Metal-Cation-Induced Tiny Ripple on Graphene
Abstract
:1. Introduction
2. Methods
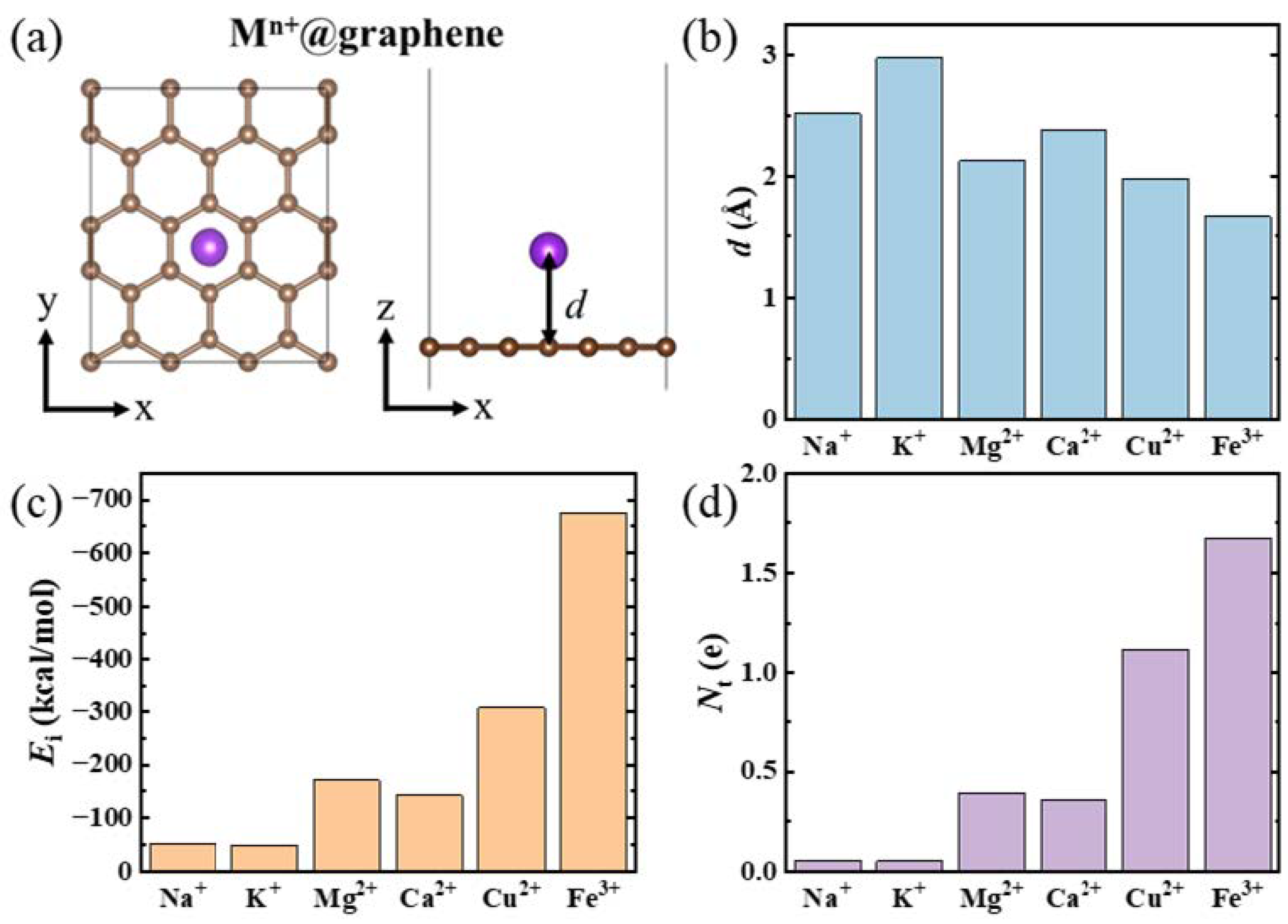
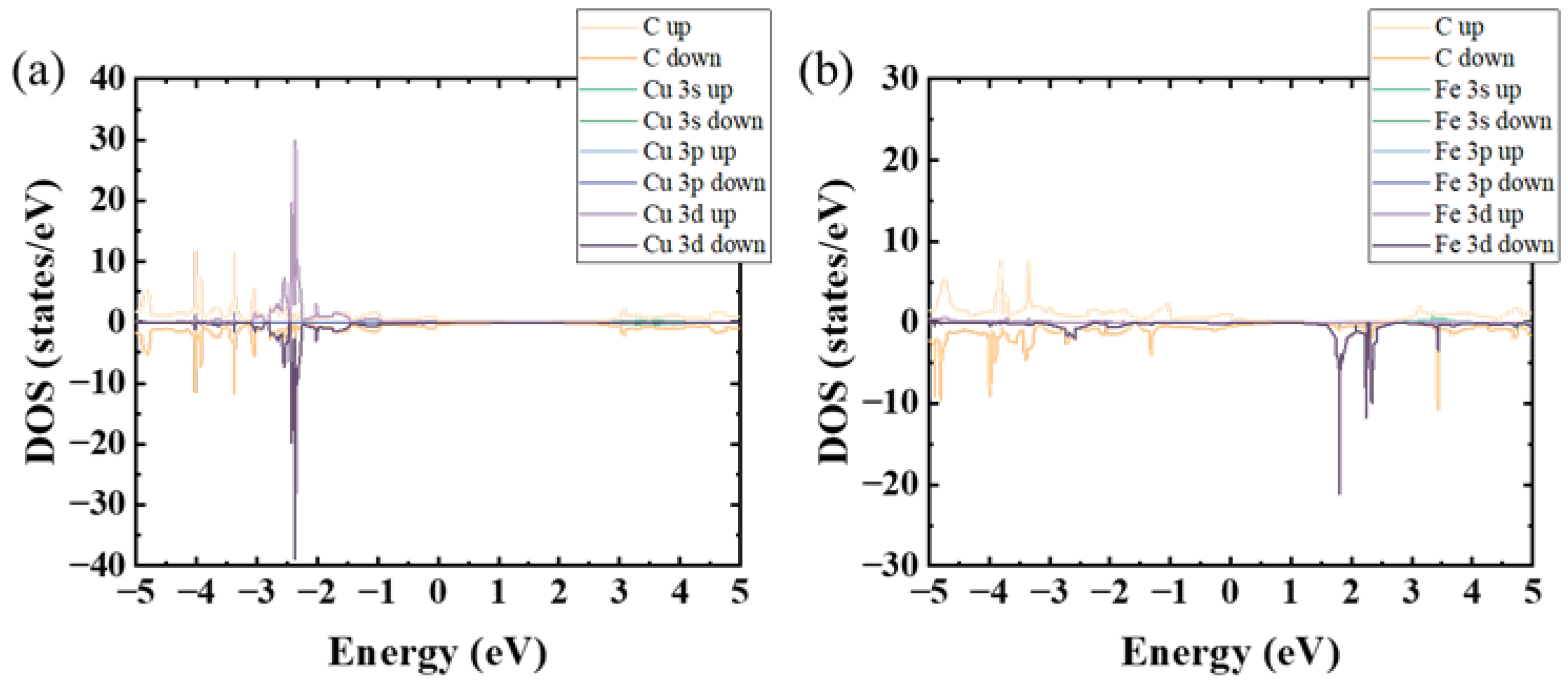
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Novoselov, K.S.; Geim, A.K.; Morozov, S.V.; Jiang, D.; Katsnelson, M.I.; Grigorieva, I.V.; Dubonos, S.V.; Firsov, A.A. Two-dimensional gas of massless Dirac fermions in graphene. Nature 2005, 438, 197–200. [Google Scholar] [CrossRef] [PubMed]
- Novoselov, K.S.; Geim, A.K.; Morozov, S.V.; Jiang, D.; Zhang, Y.; Dubonos, S.V.; Grigorieva, I.V.; Firsov, A.A. Electric field effect in atomically thin carbon films. Science 2004, 306, 666–669. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.B.; Akatyeva, E.; Dumitrică, T. Bending ultrathin graphene at the margins of continuum mechanics. Phys. Rev. Lett. 2011, 106, 255503. [Google Scholar] [CrossRef]
- Tapasztó, L.; Dumitrică, T.; Kim, S.J.; Nemes-Incze, P.; Hwang, C.; Biró, L.P. Breakdown of continuum mechanics for nanometre-wavelength rippling of graphene. Nat. Phys. 2012, 8, 739–742. [Google Scholar] [CrossRef]
- Deng, S.; Berry, V. Wrinkled, rippled and crumpled graphene: An overview of formation mechanism, electronic properties, and applications. Mater. Today 2016, 19, 197–212. [Google Scholar] [CrossRef]
- Ling, F.; Liao, R.; Yuan, C.; Shi, X.; Li, L.; Zhou, X.; Tang, X.; Jing, C.; Wang, Y.; Jiang, S. Geometric, electronic and transport properties of bulged graphene: A theoretical study. J. Chem. Phys. 2023, 158, 084702. [Google Scholar] [CrossRef]
- Sun, P.Z.; Xiong, W.Q.; Bera, A.; Timokhin, I.; Wu, Z.F.; Mishchenko, A.; Sellers, M.C.; Liu, B.L.; Cheng, H.M.; Janzen, E.; et al. Unexpected catalytic activity of nanorippled graphene. Proc. Natl. Acad. Sci. USA 2023, 120, e2300481120. [Google Scholar] [CrossRef]
- Dobrik, G.; Nemes-Incze, P.; Majerus, B.; Sule, P.; Vancso, P.; Piszter, G.; Menyhard, M.; Kalas, B.; Petrik, P.; Henrard, L.; et al. Large-area nanoengineering of graphene corrugations for visible-frequency graphene plasmons. Nat. Nanotechnol. 2022, 17, 61–66. [Google Scholar] [CrossRef]
