Ordered versus Non-Ordered Mesoporous CeO2-Based Systems for the Direct Synthesis of Dimethyl Carbonate from CO2
Abstract
:1. Introduction
2. Materials and Methods
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mikulčić, H.; Ridjan Skov, I.; Dominković, D.F.; Wan Alwi, S.R.; Manan, Z.A.; Tan, R.; Duić, N.; Hidayah Mohamad, S.N.; Wang, X. Flexible Carbon Capture and Utilization Technologies in Future Energy Systems and the Utilization Pathways of Captured CO2. Renew. Sustain. Energy Rev. 2019, 114, 109338. [Google Scholar] [CrossRef]
- Fu, L.; Ren, Z.; Si, W.; Ma, Q.; Huang, W.; Liao, K.; Huang, Z.; Wang, Y.; Li, J.; Xu, P. Research Progress on CO2 Capture and Utilization Technology. J. CO2 Util. 2022, 66, 102260. [Google Scholar] [CrossRef]
- Ghiat, I.; Al-Ansari, T. A Review of Carbon Capture and Utilisation as a CO2 Abatement Opportunity within the EWF Nexus. J. CO2 Util. 2021, 45, 101432. [Google Scholar] [CrossRef]
- Álvarez, A.; Bansode, A.; Urakawa, A.; Bavykina, A.V.; Wezendonk, T.A.; Makkee, M.; Gascon, J.; Kapteijn, F. Challenges in the Greener Production of Formates/Formic Acid, Methanol, and DME by Heterogeneously Catalyzed CO2 Hydrogenation Processes. Chem. Rev. 2017, 117, 9804–9838. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Shi, F.; Wang, L. A Review of Catalysts for Synthesis of Dimethyl Carbonate. Catalysts 2024, 14, 259. [Google Scholar] [CrossRef]
- Zhang, M.; Xu, Y.; Williams, B.L.; Xiao, M.; Wang, S.; Han, D.; Sun, L.; Meng, Y. Catalytic Materials for Direct Synthesis of Dimethyl Carbonate (DMC) from CO2. J. Clean. Prod. 2021, 279, 123344. [Google Scholar] [CrossRef]
- Ashok, J.; Pati, S.; Hongmanorom, P.; Tianxi, Z.; Junmei, C.; Kawi, S. A Review of Recent Catalyst Advances in CO2 Methanation Processes. Catal. Today 2020, 356, 471–489. [Google Scholar] [CrossRef]
- Atzori, L.; Cutrufello, M.G.; Meloni, D.; Secci, F.; Cannas, C.; Rombi, E. Soft-Templated NiO–CeO2 Mixed Oxides for Biogas Upgrading by Direct CO2 Methanation. Int. J. Hydrogen Energy 2023, 48, 25031–25043. [Google Scholar] [CrossRef]
- Guil-López, R.; Mota, N.; Llorente, J.; Millán, E.; Pawelec, B.; Fierro, J.L.G.; Navarro, R.M. Methanol Synthesis from CO2: A Review of the Latest Developments in Heterogeneous Catalysis. Materials 2019, 12, 3902. [Google Scholar] [CrossRef]
