Pressure-Promoted Triplet-Pair Separation in Singlet-Fission TIPS-Pentacene Nanofilms Revealed by Ultrafast Spectroscopy
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials and Preparation
2.2. Experiential Instrumentation
2.2.1. High-Pressure Device: Diamond Anvil Cell
2.2.2. Femtosecond Time-Resolved Transient Absorption System
3. Results and Discussion
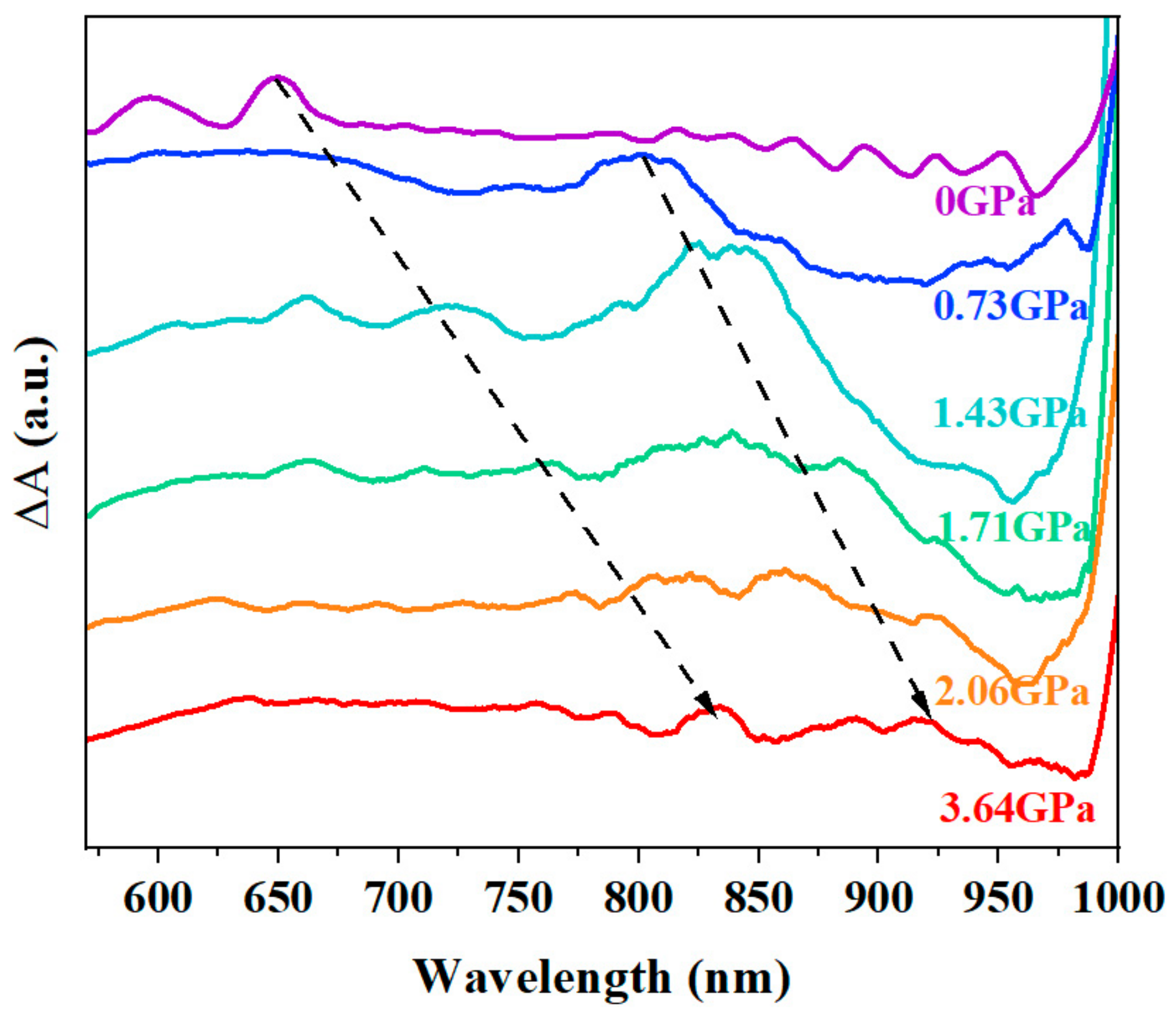
3.1. Steady-State Spectra in Amorphous TPN Films under High-Pressure
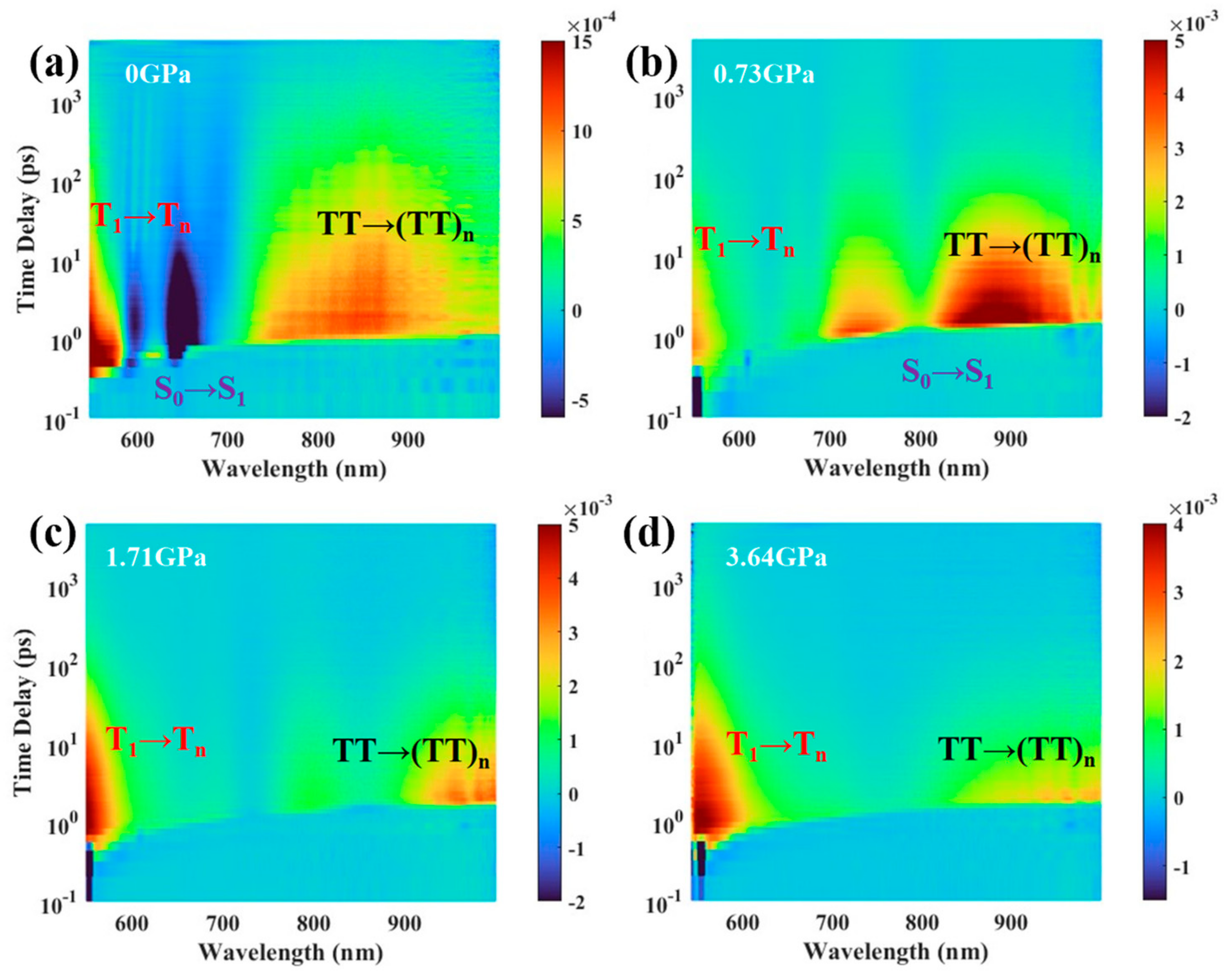
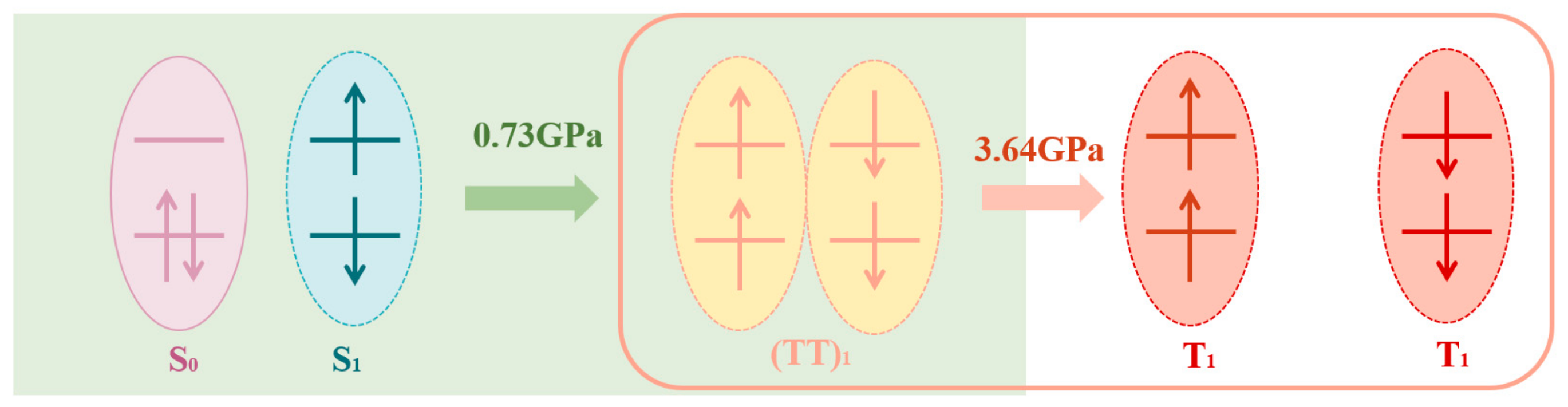
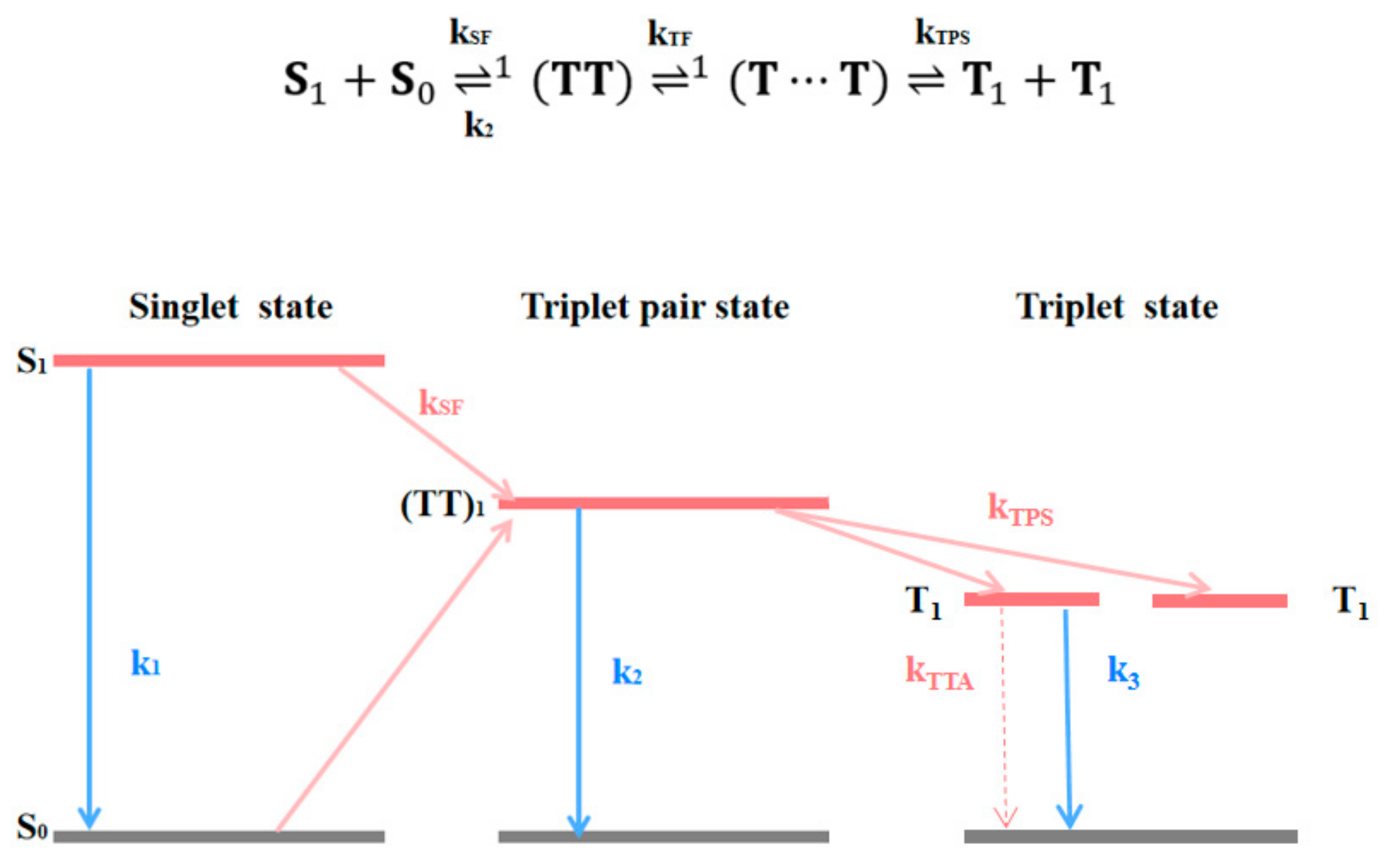
3.2. Transient Absorption Spectra in Amorphous TPN Films under High Pressure
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Smith, M.B.; Michl, J. Singlet Fission. Chem. Rev. 2010, 110, 6891–6936. [Google Scholar] [CrossRef] [PubMed]
- Hanna, M.C.; Nozik, A.J. Solar conversion efficiency of photovoltaic and photoelectrolysis cells with carrier multiplication absorbers. J. Appl. Phys. 2006, 100, 074510. [Google Scholar] [CrossRef]
- Ehrler, B.; Wilson, M.W.B.; Rao, A.; Friend, R.H.; Greenham, N.C. Singlet Exciton Fission-Sensitized Infrared Quantum Dot Solar Cells. Nano Lett. 2012, 12, 1053–1057. [Google Scholar] [CrossRef] [PubMed]
- Smith, M.B.; Michl, J. Recent Advances in Singlet Fission. Annu. Rev. Phys. Chem. 2013, 64, 361–386. [Google Scholar] [CrossRef] [PubMed]
- Pensack, R.D.; Ostroumov, E.E.; Tilley, A.J.; Mazza, S.; Grieco, C.; Thorley, K.J.; Asbury, J.B.; Seferos, D.S.; Anthony, J.E.; Scholes, G.D. Observation of Two Triplet-Pair Intermediates in Singlet Exciton Fission. J. Phys. Chem. Lett. 2016, 7, 2370–2375. [Google Scholar] [CrossRef] [PubMed]
