Enhancing the Performance of Nanocrystalline SnO2 for Solar Cells through Photonic Curing Using Impedance Spectroscopy Analysis
Abstract
:1. Introduction
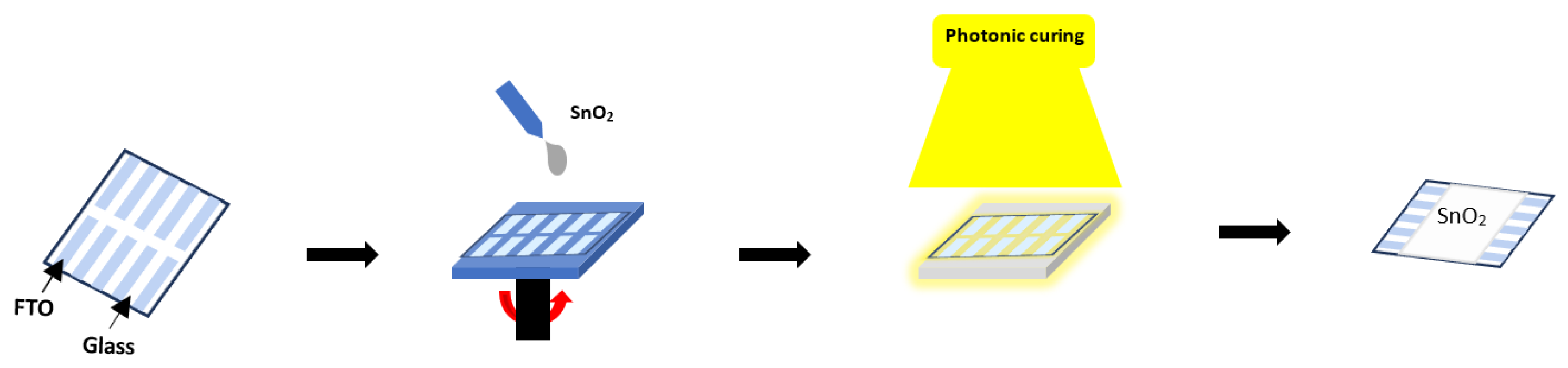
2. Experimental Section
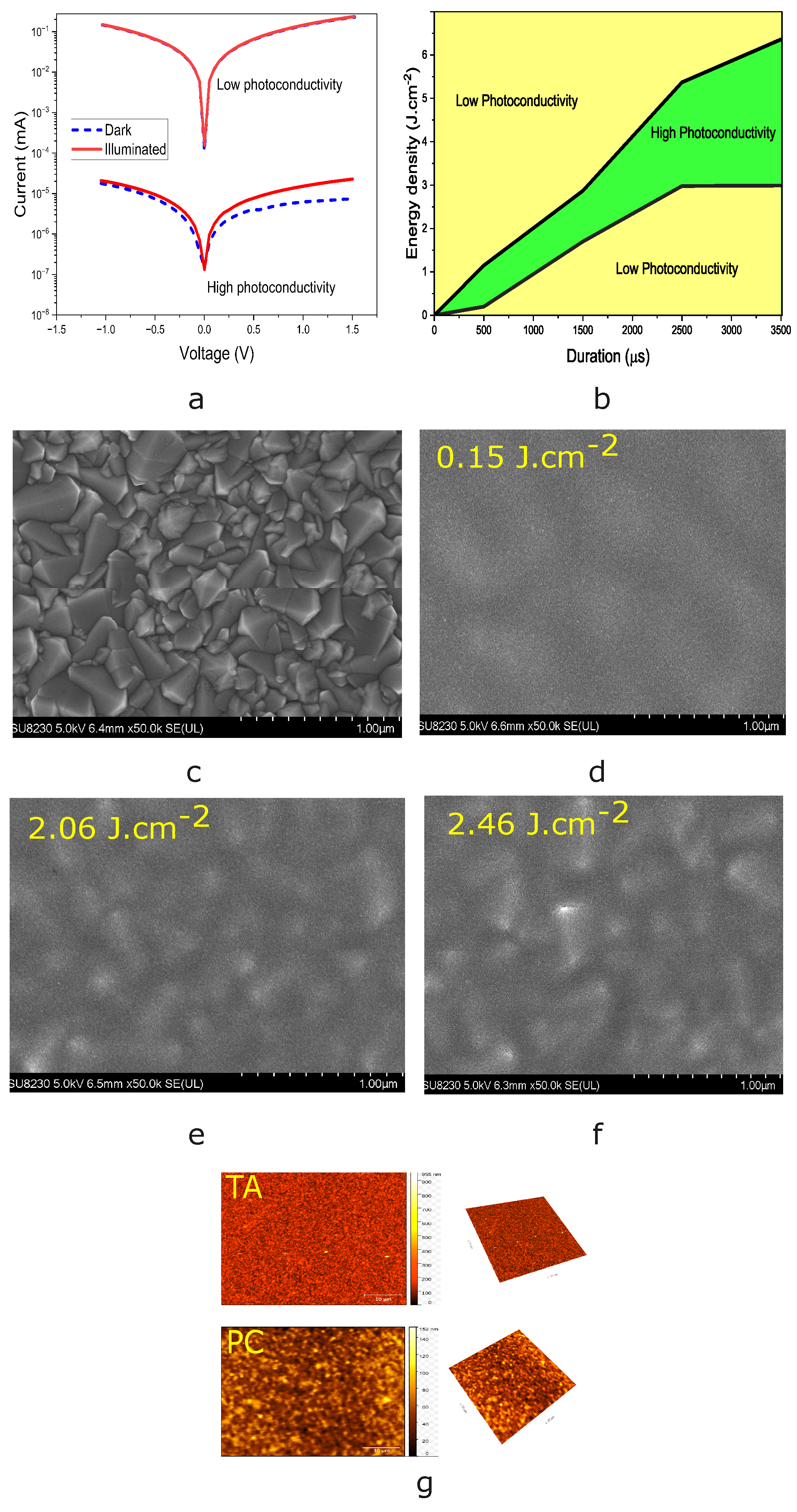
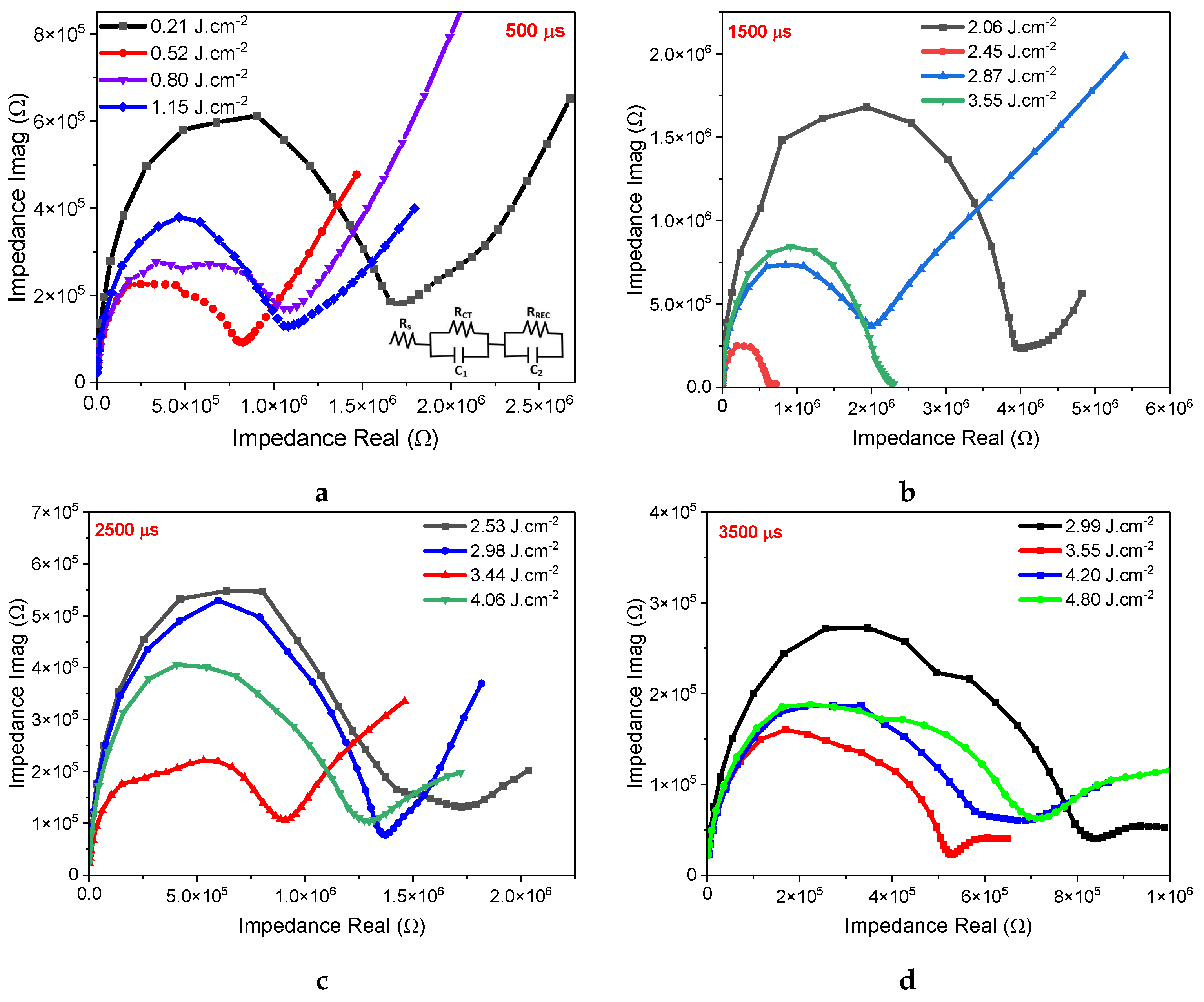
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| ETL | Electron-transporting layer |
| PSC | Perovskite solar cell |
| TA | Thermal annealing |
| PC | Photonic curing |
References
- Wei, Z.; Zhao, Y.; Jiang, J.; Yan, W.; Feng, Y.; Ma, J. Research progress on hybrid organic–inorganic perovskites for photo-applications. Chin. Chem. Lett. 2020, 31, 3055–3064. [Google Scholar] [CrossRef]
- Jing, H.; Zhu, Y.; Peng, R.W.; Li, C.Y.; Xiong, B.; Wang, Z.; Liu, Y.; Wang, M. Hybrid organic-inorganic perovskite metamaterial for light trapping and photon-to-electron conversion. Nanophotonics 2020, 9, 3323–3333. [Google Scholar] [CrossRef]
- Yang, Z.; Lai, J.; Zhu, R.; Tan, J.; Luo, Y.; Ye, S. Electronic Disorder Dominates the Charge-Carrier Dynamics in Two-Dimensional/Three-Dimensional Organic–Inorganic Perovskite Heterostructure. J. Phys. Chem. C 2022, 126, 12689–12695. [Google Scholar] [CrossRef]
- Jiang, Q.; Chu, Z.; Wang, P.; Yang, X.; Liu, H.; Wang, Y.; Yin, Z.; Wu, J.; Zhang, X.; You, J. Planar-structure perovskite solar cells with efficiency beyond 21%. Adv. Mater. 2017, 29, 1703852. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Hu, M.; Zhou, X.; Wu, J.; Zhang, L.; Kong, W.; Li, X.; Zhao, X.; Dai, S.; Xu, B. Efficiency and stability enhancement of perovskite solar cells by introducing CsPbI3 quantum dots as an interface engineering layer. NPG Asia Mater. 2018, 10, 552–561. [Google Scholar] [CrossRef]
- Schroder, K.A. Mechanisms of photonic curing™: Processing high temperature films on low temperature substrates. Nanotechnology 2011, 2, 220–223. [Google Scholar]
- Akhavan, V.; Schroder, K.; Farnsworth, S. Photonic Curing. Inkjet Print. Ind. Mater. Technol. Syst. Appl. 2022, 2, 1051–1064. [Google Scholar]
- Secor, E.B.; Ahn, B.Y.; Gao, T.Z.; Lewis, J.A.; Hersam, M.C. Rapid and versatile photonic annealing of graphene inks for flexible printed electronics. Adv. Mater. 2015, 27, 6683–6688. [Google Scholar] [CrossRef]