- Yildiz, G.; Bolton-Warberg, M.; Awaja, F. Graphene and graphene oxide for bio-sensing: General properties and the effects of graphene ripples. Acta Biomater. 2021, 131, 62–79. [Google Scholar] [CrossRef]
- Kun, P.; Kukucska, G.; Dobrik, G.; Koltai, J.; Kürti, J.; Biró, L.P.; Tapasztó, L.; Nemes-Incze, P. Large intravalley scattering due to pseudo-magnetic fields in crumpled graphene. npj 2D Mater. Appl. 2019, 3, 11. [Google Scholar] [CrossRef]
- Kim, C.-E.; Lee, J.; Walsh, A.; Lordi, V.; Bahr, D.F. Role of ripples in altering the electronic and chemical properties of graphene. J. Chem. Phys. 2022, 156, 054708. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.K.; Yamazaki, S.; Yun, H.; Park, J.; Kennedy, G.P.; Kim, G.T.; Pietzsch, O.; Wiesendanger, R.; Lee, S.; Hong, S.; et al. Modification of electrical properties of graphene by substrate-induced nanomodulation. Nano Lett. 2013, 13, 3494–3500. [Google Scholar] [CrossRef] [PubMed]
- Pereira, V.M.; Castro Neto, A.H.; Liang, H.Y.; Mahadevan, L. Geometry, mechanics, and electronics of singular structures and wrinkles in graphene. Phys. Rev. Lett. 2010, 105, 156603. [Google Scholar] [CrossRef] [PubMed]
- Yan, W.; He, W.Y.; Chu, Z.D.; Liu, M.; Meng, L.; Dou, R.F.; Zhang, Y.; Liu, Z.; Nie, J.C.; He, L. Strain and curvature induced evolution of electronic band structures in twisted graphene bilayer. Nat. Commun. 2013, 4, 2159. [Google Scholar] [CrossRef] [PubMed]
- Gui, G.; Zhong, J.; Ma, Z. Electronic properties of rippled graphene. J. Phys. Conf. Ser. 2012, 402, 012004. [Google Scholar] [CrossRef]
- Guinea, F.; Katsnelson, M.I.; Geim, A.K. Energy gaps and a zero-field quantum Hall effect in graphene by strain engineering. Nat. Phys. 2010, 6, 30–33. [Google Scholar] [CrossRef]
- Zhu, W.; Low, T.; Perebeinos, V.; Bol, A.A.; Zhu, Y.; Yan, H.; Tersoff, J.; Avouris, P. Structure and electronic transport in graphene wrinkles. Nano Lett. 2012, 12, 3431–3436. [Google Scholar] [CrossRef]