- Catizzone, E.; Migliori, M.; Purita, A.; Giordano, G. Ferrierite vs. γ-Al2O3: The Superiority of Zeolites in Terms of Water-Resistance in Vapour-Phase Dehydration of Methanol to Dimethyl Ether. J. Energy Chem. 2019, 30, 162–169. [Google Scholar] [CrossRef]
- Catizzone, E.; Freda, C.; Braccio, G.; Frusteri, F.; Bonura, G. Dimethyl Ether as Circular Hydrogen Carrier: Catalytic Aspects of Hydrogenation/Dehydrogenation Steps. J. Energy Chem. 2021, 58, 55–77. [Google Scholar] [CrossRef]
- Secci, F.; Mameli, V.; Rombi, E.; Lai, S.; Angotzi, M.S.; Russo, P.A.; Pinna, N.; Mureddu, M.; Cannas, C. On the Role of the Nature and Density of Acid Sites on Mesostructured Aluminosilicates Dehydration Catalysts for Dimethyl Ether Production from CO2. J. Environ. Chem. Eng. 2023, 11, 110018. [Google Scholar] [CrossRef]
- Cara, C.; Secci, F.; Lai, S.; Mameli, V.; Skrodczky, K.; Russo, P.A.; Ferrara, F.; Rombi, E.; Pinna, N.; Mureddu, M.; et al. On the Design of Mesostructured Acidic Catalysts for the One-Pot Dimethyl Ether Production from CO2. J. CO2 Util. 2022, 62, 102066. [Google Scholar] [CrossRef]
- Pyo, S.-H.; Park, J.H.; Chang, T.-S.; Hatti-Kaul, R. Dimethyl Carbonate as a Green Chemical. Curr. Opin. Green Sustain. Chem. 2017, 5, 61–66. [Google Scholar] [CrossRef]
- Tan, H.Z.; Wang, Z.Q.; Xu, Z.N.; Sun, J.; Xu, Y.P.; Chen, Q.S.; Chen, Y.; Guo, G.C. Review on the Synthesis of Dimethyl Carbonate. Catal. Today 2018, 316, 2–12. [Google Scholar] [CrossRef]
- Raza, A.; Ikram, M.; Guo, S.; Baiker, A.; Li, G. Green Synthesis of Dimethyl Carbonate from CO2 and Methanol: New Strategies and Industrial Perspective. Adv. Sustain. Syst. 2022, 6, 2200087. [Google Scholar] [CrossRef]
- Yang, Z.; Zheng, J.T.; Lu, X.; Lin, M.M.; Cai, D.; Wang, Y.; Yu, W.-Y.; Zhu, Y.; Xia, Y. Porous Ceria Materials for Efficient Direct Conversion of Carbon Dioxide and Methanol to Dimethyl Carbonate. Mater. Adv. 2024, 5, 6605–6617. [Google Scholar] [CrossRef]
- Hou, G.; Wang, Q.; Xu, D.; Fan, H.; Liu, K.; Li, Y.; Gu, X.; Ding, M. Dimethyl Carbonate Synthesis from CO2 over CeO2 with Electron-Enriched Lattice Oxygen Species. Angew. Chem. Int. Ed. 2024, 63, e202402053. [Google Scholar] [CrossRef]