- Walker, B.J.; Musser, A.J.; Beljonne, D.; Friend, R.H. Singlet exciton fission in solution. Nat. Chem. 2013, 5, 1019–1024. [Google Scholar] [CrossRef] [PubMed]
- Pensack, R.D.; Tilley, A.J.; Grieco, C.; Purdum, G.E.; Ostroumov, E.E.; Granger, D.B.; Oblinsky, D.G.; Dean, J.C.; Doucette, G.S.; Asbury, J.B.; et al. Striking the right balance of intermolecular coupling for high-efficiency singlet fission. Chem. Sci. 2018, 9, 6240–6259. [Google Scholar] [CrossRef]
- Gilligan, A.T.; Miller, E.G.; Sammakia, T.; Damrauer, N.H. Using Structurally Well-Defined Norbornyl-Bridged Acene Dimers to Map a Mechanistic Landscape for Correlated Triplet Formation in Singlet Fission. J. Am. Chem. Soc. 2019, 141, 5961–5971. [Google Scholar] [CrossRef]
- Sakai, H.; Inaya, R.; Nagashima, H.; Nakamura, S.; Kobori, Y.; Tkachenko, N.V.; Hasobe, T. Multiexciton Dynamics Depending on Intramolecular Orientations in Pentacene Dimers: Recombination and Dissociation of Correlated Triplet Pairs. J. Phys. Chem. Lett. 2018, 9, 3354–3360. [Google Scholar] [CrossRef]
- Zirzlmeier, J.; Lehnherr, D.; Coto, P.B.; Chernick, E.T.; Casillas, R.; Basel, B.S.; Thoss, M.; Tykwinski, R.R.; Guldi, D.M. Singlet fission in pentacene dimers. Proc. Natl. Acad. Sci. USA 2015, 112, 5325–5330. [Google Scholar] [CrossRef]
- Dillon, R.J.; Piland, G.B.; Bardeen, C.J. Different Rates of Singlet Fission in Monoclinic versus Orthorhombic Crystal Forms of Diphenylhexatriene. J. Am. Chem. Soc. 2013, 135, 17278–17281. [Google Scholar] [CrossRef] [PubMed]