- Katsnelson, M.I.; Geim, A.K. Electron scattering on microscopic corrugations in graphene. Philos. Trans. A Math. Phys. Eng. Sci. 2008, 366, 195–204. [Google Scholar] [CrossRef]
- Partovi-Azar, P.; Nafari, N.; Tabar, M.R.R. Interplay between geometrical structure and electronic properties in rippled free-standing graphene. Phys. Rev. B 2011, 83, 165434. [Google Scholar] [CrossRef]
- Martin, J.; Akerman, N.; Ulbricht, G.; Lohmann, T.; Smet, J.H.; von Klitzing, K.; Yacoby, A. Observation of electron–hole puddles in graphene using a scanning single-electron transistor. Nat. Phys. 2008, 4, 144–148. [Google Scholar] [CrossRef]
- Xu, X.; Pereira, L.F.C.; Wang, Y.; Wu, J.; Zhang, K.; Zhao, X.; Bae, S.; Tinh Bui, C.; Xie, R.; Thong, J.T.L.; et al. Length-dependent thermal conductivity in suspended single-layer graphene. Nat. Commun. 2014, 5, 3689. [Google Scholar] [CrossRef] [PubMed]
- Zhao, S.; Zhang, Y.; Yang, J.; Kitipornchai, S. Improving interfacial shear strength between graphene sheets by strain-induced wrinkles. Carbon 2020, 168, 135–143. [Google Scholar] [CrossRef]
- Levy, N.; Burke, S.A.; Meaker, K.L.; Panlasigui, M.; Zettl, A.; Guinea, F.; Castro Neto, A.H.; Crommie, M.F. Strain-induced pseudo-magnetic fields greater than 300 tesla in graphene nanobubbles. Science 2010, 329, 544–547. [Google Scholar] [CrossRef]
- Bao, W.; Miao, F.; Chen, Z.; Zhang, H.; Jang, W.; Dames, C.; Lau, C.N. Controlled ripple texturing of suspended graphene and ultrathin graphite membranes. Nat. Nanotechnol. 2009, 4, 562–566. [Google Scholar] [CrossRef] [PubMed]
- Lee, S. Effect of intrinsic ripples on elasticity of the graphene monolayer. Nanoscale Res. Lett. 2015, 10, 422. [Google Scholar] [CrossRef]
- Meyer, J.C.; Geim, A.K.; Katsnelson, M.I.; Novoselov, K.S.; Booth, T.J.; Roth, S. The structure of suspended graphene sheets. Nature 2007, 446, 60–63. [Google Scholar] [CrossRef]
- Hu, J.; Vanacore, G.M.; Cepellotti, A.; Marzari, N.; Zewail, A.H. Rippling ultrafast dynamics of suspended 2D monolayers, graphene. Proc. Natl. Acad. Sci. USA 2016, 113, E6555–E6561. [Google Scholar] [CrossRef]