- Marciniak, A.A.; Alves, O.C.; Appel, L.G.; Mota, C.J.A. Synthesis of Dimethyl Carbonate from CO2 and Methanol over CeO2: Role of Copper as Dopant and the Use of Methyl Trichloroacetate as Dehydrating Agent. J. Catal. 2019, 371, 88–95. [Google Scholar] [CrossRef]
- Marciniak, A.A.; Santos, E.C.S.; Caraballo-Vivas, R.J.; Alves, O.C.; Maia da Costa, M.E.H.; Garcia, F.; Mota, C.J.A. CeO2-Decorated α-Fe2O3 Nanorings for the Direct Synthesis of Dimethyl Carbonate from CO2 and Methanol. Energy Fuels 2024, 38, 628–636. [Google Scholar] [CrossRef]
- Seeharaj, P.; Saenman, T.; Phiwhom, T.; Muangsuwan, C.; Srinives, S.; Kim-Lohsoontorn, P. Improvement of Surface Properties of Metal Doped-CeO2 Nanospindle Catalysts for Direct Synthesis of Dimethyl Carbonate from CO2 and Methanol. J. Environ. Chem. Eng. 2023, 11, 109813. [Google Scholar] [CrossRef]
- Kulthananat, T.; Kim-Lohsoontorn, P.; Seeharaj, P. Ultrasonically Assisted Surface Modified CeO2 Nanospindle Catalysts for Conversion of CO2 and Methanol to DMC. Ultrason. Sonochem. 2022, 90, 106164. [Google Scholar] [CrossRef]
- Dubey, M.; Wadhwa, S.; Mathur, A.; Kumar, R. Progress in Mesoporous Ceria: A Review on Synthesis Strategies and Catalytic Applications. Appl. Surf. Sci. Adv. 2022, 12, 100340. [Google Scholar] [CrossRef]
- Sakina, F.; Muñoz-Ocaña, J.M.; Bouziane, A.; Lopez-Haro, M.; Baker, R.T. Synthesis of Mesoporous Ceria Using Metal- and Halogen-Free Ordered Mesoporous Carbon as a Hard Template. Nanoscale Adv. 2019, 1, 4772–4782. [Google Scholar] [CrossRef] [PubMed]
- Roggenbuck, J.; Schäfer, H.; Tsoncheva, T.; Minchev, C.; Hanss, J.; Tiemann, M. Mesoporous CeO2: Synthesis by Nanocasting, Characterisation and Catalytic Properties. Microporous Mesoporous Mater. 2007, 101, 335–341. [Google Scholar] [CrossRef]
- Liang, X.; Xiao, J.; Chen, B.; Li, Y. Catalytically Stable and Active CeO2 Mesoporous Spheres. Inorg. Chem. 2010, 49, 8188–8190. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Sel, O.; Djerdj, I.; Smarsly, B. Preparation of a Large Mesoporous CeO2 with Crystalline Walls Using PMMA Colloidal Crystal Templates. Colloid Polym. Sci. 2006, 285, 1–9. [Google Scholar] [CrossRef]
