Electrospun Amorphous Solid Dispersions with Lopinavir and Ritonavir for Improved Solubility and Dissolution Rate
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Solutions for Electrospinning
2.3. Electrospinning Process
2.4. Characterization of the ASDs
2.4.1. Morphological Assessment
2.4.2. Differential Scanning Calorimetry (DSC)
2.4.3. X-ray Diffraction (XRD)
2.4.4. Wettability Study
2.4.5. High-Performance Liquid Chromatography (HPLC)
2.4.6. Drug Loading and Encapsulation Efficiency
2.4.7. Equilibrium Solubility
2.4.8. Dissolution Studies
3. Results and Discussion
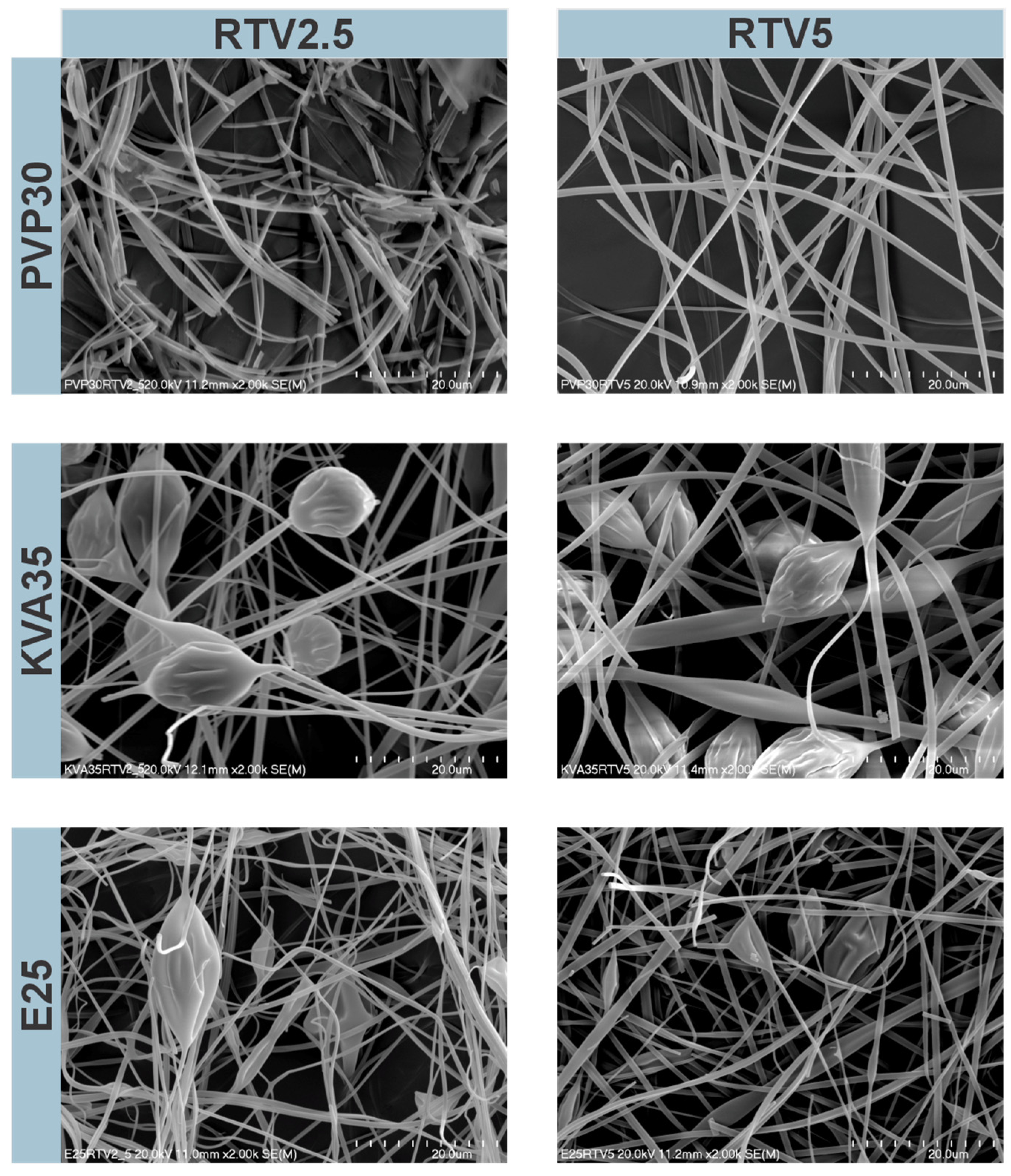
3.1. Morphology of the Electrospun Fibers
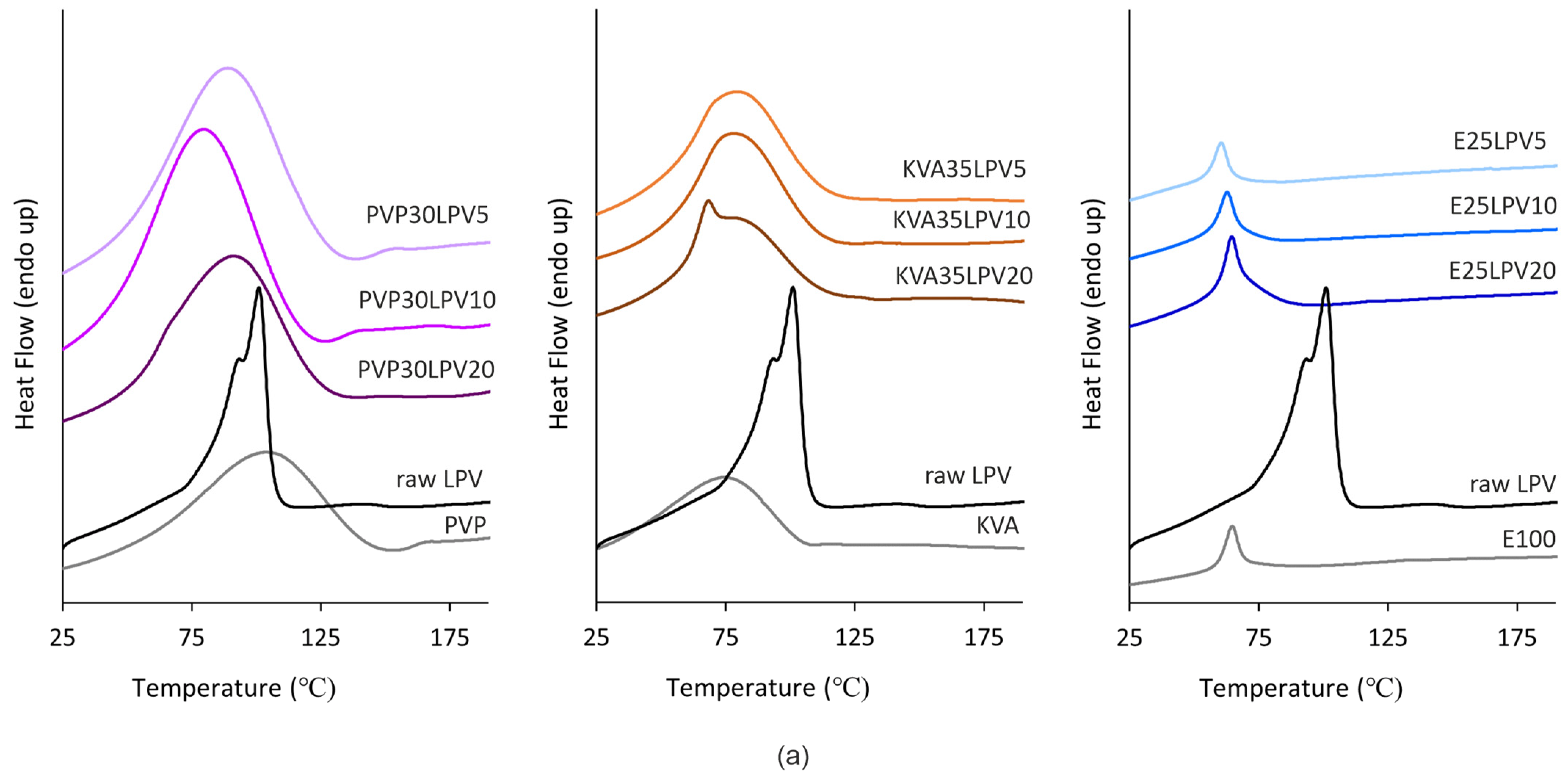
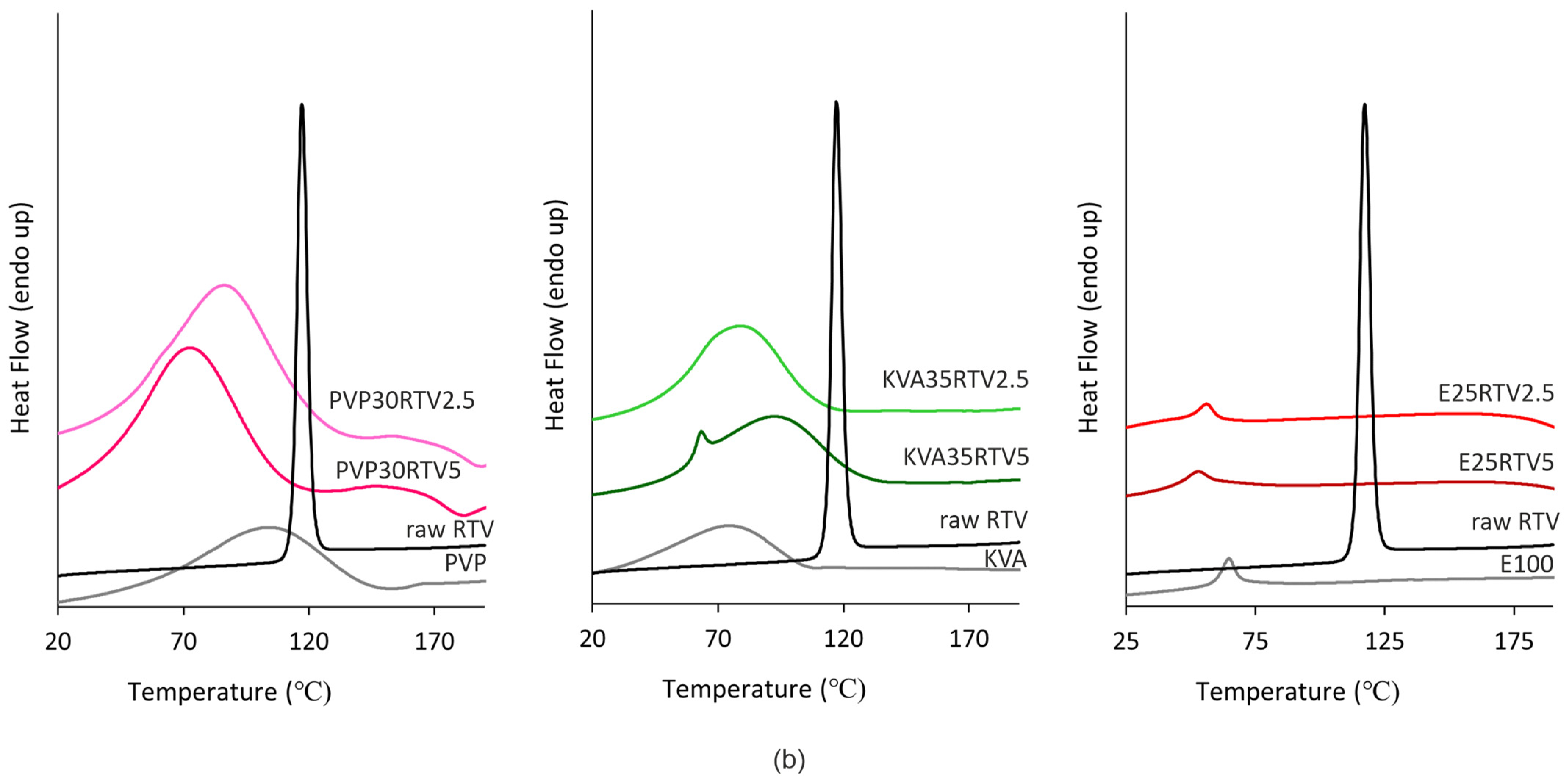
3.2. Differential Scanning Calorimetry (DSC)
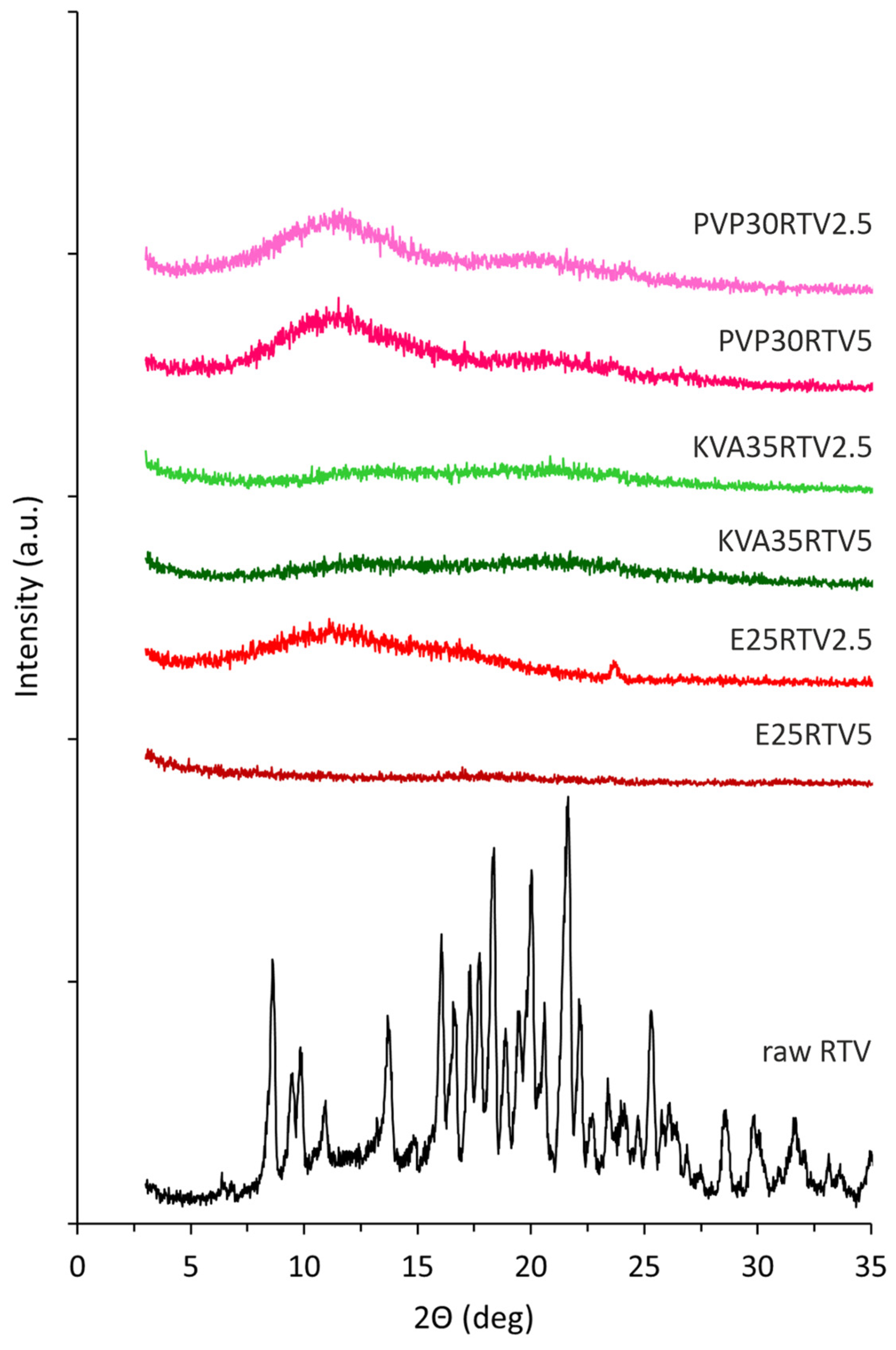
3.3. X-ray Diffraction (XRD)
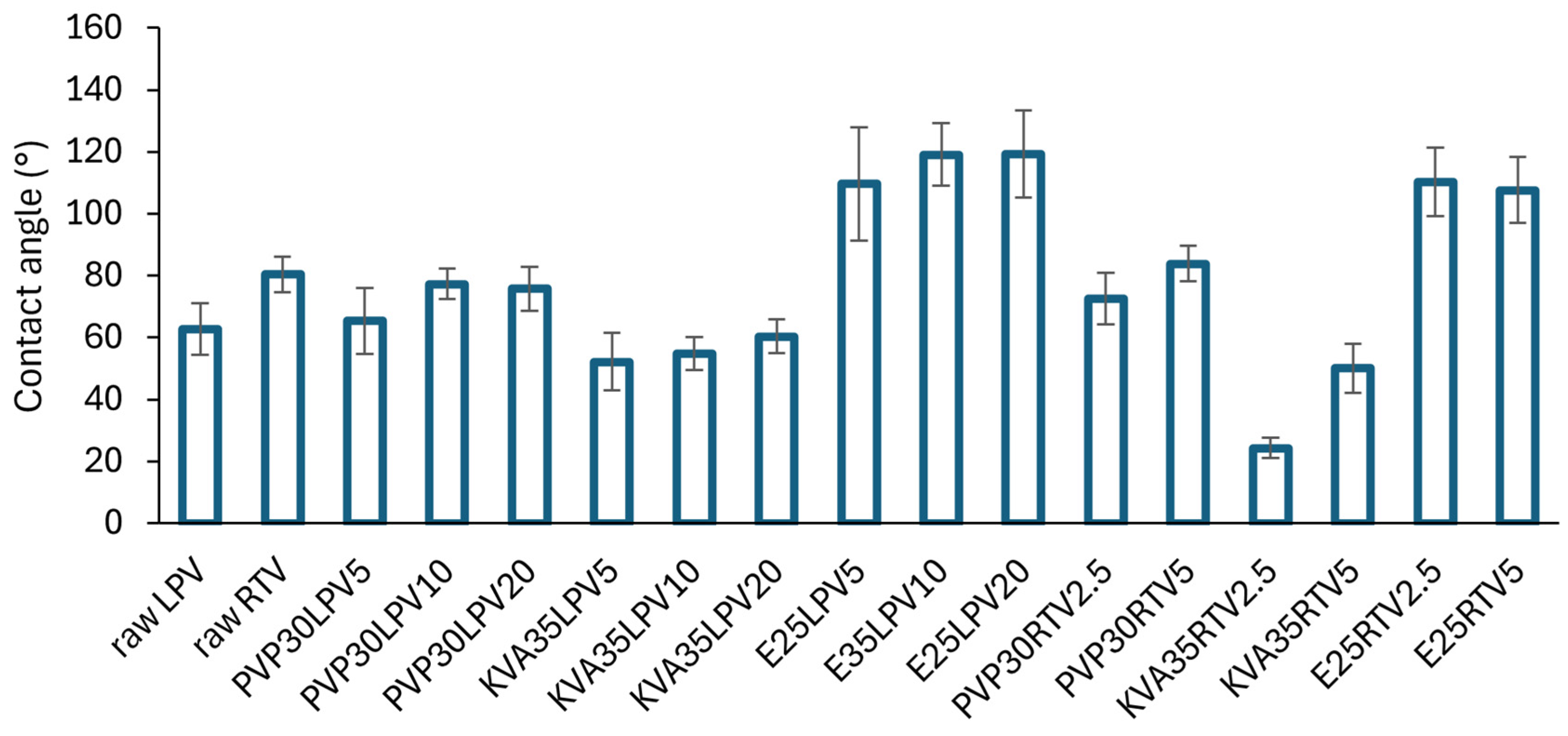
3.4. Wettability Study
3.5. Drug Loading (DL) and Encapsulation Efficiency (EE%)
3.6. Equilibrium Solubility Study
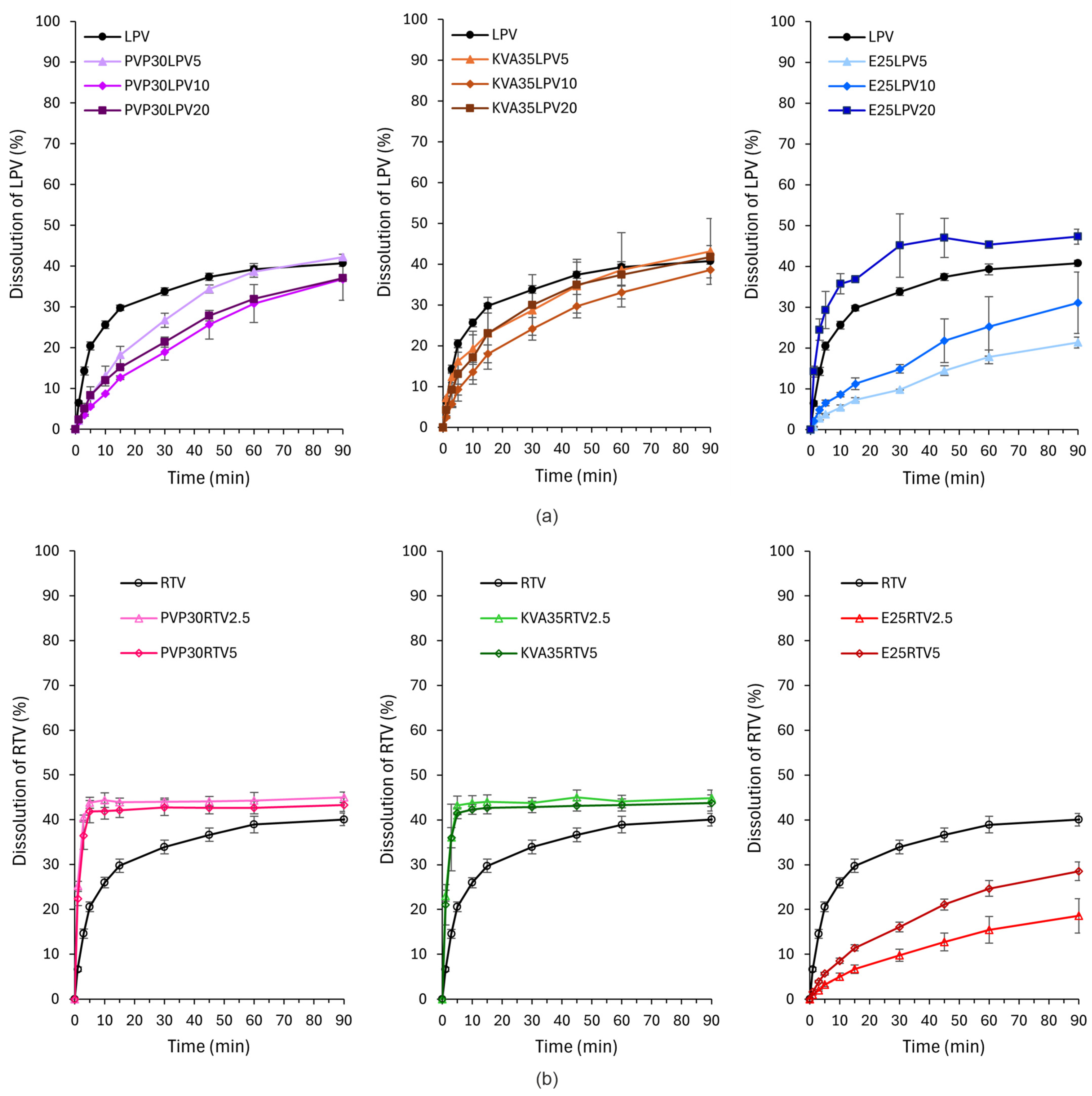
3.7. Dissolution Study
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Ghadi, R.; Dand, N. BCS Class IV Drugs: Highly Notorious Candidates for Formulation Development. J. Control. Release 2017, 248, 71–95. [Google Scholar] [CrossRef] [PubMed]
- De Espíndola, B.; Beringhs, A.O.R.; Sonaglio, D.; Stulzer, H.K.; Silva, M.A.S.; Ferraz, H.G.; Pezzini, B.R. Liquisolid Pellets: A Pharmaceutical Technology Strategy to Improve the Dissolution Rate of Ritonavir. Saudi Pharm. J. 2019, 27, 702–712. [Google Scholar] [CrossRef]