- Fasolino, A.; Los, J.H.; Katsnelson, M.I. Intrinsic ripples in graphene. Nat. Mater. 2007, 6, 858–861. [Google Scholar] [CrossRef]
- Martinez-Asencio, J.; Ruestes, C.J.; Bringa, E.M.; Caturla, M.J. Controlled rippling of graphene via irradiation and applied strain modify its mechanical properties: A nanoindentation simulation study. Phys. Chem. Chem. Phys. 2016, 18, 13897–13903. [Google Scholar] [CrossRef]
- Obraztsov, A.N.; Obraztsova, E.A.; Tyurnina, A.V.; Zolotukhin, A.A. Chemical vapor deposition of thin graphite films of nanometer thickness. Carbon 2007, 45, 2017–2021. [Google Scholar] [CrossRef]
- Rhee, D.; Paci, J.T.; Deng, S.; Lee, W.-K.; Schatz, G.C.; Odom, T.W. Soft Skin layers enable area-specific, multiscale graphene wrinkles with switchable orientations. ACS Nano 2020, 14, 166–174. [Google Scholar] [CrossRef] [PubMed]
- Koenig, S.P.; Boddeti, N.G.; Dunn, M.L.; Bunch, J.S. Ultrastrong adhesion of graphene membranes. Nat. Nanotechnol. 2011, 6, 543–546. [Google Scholar] [CrossRef] [PubMed]
- Bai, K.K.; Zhou, Y.; Zheng, H.; Meng, L.; Peng, H.; Liu, Z.; Nie, J.C.; He, L. Creating one-dimensional nanoscale periodic ripples in a continuous mosaic graphene monolayer. Phys. Rev. Lett. 2014, 113, 086102. [Google Scholar] [CrossRef] [PubMed]
- Sunner, J.; Nishizawa, K.; Kebarle, P. Ion-solvent molecule interactions in the gas phase. The potassium ion and benzene. J. Phys. Chem. 1981, 85, 1814–1820. [Google Scholar] [CrossRef]
- Georgakilas, V.; Otyepka, M.; Bourlinos, A.B.; Chandra, V.; Kim, N.; Kemp, K.C.; Hobza, P.; Zboril, R.; Kim, K.S. Functionalization of graphene: Covalent and non-covalent approaches, derivatives and applications. Chem. Rev. 2012, 112, 6156–6214. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.; Camilli, L.; Caridad, J.M.; Santos, J.E.; Liu, Y. Spontaneous adsorption of ions on graphene at the electrolyte–graphene interface. Appl. Phys. Lett. 2020, 117, 203102. [Google Scholar] [CrossRef]
- Shi, G.; Liu, J.; Wang, C.; Song, B.; Tu, Y.; Hu, J.; Fang, H. Ion enrichment on the hydrophobic carbon-based surface in aqueous salt solutions due to cation-π interactions. Sci. Rep. 2013, 3, 3436. [Google Scholar] [CrossRef]