- Junais, P.M.; Athika, M.; Govindaraj, G.; Elumalai, P. Supercapattery Performances of Nanostructured Cerium Oxide Synthesized Using Polymer Soft-Template. J. Energy Storage 2020, 28, 101241. [Google Scholar] [CrossRef]
- Strunk, J.; Vining, W.C.; Bell, A.T. Synthesis of Different CeO2 Structures on Mesoporous Silica and Characterization of Their Reduction Properties. J. Phys. Chem. C 2011, 115, 4114–4126. [Google Scholar] [CrossRef]
- Dhall, A.; Self, W. Cerium Oxide Nanoparticles: A Brief Review of Their Synthesis Methods and Biomedical Applications. Antioxidants 2018, 7, 97. [Google Scholar] [CrossRef]
- Zagaynov, I.V.; Kutsev, S.V. Formation of Mesoporous Nanocrystalline Ceria from Cerium Nitrate, Acetate or Acetylacetonate. Appl. Nanosci. 2014, 4, 339–345. [Google Scholar] [CrossRef]
- Borjas-García, S.E.; Medina-Flores, A.; Béjar, L.; Martínez-Torres, P.; Dasgupta-Schubert, N.; Bernal, J.L. Synthesis of Mesoporous Ceria by Using CTAB as Template. Microsc. Microanal. 2016, 22, 1918–1919. [Google Scholar] [CrossRef]
- Kurajica, S.; Minga, I.; Guliš, M.; Mandić, V.; Simčić, I. High Surface Area Ceria Nanoparticles via Hydrothermal Synthesis Experiment Design. J. Nanomater. 2016, 2016, 1–8. [Google Scholar] [CrossRef]
- Zhang, G.; Shen, Z.; Liu, M.; Guo, C.; Sun, P.; Yuan, Z.; Li, B.; Ding, D.; Chen, T. Synthesis and Characterization of Mesoporous Ceria with Hierarchical Nanoarchitecture Controlled by Amino Acids. J. Phys. Chem. B 2006, 110, 25782–25790. [Google Scholar] [CrossRef]
- Wang, M.M.; He, L.; Liu, Y.M.; Cao, Y.; He, H.Y.; Fan, K.N. Gold Supported on Mesostructured Ceria as an Efficient Catalyst for the Chemoselective Hydrogenation of Carbonyl Compounds in Neat Water. Green Chem. 2011, 13, 602–607. [Google Scholar] [CrossRef]
- Liu, H.; Zhu, D.; Jia, B.; Huang, Y.; Cheng, Y.; Luo, X.; Liang, Z. Study on Catalytic Performance and Kinetics of High Efficiency CeO2 Catalyst Prepared by Freeze Drying for the Synthesis of Dimethyl Carbonate from CO2 and Methanol. Chem. Eng. Sci. 2022, 254, 117614. [Google Scholar] [CrossRef]
- Hu, L.; Hu, K.; Xu, Z.; Yao, W.; Wang, A.; Wu, G.; Xu, W. Preparation and Characterization of Hollow CeO2 Nanoparticles for the Efficient Conversion of CO2 into Dimethyl Carbonate. ChemCatChem 2023, 15, e202300786. [Google Scholar] [CrossRef]