- Zhu, M.; Liu, W.; Ke, W.; Clark, S.; Secor, E.B.; Song, T.B.; Kanatzidis, M.G.; Li, X.; Hersam, M.C. Millisecond-pulsed photonically-annealed tin oxide electron transport layers for efficient perovskite solar cells. J. Mater. Chem. A 2017, 5, 24110–24115. [Google Scholar] [CrossRef]
- Altay, B.N.; Turkani, V.S.; Pekarovicova, A.; Fleming, P.D.; Atashbar, M.Z.; Bolduc, M.; Cloutier, S.G. One-step photonic curing of screen-printed conductive Ni flake electrodes for use in flexible electronics. Sci. Rep. 2021, 11, 3393. [Google Scholar] [CrossRef]
- Piper, R.T.; Daunis, T.B.; Xu, W.; Schroder, K.A.; Hsu, J.W. Photonic curing of nickel oxide transport layer and perovskite active layer for flexible perovskite solar cells: A path towards high-throughput manufacturing. Front. Energy Res. 2021, 9, 640960. [Google Scholar] [CrossRef]
- Maskey, B.B.; Koirala, G.R.; Kim, Y.; Park, H.; Yadav, P.; Park, J.; Sun, J.; Cho, G. Photonic Curing for Enhancing the Performance of Roll-to-Roll Printed Electronic Devices. Oklahoma State University, USA. 2019. Available online: https://core.ac.uk/reader/215230370 (accessed on 31 July 2024).
- Dong, Q.; Shi, Y.; Wang, K.; Li, Y.; Wang, S.; Zhang, H.; Xing, Y.; Du, Y.; Bai, X.; Ma, T. Insight into perovskite solar cells based on SnO2 compact electron-selective layer. J. Phys. Chem. C 2015, 119, 10212–10217. [Google Scholar] [CrossRef]
- Matacena, I.; Guerriero, P.; Lancellotti, L.; Alfano, B.; De Maria, A.; La Ferrara, V.; Mercaldo, L.V.; Miglietta, M.L.; Polichetti, T.; Rametta, G. Impedance spectroscopy analysis of perovskite solar cell stability. Energies 2023, 16, 4951. [Google Scholar] [CrossRef]
- Hu, M.; Zhang, L.; She, S.; Wu, J.; Zhou, X.; Li, X.; Wang, D.; Miao, J.; Mi, G.; Chen, H. Electron transporting bilayer of SnO2 and TiO2 nanocolloid enables highly efficient planar perovskite solar cells. Sol. RRL 2020, 4, 1900331. [Google Scholar] [CrossRef]
- Irfan, M.; Ünlü, F.; Lê, K.; Fischer, T.; Ullah, H.; Mathur, S. Electrospun Networks of ZnO-SnO2 Composite Nanowires as Electron Transport Materials for Perovskite Solar Cells. J. Nanomater. 2022, 2022, 6043406. [Google Scholar] [CrossRef]