- Munson, K.T.; Gan, J.N.; Grieco, C.; Doucette, G.S.; Anthony, J.E.; Asbury, J.B. Ultrafast Triplet Pair Separation and Triplet Trapping following Singlet Fission in Amorphous Pentacene Films. J. Phys. Chem. C 2020, 124, 23567–23578. [Google Scholar] [CrossRef]
- Jia, W.Y.; Chen, Q.S.; Chen, L.X.; Yuan, D.; Xiang, J.; Chen, Y.B.; Xiong, Z.H. Molecular Spacing Modulated Conversion of Singlet Fission to Triplet Fusion in Rubrene-Based Organic Light-Emitting Diodes at Ambient Temperature. J. Phys. Chem. C 2016, 120, 8380–8386. [Google Scholar] [CrossRef]
- Stuart, A.N.; Tapping, P.C.; Schrefl, E.; Huang, D.M.; Kee, T.W. Controlling the Efficiency of Singlet Fission in TIPS-Pentacene/Polymer Composite Nanoparticles. J. Phys. Chem. C 2019, 123, 5813–5825. [Google Scholar] [CrossRef]
- Grieco, C.; Doucette, G.S.; Munro, J.M.; Kennehan, E.R.; Lee, Y.; Rimshaw, A.; Payne, M.M.; Wonderling, N.; Anthony, J.E.; Dabo, I.; et al. Triplet Transfer Mediates Triplet Pair Separation during Singlet Fission in 6,13-Bis(triisopropylsilylethynyl)-Pentacene. Adv. Funct. Mater. 2017, 27, 1703929. [Google Scholar] [CrossRef]
- Anthony, J.E. The Larger Acenes: Versatile Organic Semiconductors. Angew. Chem. Int. Ed. 2008, 47, 452–483. [Google Scholar] [CrossRef] [PubMed]
- Jones, A.C.; Kearns, N.M.; Ho, J.J.; Flach, J.T.; Zanni, M.T. Impact of non-equilibrium molecular packings on singlet fission in microcrystals observed using 2D white-light microscopy. Nat. Chem. 2020, 12, 40–47. [Google Scholar] [CrossRef] [PubMed]