- Pouretedal, H.R.; Basati, S. Synthesis, Characterization and Photocatalytic Activity of CeO2-SBA-15. Iran. J. Catal. 2012, 2, 51–55. [Google Scholar]
- Mitran, R.-A.; Culita, D.C.; Atkinson, I. Thermal Stability Enhancement of Mesoporous SBA-15 Silica through Nanoconfinement of Ceria Nanoparticles. Microporous Mesoporous Mater. 2020, 306, 110484. [Google Scholar] [CrossRef]
- Pu, Y.; Xuan, K.; Wang, F.; Li, A.; Zhao, N.; Xiao, F. Synthesis of Dimethyl Carbonate from CO2 and Methanol over a Hydrophobic Ce/SBA-15 Catalyst. RSC Adv. 2018, 8, 27216–27226. [Google Scholar] [CrossRef]
- Shen, J.; Hess, C. Controlling the Dispersion of Ceria Using Nanoconfinement: Application to CeO2/SBA-15 Catalysts for NH3-SCR. Mater. Adv. 2021, 2, 7400–7412. [Google Scholar] [CrossRef]
- Yang, J.; Jia, Y.; Huang, B.; Li, X.; Guo, L.; Zheng, A.; Luque, R.; Sun, Y. Functionalized CeO2/SBA-15 Materials as Efficient Catalysts for Aqueous Room Temperature Mono-Dehydration of Sugar Alcohols. ACS Sustain. Chem. Eng. 2020, 8, 6371–6380. [Google Scholar] [CrossRef]
- Saadati-Moshtaghin, H.R.; Zonoz, F.M. In Situ Preparation of CeO2 Nanoparticles on the MCM-41 with Magnetic Core as a Novel and Efficient Catalyst for the Synthesis of Substituted Pyran Derivatives. Inorg. Chem. Commun. 2019, 99, 44–51. [Google Scholar] [CrossRef]
- Ngomade, S.B.L.; Fotsop, C.G.; Nguena, K.L.T.; Tchummegne, I.K.; Ngueteu, M.L.T.; Tamo, A.K.; Nche, G.N.A.; Anagho, S.G. Catalytic Performances of CeO2@SBA-15 as Nanostructured Material for Biodiesel Production from Podocarpus Falcatus Oil. Chem. Eng. Res. Des. 2023, 194, 789–800. [Google Scholar] [CrossRef]
- Ma, K.; Zhang, H.; Tang, C.; Dong, L. One-Pot Synthesis of CeO2 Modified SBA-15 With No Pore Clogging for NO Reduction by CO. Front. Environ. Chem. 2021, 2, 670431. [Google Scholar] [CrossRef]
- Mureddu, M.; Ferino, I.; Musinu, A.; Ardu, A.; Rombi, E.; Cutrufello, M.G.; Deiana, P.; Fantauzzi, M.; Cannas, C. MeOx/SBA-15 (Me = Zn, Fe): Highly Efficient Nanosorbents for Mid-Temperature H2S Removal. J. Mater. Chem. A 2014, 2, 19396–19406. [Google Scholar] [CrossRef]
- Mureddu, M.; Ferino, I.; Rombi, E.; Cutrufello, M.G.; Deiana, P.; Ardu, A.; Musinu, A.; Piccaluga, G.; Cannas, C. ZnO/SBA-15 Composites for Mid-Temperature Removal of H2S: Synthesis, Performance and Regeneration Studies. Fuel 2012, 102, 691–700. [Google Scholar] [CrossRef]
