Efficient Formaldehyde Gas Sensing Performance via Promotion of Oxygen Vacancy on In-Doped LaFeO3 Nanofibers
Abstract
:1. Introduction
2. Experimental Section
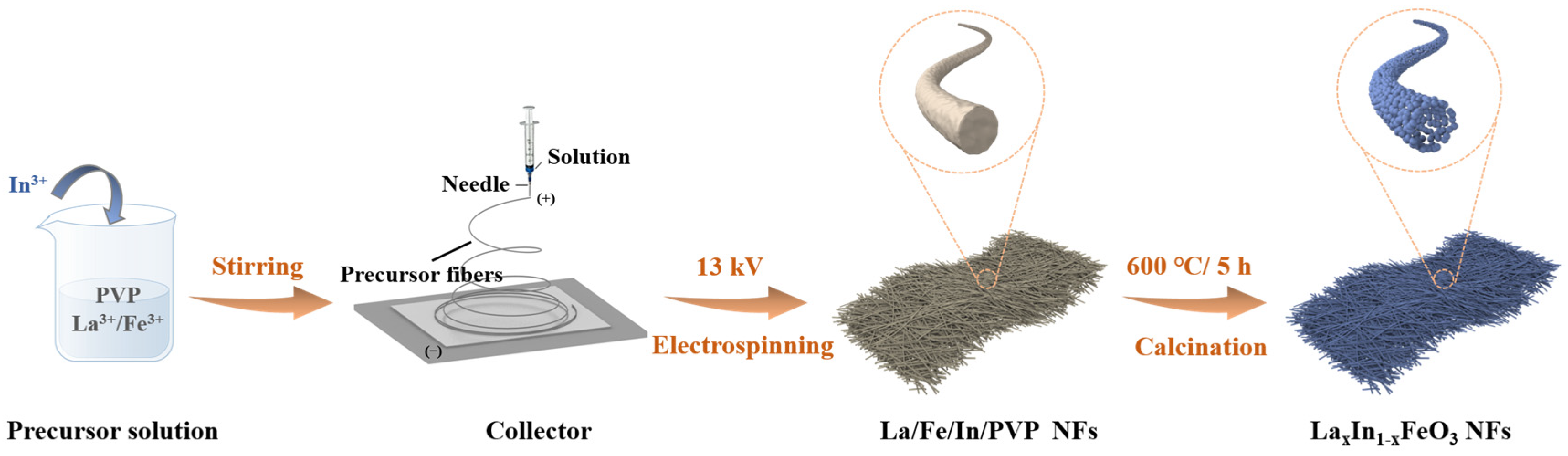
2.1. Synthesis of LaxIn1-xFeO3 Nanofibers (LxIn1-xFO NFs)
2.2. Characterizations
2.3. Gas Sensing Measurements
3. Results and Discussion
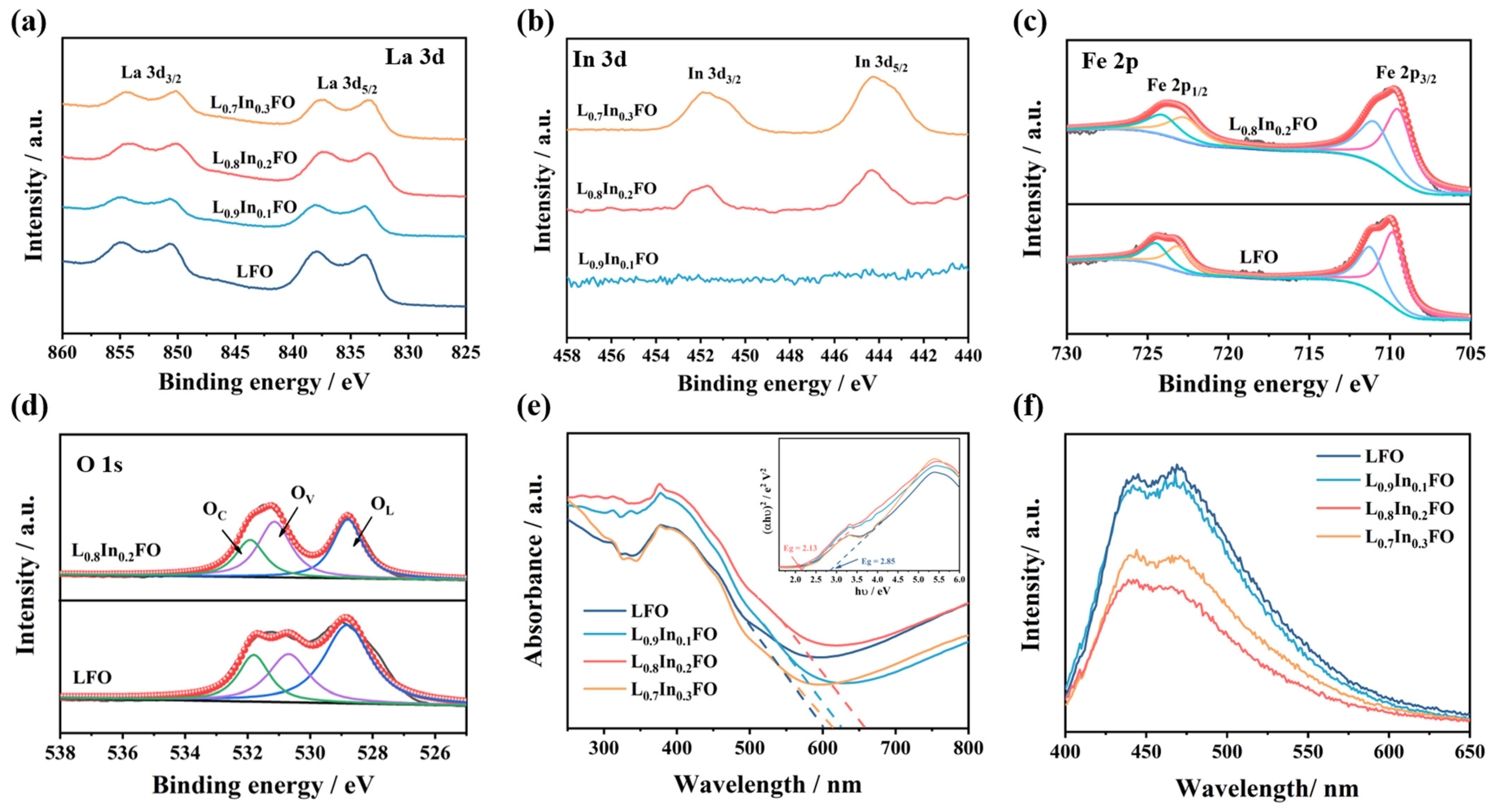
3.1. Microstructures and Composition
3.2. Gas Sensing Properties
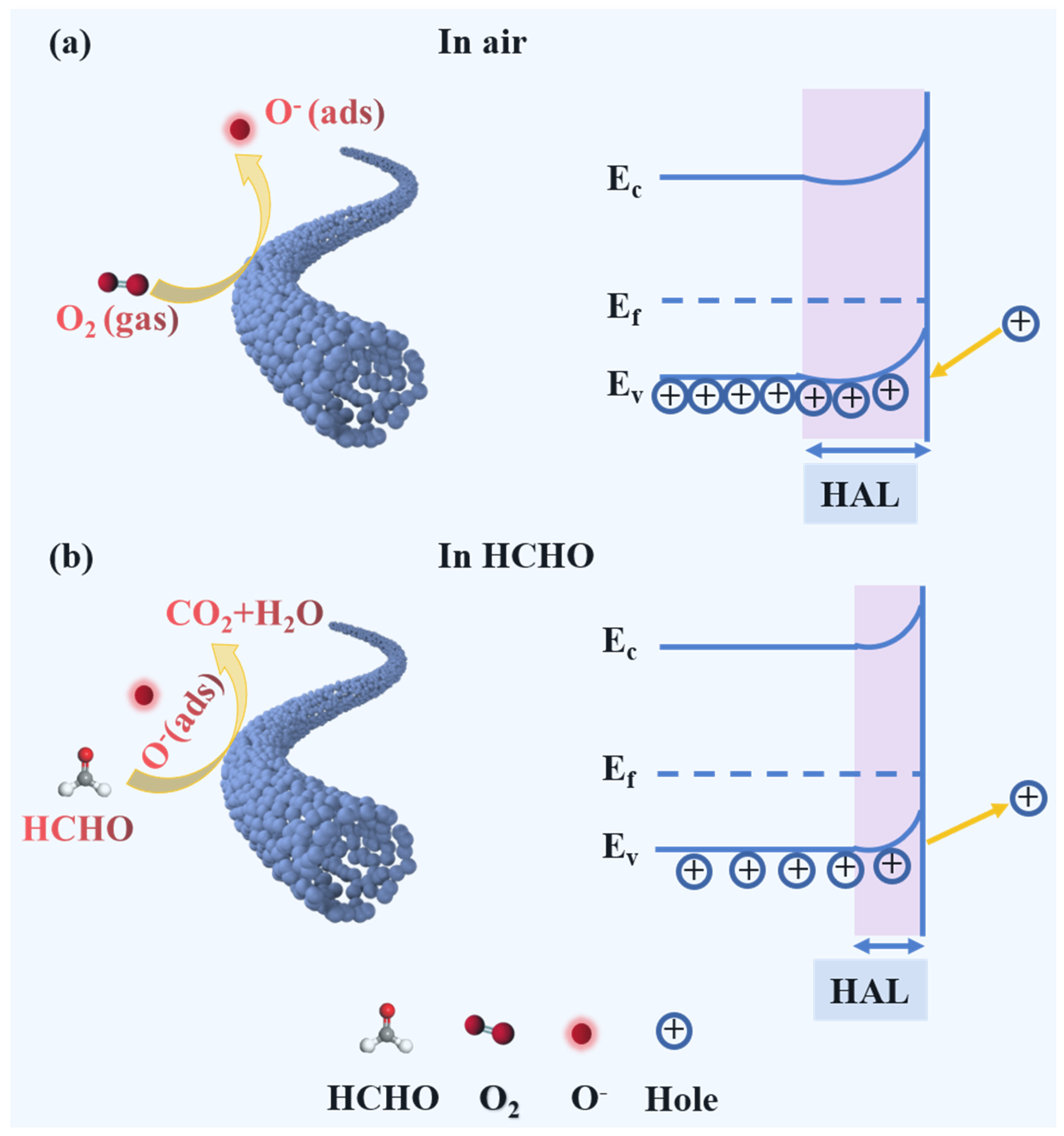
3.3. Gas Sensing Mechanism
- (i)
- The enhanced oxygen vacancies generated by the doping of In3+ ions. Oxygen vacancies commonly serve as positive charge centers, and the electrons around oxygen vacancies are more susceptible to being captured by oxygen molecules (reactions (10) and (11)). The presence of a moderate number of oxygen vacancies is beneficial for the adsorption of oxygen onto the surface of the sensing material and enables the formation of more chemisorbed oxygen species [47]. As can be seen from the O 1s XPS spectra (Figure 4d and Figure S4) and Table S1, the proportion of the Ov component rises with the increase of In3+ doping and reaches the maximum value of 38.9% in the L0.8In0.2FO NFs. The reaction process can be described as follows:
- (ii)
- The large surface area and porous structure. The BET analysis indicates that the L0.8In0.2FO NFs possess a larger specific surface area compared to the pristine LFO NFs, which increases the number of active adsorption sites and is conducive to the adsorption of oxygen and HCHO gas molecules [50]. In addition, the mesoporous structure of the L0.8In0.2FO NFs enhances the diffusive transport of the target gases into the material, thereby contributing to more effective interaction with the active sites on the sensor’s surface and achieving a faster response time and higher sensitivity.