- Martínez-Denegri, G.; Colodrero, S.; Kramarenko, M.; Martorell, J. All-nanoparticle SnO2/TiO2 electron-transporting layers processed at low temperature for efficient thin-film perovskite solar cells. ACS Appl. Energy Mater. 2018, 1, 5548–5556. [Google Scholar] [CrossRef]
- Magar, H.S.; Hassan, R.Y.; Mulchandani, A. Electrochemical impedance spectroscopy (EIS): Principles, construction, and biosensing applications. Sensors 2021, 21, 6578. [Google Scholar] [CrossRef]
- Pascoe, A.R.; Duffy, N.W.; Scully, A.D.; Huang, F.; Cheng, Y.B. Insights into planar CH3NH3PbI3 perovskite solar cells using impedance spectroscopy. J. Phys. Chem. C 2015, 119, 4444–4453. [Google Scholar] [CrossRef]
- Sinclair, D.C. Characterisation of electro-materials using ac impedance spectroscopy. Boletín Soc. Española Cerámica Vidr. 1995, 34, 55–65. [Google Scholar]
- Middlemiss, L.A.; Rennie, A.J.; Sayers, R.; West, A.R. Characterisation of batteries by electrochemical impedance spectroscopy. Energy Rep. 2020, 6, 232–241. [Google Scholar] [CrossRef]
- Shohan, S.; Harm, J.; Hasan, M.; Starly, B.; Shirwaiker, R. Non-destructive quality monitoring of 3D printed tissue scaffolds via dielectric impedance spectroscopy and supervised machine learning. Procedia Manuf. 2021, 53, 636–643. [Google Scholar] [CrossRef]
- Cherian, C.T.; Zheng, M.; Reddy, M.; Chowdari, B.; Sow, C.H. Zn2SnO4 nanowires versus nanoplates: Electrochemical performance and morphological evolution during Li-cycling. ACS Appl. Mater. Interfaces 2013, 5, 6054–6060. [Google Scholar] [CrossRef] [PubMed]
- Suo, Z.; Xiao, Z.; Li, S.; Liu, J.; Xin, Y.; Meng, L.; Liang, H.; Kan, B.; Yao, Z.; Li, C.; et al. Efficient and stable inverted structure organic solar cells utilizing surface-modified SnO2 as the electron transport layer. Nano Energy 2023, 118, 109032. [Google Scholar] [CrossRef]
- Dıaz-Flores, L.; Ramırez-Bon, R.; Mendoza-Galvan, A.; Prokhorov, E.; Gonzalez-Hernandez, J. Impedance spectroscopy studies on SnO2 films prepared by the sol–gel process. J. Phys. Chem. Solids 2003, 64, 1037–1042. [Google Scholar] [CrossRef]