- Cara, C.; Rombi, E.; Mameli, V.; Ardu, A.; Sanna Angotzi, M.; Niznansky, D.; Musinu, A.; Cannas, C. γ-Fe2O3-M41S Sorbents for H2S Removal: Effect of Different Porous Structures and Silica Wall Thickness. J. Phys. Chem. C 2018, 122, 12231–12242. [Google Scholar] [CrossRef]
- Varma, A.; Mukasyan, A.S.; Rogachev, A.S.; Manukyan, K.V. Solution Combustion Synthesis of Nanoscale Materials. Chem. Rev. 2016, 116, 14493–14586. [Google Scholar] [CrossRef]
- Cannas, C.; Musinu, A.; Peddis, D.; Piccaluga, G. Synthesis and Characterization of CoFe2O4 Nanoparticles Dispersed in a Silica Matrix by a Sol-Gel Autocombustion Method. Chem. Mater. 2006, 18, 3835–3842. [Google Scholar] [CrossRef]
- Cannas, C.; Ardu, A.; Niznansky, D.; Peddis, D.; Piccaluga, G.; Musinu, A. Simple and Fast Preparation of Pure Maghemite Nanopowders through Sol-Gel Self-Combustion. J. Solgel Sci. Technol. 2011, 60, 266–274. [Google Scholar] [CrossRef]
- Cannas, C.; Musinu, A.; Peddis, D.; Piccaluga, G. New Synthesis of Ferrite-Silica Nanocomposites by a Sol-Gel Auto-Combustion. J. Nanopart. Res. 2004, 6, 223–232. [Google Scholar] [CrossRef]
- Cannas, C.; Falqui, A.; Musinu, A.; Peddis, D.; Piccaluga, G. CoFe2O4 Nanocrystalline Powders Prepared by Citrate-Gel Methods: Synthesis, Structure and Magnetic Properties. J. Nanopart. Res. 2006, 8, 255–267. [Google Scholar] [CrossRef]
- Secci, F.; Sanna Angotzi, M.; Mameli, V.; Lai, S.; Russo, P.A.; Pinna, N.; Mureddu, M.; Rombi, E.; Cannas, C. Mesostructured γ-Al2O3-Based Bifunctional Catalysts for Direct Synthesis of Dimethyl Ether from CO2. Catalysts 2023, 13, 505. [Google Scholar] [CrossRef]
- Mureddu, M.; Ferrara, F.; Pettinau, A. Highly Efficient CuO/ZnO/ZrO2@SBA-15 Nanocatalysts for Methanol Synthesis from the Catalytic Hydrogenation of CO2. Appl. Catal. B 2019, 258, 117941. [Google Scholar] [CrossRef]
- Zhao, D.; Wan, Y.; Zhou, W. Ordered Mesoporous Materials; Wiley-VCH: Weinheim, Germany, 2013; ISBN 9783527326358. [Google Scholar]
- Zhao, D.; Feng, J.; Huo, Q.; Melosh, N.; Fredrickson, G.H.; Chmelka, B.F.; Stucky, G.D. Triblock Copolymer Syntheses of Mesoporous Silica with Periodic 50 to 300 Angstrom Pores. Science 1998, 279, 548–552. [Google Scholar] [CrossRef]
