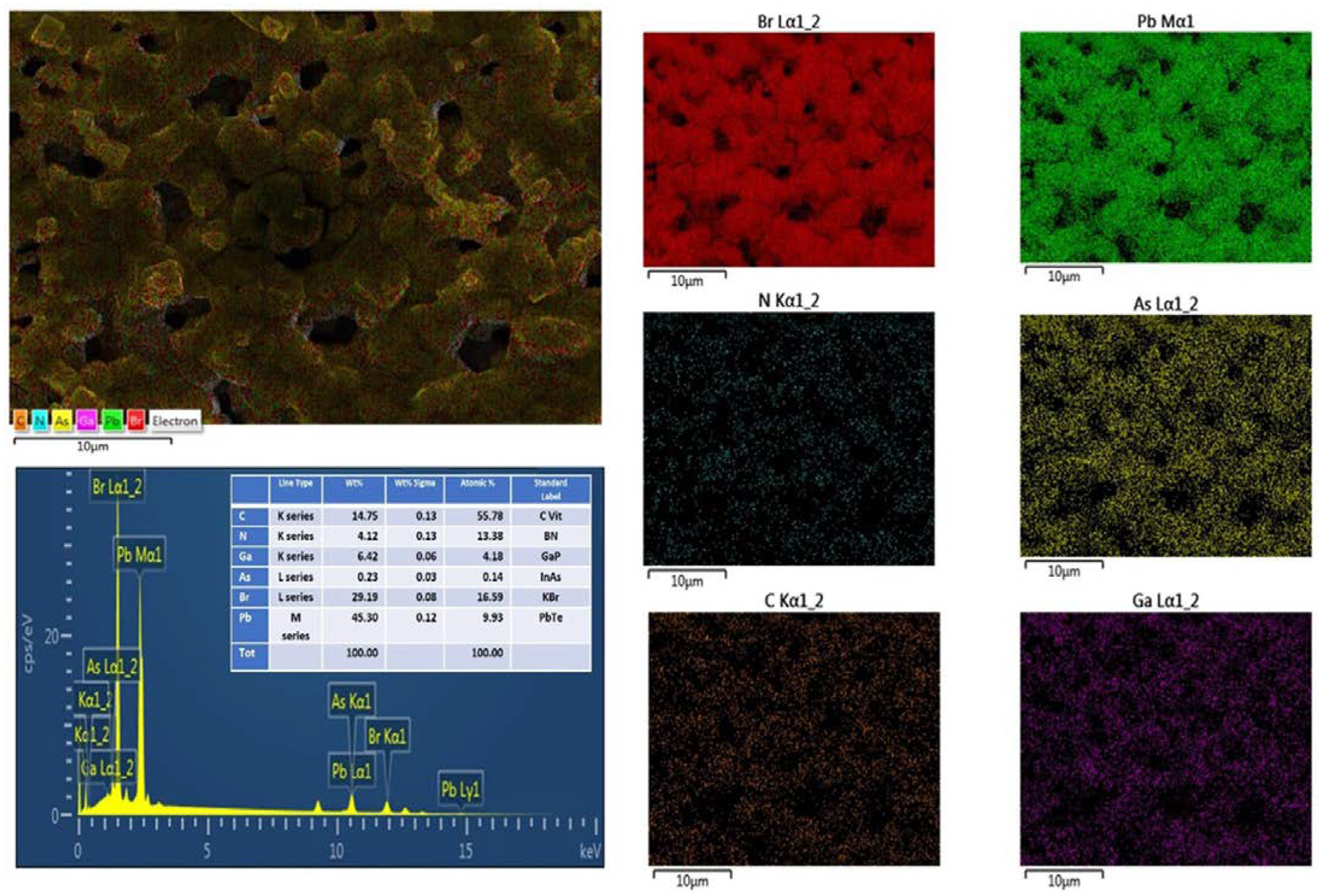
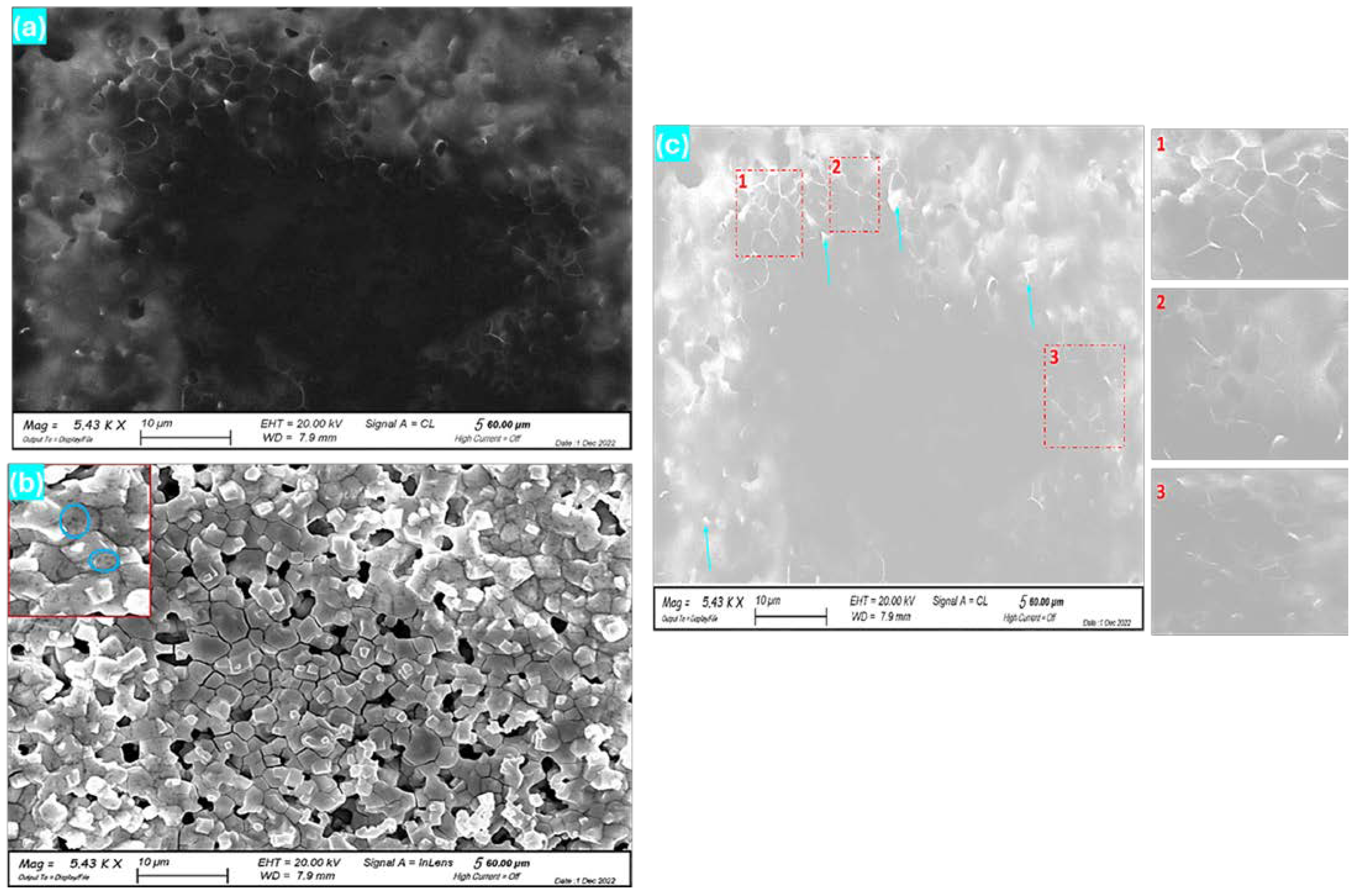
3.1. Homogeneity of MAPbBr3 Films on GaAs
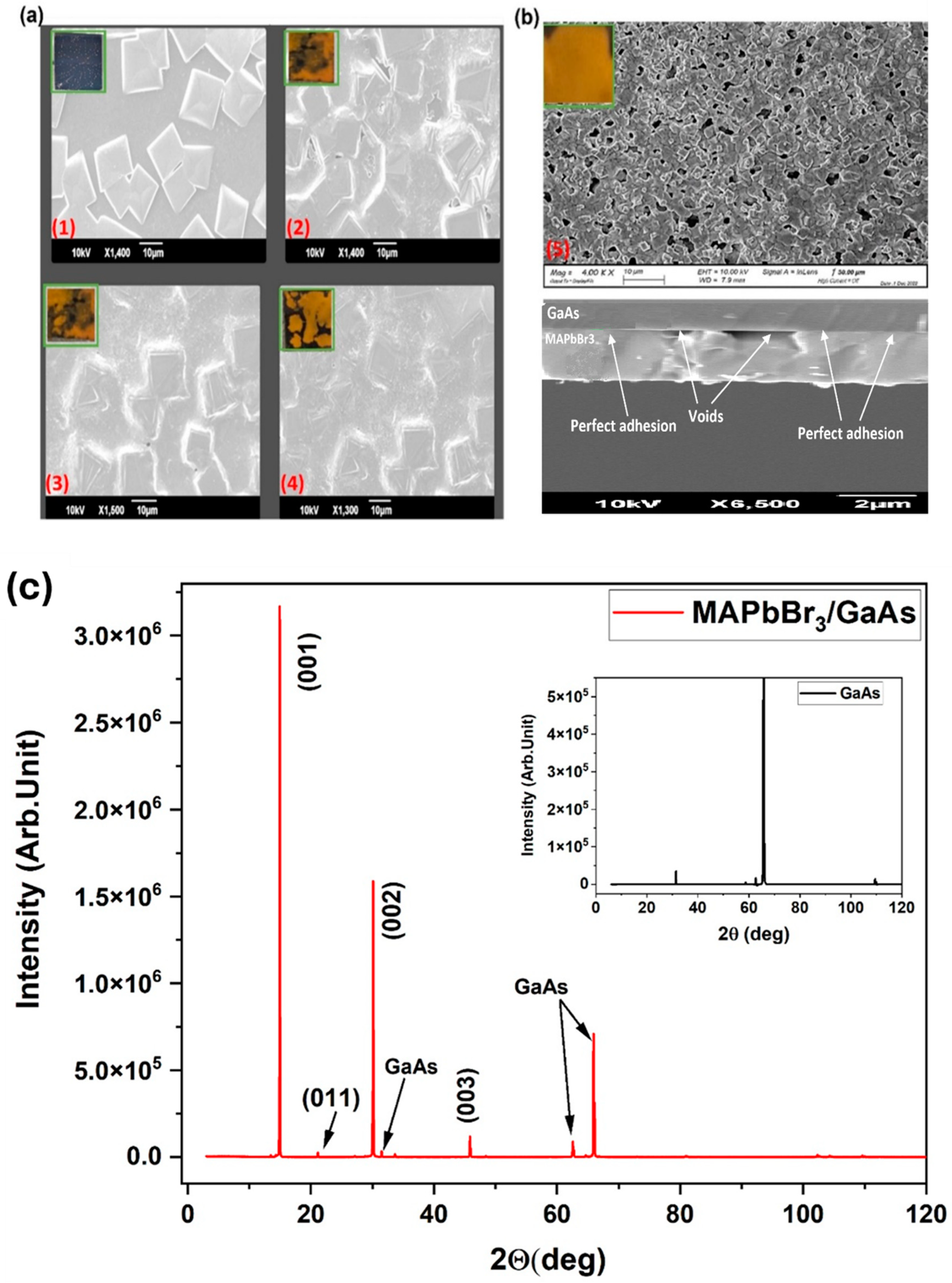
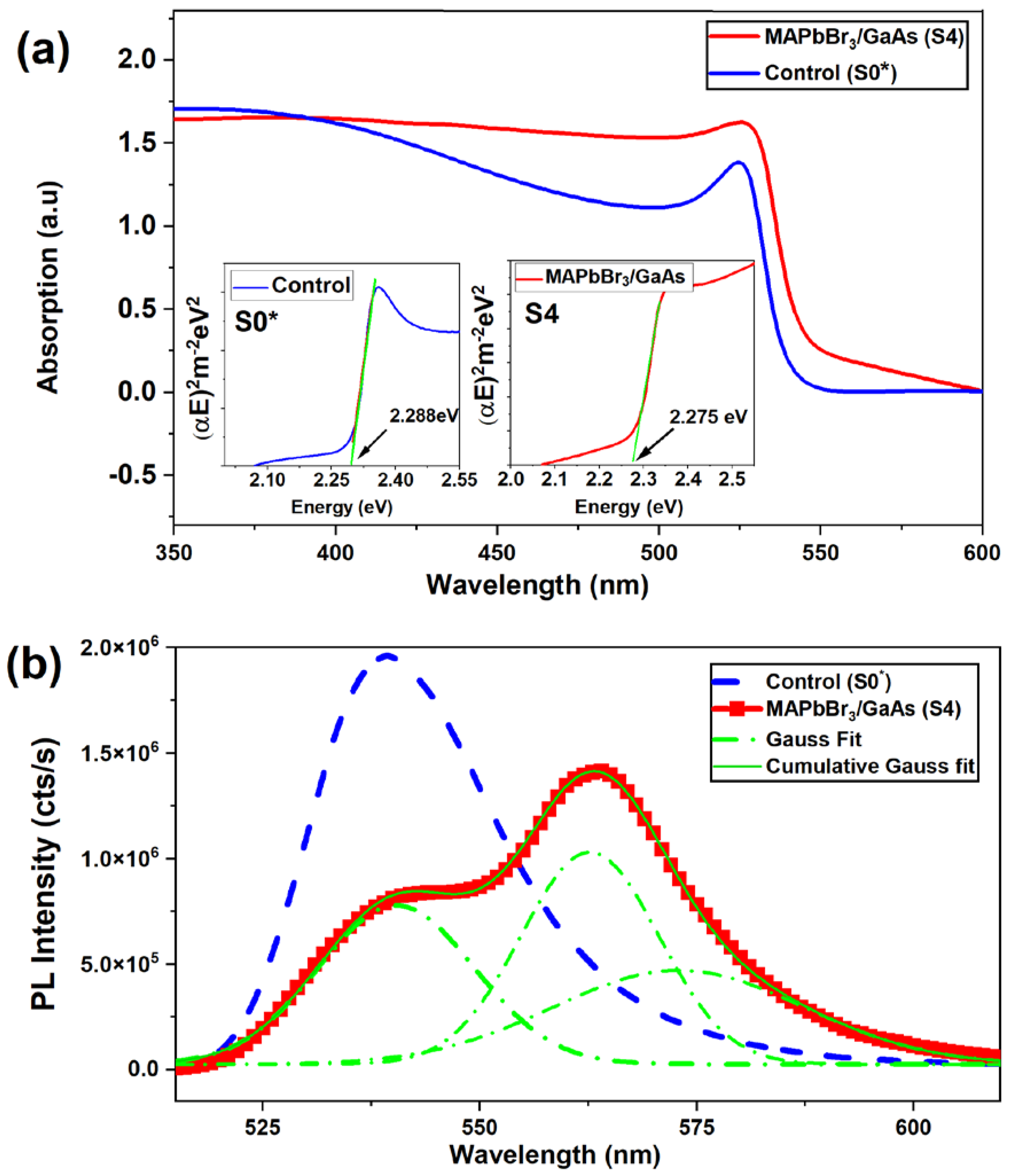
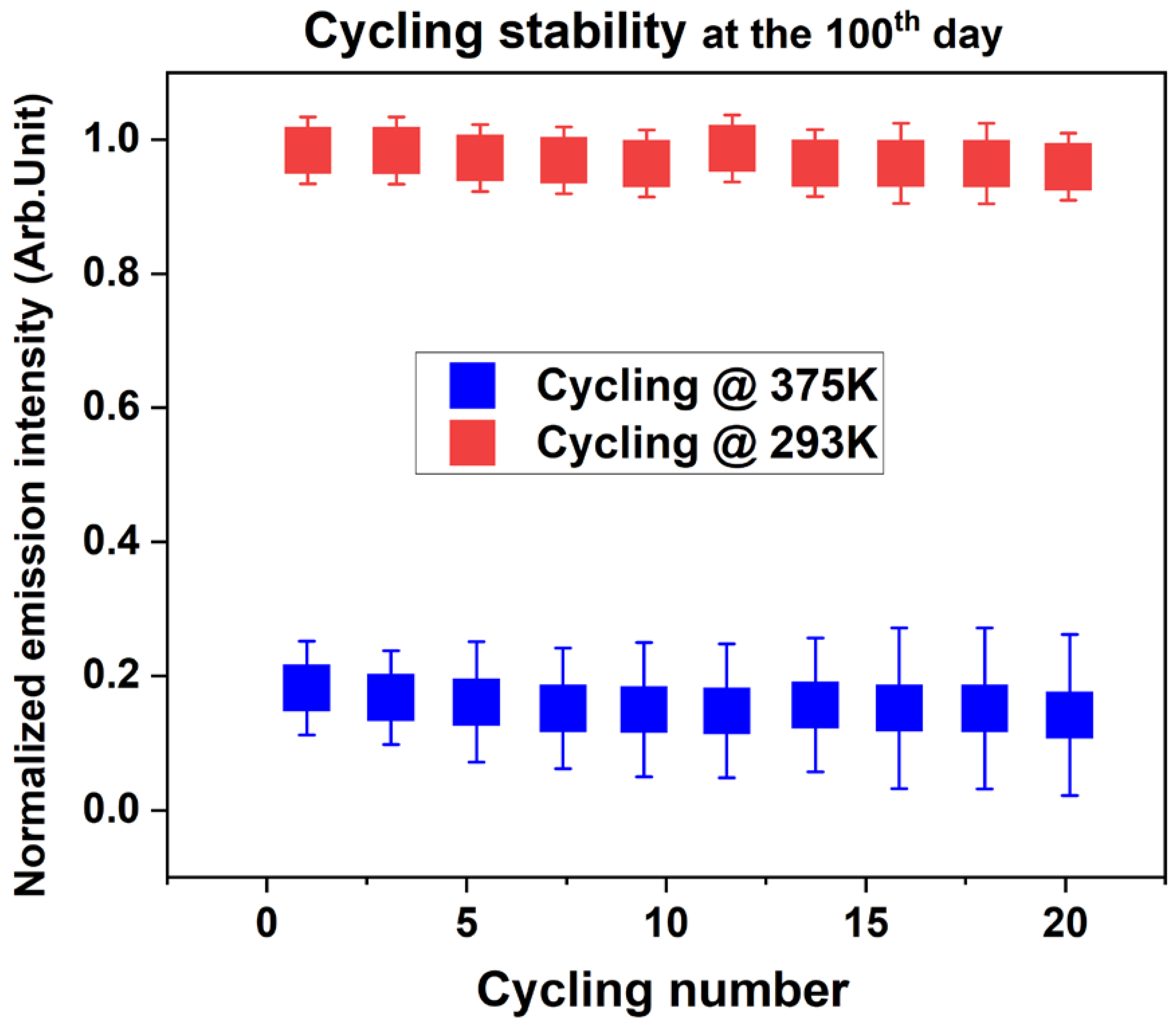
3.2. Structural and Photo-Stability
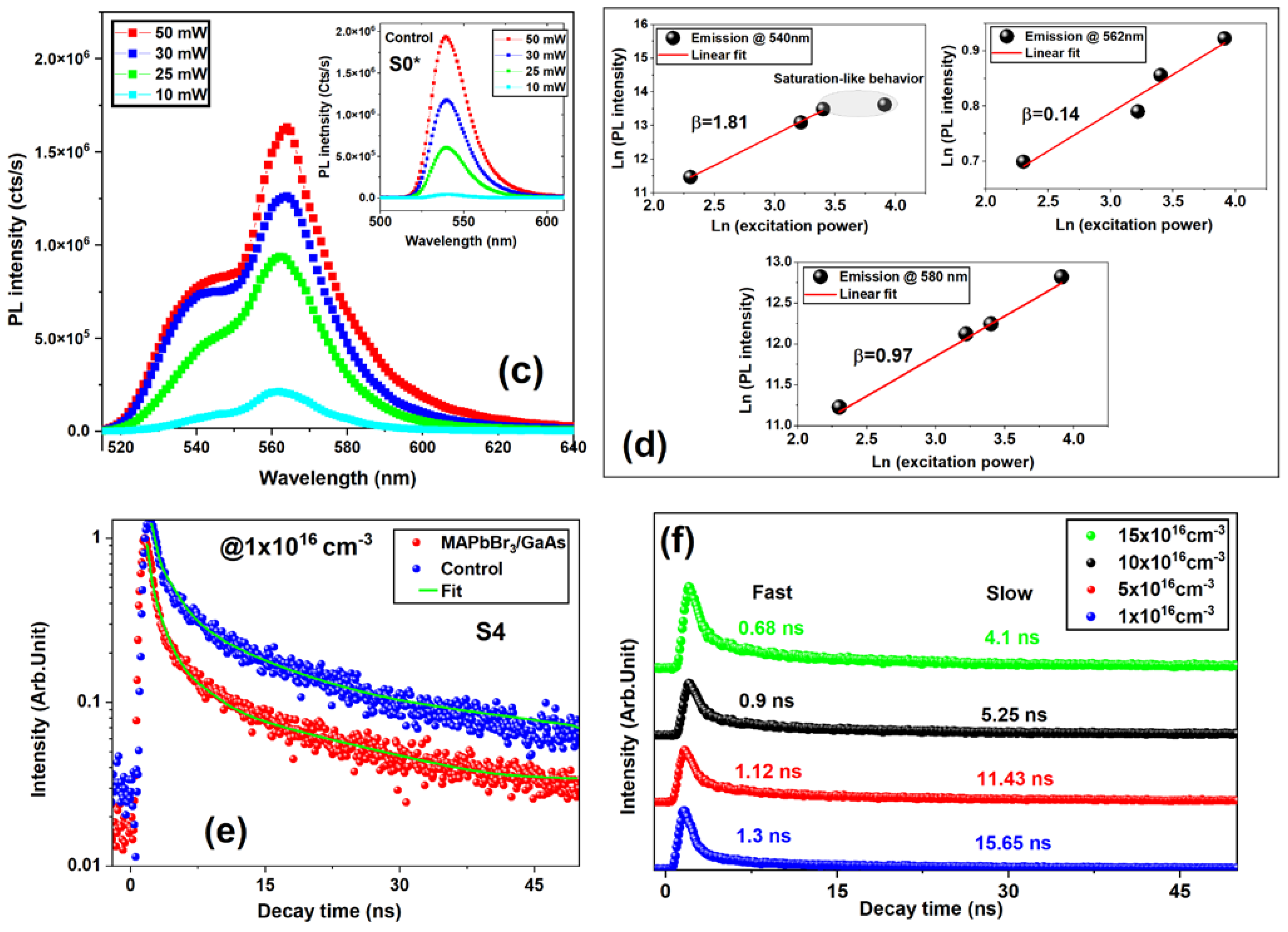
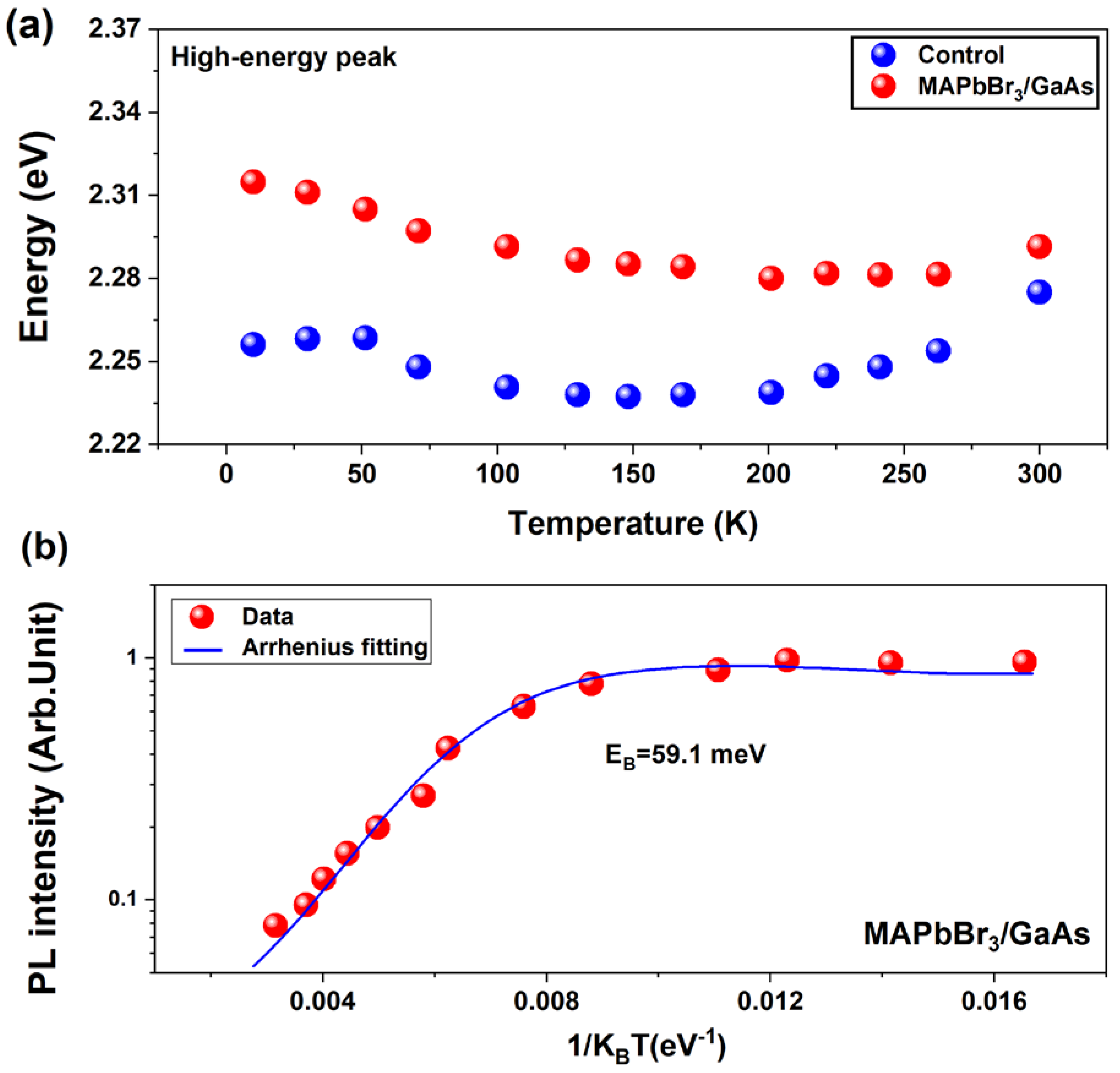
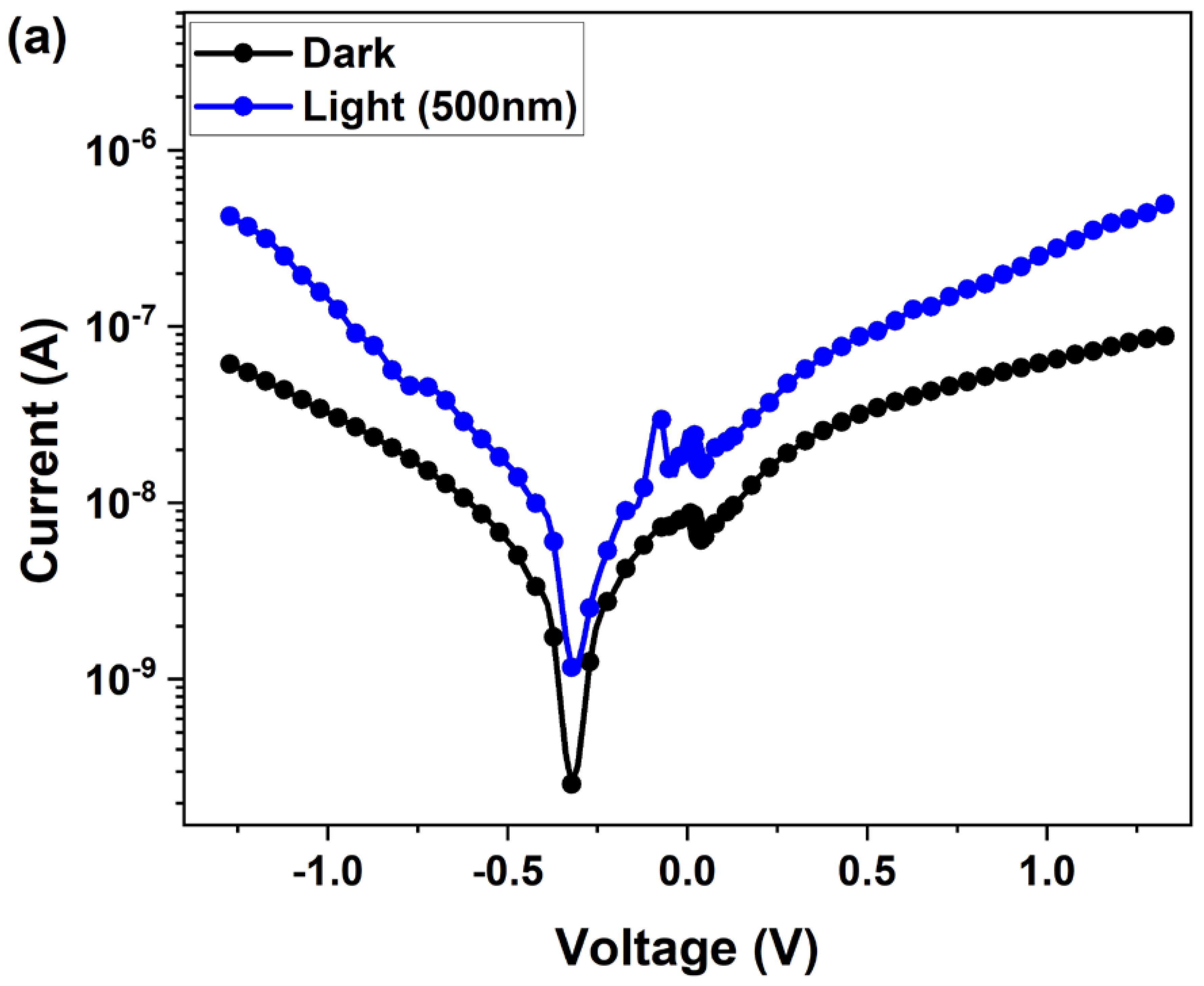
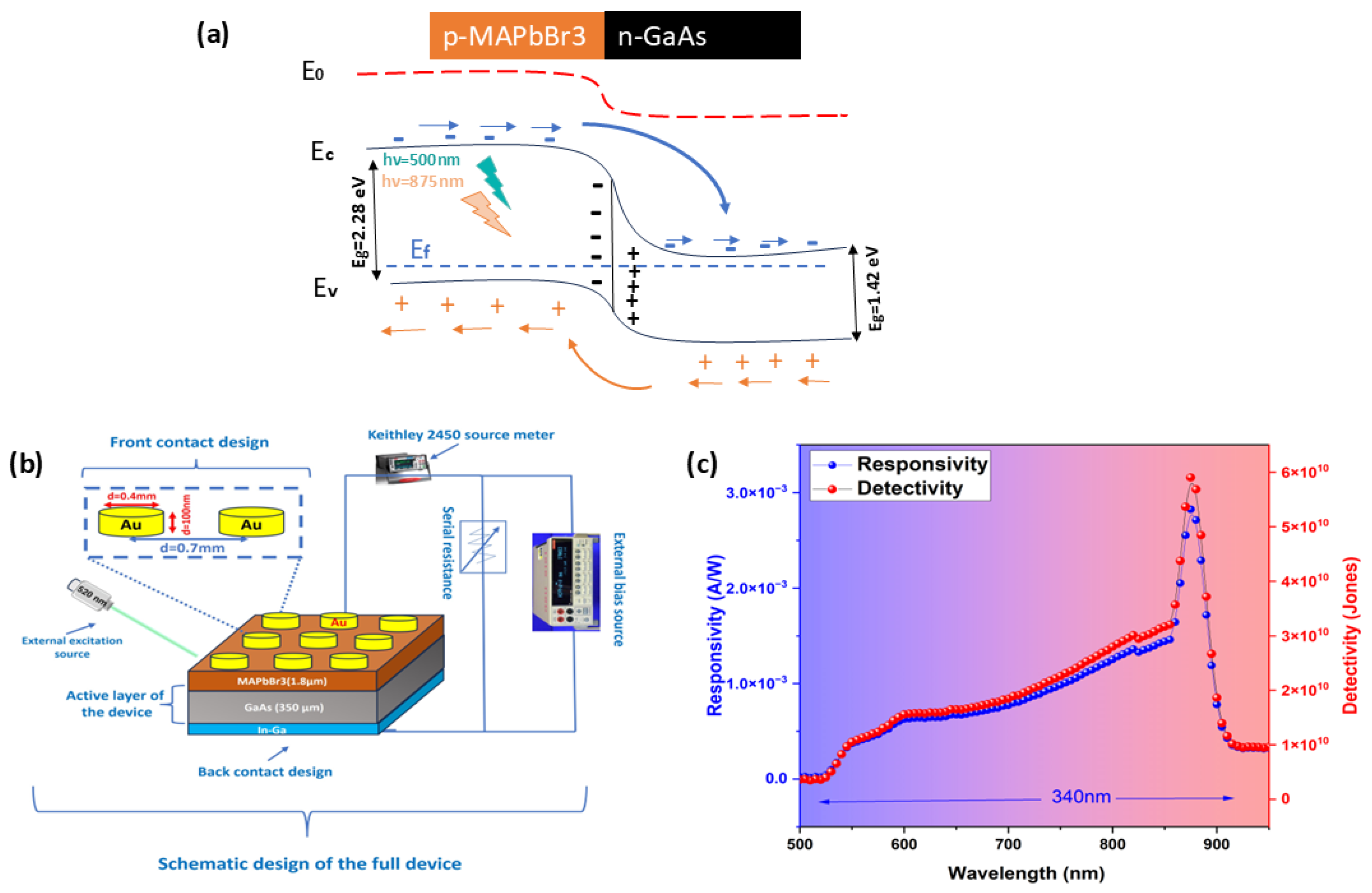
3.3. Photoelectrical Measurements
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Huang, J.; Yuan, Y.; Shao, Y.; Yan, Y. Understanding the physical properties of hybrid perovskites for photovoltaic applications. Nat. Rev. Mater. 2017, 2, 17042. [Google Scholar] [CrossRef]
- Shi, D.; Adinolfi, V.; Comin, R.; Yuan, M.; Alarousu, E.; Buin, A.; Chen, Y.; Hoogland, S.; Rothenberger, A.; Katsiev, K.; et al. Low trap-state density and long carrier diffusion in organolead trihalide perovskite single crystals. Science 2015, 347, 519–522. [Google Scholar] [CrossRef]
- Zhang, W.; Eperon, G.E.; Snaith, H.J. Metal halide perovskites for energy applications. Nat. Energy 2016, 1, 16048. [Google Scholar] [CrossRef]
- He, H.; Yu, Q.; Li, H.; Li, J.; Si, J.; Jin, Y.; Wang, N.; Wang, J.; He, J.; Wang, X.; et al. Exciton Localization in Solution-Processed Organolead Trihalide Perovskites. Nat. Commun. 2016, 7, 10896. [Google Scholar] [CrossRef]
- Liang, F.-X.; Jiang, J.-J.; Zhao, Y.-Z.; Zhang, Z.-X.; Wu, D.; Zeng, L.-H.; Tsang, Y.H.; Luo, L.-B. Fabrication of MAPbBr3 Single Crystal p-n Photodiode and n-p-n Phototriode for Sensitive Light Detection Application. Adv. Funct. Mater. 2020, 30, 2001033. [Google Scholar] [CrossRef]