- Wong, C.Y.; Cotts, B.L.; Wu, H.; Ginsberg, N.S. Exciton dynamics reveal aggregates with intermolecular order at hidden interfaces in solution-cast organic semiconducting films. Nat. Commun. 2015, 6, 5946. [Google Scholar] [CrossRef] [PubMed]
- Grieco, C.; Doucette, G.S.; Pensack, R.D.; Payne, M.M.; Rimshaw, A.; Scholes, G.D.; Anthony, J.E.; Asbury, J.B. Dynamic Exchange During Triplet Transport in Nanocrystalline TIPS-Pentacene Films. J. Am. Chem. Soc. 2016, 138, 16069–16080. [Google Scholar] [CrossRef]
- Lu, D.X.; Wang, Y.H.; Li, F.F.; Huang, X.L.; Pan, L.Y.; Gong, Y.B.; Han, B.; Zhou, Q.; Cui, T. Pressure-Dependent Relaxation Dynamics of Excitons in Conjugated Polymer Film. J. Phys. Chem. C 2015, 119, 13194–13199. [Google Scholar] [CrossRef]
- Doucette, G.S.; Huang, H.T.; Munro, J.M.; Munson, K.T.; Park, C.; Anthony, J.E.; Strobel, T.; Dabo, I.; Badding, J.V.; Asbury, J.B. Tuning Triplet-Pair Separation versus Relaxation Using a Diamond Anvil Cell. Cell Rep. Phys. Sci. 2020, 1, 100005. [Google Scholar] [CrossRef]
- Kinoshita, T.; Nakamura, S.; Harada, M.; Hasobe, T.; Fukuhara, G. Control of intramolecular singlet fission in a pentacene dimer by hydrostatic pressure. Chem. Sci. 2023, 14, 3293–3301. [Google Scholar] [CrossRef] [PubMed]
- Mao, H.K.; Xu, J.; Bell, P.M. Calibration of the ruby pressure gauge to 800 kbar under quasi-hydrostatic conditions. J. Geophys. Res. Solid Earth. 1986, 91, 4673–4676. [Google Scholar] [CrossRef]
- Mao, H.K.; Chen, X.J.; Ding, Y.; Li, B.; Wang, L. Solids, liquids, and gases under high pressure. Rev. Mod. Phys. 2018, 90, 015007. [Google Scholar] [CrossRef]