- Kumar, S.; Narayan, R.; Ahammed, V.; Nayak, Y.; Naha, A.; Nayak, U.Y. Development of Ritonavir Solid Lipid Nanoparticles by Box Behnken Design for Intestinal Lymphatic Targeting. J. Drug Deliv. Sci. Technol. 2018, 44, 181–189. [Google Scholar] [CrossRef]
- Fayed, N.D.; Arafa, M.F.; Essa, E.A.; El Maghraby, G.M. Lopinavir-Menthol Co-Crystals for Enhanced Dissolution Rate and Intestinal Absorption. J. Drug Deliv. Sci. Technol. 2022, 74, 103587. [Google Scholar] [CrossRef]
- Shinde, A.; Jadhav, N.; More, H.; Naikwadi, H. Design and Characterisation of Lopinavir Nanocrystals for Solubility and Dissolution Enhancement. Pharm. Sci. Asia 2019, 46, 193–205. [Google Scholar] [CrossRef]
- Rathod, V.; Stagner, W.C.; Gajera, B.; Haware, R.V. Hybridized Nanoamorphous Micellar Dispersion Using a QbD–DM3 Linked Rational Product Design Strategy for Ritonavir: A BCS IV Drug. Int. J. Pharm. 2020, 588, 119727. [Google Scholar] [CrossRef]
- Zi, P.; Zhang, C.; Ju, C.; Su, Z.; Bao, Y.; Gao, J.; Sun, J.; Lu, J.; Zhang, C. Solubility and Bioavailability Enhancement Study of Lopinavir Solid Dispersion Matrixed with a Polymeric Surfactant-Soluplus. Eur. J. Pharm. Sci. 2019, 134, 233–245. [Google Scholar] [CrossRef]
- Hamed, R.; Mohamed, E.M.; Sediri, K.; Khan, M.A.; Rahman, Z. Development of Stable Amorphous Solid Dispersion and Quantification of Crystalline Fraction of Lopinavir by Spectroscopic-Chemometric Methods. Int. J. Pharm. 2021, 602, 120657. [Google Scholar] [CrossRef]
- Kokott, M.; Klinken, S.; Breitkreutz, J.; Wiedey, R. Downstream Processing of Amorphous Solid Dispersions into Orodispersible Tablets. Int. J. Pharm. 2023, 631, 122493. [Google Scholar] [CrossRef]