- Piper, R.T.; Xu, W.; Hsu, J.W. How Optical and Electrical Properties of ITO Coated Willow Glass Affect Photonic Curing Outcome for Upscaling Perovskite Solar Cell Manufacturing. IEEE J. Photovoltaics 2022, 12, 722–727. [Google Scholar] [CrossRef]
- Albrecht, A.; Rivadeneyra, A.; Abdellah, A.; Lugli, P.; Salmerón, J.F. Inkjet printing and photonic sintering of silver and copper oxide nanoparticles for ultra-low-cost conductive patterns. J. Mater. Chem. C 2016, 4, 3546–3554. [Google Scholar] [CrossRef]
- Pan, D.; Fan, H.; Li, Z.; Wang, S.; Huang, Y.; Jiao, Y.; Yao, H. Influence of substrate on structural properties and photocatalytic activity of TiO2 films. Micro Nano Lett. 2017, 12, 82–86. [Google Scholar] [CrossRef]
- Hamdi, M.; Saleh, M.N.; Poulis, J.A. Improving the adhesion strength of polymers: Effect of surface treatments. J. Adhes. Sci. Technol. 2020, 34, 1853–1870. [Google Scholar] [CrossRef]
- Bandara, T.; Aththanayake, A.; Kumara, G.; Samarasekara, P.; DeSilva, L.A.; Tennakone, K. Transparent and conductive F-Doped SnO2 nanostructured thin films by sequential nebulizer spray pyrolysis. MRS Adv. 2021, 6, 417–421. [Google Scholar] [CrossRef]
- Ghahremani, A.H.; Martin, B.; Gupta, A.; Bahadur, J.; Ankireddy, K.; Druffel, T. Rapid fabrication of perovskite solar cells through intense pulse light annealing of SnO2 and triple cation perovskite thin films. Mater. Des. 2020, 185, 108237. [Google Scholar] [CrossRef]
- Mukhamedshina, D.M.; Beisenkhanov, N.B. Influence of crystallization on the properties of SnO2 thin films. In Advances in Crystallization Processes; IntechOpen: London, UK, 2012. [Google Scholar]
- Zhu, Z.; Bai, Y.; Liu, X.; Chueh, C.; Yang, S.; Jen, A.K. Enhanced efficiency and stability of inverted perovskite solar cells using highly crystalline SnO2 nanocrystals as the robust electron-transporting layer. Adv. Mater. 2016, 28, 6478–6484. [Google Scholar] [CrossRef] [PubMed]
- Creager, S.E.; Hockett, L.A.; Rowe, G.K. Consequences of microscopic surface roughness for molecular self-assembly. Langmuir 1992, 8, 854–861. [Google Scholar] [CrossRef]
- Keshtmand, R.; Zamani-Meymian, M.R.; Mohamadkhani, F.; Taghavinia, N. Smoothing and coverage improvement of SnO2 electron transporting layer by NH4F treatment: Enhanced fill factor and efficiency of perovskite solar cells. Sol. Energy 2021, 228, 253–262. [Google Scholar] [CrossRef]