- Ambrosio, F.; Meggiolaro, D.; Mosconi, E.; De Angelis, F. Charge localization and trapping at surfaces in lead-iodide perovskites: The role of polarons and defects. Mater. Chem. A 2020, 8, 6882–6892. [Google Scholar] [CrossRef]
- Yang, W.S.; Park, B.-W.; Jung, E.H.; Jeon, N.J.; Kim, Y.C.; Lee, D.U.; Shin, S.S.; Seo, J.; Kim, E.K.; Noh, J.H.; et al. Iodide management in formamidinium-lead-halide–based perovskite layers for efficient solar cells. Science 2017, 356, 1376–1379. [Google Scholar] [CrossRef]
- Xiao, Z.; Kerner, R.A.; Zhao, L.; Tran, N.L.; Lee, K.M.; Koh, T.-W.; Scholes, G.D.; Rand, B.P. Efficient perovskite light-emitting diodes featuring nanometre-sized crystallites. Nat. Photon 2017, 11, 108–115. [Google Scholar] [CrossRef]
- Xia, H.-R.; Li, J.; Sun, W.-T.; Peng, L.-M. Organohalide lead perovskite based photodetectors with much enhanced performance. Chem. Commun. 2014, 50, 13695–13697. [Google Scholar] [CrossRef]
- Dou, L.; Yang, Y.; You, J.; Hong, Z.; Chang, W.-H.; Li, G.; Yang, Y. Solution-processed hybrid perovskite photodetectors with high detectivity. Nat. Commun. 2014, 5, 5404. [Google Scholar] [CrossRef]
- Wei, W.; Zhang, Y.; Xu, Q.; Wei, H.; Fang, Y.; Wang, Q.; Deng, Y.; Li, T.; Gruverman, A.; Cao, L.; et al. Monolithic integration of hybrid perovskite single crystals with heterogenous substrate for highly sensitive X-ray imaging. Nat. Photonics 2017, 11, 315–321. [Google Scholar] [CrossRef]
- Turedi, B.; Yeddu, V.; Zheng, X.; Kim, D.Y.; Bakr, O.M.; Saidaminov, M.I. Perovskite Single-Crystal Solar Cells: Going Forward. ACS Energy Lett. 2021, 6, 631–642. [Google Scholar] [CrossRef]
- Zhang, Y.; Xu, W.X.; Xu, X.J.; Cai, J.; Yang, W.; Fang, X.S. Self-powered dual-color UV–green photodetectors based on SnO2 millimeter wire and microwires/CsPbBr3 particle heterojunctions. J. Phys. Chem. Lett. 2019, 10, 836–841. [Google Scholar] [CrossRef]