- Siriwannakij, N.; Heimbach, T.; Serajuddin, A.T.M. Aqueous Dissolution and Dispersion Behavior of Polyvinylpyrrolidone Vinyl Acetate-Based Amorphous Solid Dispersion of Ritonavir Prepared by Hot-Melt Extrusion with and without Added Surfactants. J. Pharm. Sci. 2021, 110, 1480–1494. [Google Scholar] [CrossRef]
- Denninger, A.; Westedt, U.; Rosenberg, J.; Wagner, K.G. A Rational Design of a Biphasic Dissolution Setup—Modelling of Biorelevant Kinetics for a Ritonavir Hot-Melt Extruded Amorphous Solid Dispersion. Pharmaceutics 2020, 12, 237. [Google Scholar] [CrossRef] [PubMed]
- Dhore, P.W.; Dave, V.S.; Saoji, S.D.; Bobde, Y.S.; Mack, C.; Raut, N.A. Enhancement of the Aqueous Solubility and Permeability of a Poorly Water Soluble Drug Ritonavir via Lyophilized Milk-Based Solid Dispersions. Pharm. Dev. Technol. 2017, 22, 90–102. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Guo, M.; Luo, M.; Cai, T. Advances in the Development of Amorphous Solid Dispersions: The Role of Polymeric Carriers. Asian J. Pharm. Sci. 2023, 18, 100834. [Google Scholar] [CrossRef]
- Cid, A.G.; Simonazzi, A.; Palma, S.D.; Bermúdez, J.M. Solid Dispersion Technology as a Strategy to Improve the Bioavailability of Poorly Soluble Drugs. Ther. Deliv. 2019, 10, 363–382. [Google Scholar] [CrossRef]
- Yu, D.G.; Li, J.J.; Williams, G.R.; Zhao, M. Electrospun Amorphous Solid Dispersions of Poorly Water-Soluble Drugs: A Review. J. Control. Release 2018, 292, 91–110. [Google Scholar] [CrossRef]
- Becelaere, J.; Van Den Broeck, E.; Schoolaert, E.; Vanhoorne, V.; Van Guyse, J.F.R.; Vergaelen, M.; Borgmans, S.; Creemers, K.; Van Speybroeck, V.; Vervaet, C.; et al. Stable Amorphous Solid Dispersion of Flubendazole with High Loading via Electrospinning. J. Control. Release 2022, 351, 123–136. [Google Scholar] [CrossRef]