- Salado, M.; Contreras-Bernal, L.; Caliò, L.; Todinova, A.; López-Santos, C.; Ahmad, S.; Borras, A.; Idígoras, J.; Anta, J.A. Impact of moisture on efficiency-determining electronic processes in perovskite solar cells. J. Mater. Chem. A 2017, 5, 10917–10927. [Google Scholar] [CrossRef]
- Li, N.; Yan, J.; Ai, Y.; Jiang, E.; Lin, L.; Shou, C.; Yan, B.; Sheng, J.; Ye, J. A low-temperature TiO2/SnO2 electron transport layer for high-performance planar perovskite solar cells. Sci. China Mater 2020, 63, 207–215. [Google Scholar] [CrossRef]
- Shibuya, H.; Inoue, S.; Ihara, M. Evaluation of dye-sensitized solar cells using forward bias applied impedance spectroscopy under dark. ECS Trans. 2009, 16, 93. [Google Scholar] [CrossRef]
- Abdulrahim, S.M.; Ahmad, Z.; Bahadra, J.; Al-Thani, N.J. Electrochemical impedance spectroscopy analysis of hole transporting material free mesoporous and planar perovskite solar cells. Nanomaterials 2020, 10, 1635. [Google Scholar] [CrossRef]
- Bredar, A.R.; Chown, A.L.; Burton, A.R.; Farnum, B.H. Electrochemical impedance spectroscopy of metal oxide electrodes for energy applications. ACS Appl. Energy Mater. 2020, 3, 66–98. [Google Scholar] [CrossRef]
- Chang, B.Y.; Park, S.M. Integrated description of electrode/electrolyte interfaces based on equivalent circuits and its verification using impedance measurements. Anal. Chem. 2006, 78, 1052–1060. [Google Scholar] [CrossRef]
- Matacena, I. Equivalent circuit extraction procedure from Nyquist plots for graphene-silicon solar cells. In Proceedings of the 2019 15th Conference on Ph.D Research in Microelectronics and Electronics (PRIME), Lausanne, Switzerland, 15–18 July 2019; pp. 273–276. [Google Scholar] [CrossRef]
- Prochowicz, D.; Trivedi, S.; Parikh, N.; Saliba, M.; Kalam, A.; Mahdi Tavakoli, M.; Yadav, P. In the Quest of Low-Frequency Impedance Spectra of Efficient Perovskite Solar Cells. Energy Technol. 2021, 9, 2100229. [Google Scholar] [CrossRef]
- Alvarez, A.O.; Arcas, R.; Aranda, C.A.; Bethencourt, L.; Mas-Marzá, E.; Saliba, M.; Fabregat-Santiago, F. Negative capacitance and inverted hysteresis: Matching features in perovskite solar cells. J. Phys. Chem. Lett. 2020, 11, 8417–8423. [Google Scholar] [CrossRef] [PubMed]
