Förster Resonance Energy Transfer and Enhanced Emission in Cs4PbBr6 Nanocrystals Encapsulated in Silicon Nano-Sheets for Perovskite Light Emitting Diode Applications
Abstract
:1. Introduction
2. Materials and Methods
3. Results
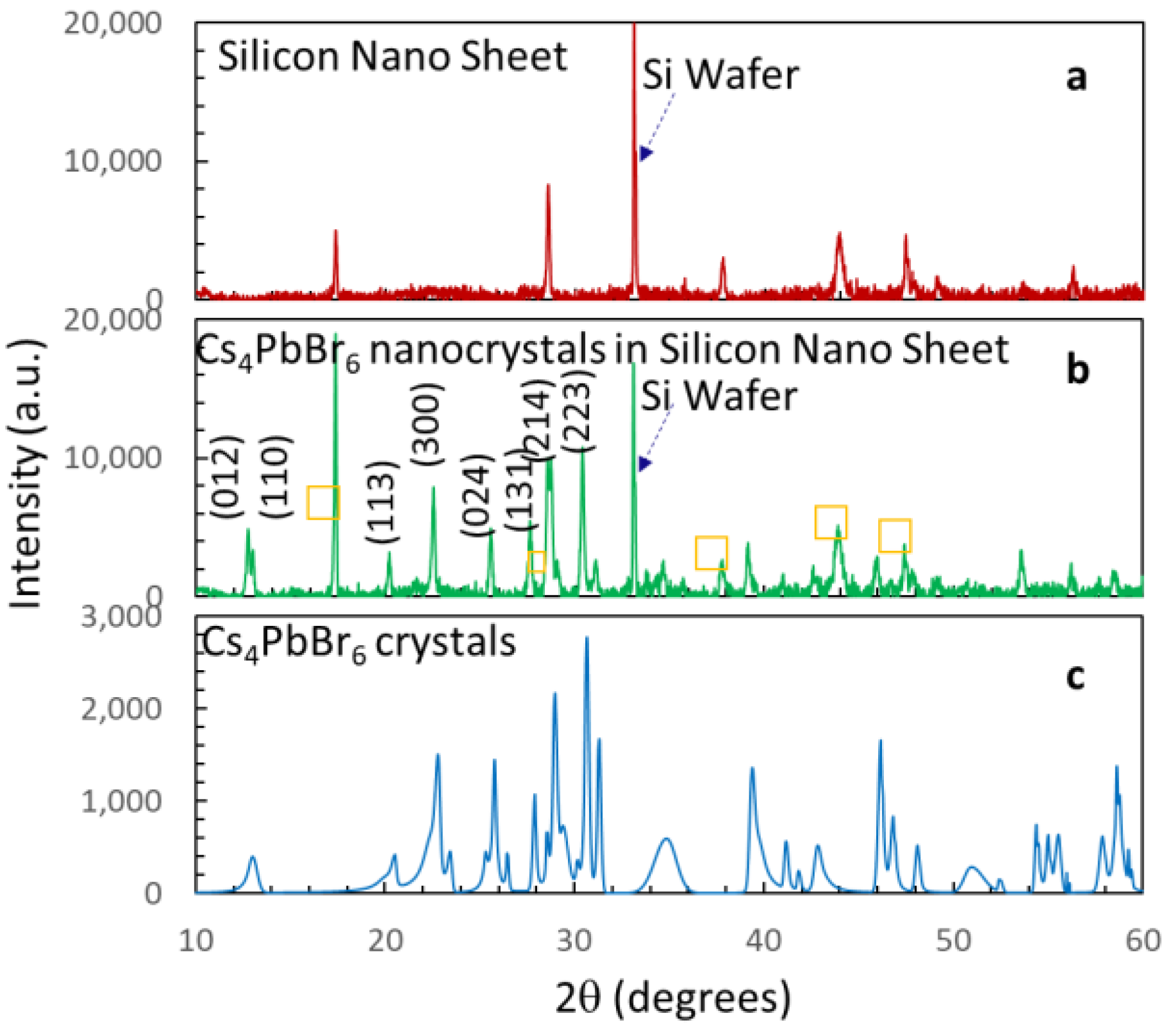
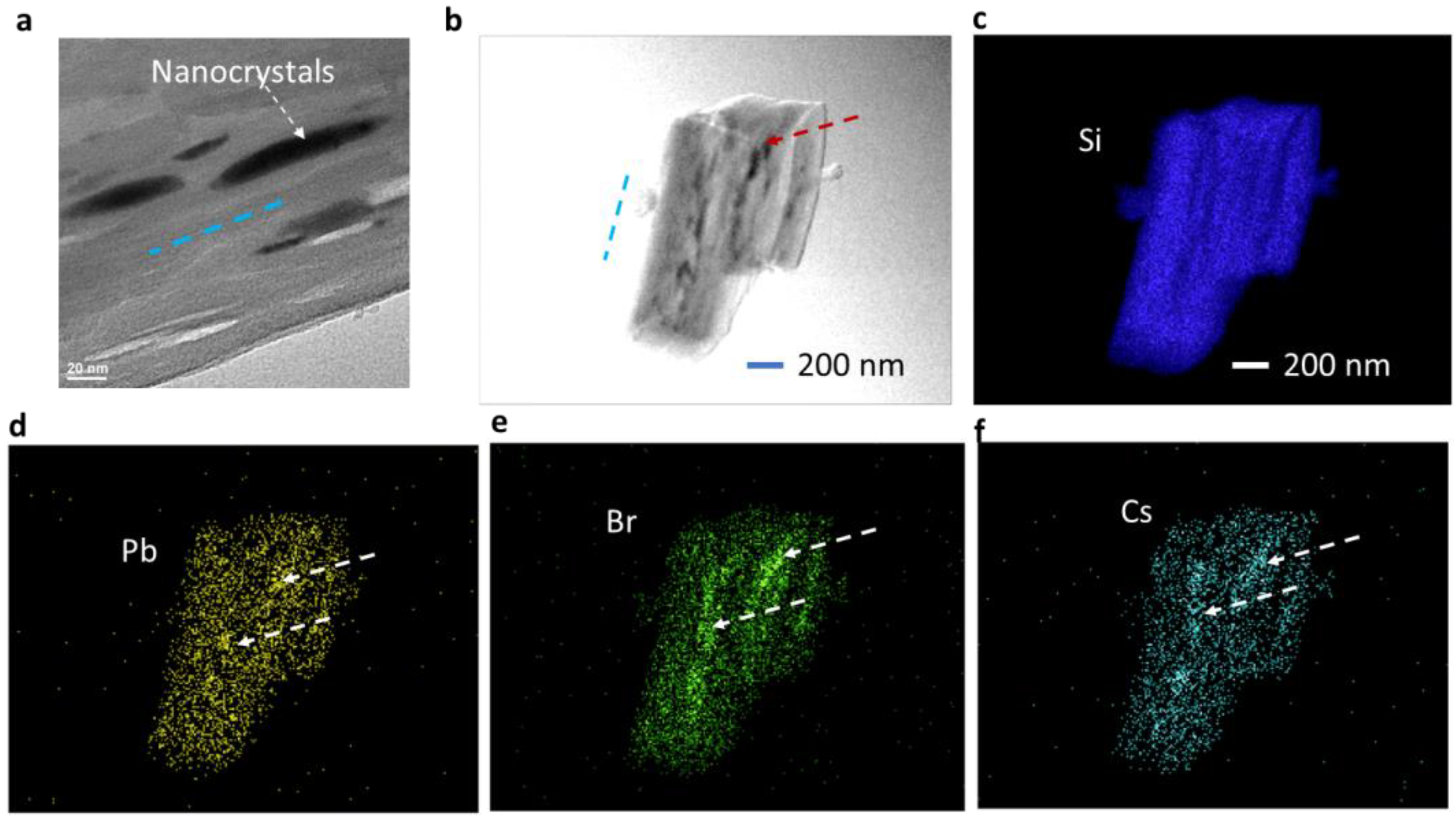
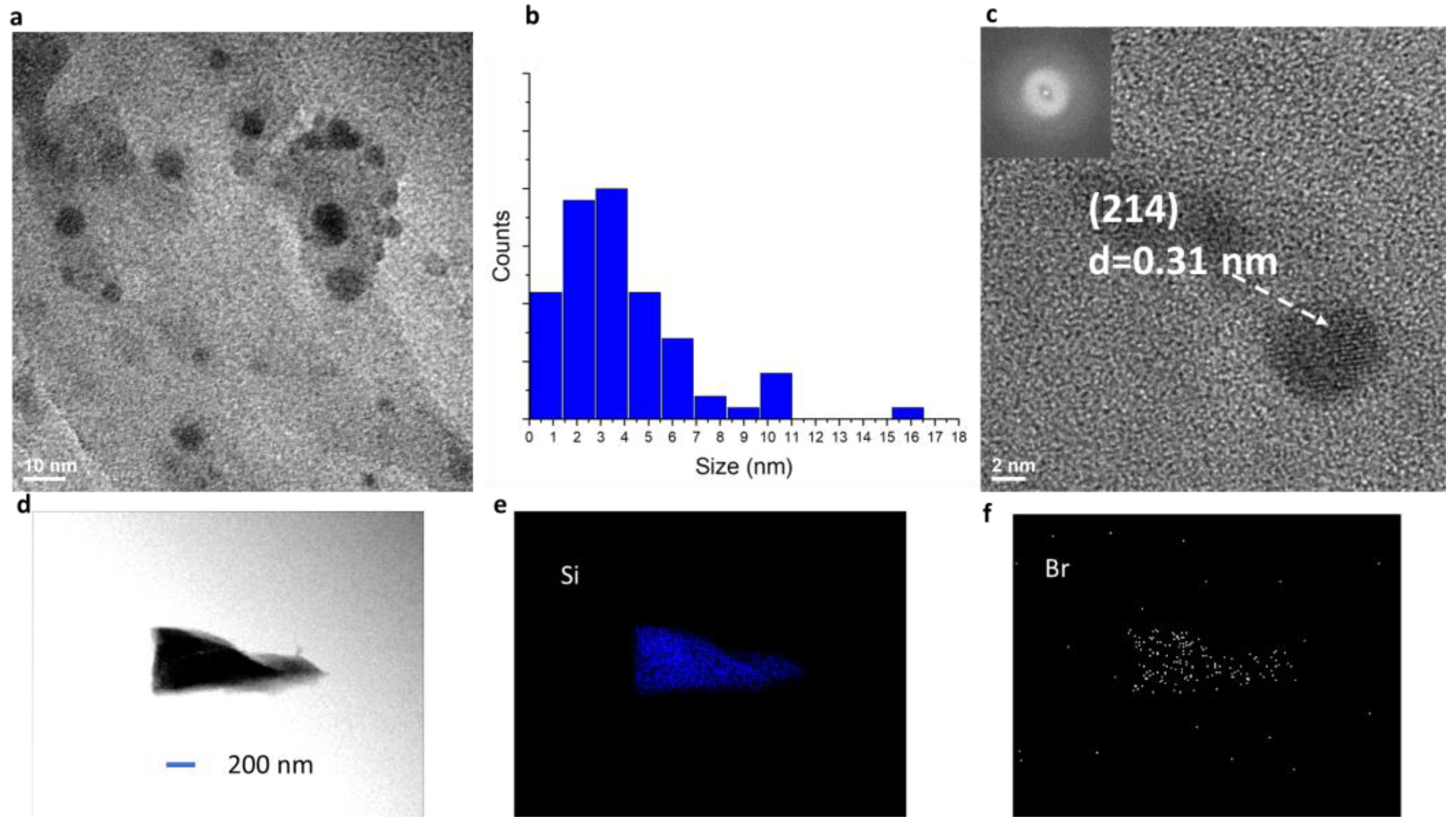