- Kramarczyk, D.; Knapik-Kowalczuk, J.; Kurek, M.; Jamróz, W.; Jachowicz, R.; Paluch, M. Hot Melt Extruded Posaconazole-Based Amorphous Solid Dispersions—The Effect of Different Types of Polymers. Pharmaceutics 2023, 15, 799. [Google Scholar] [CrossRef]
- Łyszczarz, E.; Hofmanová, J.; Szafraniec-Szczęsny, J.; Jachowicz, R. Orodispersible Films Containing Ball Milled Aripiprazole-Poloxamer® 407 Solid Dispersions. Int. J. Pharm. 2020, 575, 118955. [Google Scholar] [CrossRef]
- Krupa, A.; Descamps, M.; Willart, J.F.; Strach, B.; Wyska, E.; Jachowicz, R.; Danède, F. High-Energy Ball Milling as Green Process to Vitrify Tadalafil and Improve Bioavailability. Mol. Pharm. 2016, 13, 3891–3902. [Google Scholar] [CrossRef]
- Krupa, A.; Danède, F.; Majda, D.; Węgrzyn, A.; Strojewski, D.; Kondera, I.; Willart, J.F. High Energy Ball Milling vs. Nano Spray Drying in the Development of Supersaturated Systems Loaded with Bosentan. Eur. J. Pharm. Biopharm. 2023, 188, 137–146. [Google Scholar] [CrossRef]
- Szafraniec, J.; Antosik, A.; Knapik-Kowalczuk, J.; Kurek, M.; Syrek, K.; Chmiel, K.; Paluch, M.; Jachowicz, R. Planetary Ball Milling and Supercritical Fluid Technology as a Way to Enhance Dissolution of Bicalutamide. Int. J. Pharm. 2017, 533, 470–479. [Google Scholar] [CrossRef] [PubMed]
- Szafraniec, J.; Antosik, A.; Knapik-Kowalczuk, J.; Chmiel, K.; Kurek, M.; Gawlak, K.; Odrobińska, J.; Paluch, M.; Jachowicz, R. The Self-Assembly Phenomenon of Poloxamers and Its Effect on the Dissolution of a Poorly Soluble Drug from Solid Dispersions Obtained by Solvent Methods. Pharmaceutics 2019, 11, 130. [Google Scholar] [CrossRef] [PubMed]