- Guo, S.; Qiao, S.; Liu, J.; Ma, J.; Wang, S. Greatly improved photoresponse in the MAPbBr3/Si heterojunction by introducing an ITO layer and optimizing MAPbBr3 layer thickness. Opt. Express. 2022, 28, 11536–11548. [Google Scholar] [CrossRef]
- Zhao, L.; Gao, Y.; Su, M.; Shang, Q.; Liu, Z.; Li, Q.; Wei, Q.; Li, M.; Fu, L.; Zhong, Y.; et al. Vapor-phase incommensurate heteroepitaxy of oriented single-crystal CsPbBr3 on GaN: Toward integrated optoelectronic applications. ACS Nano 2019, 13, 10085–10094. [Google Scholar] [CrossRef]
- Wu, Z.; Bi, E.; Li, C.; Chen, L.; Song, Z.; Yan, Y. Scalable Two-Step Production of High-Efficiency Perovskite Solar Cells and Modules. Sol. RRL 2023, 7, 2200571. [Google Scholar] [CrossRef]
- Geng, X.; Wang, F.; Tian, H.; Feng, Q.; Zhang, H.; Liang, R.; Shen, Y.; Ju, Z.; Gou, G.-Y.; Deng, N.; et al. Ultrafast photodetector by integrating perovskite directly on silicon wafer. ACS Nano 2020, 14, 2860–2868. [Google Scholar] [CrossRef]
- Zhang, Z.; Chen, K.; Zuo, Z. MAPbBr3 single crystal based metal-semiconductor-metal photodetector enhanced by localized surface plasmon. Mater. Res. Express 2020, 7, 125902. [Google Scholar] [CrossRef]
- Dong, L.; Pang, T.; Yu, J.; Wang, Y.; Zhu, W.; Zheng, H.; Chen, Z. Performance-enhanced solar-blind photodetector based on a CH3NH3PbI3/β-Ga2O3 hybrid structure. J. Mater. Chem. C 2019, 7, 14205–14211. [Google Scholar] [CrossRef]
- Zdanowicz, E.; Herman, A.P.; Przypis, Ł.; Opołczyńska, K.; Serafińczuk, J.; Chlipała, M.; Skierbiszewski, C.; Kudrawiec, R. The influence of Fermi level position at the GaN surface on carrier transfer across the MAPbI3/GaN interface. Phys. Chem. Chem. Phys. 2023, 25, 16492–16498. [Google Scholar] [CrossRef]