- Laschuk, N.O.; Easton, E.B.; Zenkina, O.V. Reducing the resistance for the use of electrochemical impedance spectroscopy analysis in materials chemistry. RSC Adv. 2021, 11, 27925–27936. [Google Scholar] [CrossRef] [PubMed]
- Hernández, H.H.; Reynoso, A.R.; González, J.T.; Morán, C.G.; Hernández, J.M.; Ruiz, A.M.; Hernández, J.M.; Cruz, R.O. Electrochemical impedance spectroscopy (EIS): A review study of basic aspects of the corrosion mechanism applied to steels. In Electrochemical Impedance Spectroscopy; IntechOpen: London, UK, 2020; pp. 137–144. [Google Scholar]
- Deva Arun Kumar, K.; Valanarasu, S.; Capelle, A.; Nar, S.; Karim, W.; Stolz, A.; Aspe, B.; Semmar, N. Nanostructured Oxide (SnO2, FTO) Thin Films for Energy Harvesting: A Significant Increase in Thermoelectric Power at Low Temperature. Micromachines 2024, 15, 188. [Google Scholar] [CrossRef] [PubMed]
- Patil, G.E.; Kajale, D.D.; Gaikwad, V.B.; Jain, G.H. Preparation and characterization of SnO2 nanoparticles by hydrothermal route. Int. Nano Lett. 2012, 2, 17. [Google Scholar] [CrossRef]
- Peiris, T.N.; Benitez, J.; Sutherland, L.; Sharma, M.; Michalska, M.; Scully, A.D.; Vak, D.; Gao, M.; Weerasinghe, H.C.; Jasieniak, J. A stable aqueous SnO2 nanoparticle dispersion for roll-to-Roll fabrication of flexible perovskite solar cells. Coatings 2022, 12, 1948. [Google Scholar] [CrossRef]
- Guerrero, A.; Garcia-Belmonte, G.; Mora-Sero, I.; Bisquert, J.; Kang, Y.S.; Jacobsson, T.J.; Correa-Baena, J.P.; Hagfeldt, A. Properties of contact and bulk impedances in hybrid lead halide perovskite solar cells including inductive loop elements. J. Phys. Chem. C 2016, 120, 8023–8032. [Google Scholar] [CrossRef]
- Almora, O.; Zarazua, I.; Mas-Marza, E.; Mora-Sero, I.; Bisquert, J.; Garcia-Belmonte, G. Capacitive dark currents, hysteresis, and electrode polarization in lead halide perovskite solar cells. J. Phys. Chem. Lett. 2015, 6, 1645–1652. [Google Scholar] [CrossRef]