- Tu, H.Y.; Pan, L.Y.; Qi, H.J.; Zhang, S.H.; Li, F.F.; Sun, C.L.; Wang, X.; Cui, T. Ultrafast dynamics under high-pressure. J. Phys. condens. Mat. 2023, 35, 253002. [Google Scholar] [CrossRef] [PubMed]
- Mulazzi, E.; Ripamonti, A.; Wery, J.; Dulieu, B.; Lefrant, S. Theoretical and experimental investigation of absorption and Raman spectra of poly(paraphenylene vinylene). Phys. Rev. B. 1999, 60, 16519–16525. [Google Scholar] [CrossRef]
- Hetzer, C.; Guldi, D.M.; Tykwinski, R.R. Pentacene Dimers as a Critical Tool for the Investigation of Intramolecular Singlet Fission. Chem. Eur. J. 2018, 24, 8245–8257. [Google Scholar] [CrossRef] [PubMed]
- Trinh, M.T.; Pinkard, A.; Pun, A.B.; Sanders, S.N.; Kumarasamy, E.; Sfeir, M.Y.; Campos, L.M.; Roy, X.; Zhu, X.Y. Distinct properties of the triplet pair state from singlet fission. Sci. Adv. 2017, 3, e1700241. [Google Scholar] [CrossRef]
- Grieco, C.; Kennehan, E.R.; Kim, H.; Pensack, R.D.; Brigeman, A.N.; Rimshaw, A.; Payne, M.M.; Anthony, J.E.; Giebink, N.C.; Scholes, G.D.; et al. Direct Observation of Correlated Triplet Pair Dynamics during Singlet Fission Using Ultrafast Mid-IR Spectroscopy. J. Phys. Chem. C 2018, 122, 2012–2022. [Google Scholar] [CrossRef]
- Huang, G.; Li, J.; Zhou, Z.; Huang, Z.; Kong, W.; Zhang, F.; Zeng, Y.; Liu, G.; He, T.; Ma, L. Aggregation Regulated Ultrafast Singlet Fission Pathways in TIPS-Pentacene Films. Ultrafast Sci. 2024, 4, 0057. [Google Scholar] [CrossRef]
- Zhou, Z.; Ma, L.; Guo, D.; Zhao, X.; Wang, C.; Lin, D.; Zhang, F.; Zhang, J.; Nie, Z. Ultrafast Dynamics of Long-Lived Bound Triplet Pair Generated by Singlet Fission in 6,13-Bis(triisopropylsilylethynyl) Pentacene. J. Phys. Chem. C 2020, 124, 14503–14509. [Google Scholar] [CrossRef]