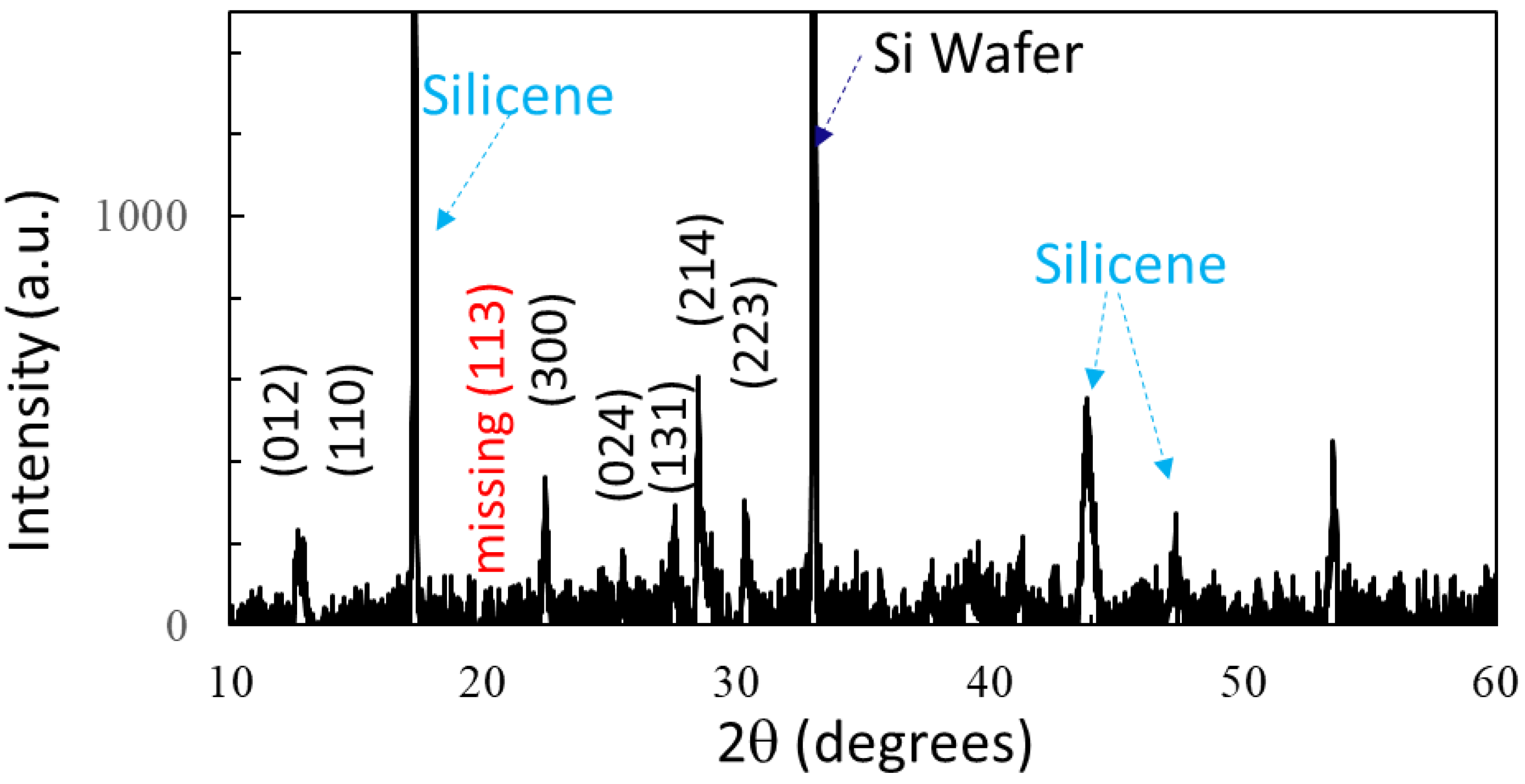
3.1. Material and Structural Characteristics
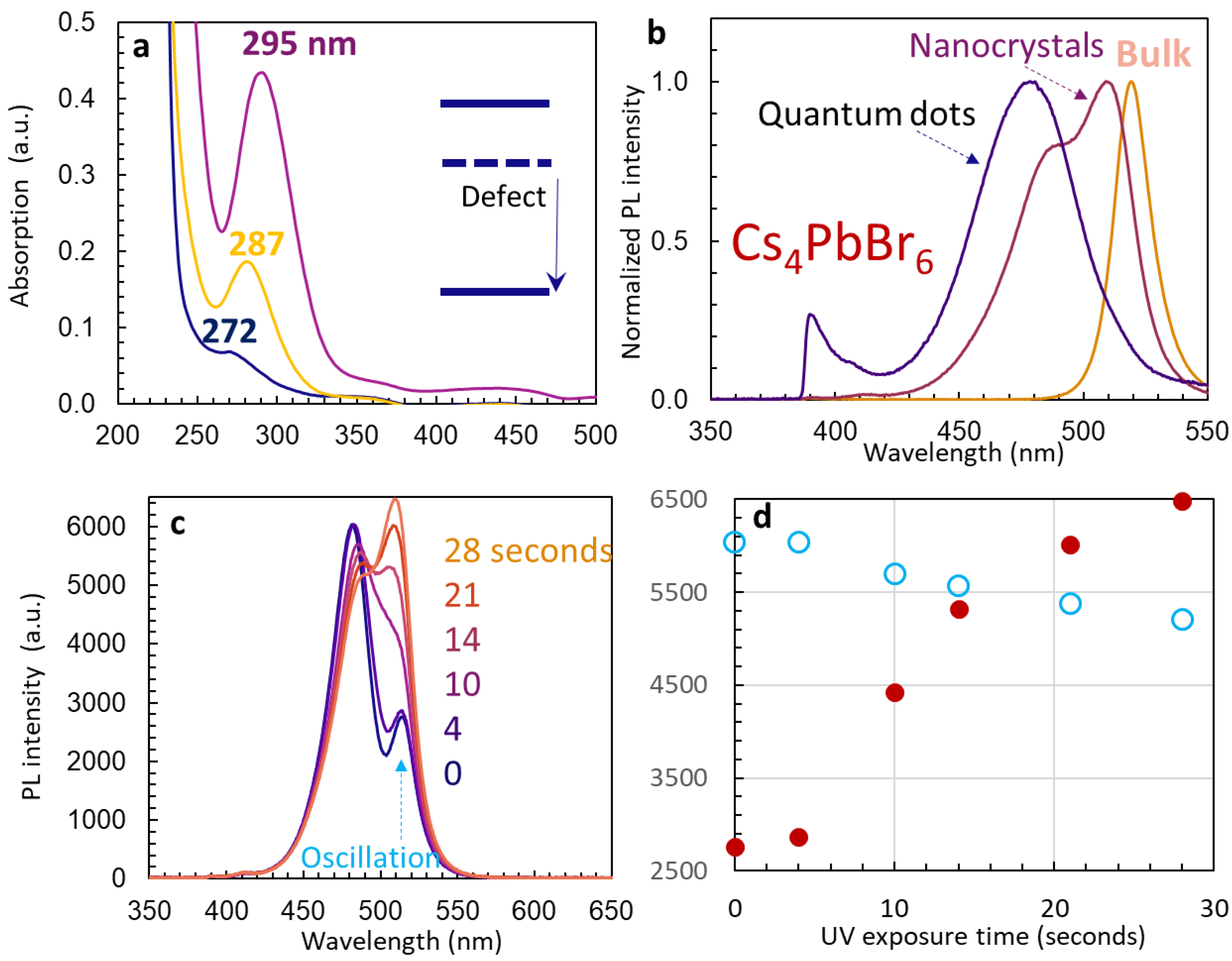
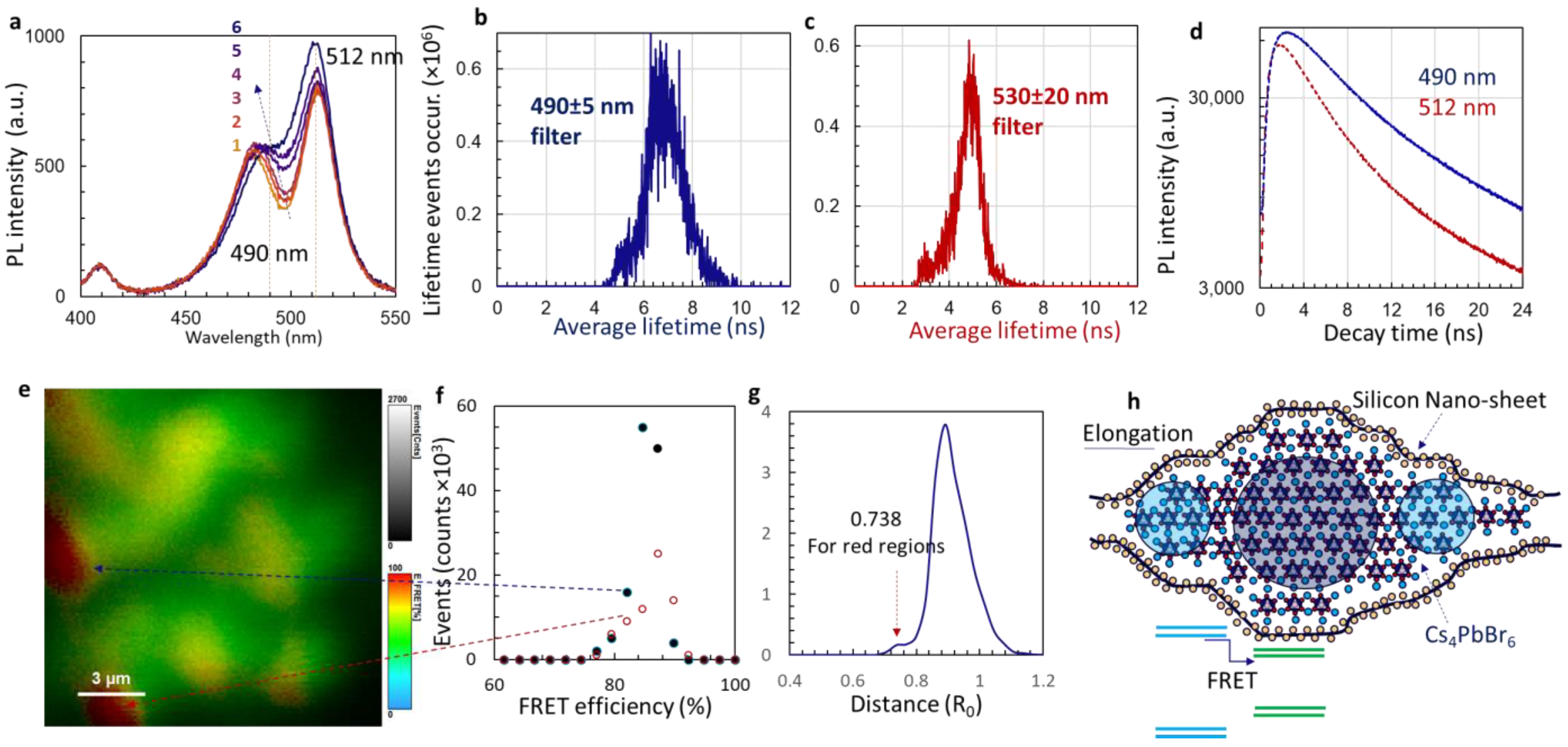
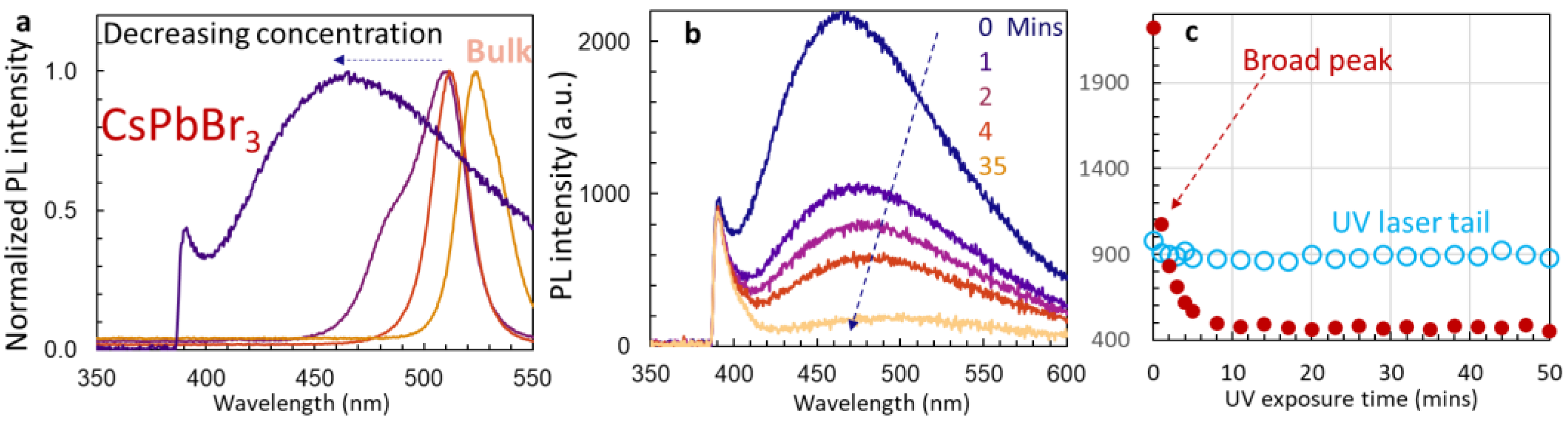
3.2. PL Spectra and Förster Resonance Energy Transfer
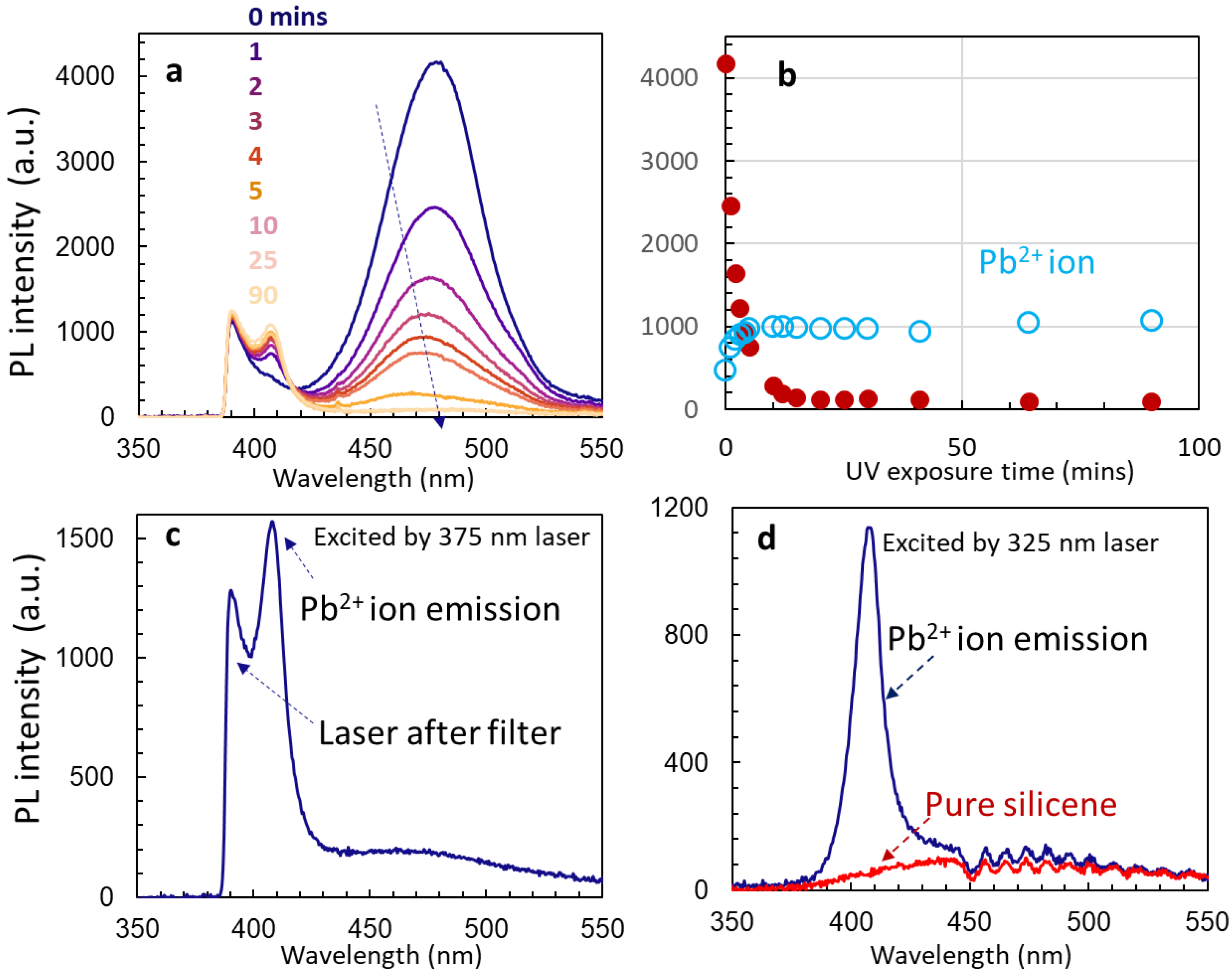