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Min, Y.; Yuan, C.; Fu, D.; Liu, J. Formaldehyde Gas Sensors Fabricated with Polymer-Based Materials: A Review. Chemosensors 2023, 11, 134. [Google Scholar] [CrossRef]
- Chen, E.X.; Yang, H.; Zhang, J. Zeolitic imidazolate framework as formaldehyde gas sensor. Inorg. Chem. 2014, 53, 5411–5413. [Google Scholar] [CrossRef] [PubMed]
- Chung, P.R.; Tzeng, C.T.; Ke, M.T.; Lee, C.Y. Formaldehyde gas sensors: A review. Sensors 2013, 13, 4468–4484. [Google Scholar] [CrossRef] [PubMed]
- Castro-Hurtado, I.; Mandayo, G.G.; Castaño, E. Conductometric formaldehyde gas sensors. A review: From conventional films to nanostructured materials. Thin Solid Films 2013, 548, 665–676. [Google Scholar] [CrossRef]
- Souri, M.; Salar Amoli, H.; Yamini, Y. Three-dimensionally ordered porous In-doped SmFeO3 perovskite gas sensor for highly sensitive and selective detection of formaldehyde. Sens. Actuators B 2024, 404, 135213. [Google Scholar] [CrossRef]
- WHO-ROE. Air Quality Guidelines for Europe; WHO Regional Publications: Geneva, Switzerland, 2000. [Google Scholar]
- Lee, J.H. Gas sensors using hierarchical and hollow oxide nanostructures: Overview. Sens. Actuators B 2009, 140, 319–336. [Google Scholar] [CrossRef]
- Paul, R.; Das, B.; Ghosh, R. Novel approaches towards design of metal oxide based hetero-structures for room temperature gas sensor and its sensing mechanism: A recent progress. J. Alloys Compd. 2023, 941, 168943. [Google Scholar] [CrossRef]
- Chizhov, A.; Rumyantseva, M.; Gaskov, A. Light Activation of Nanocrystalline Metal Oxides for Gas Sensing: Principles, Achievements, Challenges. Nanomaterials 2021, 11, 892. [Google Scholar] [CrossRef]
- Souri, M.; Salar Amoli, H. Gas sensing mechanisms in ABO3 perovskite materials at room temperature: A review. Mater. Sci. Semicond. Process. 2023, 156, 107271. [Google Scholar] [CrossRef]
- Ji, Q.; Bi, L.; Zhang, J.; Cao, H.; Zhao, X.S. The role of oxygen vacancies of ABO3 perovskite oxides in the oxygen reduction reaction. Energy Environ. Sci. 2020, 13, 1408–1428. [Google Scholar] [CrossRef]
- Zhang, Y.; Jiang, B.; Yuan, M.; Li, P.; Li, W.; Zheng, X. Formaldehyde-sensing properties of LaFeO3 particles synthesized by citrate sol-gel method. J. Sol-Gel Sci. Technol. 2016, 79, 167–175. [Google Scholar] [CrossRef]
- Sun, B.; Liu, M.; Liu, L.; Wang, Q.; Song, P. Synthesis of 1D LaFeO3 nanofibers/2D MXene heterostructures for formaldehyde detection at low temperature. Sens. Actuators B 2024, 415, 136011. [Google Scholar] [CrossRef]
- Di, J.; Chen, C.; Zhu, C.; Ji, M.; Xia, J.; Yan, C.; Hao, W.; Li, S.; Li, H.; Liu, Z. Bismuth vacancy mediated single unit cell Bi2WO6 nanosheets for boosting photocatalytic oxygen evolution. Appl. Catal. B 2018, 238, 119–125. [Google Scholar] [CrossRef]
- He, N.; Yu, Z.; Yang, G.; Tan, Q.; Wang, J.; Chen, Y. Designing with A-site cation defects in LaFeO3: Removal of tetracycline hydrochloride in complex environments using photo-Fenton synergy. Chem. Eng. J. 2024, 484, 149613. [Google Scholar] [CrossRef]
- Jing, J.; Pervez, M.N.; Sun, P.; Cao, C.; Li, B.; Naddeo, V.; Jin, W.; Zhao, Y. Highly efficient removal of bisphenol A by a novel Co-doped LaFeO3 perovskite/PMS system in salinity water. Sci. Total Environ. 2021, 801, 149490. [Google Scholar] [CrossRef] [PubMed]