- Wang, G.; Pang, T.; Sun, K.; Luan, S.; Zhang, Y.; Yuan, L.; Jia, R. High-performance layer-structured Si/Ga2O3/CH3NH3PbI3 heterojunction photodetector based on a Ga2O3 buffer interlayer. Appl Opt. 2023, 62, A76–A82. [Google Scholar] [CrossRef] [PubMed]
- Ma, J.; Xia, X.; Yan, S.; Li, Y.; Liang, W.; Yan, J.; Chen, X.; Wu, D.; Li, X.; Shi, Z. Stable and self-powered solar-blind ultraviolet photodetectors based on a Cs3Cu2I5/β-Ga2O3 heterojunction prepared by dual-source vapor codeposition. ACS Appl. Mater. Interfaces 2021, 13, 15409–15419. [Google Scholar] [CrossRef] [PubMed]
- Siekmann, J.; Ravishankar, S.; Kirchartz, T. Apparent defect densities in halide perovskite thin films and single crystals. ACS Energy Lett. 2021, 6, 3244–3251. [Google Scholar] [CrossRef]
- Burschka, J.; Pellet, N.; Moon, S.-J.; Humphry-Baker, R.; Gao, P.; Nazeeruddin, M.K.; Grätzel, M. Sequential deposition as a route to high-performance perovskite-sensitized solar cells. Natute 2013, 49, 316–319. [Google Scholar] [CrossRef] [PubMed]
- Zhou, S.; Wang, Q.; Xu, Z.; Xu, S.; Yang, P.; Deng, H.; Li, B.; Dong, Y.; Han, P.; Su, Y. Antisolvent solvothermal synthesis of MAPbBr3 nanocrystals for efficient solar photodecomposition of methyl orange. J. Colloid Interface Sci. 2021, 595, 98–106. [Google Scholar] [CrossRef]
- Li, B.; Zhang, Y.; Fu, L.; Yu, T.; Zhou, S.; Zhang, L.; Yin, L. Surface passivation engineering strategy to fully-inorganic cubic CsPbI3 perovskites for high-performance solar cells. Nat. Commun. 2018, 9, 1076. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.-H.; Li, L.-C.; Shellaiah, M.; Sun, K.W. Structural and Photophysical Properties of Methylammonium Lead Tribromide (MAPbBr3) Single Crystals. Sci. Rep. 2017, 7, 13643. [Google Scholar] [CrossRef]