- Chen, L.; Guo, Y.; Xu, Z.; Yang, X. Multiscale simulation of the interaction and adsorption of ions on a hydrophobic graphene surface. Chem. Phys. Chem. 2018, 19, 2954–2960. [Google Scholar] [CrossRef]
- Williams, C.D.; Dix, J.; Troisi, A.; Carbone, P. Effective polarization in pairwise potentials at the graphene-electrolyte interface. J. Phys. Chem. Lett. 2017, 8, 703–708. [Google Scholar] [CrossRef]
- Hafner, J. Materials simulations using VASP—A quantum perspective to materials science. Comput. Phys. Commun. 2007, 177, 6–13. [Google Scholar] [CrossRef]
- Hafner, J. Ab-initio simulations of materials using VASP: Density-functional theory and beyond. J. Comput. Chem. 2008, 29, 2044–2078. [Google Scholar] [CrossRef] [PubMed]
- Kresse, G.; Furthmüller, J. Efficient iterative schemes for ab initio total-energy calculations using a plane-wave basis set. Phys. Rev. B 1996, 54, 11169–11186. [Google Scholar] [CrossRef] [PubMed]
- Kresse, G.; Joubert, D. From ultrasoft pseudopotentials to the projector augmented-wave method. Phys. Rev. B 1999, 59, 1758–1775. [Google Scholar] [CrossRef]
- Perdew, J.P.; Burke, K.; Ernzerhof, M. Generalized gradient approximation made simple. Phys. Rev. Lett. 1996, 77, 3865–3868. [Google Scholar] [CrossRef]
- Grimme, S.; Antony, J.; Ehrlich, S.; Krieg, H. A consistent and accurate ab initio parametrization of density functional dispersion correction (DFT-D) for the 94 elements H-Pu. J. Chem. Phys. 2010, 132, 154104. [Google Scholar] [CrossRef]
- Wang, V.; Xu, N.; Liu, J.-C.; Tang, G.; Geng, W.-T. VASPKIT: A user-friendly interface facilitating high-throughput computing and analysis using VASP code. Comput. Phys. Commun. 2021, 267, 108033. [Google Scholar] [CrossRef]
- Paier, J.; Marsman, M.; Hummer, K.; Kresse, G.; Gerber, I.C.; Ángyán, J.G. Screened hybrid density functionals applied to solids. J. Chem. Phys. 2006, 124, 154709. [Google Scholar] [CrossRef]