- Wu, M.D.; Chen, S.Y.; Xiang, W.G. Oxygen vacancy induced performance enhancement of toluene catalytic oxidation using LaFeO3 perovskite oxides. Chem. Eng. J. 2020, 387, 124101–124113. [Google Scholar] [CrossRef]
- Ji, Y.; Xie, Y.; Zheng, L.; Xu, F. Efficient activation of peroxymonosulfate by porous Co-doped LaFeO3 for organic pollutants degradation in water. J. Solid State Chem. 2021, 297, 122077. [Google Scholar] [CrossRef]
- Sun, L.; Li, H.; Zhao, J.; Wang, G.; Huo, L.; Zhao, H. Effects of calcium doping to oxygen reduction activity on Pr2−xCaxNiMnO6 cathode. J. Alloys Compd. 2019, 777, 1319–1326. [Google Scholar] [CrossRef]
- Duan, X.; Jiang, Y.; Liu, B.; Duan, Z.; Zhang, Y.; Yuan, Z.; Tai, H. Enhancing the carbon dioxide sensing performance of LaFeO3 by Co doping. Sens. Actuators B 2024, 402, 135136. [Google Scholar] [CrossRef]
- Wang, Z.; Zhu, L.; Sun, S.Y.; Wang, J.N.; Yan, W. One-dimensional nanomaterials in resistive gas sensor: From material design to application. Chemosensors 2021, 9, 198. [Google Scholar] [CrossRef]
- Yang, J.Q.; Han, W.J.; Ma, J.; Wang, C.; Shimanoe, K.; Zhang, S.M.; Sun, Y.F.; Cheng, P.F.; Wang, Y.L.; Zhang, H.; et al. Sn doping effect on NiO hollow nanofibers based gas sensors about the humidity dependence for triethylamine detection. Sens. Actuators B 2021, 340, 129971. [Google Scholar] [CrossRef]
- Zhu, L.; Wang, J.; Liu, J.; Chen, X.; Xu, Z.; Ma, Q.; Wang, Z.; Liang, J.; Li, S.; Yan, W. Designing highly sensitive formaldehyde sensors via A-site cation deficiency in LaFeO3 hollow nanofibers. Appl. Surf. Sci. 2022, 590, 153085. [Google Scholar] [CrossRef]
- Guo, Y.; Sui, M.; Liu, S.; Li, T.; Lv, X.; Yu, M.; Mo, Y. Insight into cobalt substitution in LaFeO3-based catalyst for enhanced activation of peracetic acid: Reactive species and catalytic mechanism. J. Hazard. Mater. 2024, 461, 132662. [Google Scholar] [CrossRef] [PubMed]
- Huang, B.; Wu, L.; Ren, H.; Zhang, Q.; Lin, H.; Xu, S.; Xiong, D.; Deng, W. Effect of Y3+ doping of rare earth element on microstructure and antiferromagnetism of CuFeO2 ceramics. Ceram. Int. 2024, 50, 9372–9379. [Google Scholar] [CrossRef]
- Sharma, S.; Khan, S. Tailoring the structural, optical, electrical and multiferroic properties of Sm1−xRxFeO3 (x = 0.0 and 0.5; R = Pr, Nd, and Gd) and their synergistic photocatalytic activity. Ceram. Int. 2024, 50, 9185–9205. [Google Scholar] [CrossRef]
- Zhang, Y.; Xu, D.; Zhou, T.; Song, Z.; Deng, Z.; Zi, B.; Zhang, J.; Zhao, J.; Liu, Q.; Hu, G. Nonstoichiometric Doping of La0.9FexSn1−xO3 Hollow Microspheres for an Ultrasensitive Formaldehyde Sensor. ACS Sensors 2023, 8, 4334–4343. [Google Scholar] [CrossRef]
- Fang, F.; Feng, N.J.; Wang, L.; Meng, J.; Liu, G.; Zhao, P.; Gao, P.F.; Ding, J.; Wan, H.; Guan, G.F. Fabrication of perovskite-type macro/mesoporous La1−xKxFeO3−δ nanotubes as an efficient catalyst for soot combustion. Appl. Catal. B 2018, 236, 184–194. [Google Scholar] [CrossRef]
- Zhu, L.; Wang, J.; Liu, J.; Wang, Z.; Sun, S.; Li, M.; Yan, W. ZIF-L(Co) derived cobalt doped In2O3 hollow nanofibers with high surface activity for efficient formaldehyde gas sensing. Sens. Actuators B 2024, 403, 135129. [Google Scholar] [CrossRef]
- Eyméoud, P.; Turquat, C.; Pardanaud, C.; Leroux, C.; Merlen, A. Raman spectroscopic detection of vacancies in LaFeO3. Mater. Lett. 2023, 330, 133296. [Google Scholar] [CrossRef]