- McGovern, L.; Koschany, I.; Grimaldi, G.; Muscarella, L.A.; Ehrler, B. Grain size influences activation energy and migration pathways in MAPbBr3 perovskite solar cells. J. Phys. Chem. Lett. 2021, 12, 2423–2428. [Google Scholar] [CrossRef]
- Chen, J.; Morrow, D.J.; Fu, Y.; Zheng, W.; Zhao, Y.; Dang, L.; Stolt, M.J.; Kohler, D.D.; Wang, X.; Czech, K.J.; et al. Single-crystal thin films of cesium lead bromide perovskite epitaxially grown on metal oxide perovskite (SrTiO3). J. Am. Chem. Soc. 2017, 139, 13525–13532. [Google Scholar] [CrossRef]
- Fu, Y.; Meng, F.; Rowley, M.B.; Thompson, B.J.; Shearer, M.J.; Ma, D.; Hamers, R.J.; Wright, J.C.; Jin, S. Solution growth of single crystal methylammonium lead halide perovskite nanostructures for optoelectronic and photovoltaic applications. J. Am. Chem. Soc. 2015, 137, 5810–5818. [Google Scholar] [CrossRef]
- Zhu, H.; Fu, Y.; Meng, F.; Wu, X.; Gong, Z.; Ding, Q.; Gustafsson, M.V.; Trinh, M.T.; Jin, S.; Zhu, X.-Y. Lead halide perovskite nanowire lasers with low lasing thresholds and high quality factors. Nat. Mater. 2015, 14, 636–642. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Luo, Z.; Zhao, C.; Guo, Q.; Wang, Y.; Wang, F.; Tan, Z.A. Efficient and stable pure green all-inorganic perovskite CsPbBr3 light-emitting diodes with a solution-processed NiOx interlayer. J. Phys. Chem. C 2017, 121, 28132–28138. [Google Scholar] [CrossRef]
- Karmo, M.; Alvarado, I.A.R.; Schmidt, W.G.; Runge, E. Reconstructions of the As-Terminated GaAs(001) Surface Exposed to Atomic Hydrogen. ACS Omega 2022, 7, 5064–5068. [Google Scholar] [CrossRef]