- Casian, T.; Borbás, E.; Ilyés, K.; Démuth, B.; Farkas, A.; Rapi, Z.; Bogdan, C.; Iurian, S.; Toma, V.; Știufiuc, R.; et al. Electrospun Amorphous Solid Dispersions of Meloxicam: Influence of Polymer Type and Downstream Processing to Orodispersible Dosage Forms. Int. J. Pharm. 2019, 569, 118593. [Google Scholar] [CrossRef] [PubMed]
- Sóti, P.L.; Bocz, K.; Pataki, H.; Eke, Z.; Farkas, A.; Verreck, G.; Kiss, É.; Fekete, P.; Vigh, T.; Wagner, I.; et al. Comparison of Spray Drying, Electroblowing and Electrospinning for Preparation of Eudragit E and Itraconazole Solid Dispersions. Int. J. Pharm. 2015, 494, 23–30. [Google Scholar] [CrossRef]
- Szabó, E.; Záhonyi, P.; Gyürkés, M.; Nagy, B.; Galata, D.L.; Madarász, L.; Hirsch, E.; Farkas, A.; Andersen, S.K.; Vígh, T.; et al. Continuous Downstream Processing of Milled Electrospun Fibers to Tablets Monitored by Near-Infrared and Raman Spectroscopy. Eur. J. Pharm. Sci. 2021, 164, 105907. [Google Scholar] [CrossRef]
- Yu, D.G.; Branford-White, C.; Shen, X.X.; Zhang, X.F.; Zhu, L.M. Solid Dispersions of Ketoprofen in Drug-Loaded Electrospun Nanofibers. J. Dispers. Sci. Technol. 2010, 31, 902–908. [Google Scholar] [CrossRef]
- Ahmadi Bonakdar, M.; Rodrigue, D. Electrospinning: Processes, Structures, and Materials. Macromol 2024, 4, 58–103. [Google Scholar] [CrossRef]
- United States Pharmacopeia. USP 43-NF38 Lopinavir and Ritonavir Tablets Lopinavir and Ritonavir Tablets; United States Pharmacopeia: Rockville, MD, USA, 2024. [Google Scholar] [CrossRef]
- Xue, J.; Wu, T.; Dai, Y.; Xia, Y. Electrospinning and Electrospun Nanofibers: Methods, Materials, and Applications. Chem. Rev. 2019, 119, 5298–5415. [Google Scholar] [CrossRef]