- Shi, J.M.; Chang, Y.; Tang, Y.S.; Wang, X.B.; Wang, X.F.; Zhang, X.C.; Cao, J.L. Hydrogenated LaFeO3 with oxygen vacancies for Enhanced Visible light photocatalytic performance. Ceram. Int. 2020, 46, 5315–5322. [Google Scholar] [CrossRef]
- Ma, Z.; Yang, B.; Song, Y.; Song, Y.; Sun, J. Highly conductive ZIF-67 derived La-doped hollow structure for H2S detection. Sens. Actuators B 2023, 379, 133139. [Google Scholar] [CrossRef]
- Zhu, L.; Wang, Z.; Wang, J.; Liu, J.; Zhao, W.; Zhang, J.; Yan, W. Synergistic Effect of ZIF-8 and Pt-Functionalized NiO/In2O3 Hollow Nanofibers for Highly Sensitive Detection of Formaldehyde. Nanomaterials 2024, 14, 841. [Google Scholar] [CrossRef] [PubMed]
- Qiu, C.; Wang, L.; Chen, R.; Zhang, J.; Ding, J.; Zhang, J.; Wan, H.; Guan, G. Insight of the State for Deliberately Introduced A-Site Defect in Nanofibrous LaFeO3 for Boosting Artificial Photosynthesis of CH3OH. ACS Appl. Mater. Interfaces 2023, 15, 56945–56956. [Google Scholar] [CrossRef] [PubMed]
- Shao, J.; Sun, C.; Pan, G.; Yang, X. Conductometric n-butanol sensor based on Pt-loaded LaFeO3 with 3D hierarchical structure. Sens. Actuators B 2024, 417, 136086. [Google Scholar] [CrossRef]
- Cheng, C.; Gao, S.; Zhu, J.; Wang, G.; Wang, L.; Xia, X. Enhanced performance of LaFeO3 perovskite for peroxymonosulfate activation through strontium doping towards 2,4-D degradation. Chem. Eng. J. 2020, 384, 123377. [Google Scholar] [CrossRef]
- Zhao, L.; Gong, X.; Tao, W.; Wang, T.; Liu, X.; Liu, F.; Yan, X.; Wang, C.; Sun, P.; Lu, G. Homovalent cationic Se dopant with noble metal-like activation properties to enhance the sensitivity of SnO2 sensors. Sens. Actuators B 2023, 378, 133114. [Google Scholar] [CrossRef]
- Yadav, R.; Modi, A.; Dubey, K.; Malik, M.M. Optical and photoluminescence response driven by sintering temperature in LaFeO3 nano-perovskites. J. Mater. Sci. Mater. Electron. 2023, 34, 1243. [Google Scholar] [CrossRef]
- Prasad, P.; Sivanandan, V.T.; Prasad, A.S. Room Temperature Ferromagnetic Behavior of Nanocrystalline LaFeO3 Perovskite Oxide Synthesized through Phytochemical Mediated Combustion Technique. J. Cluster Sci. 2023, 35, 929–940. [Google Scholar] [CrossRef]
- Manchala, S.; Gandamalla, A.; Vempuluru, N.R.; Muthukonda Venkatakrishnan, S.; Shanker, V. High potential and robust ternary LaFeO3/CdS/carbon quantum dots nanocomposite for photocatalytic H2 evolution under sunlight illumination. J. Colloid Interface Sci. 2021, 583, 255–266. [Google Scholar] [CrossRef]
- Xiao, C.; Tang, Z.; Ma, Z.; Gao, X.; Wang, H.; Jia, L. High performance porous LaFeO3 gas sensor with embedded p-n junctions enabling ppb-level formaldehyde detection. Sens. Actuators B 2023, 397, 134670. [Google Scholar] [CrossRef]
- Yang, K.; Ma, J.Z.; Qiao, X.K.; Cui, Y.W.; Jia, L.C.; Wang, H.Q. Hierarchical porous LaFeO3 nanostructure for efficient trace detection of formaldehyde. Sens. Actuators B 2020, 313, 128022. [Google Scholar] [CrossRef]
- Lou, C.; Huang, Q.; Li, Z.; Lei, G.; Liu, X.; Zhang, J. Fe2O3-sensitized SnO2 nanosheets via atomic layer deposition for sensitive formaldehyde detection. Sens. Actuators B 2021, 345, 130429. [Google Scholar] [CrossRef]
- Xiao, C.; Zhang, X.; Ma, Z.; Yang, K.; Gao, X.; Wang, H.; Jia, L. Formaldehyde gas sensor with 1 ppb detection limit based on In-doped LaFeO3 porous structure. Sens. Actuators B 2022, 371, 132558. [Google Scholar] [CrossRef]