- Li, W.; Rothmann, M.U.; Liu, A.; Wang, Z.; Zhang, Y.; Pascoe, A.R.; Lu, J.; Jiang, L.; Chen, Y.; Huang, F.; et al. Phase segregation enhanced ion movement in efficient inorganic CsPbIBr2 solar cells. Adv. Energy Mater. 2017, 7, 1700946. [Google Scholar] [CrossRef]
- Sherkar, T.S.; Momblona, C.; Gil-Escrig, L.; Ávila, J.; Sessolo, M.; Bolink, H.J.; Jan Anton Koster, L. Recombination in perovskite solar cells: Significance of grain boundaries, interface traps, and defect ions. ACS Energy Lett. 2017, 2, 1214–1222. [Google Scholar] [CrossRef]
- Saidaminov, M.I.; Abdelhady, A.L.; Murali, B.; Alarousu, E.; Burlakov, V.M.; Peng, W.; Dursun, I.; Wang, L.; He, Y.; Maculan, G.; et al. High-quality bulk hybrid perovskite single crystals within minutes by inverse temperature crystallization. Nat. Commun. 2015, 6, 7586. [Google Scholar] [CrossRef]
- Grisorio, R.; Conelli, D.; Giannelli, R.; Fanizza, E.; Striccoli, M.; Altamura, D.; Giannini, C.; Allegretta, I.; Terzano, R.; Suranna, G.P. A new route for the shape differentiation of cesium lead bromide perovskite nanocrystals with near-unity photoluminescence quantum yield. Nanoscale 2020, 12, 17053–17063. [Google Scholar] [CrossRef] [PubMed]
- Wu, B.; Nguyen, H.T.; Ku, Z.; Han, G.; Giovanni, D.; Mathews, N.; Fan, H.J.; Sum, T.C. Discerning the Surface and Bulk Recombination Kinetics of Organic–Inorganic Halide Perovskite Single Crystals. Adv. Energy Mater. 2016, 6, 1600551. [Google Scholar] [CrossRef]
- Li, D.; Wang, G.; Cheng, H.-C.; Chen, C.-Y.; Wu, H.; Liu, Y.; Huang, Y.; Duan, X. Size-dependent phase transition in methylammonium lead iodide perovskite microplate crystals. Nat. Commun. 2016, 7, 11330. [Google Scholar] [CrossRef]