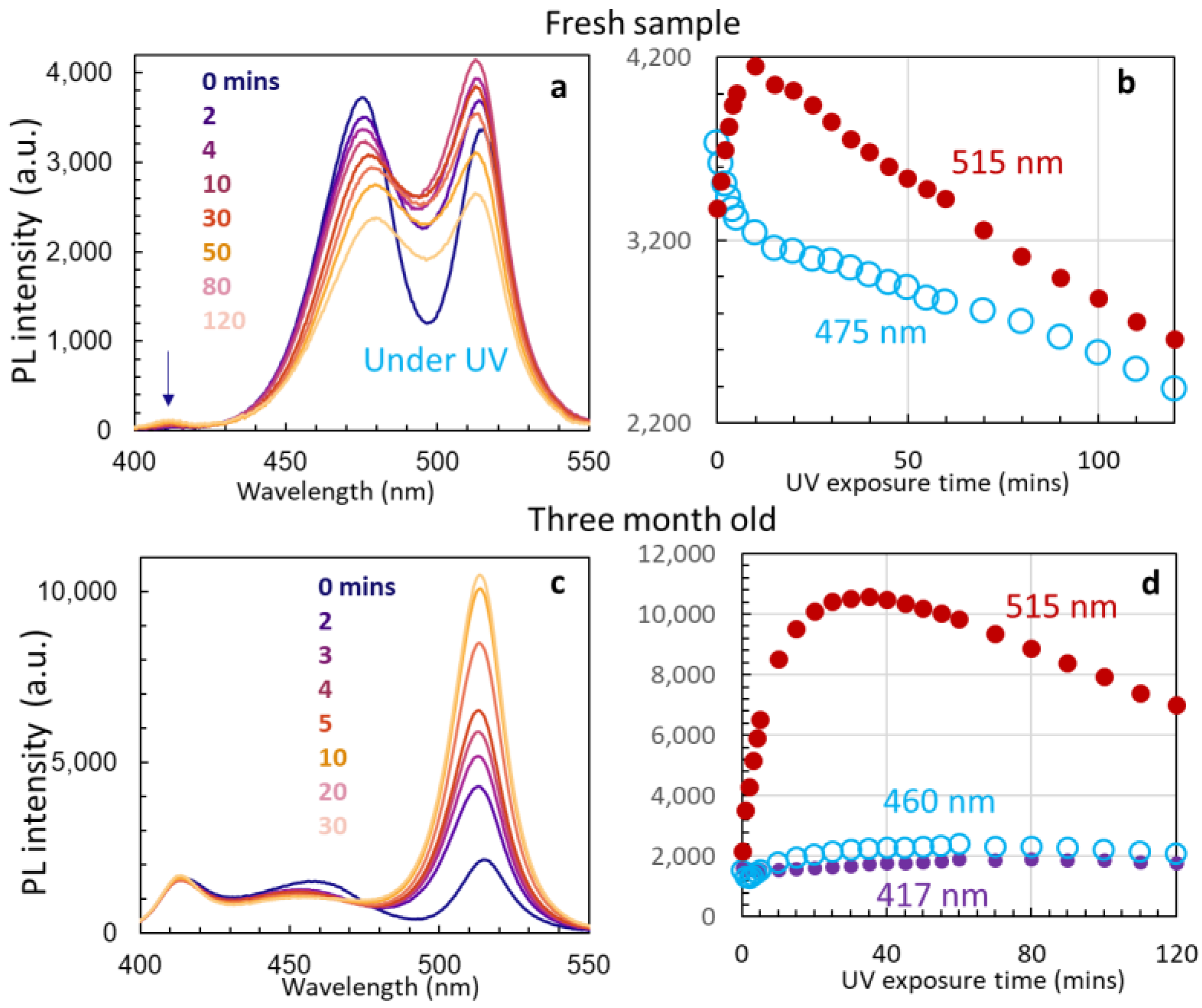
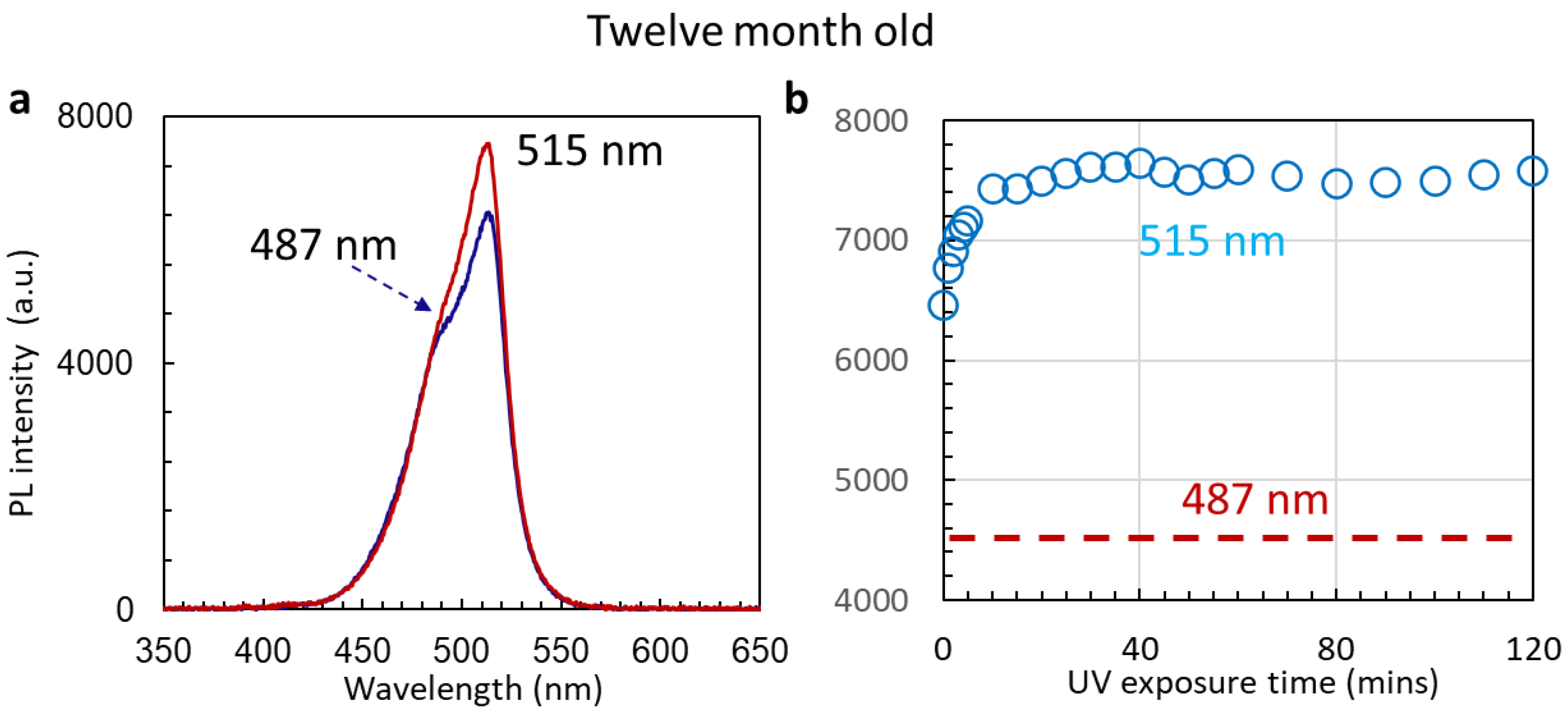
3.3. Stability of Un-Passivated Cs4PbBr6 and Enhanced PL Emissions
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Xing, G.; Mathews, N.; Lim, S.S.; Yantara, N.; Liu, X.; Sabba, D.; Grätzel, M.; Mhaisalkar, S.; Sum, T.C. Low-Temperature Solution-Processed