- Mahapatra, A.; Parikh, N.; Kumar, P.; Kumar, M.; Prochowicz, D.; Kalam, A.; Tavakoli, M.M.; Yadav, P. Changes in the electrical characteristics of perovskite solar cells with aging time. Molecules 2020, 25, 2299. [Google Scholar] [CrossRef]
- Todinova, A.; Contreras-Bernal, L.; Salado, M.; Ahmad, S.; Morillo, N.; Idígoras, J.; Anta, J.A. Towards a universal approach for the analysis of impedance spectra of perovskite solar cells: Equivalent circuits and empirical analysis. ChemElectroChem 2017, 4, 2891–2901. [Google Scholar] [CrossRef]
- Zarazua, I.; Han, G.; Boix, P.P.; Mhaisalkar, S.; Fabregat-Santiago, F.; Mora-Seró, I.; Bisquert, J.; Garcia-Belmonte, G. Surface recombination and collection efficiency in perovskite solar cells from impedance analysis. J. Phys. Chem. Lett. 2016, 7, 5105–5113. [Google Scholar] [CrossRef]
- Zarazua, I.; Bisquert, J.; Garcia-Belmonte, G. Light-induced space-charge accumulation zone as photovoltaic mechanism in perovskite solar cells. J. Phys. Chem. Lett. 2016, 7, 525–528. [Google Scholar] [CrossRef] [PubMed]
- Li, J.V.; Ferrari, G. Capacitance Spectroscopy of Semiconductors; CRC Press: Boca Raton, FL, USA, 2018. [Google Scholar]
- Heeger, A.J.; MacDiarmid, A.G.; Shirakawa, H. The Nobel Prize in Chemistry, 2000: Conductive Polymers; Royal Swedish Academy of Sciences: Stockholm, Sweden, 2000; pp. 1–16. [Google Scholar]
- Namsheer, K.; Rout, C.S. Conducting polymers: A comprehensive review on recent advances in synthesis, properties and applications. RSC Adv. 2021, 11, 5659–5697. [Google Scholar]
- Wang, Y.; Su, N.; Liu, J.; Lin, Y.; Wang, J.; Guo, X.; Zhang, Y.; Qin, Z.; Liu, J.; Zhang, C.; et al. Enhanced visible-light photocatalytic properties of SnO2 quantum dots by niobium modification. Results Phys. 2022, 37, 105515. [Google Scholar] [CrossRef]
- Esward, T.; Knox, S.; Jones, H.; Brewer, P.; Murphy, C.; Wright, L.; Williams, J. A metrology perspective on the dark injection transient current method for charge mobility determination in organic semiconductors. J. Appl. Phys. 2011, 109. [Google Scholar] [CrossRef]
- Sarda, N.; Vidhan, A.; Basak, S.; Hazra, P.; Behera, T.; Ghosh, S.; Choudhary, R.J.; Chowdhury, A.; Sarkar, S.K. Photonically Cured Solution-Processed SnO2 Thin Films for High-Efficiency and Stable Perovskite Solar Cells and Minimodules. ACS Appl. Energy Mater. 2023, 6, 3996–4006. [Google Scholar] [CrossRef]