- Yamada, Y.; Furukawa, K.; Sodeyama, K.; Kikuchi, K.; Yaegashi, M.; Tateyama, Y.; Yamada, A. Unusual stability of acetonitrile-based superconcentrated electrolytes for fast-charging lithium-ion batteries. J. Am. Chem. Soc. 2014, 136, 5039–5046. [Google Scholar] [CrossRef]
- Francisco-López, A.; Charles, B.; Weber, O.J.; Alonso, M.I.; Garriga, M.; Campoy-Quiles, M.; Weller, M.T.; Goñi, A.R. Equal footing of thermal expansion and electron–phonon interaction in the temperature dependence of lead halide perovskite band gaps. J. Phys. Chem. Lett. 2019, 10, 2971. [Google Scholar] [CrossRef] [PubMed]
- Wu, B.; Yuan, H.; Xu, Q.; Steele, J.A.; Giovanni, D.; Puech, P.; Fu, J.; Ng, Y.F.; Jamaludin, N.F.; Solanki, A.; et al. Indirect tail states formation by thermal-induced polar fluctuations in halide perovskites. Nat. Commun. 2019, 10, 484. [Google Scholar] [CrossRef] [PubMed]



| Stoichiometry (MABr:PbBr2) | Soaking Time and Procedure | Sample Symbol and Role | |
|---|---|---|---|
| MAPbBr3/GaAs | (1:1) | 10 s (single shot) | S *, control |
| MAPbBr3/glass | (1:1) | 10 s (single shot) | S g |
| MAPbBr3/glass | (3:1) | 10 s (single shot) | S0, control |
| MAPbBr3/glass | (3:1) | 15 s + (4 × 5 s) = 35 s (multiple shots) | S0 *, control |
| MAPbBr3/GaAs | (3:1) | 10 s (single shot) | S1 |
| MAPbBr3/GaAs | (3:1) | 5 s + 5 s = 10 s (multiple shots) | S2 |
| MAPbBr3/GaAs | (3:1) | 10 s + 5 s = 15 s (multiple shots) | S3 |
| MAPbBr3/GaAs | (3:1) | 15 s + (4 × 5 s) = 35 s (multiple shots) | S4 (Optimized) |
| Materials and Structures | Responsivity (mA/W) | Conditions: Voltage/Light Excitation | Preparation Method | Reference |
|---|---|---|---|---|
| In-Ga/GaAs/MAPbBr3/Au | 3 16 | @ −1 V, 500 nm @ 50 V, 500 nm | Spin coating | This work |