- Avossa, J.; Herwig, G.; Toncelli, C.; Itel, F.; Rossi, R.M. Electrospinning Based on Benign Solvents: Current Definitions, Implications and Strategies. Green Chem. 2022, 24, 2347–2375. [Google Scholar] [CrossRef]
- Vlachou, M.; Kikionis, S.; Siamidi, A.; Kyriakou, S.; Tsotinis, A.; Ioannou, E.; Roussis, V. Development and Characterization of Eudragit®-Based Electrospun Nanofibrous Mats and Their Formulation into Nanofiber Tablets for the Modified Release of Furosemide. Pharmaceutics 2019, 11, 480. [Google Scholar] [CrossRef]
- Kazsoki, A.; Szabó, P.; Zelkó, R. Prediction of the Hydroxypropyl Cellulose—Poly(Vinyl Alcohol) Ratio in Aqueous Solution Containing Papaverine Hydrochloride in Terms of Drug Loaded Electrospun Fiber Formation. J. Pharm. Biomed. Anal. 2017, 138, 357–362. [Google Scholar] [CrossRef] [PubMed]
- Nagy, Z.K.; Balogh, A.; Démuth, B.; Pataki, H.; Vigh, T.; Szabó, B.; Molnár, K.; Schmidt, B.T.; Horák, P.; Marosi, G.; et al. High Speed Electrospinning for Scaled-up Production of Amorphous Solid Dispersion of Itraconazole. Int. J. Pharm. 2015, 480, 137–142. [Google Scholar] [CrossRef] [PubMed]
- Birer, M.; Acartürk, F. Electrospun Orally Disintegrating Film Formulation of Telmisartan. Pharm. Dev. Technol. 2021, 26, 661–672. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Chang, S.; Bai, Y.; Du, Y.; Yu, D.G. Electrospun Triaxial Nanofibers with Middle Blank Cellulose Acetate Layers for Accurate Dual-Stage Drug Release. Carbohydr. Polym. 2020, 243, 116477. [Google Scholar] [CrossRef]
- PubChem. Lopinavir. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/92727 (accessed on 12 July 2024).