- Musser, A.J.; Liebel, M.; Schnedermann, C.; Wende, T.; Kehoe, T.B.; Rao, A.; Kukura, P. Evidence for conical intersection dynamics mediating ultrafast singlet exciton fission. Nat. Phys. 2015, 11, 352–357. [Google Scholar] [CrossRef]
- Herz, J.; Buckup, T.; Paulus, F.; Engelhart, J.U.; Bunz, U.H.F.; Motzkus, M. Unveiling Singlet Fission Mediating States in TIPS-pentacene and its Aza Derivatives. J. Phys. Chem. A 2015, 119, 6602–6610. [Google Scholar] [CrossRef] [PubMed]
- Wilson, M.W.; Rao, A.; Clark, J.; Kumar, R.S.; Brida, D.; Cerullo, G.; Friend, R.H. Ultrafast dynamics of exciton fission in polycrystalline pentacene. J. Am. Chem. Soc. 2011, 133, 11830–11833. [Google Scholar] [CrossRef] [PubMed]
- Hudson, R.J.; Stuart, A.N.; de la Perrelle, J.M.; Huang, D.M.; Kee, T.W. Nanoparticle Size-Dependent Singlet Fission and Exciton Dynamics in Amorphous TIPS-Pentacene. J. Phys. Chem. C 2021, 125, 21559–21570. [Google Scholar] [CrossRef]
- Paudel, K.; Moghe, D.; Chandrasekhar, M.; Yu, P.; Ramasesha, S.; Scherf, U.; Guha, S. Pressure dependence of singlet and triplet excitons in amorphous polymer semiconductors. EPL 2013, 104, 27008. [Google Scholar] [CrossRef]
- Jankus, V.; Snedden, E.W.; Bright, D.W.; Arac, E.; Dai, D.C.; Monkman, A.P. Competition between polaron pair formation and singlet fission observed in amorphous rubrene films. Phys. Rev. B 2013, 87, 224202. [Google Scholar] [CrossRef]
- Li, J.; Chen, Z.H.; Lei, Y.L.; Xiong, Z.H.; Zhang, Y. Competition between singlet exciton fission, radiation, and dissociation measured in rubrene-doped amorphous films. Synth. Met. 2015, 207, 13–17. [Google Scholar] [CrossRef]