- Le Bail, A. Modelling the Silica Glass Structure by the Rietveld Method. J. Non Cryst. Solids 1995, 183, 39–42. [Google Scholar] [CrossRef]
- Ennas, G.; Marongiu, G.; Marras, S.; Piccaluga, G. Mechanochemical Route for the Synthesis of Cobalt Ferrite–Silica and Iron–Cobalt Alloy–Silica Nanocomposites. J. Nanopart. Res. 2004, 6, 99–105. [Google Scholar] [CrossRef]
- Janssen, A.H.; Yang, C.M.; Wang, Y.; Schüth, F.; Koster, A.J.; De Jong, K.P. Localization of Small Metal (Oxide) Particles in SBA-15 Using Bright-Field Electron Tomography. J. Phys. Chem. B 2003, 107, 10552–10556. [Google Scholar] [CrossRef]
- Delahaye, E.; Escax, V.; El Hassan, N.; Davidson, A.; Aquino, R.; Dupuis, V.; Perzynski, R.; Raikher, Y.L. “Nanocasting”: Using SBA-15 Silicas as Hard Templates to Obtain Ultrasmall Monodispersed γ-Fe2O3 Nanoparticles. J. Phys. Chem. B 2006, 110, 26001–26011. [Google Scholar] [CrossRef]
- Liu, B.; Li, C.; Zhang, G.; Yao, X.; Chuang, S.S.C.; Li, Z. Oxygen Vacancy Promoting Dimethyl Carbonate Synthesis from CO2 and Methanol over Zr-Doped CeO2 Nanorods. ACS Catal. 2018, 8, 10446–10456. [Google Scholar] [CrossRef]
- Wang, F.; Wan, T.; Xue, Y.; Cui, L.; Da, B.; Liu, N.; Ma, Q.; Xu, J.; Xue, B. Ga-Doped CeO2 Nanorods as Highly Active Catalysts for the Synthesis of Dimethyl Carbonate from CO2 and Methanol. React. Kinet. Mech. Catal. 2023, 136, 2941–2954. [Google Scholar] [CrossRef]
- Kuan, W.-F.; Yu, W.-Y.; Tu, F.-Y.; Chung, C.-H.; Chang, Y.-C.; Lin, M.M.; Yu, T.-H.; Chen, L.-J. Facile Reflux Preparation of Defective Mesoporous Ceria Nanorod with Superior Catalytic Activity for Direct Carbon Dioxide Conversion into Dimethyl Carbonate. Chem. Eng. J. 2022, 430, 132941. [Google Scholar] [CrossRef]
- Chen, L.; Wang, S.; Zhou, J.; Shen, Y.; Zhao, Y.; Ma, X. Dimethyl Carbonate Synthesis from Carbon Dioxide and Methanol over CeO2 versus over ZrO2: Comparison of Mechanisms. RSC Adv. 2014, 4, 30968–30975. [Google Scholar] [CrossRef]
- Kabra, S.K.; Turpeinen, E.; Keiski, R.L.; Yadav, G.D. Direct Synthesis of Dimethyl Carbonate from Methanol and Carbon Dioxide: A Thermodynamic and Experimental Study. J. Supercrit. Fluids 2016, 117, 98–107. [Google Scholar] [CrossRef]