- DrugBank. Lopinavir. Available online: https://go.drugbank.com/drugs/DB01601 (accessed on 12 July 2024).
- PubChem. Ritonavir. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/392622 (accessed on 12 July 2024).
- DrugBank. Ritonavir. Available online: https://go.drugbank.com/drugs/DB00503 (accessed on 12 July 2024).
- He, H.; Kara, Y.; Molnar, K. Effect of Needle Characteristic on Fibrous PEO Produced by Electrospinning. Resolut. Discov. 2019, 4, 7–11. [Google Scholar] [CrossRef]
- Abdelhakim, H.E.; Coupe, A.; Tuleu, C.; Edirisinghe, M.; Craig, D.Q.M. Electrospinning Optimization of Eudragit e PO with and without Chlorpheniramine Maleate Using a Design of Experiment Approach. Mol. Pharm. 2019, 16, 2557–2568. [Google Scholar] [CrossRef]
- Wu, H.; Wang, Z.; Zhao, Y.; Gao, Y.; Wang, L.; Zhang, H.; Bu, R.; Ding, Z.; Han, J. Effect of Different Seed Crystals on the Supersaturation State of Ritonavir Tablets Prepared by Hot-Melt Extrusion. Eur. J. Pharm. Sci. 2023, 185, 106440. [Google Scholar] [CrossRef]
- Cardoso, P.H.N.; Oliveira, C.Y.B.; Nunes, M.; Tavares, G.F.; Faia, P.M.; Araújo, E.S. Eudragit E100/Hesperidin 3D Printing Filaments: Preparation, Characterization, and In Vitro Release Studies. Appl. Sci. 2023, 13, 11558. [Google Scholar] [CrossRef]
- Strojewski, D.; Krupa, A. Kollidon® VA 64 and Soluplus® as Modern Polymeric Carriers for Amorphous Solid Dispersions. Polim. Med. 2022, 52, 17–27. [Google Scholar] [CrossRef]
- Setianto, A.B.; Nugraha, Y.P.; Suendo, V.; Ainurofiq, A.; Uekusa, H.; Soewandhi, S.N. X-ray Diffraction and Vibrational Spectroscopic Studies of the Intermolecular Interactions on the Grinding and Compaction Behaviours of Lopinavir and Ritonavir Crystals. Acta Pol. Pharm. 2020, 77, 259–269. [Google Scholar] [CrossRef]
- Li, S.; Liu, B.; Chen, Z.; Ou, X.; Rong, H.; Lu, M. Ritonavir Revisited: Melt Crystallization Can Easily Find the Late-Appearing Polymorph II and Unexpectedly Discover a New Polymorph III. Mol. Pharm. 2023, 20, 3854–3863. [Google Scholar] [CrossRef] [PubMed]
- Parent, S.D.; Smith, P.A.; Purcell, D.K.; Smith, D.T.; Bogdanowich-Knipp, S.J.; Bhavsar, A.S.; Chan, L.R.; Croom, J.M.; Bauser, H.C.; McCalip, A.; et al. Ritonavir Form III: A Coincidental Concurrent Discovery. Cryst. Growth Des. 2023, 23, 320–325. [Google Scholar] [CrossRef]
- Dezena, R.M.B. Ritonavir Polymorphism: Analytical Chemistry Approach to Problem Solving in the Pharmaceutical Industry. Braz. J. Anal. Chem. 2020, 7, 12–17. [Google Scholar] [CrossRef]
- Szafraniec-Szczęsny, J.; Antosik-Rogóż, A.; Kurek, M.; Gawlak, K.; Górska, A.; Peralta, S.; Knapik-Kowalczuk, J.; Kramarczyk, D.; Paluch, M.; Jachowicz, R. How Does the Addition of Kollidon® va64 Inhibit the Recrystallization and Improve Ezetimibe Dissolution from Amorphous Solid Dispersions? Pharmaceutics 2021, 13, 147. [Google Scholar] [CrossRef]
- Dahlberg, C.; Millqvist-Fureby, A.; Schuleit, M. Surface Composition and Contact Angle Relationships for Differently Prepared Solid Dispersions. Eur. J. Pharm. Sci. 2008, 70, 478–485. [Google Scholar] [CrossRef]
- Krupa, A.; Tabor, Z.; Tarasiuk, J.; Strach, B.; Pociecha, K.; Wyska, E.; Wroński, S.; Łyszczarz, E.; Jachowicz, R. The Impact of Polymers on 3D Microstructure and Controlled Release of Sildenafil Citrate from Hydrophilic Matrices. Eur. J. Pharm. Sci. 2018, 119, 234–243. [Google Scholar] [CrossRef]
- Linares, V.; Yarce, C.J.; Echeverri, J.D.; Galeano, E.; Salamanca, C.H. Relationship between Degree of Polymeric Ionisation and Hydrolytic Degradation of Eudragit® E Polymers under Extreme Acid Conditions. Polymers 2019, 11, 1010. [Google Scholar] [CrossRef]
- Bhattarai, R.S.; Bachu, R.D.; Boddu, S.H.S.; Bhaduri, S. Biomedical Applications of Electrospun Nanofibers: Drug and Nanoparticle Delivery. Pharmaceutics 2019, 11, 5. [Google Scholar] [CrossRef]
- Xu, H.; Xu, X.; Li, S.; Song, W.L.; Yu, D.G.; Annie Bligh, S.W. The Effect of Drug Heterogeneous Distributions within Core-Sheath Nanostructures on Its Sustained Release Profiles. Biomolecules 2021, 11, 1330. [Google Scholar] [CrossRef]