- Vydrov, O.A.; Heyd, J.; Krukau, A.V.; Scuseria, G.E. Importance of short-range versus long-range Hartree-Fock exchange for the performance of hybrid density functionals. J. Chem. Phys. 2006, 125, 074106. [Google Scholar] [CrossRef]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Petersson, G.A.; Nakatsuji, H.; et al. Gaussian 09, Revision A.1; Gaussian, Inc.: Wallingford, CT, USA, 2009. [Google Scholar]
- Becke, A.D. Density-functional thermochemistry. III. The role of exact exchange. J. Chem. Phys. 1993, 98, 5648–5652. [Google Scholar] [CrossRef]
- Lu, T.; Chen, F. Multiwfn: A multifunctional wavefunction analyzer. J. Comput. Chem. 2012, 33, 580–592. [Google Scholar] [CrossRef]
- Meng, L.; Su, Y.; Geng, D.; Yu, G.; Liu, Y.; Dou, R.-F.; Nie, J.-C.; He, L. Hierarchy of graphene wrinkles induced by thermal strain engineering. Appl. Phys. Lett. 2013, 103, 251610. [Google Scholar] [CrossRef]
- Cerda, E.; Mahadevan, L. Geometry and physics of wrinkling. Phys. Rev. Lett. 2003, 90, 074302. [Google Scholar] [CrossRef] [PubMed]


| System | C11 (N/m) | C12 (N/m) | C22 (N/m) | C33 (N/m) | Young’s Modulus (N/m) |
|---|---|---|---|---|---|
| Graphene | 325.74 | 78.16 | 347.47 | 138.87 | 325.39 |
| Na+@graphene | 326.09 | 76.88 | 347.11 | 138.92 | 325.53 |
| K+@graphene | 326.41 | 77.29 | 347.61 | 138.98 | 325.83 |
| Mg2+@graphene | 342.73 | 64.06 | 357.12 | 148.78 | 342.15 |
| Ca2+@graphene | 333.94 | 72.99 | 350.89 | 140.35 | 330.84 |
| Cu2+@graphene | 344.97 | 60.50 | 362.21 | 143.20 | 340.86 |
| Fe3+@graphene | 350.08 | 68.56 | 352.71 | 142.53 | 338.81 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huang, Y.; Li, H.; Zhu, L.; Song, Y.; Fang, H. Metal-Cation-Induced Tiny Ripple on Graphene. Nanomaterials 2024, 14, 1593. https://doi.org/10.3390/nano14191593
Huang Y, Li H, Zhu L, Song Y, Fang H. Metal-Cation-Induced Tiny Ripple on Graphene. Nanomaterials. 2024; 14(19):1593. https://doi.org/10.3390/nano14191593
Chicago/Turabian StyleHuang, Yingying, Hanlin Li, Liuyuan Zhu, Yongshun Song, and Haiping Fang. 2024. "Metal-Cation-Induced Tiny Ripple on Graphene" Nanomaterials 14, no. 19: 1593. https://doi.org/10.3390/nano14191593