- Siranjeevi, R.; Vasumathi, V.; Suganya, S.; Saravanan, A.; Usha, R.; Azhagurajan, M.; Jeyalakshmi, R. Evaluation of Biosynthesized GO@CeO2 Nanocomposites as a Catalyst for UV-Assisted Degradation of Organic Dyes and Phytotoxicity Studies. Surf. Interfaces 2024, 44, 103748. [Google Scholar] [CrossRef]
- Meng, F.; Li, H.; Gong, J.; Fan, Z. Photocatalytic and Magnetic Properties of Loosened Ceria Hollow Microspheres Synthesized by a Single-Step Hydrothermal Method. J. Mater. Sci. Mater. Electron. 2016, 27, 8433–8439. [Google Scholar] [CrossRef]
- Mikheeva, N.N.; Zaikovskii, V.I.; Mamontov, G.V. Synthesis of Ceria Nanoparticles in Pores of SBA-15: Pore Size Effect and Influence of Citric Acid Addition. Microporous Mesoporous Mater. 2019, 277, 10–16. [Google Scholar] [CrossRef]
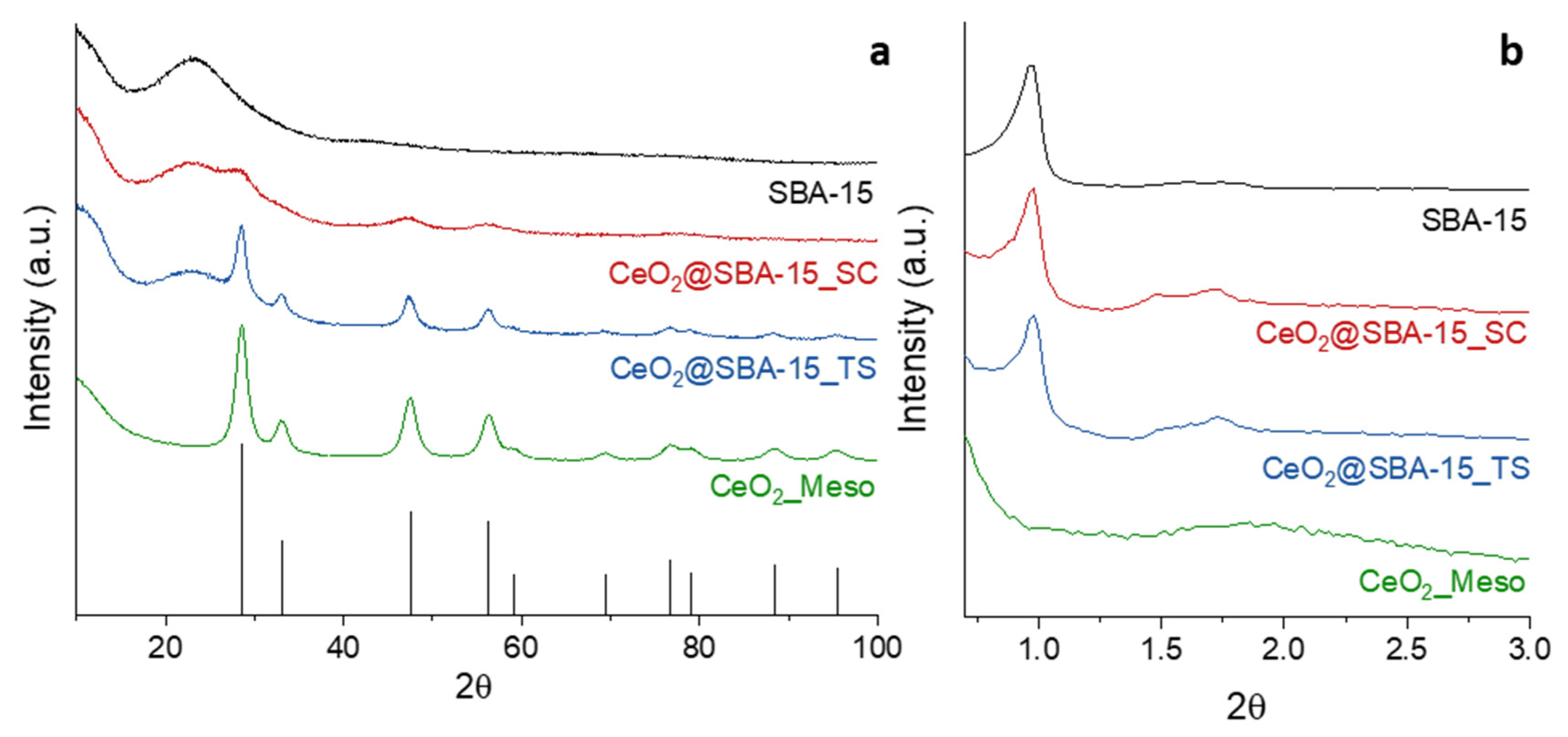

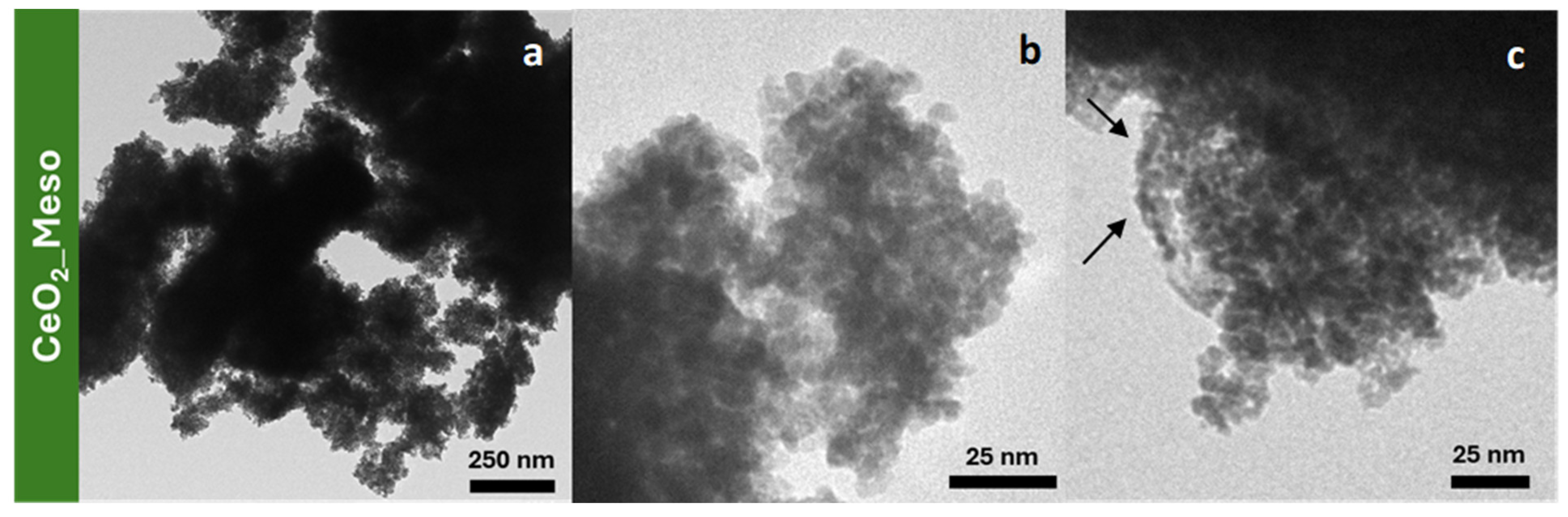

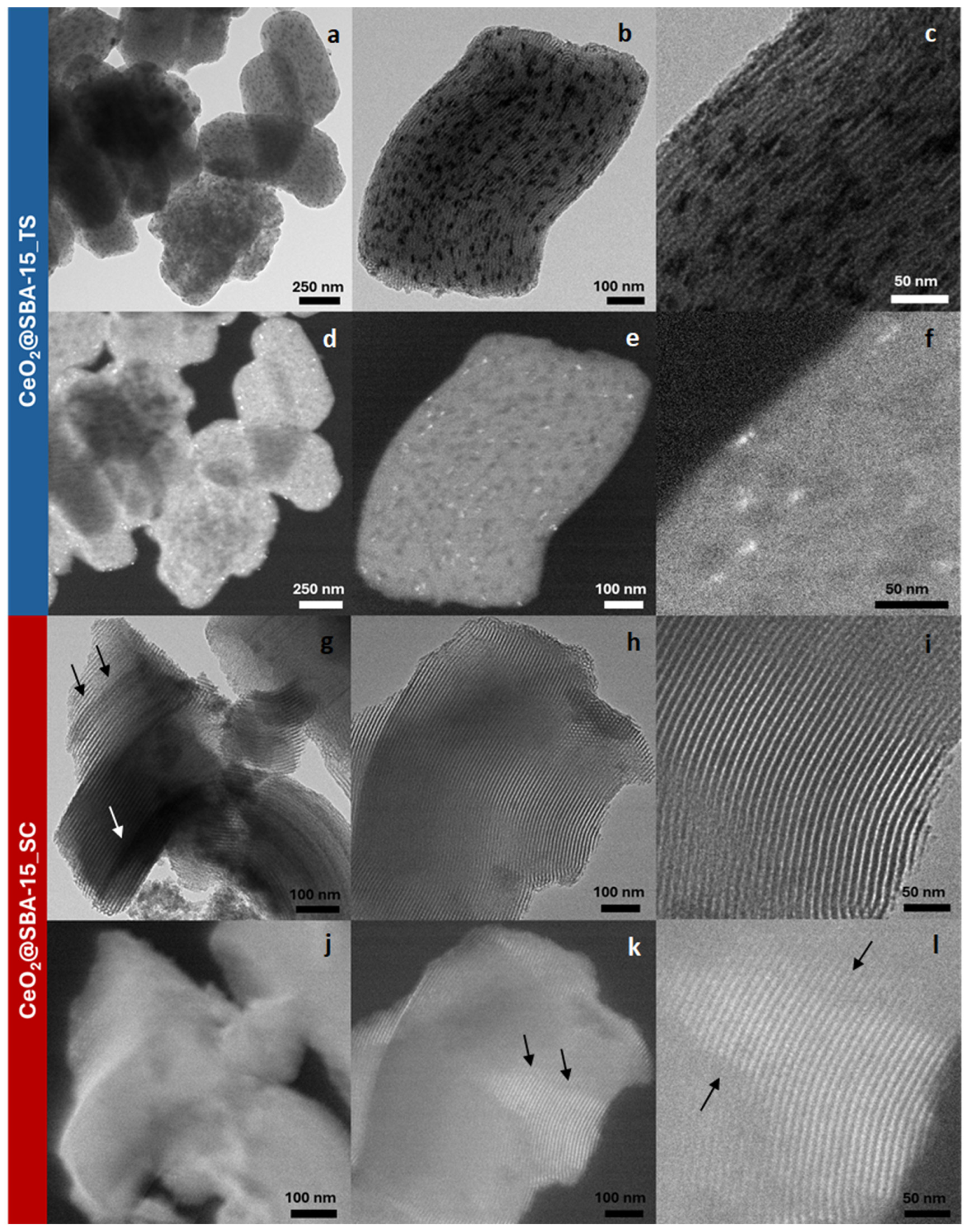
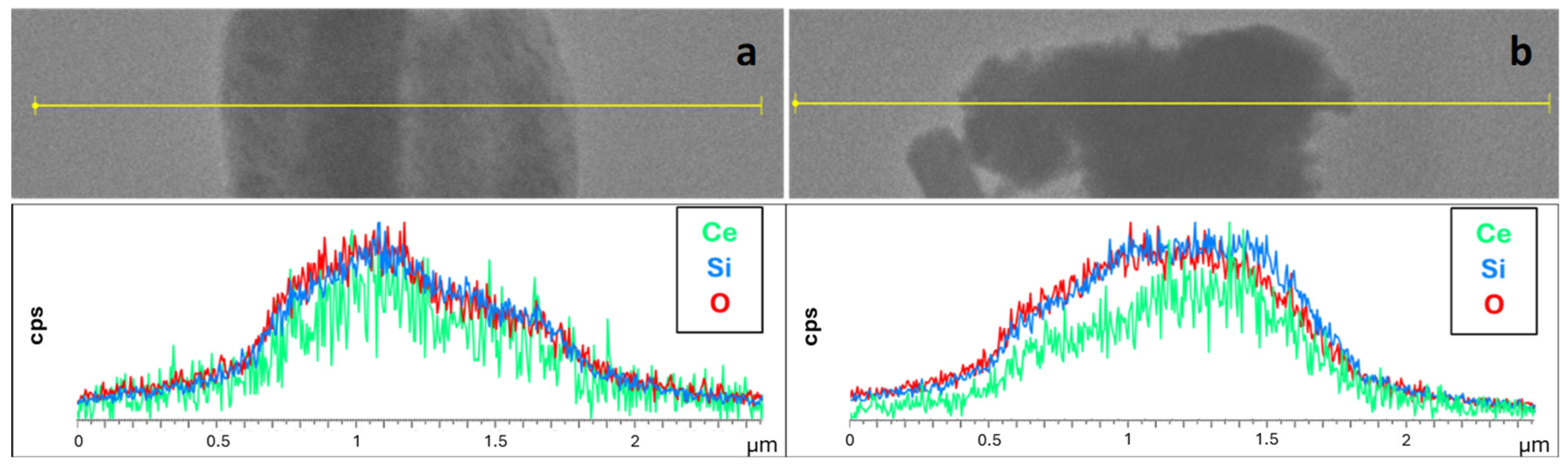

| Sample | DXRD (nm) | a0 (nm) | S.A. (m2/g) | Vp (cm3/g) | DP (nm) | Tw (nm) |
| CeO2_Meso | 7.9 (1) | - | 182 | 0.27 | 5.8 | - |
| CeO2@SBA-15_TS | 7.9 (1) | 10.4 | 722 | 0.93 | 6.1 | 4.3 |
| CeO2@SBA-15_SC | 2.6 (1) | 10.4 | 635 | 0.88 | 6.1 | 4.3 |
| SBA-15 | - | 10.5 | 853 | 0.99 | 6.3 | 4.2 |
| Catalyst | Yield (mmol/gcat) | Yield (mmol/gact.ph.) | Yield (mol%) |
|---|---|---|---|
| CeO2_Meso | 0.941 | 0.941 | 2 × 10−3 |
| CeO2@SBA-15_SC | 0.097 | 0.971 | 2 × 10−4 |
| CeO2@SBA-15_TS | 0.066 | 0.662 | 1 × 10−4 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rusta, N.; Secci, F.; Mameli, V.; Cannas, C. Ordered versus Non-Ordered Mesoporous CeO2-Based Systems for the Direct Synthesis of Dimethyl Carbonate from CO2. Nanomaterials 2024, 14, 1490. https://doi.org/10.3390/nano14181490
Rusta N, Secci F, Mameli V, Cannas C. Ordered versus Non-Ordered Mesoporous CeO2-Based Systems for the Direct Synthesis of Dimethyl Carbonate from CO2. Nanomaterials. 2024; 14(18):1490. https://doi.org/10.3390/nano14181490
Chicago/Turabian StyleRusta, Nicoletta, Fausto Secci, Valentina Mameli, and Carla Cannas. 2024. "Ordered versus Non-Ordered Mesoporous CeO2-Based Systems for the Direct Synthesis of Dimethyl Carbonate from CO2" Nanomaterials 14, no. 18: 1490. https://doi.org/10.3390/nano14181490