- Trasi, N.S.; Bhujbal, S.; Zhou, Q.T.; Taylor, L.S. Amorphous Solid Dispersion Formation via Solvent Granulation—A Case Study with Ritonavir and Lopinavir. Int. J. Pharm. X 2019, 1, 100035. [Google Scholar] [CrossRef]
- Nikam, A.; Sahoo, P.R.; Musale, S.; Pagar, R.R.; Paiva-Santos, A.C.; Giram, P.S. A Systematic Overview of Eudragit® Based Copolymer for Smart Healthcare. Pharmaceutics 2023, 15, 587. [Google Scholar] [CrossRef] [PubMed]
- Lin, X.; Su, L.; Li, N.; Hu, Y.; Tang, G.; Liu, L.; Li, H.; Yang, Z. Understanding the Mechanism of Dissolution Enhancement for Poorly Water-Soluble Drugs by Solid Dispersions Containing Eudragit® E PO. J. Drug Deliv. Sci. Technol. 2018, 48, 328–337. [Google Scholar] [CrossRef]
- Brniak, W.; Maślak, E.; Jachowicz, R. Orodispersible Films and Tablets with Prednisolone Microparticles. Eur. J. Pharm. Sci. 2015, 75, 81–90. [Google Scholar] [CrossRef]
- Vuddanda, P.R.; Montenegro-Nicolini, M.; Morales, J.O.; Velaga, S. Effect of Surfactants and Drug Load on Physico-Mechanical and Dissolution Properties of Nanocrystalline Tadalafil-Loaded Oral Films. Eur. J. Pharm. Sci. 2017, 109, 372–380. [Google Scholar] [CrossRef]
- WHO. Model List of Essential Medicines for Children 23rd List 2023; WHO: Geneva, Switzerland, 2023. [Google Scholar]




| Formulation | Content of Electrospinning Solution Components (%) | ||||
|---|---|---|---|---|---|
| 30% PVP | 35% KVA | 25% E | LPV | RTV | |
| PVP30LPV5 | 95.0 | - | - | 5.0 | - |
| PVP30LPV10 | 90.0 | - | - | 10.0 | - |
| PVP30LPV20 | 80.0 | - | - | 20.0 | - |
| KVA35LPV5 | - | 95.0 | - | 5.0 | - |
| KVA35LPV10 | - | 90.0 | - | 10.0 | - |
| KVA35LPV20 | - | 80.0 | - | 20.0 | - |
| E25LPV5 | - | - | 95.0 | 5.0 | - |
| E25LPV10 | - | - | 90.0 | 10.0 | - |
| E25LPV20 | - | - | 80.0 | 20.0 | - |
| PVP30RTV2.5 | 97.5 | - | - | - | 2.5 |
| PVP30RTV5 | 95.0 | - | - | - | 5.0 |
| KVA35RTV2.5 | - | 97.5 | - | - | 2.5 |
| KVA35RTV5 | - | 95.0 | - | - | 5.0 |
| E25RTV2.5 | - | - | 97.5 | - | 2.5 |
| E25RTV5 | - | - | 95.0 | - | 5.0 |
| Properties | Lopinavir | Ritonavir |
|---|---|---|
| Molecular weight | 628.8 g/mol | 720.9 g/mol |
| Water solubility | 1.92 µg/mL | 1.26 µg/mL |
| Ethanol solubility | freely soluble | freely soluble |
| logP | 4.69 | 5.22 |
| pKa (strongest acidic condition) | 13.39 | 13.68 |
| pKa (strongest basic condition) | −1.5 | 2.84 |
| Formulation | DL ± SD (% w/w) | EE% ± RSD (%) |
|---|---|---|
| PVP30LPV5 | 12.9 ± 0.3 | 86.9 ± 2.6 |
| PVP30LPV10 | 23.7 ± 0.7 | 87.6 ± 3.2 |
| PVP30LPV20 | 39.7 ± 1.3 | 87.2 ± 3.8 |
| KVA35LPV5 | 12.2 ± 1.0 | 93.5 ± 8.8 |
| KVA35LPV10 | 22.6 ± 0.1 | 94.0 ± 0.3 |
| KVA35LPV20 | 37.8 ± 0.3 | 90.7 ± 1.0 |
| E25LPV5 | 18.5 ± 2.1 | 106.1 ± 10.6 |
| E25LPV10 | 23.0 ± 0.5 | 97.3 ± 1.7 |
| E25LPV20 | 49.2 ± 1.3 | 98.5 ± 2.7 |
| PVP30RTV2.5 | 7.5 ± 0.2 | 94.7 ± 2.7 |
| PVP30RTV5 | 14.0 ± 0.3 | 93.6 ± 2.0 |
| KVA35RTV2.5 | 6.8 ± 0.1 | 100.4 ± 1.9 |
| KVA35RTV5 | 12.6 ± 0.2 | 96.1 ± 1.8 |
| E25RTV2.5 | 9.4 ± 0.1 | 101.0 ± 0.4 |
| E25RTV5 | 17.6 ± 0.4 | 101.4 ± 2.4 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Łyszczarz, E.; Sosna, O.; Srebro, J.; Rezka, A.; Majda, D.; Mendyk, A. Electrospun Amorphous Solid Dispersions with Lopinavir and Ritonavir for Improved Solubility and Dissolution Rate. Nanomaterials 2024, 14, 1569. https://doi.org/10.3390/nano14191569
Łyszczarz E, Sosna O, Srebro J, Rezka A, Majda D, Mendyk A. Electrospun Amorphous Solid Dispersions with Lopinavir and Ritonavir for Improved Solubility and Dissolution Rate. Nanomaterials. 2024; 14(19):1569. https://doi.org/10.3390/nano14191569
Chicago/Turabian StyleŁyszczarz, Ewelina, Oskar Sosna, Justyna Srebro, Aleksandra Rezka, Dorota Majda, and Aleksander Mendyk. 2024. "Electrospun Amorphous Solid Dispersions with Lopinavir and Ritonavir for Improved Solubility and Dissolution Rate" Nanomaterials 14, no. 19: 1569. https://doi.org/10.3390/nano14191569
APA StyleŁyszczarz, E., Sosna, O., Srebro, J., Rezka, A., Majda, D., & Mendyk, A. (2024). Electrospun Amorphous Solid Dispersions with Lopinavir and Ritonavir for Improved Solubility and Dissolution Rate. Nanomaterials, 14(19), 1569. https://doi.org/10.3390/nano14191569