- Mou, W.W.; Hattori, S.; Rajak, P.; Shimojo, F.; Nakano, A. Nanoscopic mechanisms of singlet fission in amorphous molecular solid. Appl. Phys. Lett. 2013, 102, 173301. [Google Scholar] [CrossRef]
- Li, J.; Chen, Z.H.; Zhang, Q.M.; Xiong, Z.H.; Zhang, Y. Temperature-dependent singlet exciton fission observed in amorphous rubrene films. Org. Electron. 2015, 26, 213–217. [Google Scholar] [CrossRef]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, L.; Zhu, R.; Pu, R.; Liu, W.; Lu, Y.; Weng, T.-C. Pressure-Promoted Triplet-Pair Separation in Singlet-Fission TIPS-Pentacene Nanofilms Revealed by Ultrafast Spectroscopy. Nanomaterials 2024, 14, 1487. https://doi.org/10.3390/nano14181487
Wang L, Zhu R, Pu R, Liu W, Lu Y, Weng T-C. Pressure-Promoted Triplet-Pair Separation in Singlet-Fission TIPS-Pentacene Nanofilms Revealed by Ultrafast Spectroscopy. Nanomaterials. 2024; 14(18):1487. https://doi.org/10.3390/nano14181487
Chicago/Turabian StyleWang, Lu, Ruixue Zhu, Ruihua Pu, Weimin Liu, Yang Lu, and Tsu-Chieu Weng. 2024. "Pressure-Promoted Triplet-Pair Separation in Singlet-Fission TIPS-Pentacene Nanofilms Revealed by Ultrafast Spectroscopy" Nanomaterials 14, no. 18: 1487. https://doi.org/10.3390/nano14181487
APA StyleWang, L., Zhu, R., Pu, R., Liu, W., Lu, Y., & Weng, T.-C. (2024). Pressure-Promoted Triplet-Pair Separation in Singlet-Fission TIPS-Pentacene Nanofilms Revealed by Ultrafast Spectroscopy. Nanomaterials, 14(18), 1487. https://doi.org/10.3390/nano14181487