- Sui, C.; Zhang, M.; Li, Y.; Wang, Y.; Liu, Y.; Liu, Z.; Bai, J.; Liu, F.; Lu, G. Pd@Pt Core–Shell Nanocrystal-Decorated ZnO Nanosheets for ppt-Level NO2 Detection. ACS Sens. 2024, 9, 1967–1977. [Google Scholar] [CrossRef] [PubMed]
- Lv, S.-S.; Jiang, C.-N.; Jiao, S.-Q.; Wu, Z.-Y.; Huang, Y.-T.; Jin, X.-J.; Zhang, C.; Zheng, X.-H. Improved performance of triethylamine sensors through defect formation in La-doped MoO3 nanorods. Sens. Actuators A Phys. 2024, 366, 114975. [Google Scholar] [CrossRef]
- Wang, J.; Ni, Y.; Li, X.; Jiang, X.; Jin, Q.; Zhang, X.; Wei, S.; Jin, H.; Yuan, T.; Zou, J.; et al. Enhancement of nitric oxide sensing performance via oxygen vacancy promotion on strontium-doped LaFeO3 perovskites. Sens. Actuators B 2024, 417, 136157. [Google Scholar] [CrossRef]
- Al Hashem, M.; Akbar, S.; Morris, P. Role of oxygen vacancies in nanostructured metal-oxide gas sensors: A review. Sens. Actuators B 2019, 301, 126845. [Google Scholar] [CrossRef]
- Qiao, X.; Xu, Y.; Yang, K.; Ma, J.; Li, C.; Wang, H.; Jia, L. Mo doped BiVO4 gas sensor with high sensitivity and selectivity towards H2S. Chem. Eng. J. 2020, 395, 125144. [Google Scholar] [CrossRef]
- Wang, T.; Liu, T.; Li, W.; Hao, X.; Lu, Q.; Yu, H.; Liang, X.; Liu, F.; Liu, F.; Wang, C.; et al. Triethylamine sensing with a mixed potential sensor based on Ce0.8Gd0.2O1.95 solid electrolyte and La1−xSrxMnO3 (x = 0.1, 0.2, 0.3) sensing electrodes. Sens. Actuators B 2021, 327, 128830. [Google Scholar] [CrossRef]
- Cao, J.; Zhang, N.R.; Wang, S.M.; Zhang, H.M. Electronic structure-dependent formaldehyde gas sensing performance of the In2O3/Co3O4 core/shell hierarchical heterostructure sensors. J. Colloid Interface Sci. 2020, 577, 19–28. [Google Scholar] [CrossRef]
- Zhang, S.; Song, P.; Li, J.; Zhang, J.; Yang, Z.; Wang, Q. Facile approach to prepare hierarchical Au-loaded In2O3 porous nanocubes and their enhanced sensing performance towards formaldehyde. Sens. Actuators B 2017, 241, 1130–1138. [Google Scholar] [CrossRef]
- Li, Y.; Chen, N.; Deng, D.; Xing, X.; Xiao, X.; Wang, Y. Formaldehyde detection: SnO2 microspheres for formaldehyde gas sensor with high sensitivity, fast response/recovery and good selectivity. Sens. Actuators B 2017, 238, 264–273. [Google Scholar] [CrossRef]
- Wang, X.S.; Zhang, J.B.; Wang, L.Y.; Li, S.C.; Liu, L.; Su, C.; Liu, L.L. High response gas sensors for formaldehyde based on Er-doped In2O3 nanotubes. J. Mater. Sci. Technol. 2015, 31, 1175–1180. [Google Scholar] [CrossRef]
- Wei, W.; Guo, S.; Chen, C.; Sun, L.; Chen, Y.; Guo, W.; Ruan, S. High sensitive and fast formaldehyde gas sensor based on Ag-doped LaFeO3 nanofibers. J. Alloys Compd. 2017, 695, 1122–1127. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhu, L.; Zhang, J.; Wang, J.; Liu, J.; Zhao, W.; Yan, W. Efficient Formaldehyde Gas Sensing Performance via Promotion of Oxygen Vacancy on In-Doped LaFeO3 Nanofibers. Nanomaterials 2024, 14, 1595. https://doi.org/10.3390/nano14191595
Zhu L, Zhang J, Wang J, Liu J, Zhao W, Yan W. Efficient Formaldehyde Gas Sensing Performance via Promotion of Oxygen Vacancy on In-Doped LaFeO3 Nanofibers. Nanomaterials. 2024; 14(19):1595. https://doi.org/10.3390/nano14191595
Chicago/Turabian StyleZhu, Lei, Jiaxin Zhang, Jianan Wang, Jianwei Liu, Wei Zhao, and Wei Yan. 2024. "Efficient Formaldehyde Gas Sensing Performance via Promotion of Oxygen Vacancy on In-Doped LaFeO3 Nanofibers" Nanomaterials 14, no. 19: 1595. https://doi.org/10.3390/nano14191595