- Knapp, E.; Ruhstaller, B. The role of shallow traps in dynamic characterization of organic semiconductor devices. J. Appl. Phys. 2012, 112, 024519. [Google Scholar] [CrossRef]
- Aukštuolis, A.; Girtan, M.; Mousdis, G.A.; Mallet, R.; Socol, M.; Rasheed, M.; Stanculescu, A. Measurement of charge carrier mobility in perovskite nanowire films by photo-CELIV method. Proc. Rom. Acad.-Ser. A Math. Phys. Tech. Sci. Inf. Sci. 2017, 18, 34–41. [Google Scholar]
- Stephen, M.; Genevičius, K.; Juška, G.; Arlauskas, K.; Hiorns, R.C. Charge transport and its characterization using photo-CELIV in bulk heterojunction solar cells. Polym. Int. 2017, 66, 13–25. [Google Scholar] [CrossRef]
- Sen, S.; Islam, R. Investigation of Charge Carrier Transport of Bulk Heterojunction Organic Solar Cell Using Photo-CELIV Electrical Simulation. Proc. Natl. Acad. Sci. India Sect. A Phys. Sci. 2022, 92, 713–717. [Google Scholar] [CrossRef]
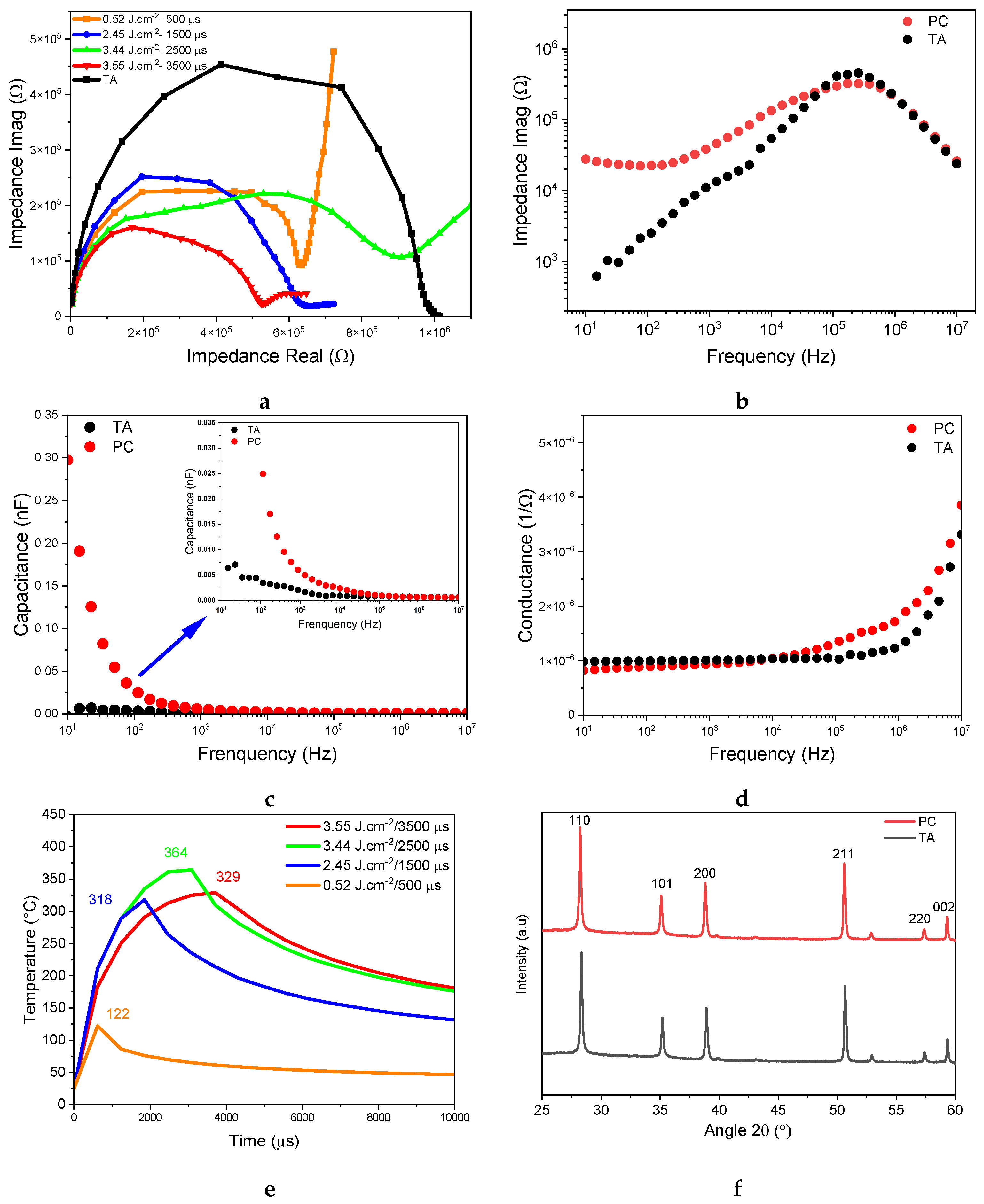
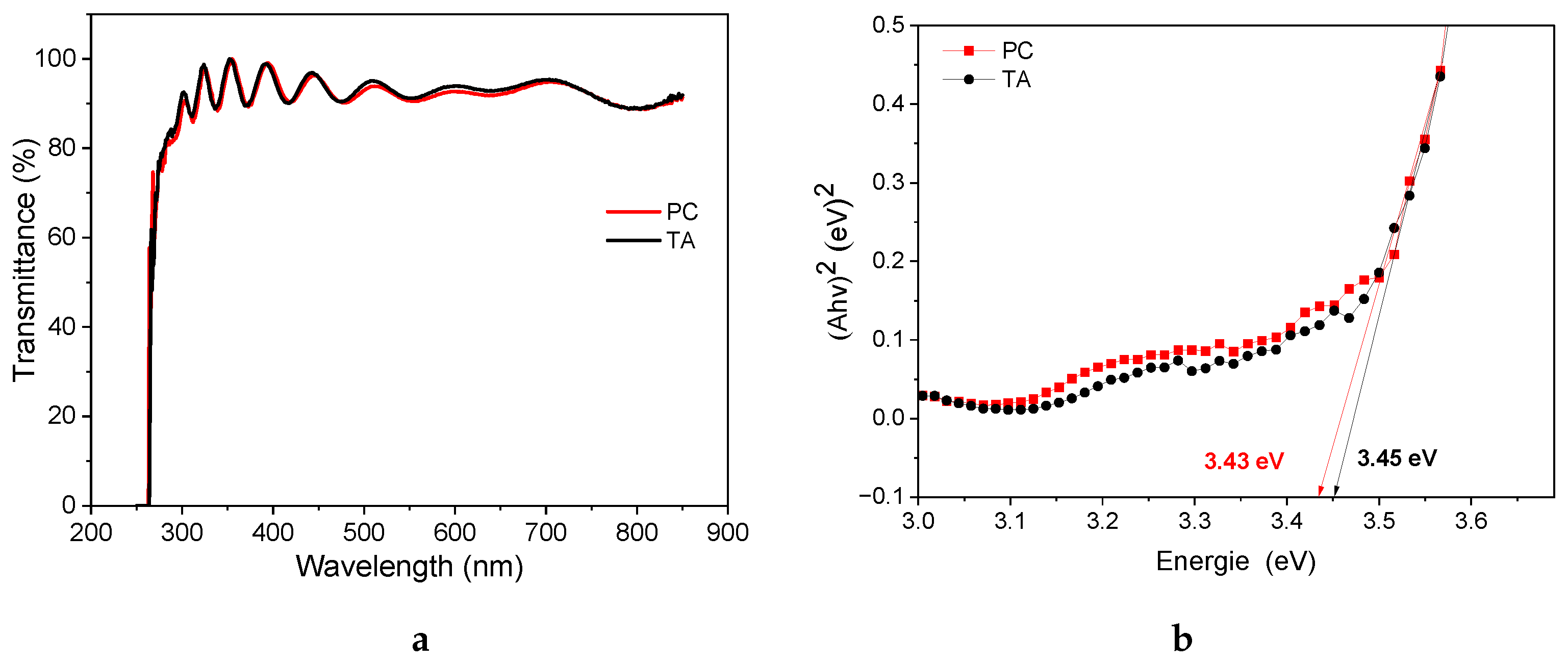
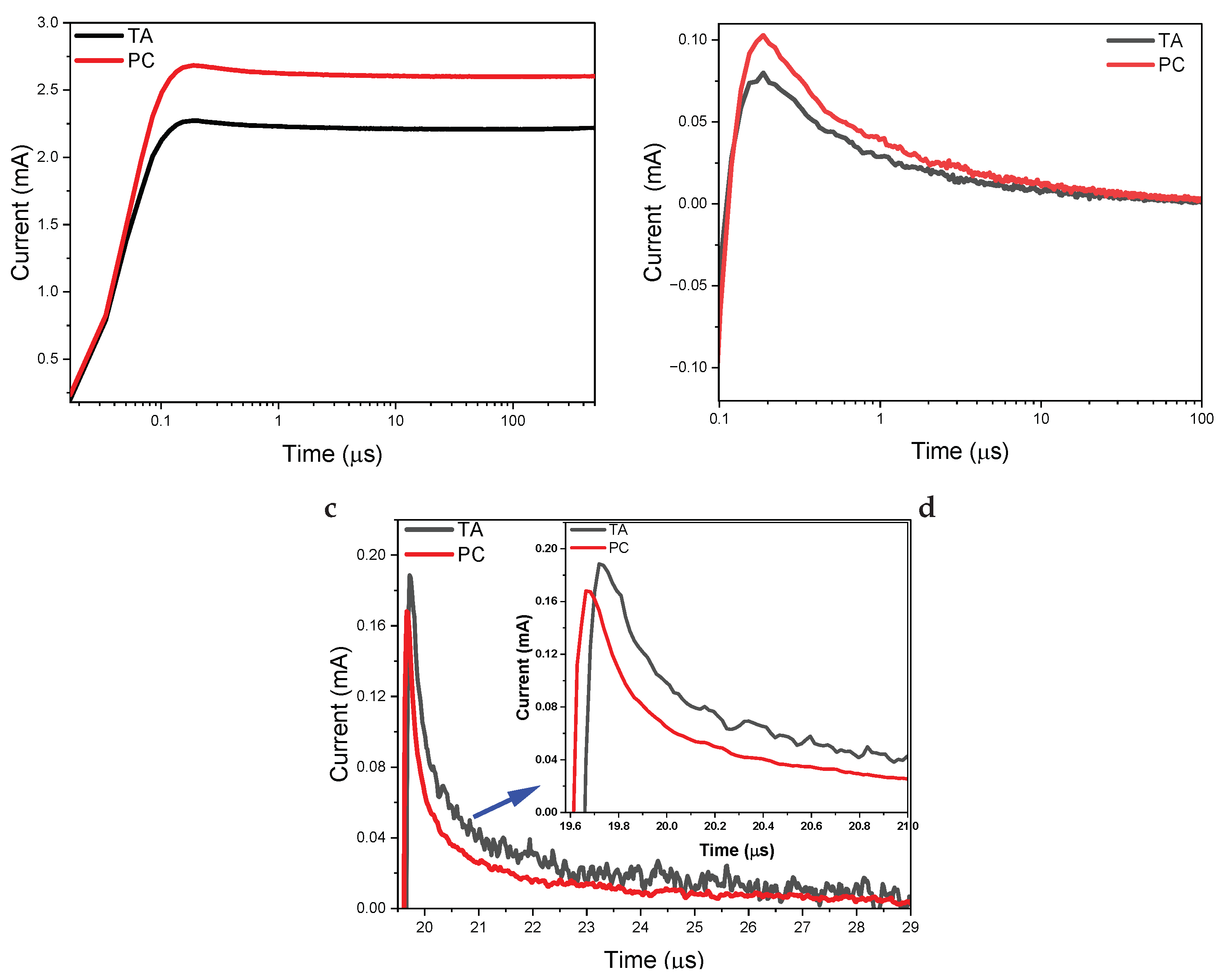
| Device | Rs () | () | Ceq () | () |
|---|---|---|---|---|
| Thermally annealed | 1.96 | 0.99 | 0.88 | 0.87 |
| Photonically treated | 3.06 | 0.49 | 0.78 | 0.38 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Slimani, M.A.; Benavides-Guerrero, J.A.; Cloutier, S.G.; Izquierdo, R. Enhancing the Performance of Nanocrystalline SnO2 for Solar Cells through Photonic Curing Using Impedance Spectroscopy Analysis. Nanomaterials 2024, 14, 1508. https://doi.org/10.3390/nano14181508
Slimani MA, Benavides-Guerrero JA, Cloutier SG, Izquierdo R. Enhancing the Performance of Nanocrystalline SnO2 for Solar Cells through Photonic Curing Using Impedance Spectroscopy Analysis. Nanomaterials. 2024; 14(18):1508. https://doi.org/10.3390/nano14181508
Chicago/Turabian StyleSlimani, Moulay Ahmed, Jaime A. Benavides-Guerrero, Sylvain G. Cloutier, and Ricardo Izquierdo. 2024. "Enhancing the Performance of Nanocrystalline SnO2 for Solar Cells through Photonic Curing Using Impedance Spectroscopy Analysis" Nanomaterials 14, no. 18: 1508. https://doi.org/10.3390/nano14181508