| Au/MAPbBr3 SC/Au Plane MSM-photodetector | 1.7 | @5 V, 532 nm | Unchanged temperature | [18] |
| Pt/MAPb(BrxI1−x)3 SC/Pt | 2.41 | @ 10 V, white light | Inverse temperature crystallization (ITC) | [2] |
| Au/MAPbI3 nanosheets/SiO2/Au | 0.5 | @ 1 V, 635 nm | Chemical vapor deposition | [14] |
| Cs3Cu2I5/β-Ga2O3 | 2.3 3 | @ 0 V, @ −3 V, 265 nm | Dual-source vapor co-deposition | [19] |
| MAPbBr3/Si/In | 0.394 | @ −1 V, 532 nm | Spin coating | [14] |
| Si/Ga2O3/MAPbI3 | 1.6 | @ 1 V, 780 nm | PLD + Spin coating | [21] |
| MAPbI3/GaN | 110 × 10−6 | @ 0 V, 405 nm | Two-step spin coating | [20] |
| β-Ga2O3/Au/MAPbBr3 | 1.63 1.40 | @ 0 V, 240 nm @ 0 V 520 nm | MBE + spin coating | [6] |
| SnO2 MWs/CsPbBr3 | 347 × 10−3 | @ 3 V, 530 nm | Vapor transport, drop casting | [13] |
| ITO-MAPbI3-TiO2-ITO | 0.49 × 10−6 | @ 3 V, white light | Spin coating | [9] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hidouri, T.; Pavesi, M.; Vaccari, M.; Parisini, A.; Jarmouni, N.; Cristofolini, L.; Fornari, R. Physical Properties of an Efficient MAPbBr3/GaAs Hybrid Heterostructure for Visible/Near-Infrared Detectors. Nanomaterials 2024, 14, 1472. https://doi.org/10.3390/nano14181472
Hidouri T, Pavesi M, Vaccari M, Parisini A, Jarmouni N, Cristofolini L, Fornari R. Physical Properties of an Efficient MAPbBr3/GaAs Hybrid Heterostructure for Visible/Near-Infrared Detectors. Nanomaterials. 2024; 14(18):1472. https://doi.org/10.3390/nano14181472
Chicago/Turabian StyleHidouri, Tarek, Maura Pavesi, Marco Vaccari, Antonella Parisini, Nabila Jarmouni, Luigi Cristofolini, and Roberto Fornari. 2024. "Physical Properties of an Efficient MAPbBr3/GaAs Hybrid Heterostructure for Visible/Near-Infrared Detectors" Nanomaterials 14, no. 18: 1472. https://doi.org/10.3390/nano14181472