Wavelength-Tunable Perovskites for Lasing. Nat. Mater. 2014, 13, 476–480. [Google Scholar] [CrossRef] [PubMed]
- Tan, H.; Jain, A.; Voznyy, O.; Lan, X.; García de Arquer, F.P.; Fan, J.Z.; Quintero-Bermudez, R.; Yuan, M.; Zhang, B.; Zhao, Y.; et al. Efficient and Stable Solution-Processed Planar Perovskite Solar Cells via Contact Passivation. Science 2017, 355, 722–726. [Google Scholar] [CrossRef] [PubMed]
- Nie, W.; Tsai, H.; Asadpour, R.; Blancon, J.-C.; Neukirch, A.J.; Gupta, G.; Crochet, J.J.; Chhowalla, M.; Tretiak, S.; Alam, M.A.; et al. High-Efficiency Solution-Processed Perovskite Solar Cells with Millimeter-Scale Grains. Science 2015, 347, 522–525. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Hu, S.; Guo, M.; Ren, Y.; Wei, L.; Li, W.; Lin, F.; Yang, Z.; Yang, Z.; Liu, C.; et al. Manipulation of Charge Dynamics for Efficient and Bright Blue Perovskite Light-Emitting Diodes with Chiral Ligands. Adv. Mater. 2023, 35, 2302059. [Google Scholar] [CrossRef]
- Finkenauer, B.P.; Akriti; Ma, K.; Dou, L. Degradation and Self-Healing in Perovskite Solar Cells. ACS Appl. Mater. Interfaces 2022, 14, 24073–24088. [Google Scholar] [CrossRef]
- Jiao, H.; Ni, Z.; Shi, Z.; Fei, C.; Liu, Y.; Dai, X.; Huang, J. Perovskite Grain Wrapping by Converting Interfaces and Grain Boundaries into Robust and Water-Insoluble Low-Dimensional Perovskites. Sci. Adv. 2022, 8, abq4524. [Google Scholar] [CrossRef]
- Chen, S.; Dai, X.; Xu, S.; Jiao, H.; Zhao, L.; Huang, J. Stabilizing Perovskite-Substrate Interfaces for High-Performance Perovskite Modules. Science 2021, 373, 902–907. [Google Scholar] [CrossRef]
- Gonzalez-Rodriguez, R.; Hathaway, E.; Lin, Y.; Coffer, J.L.; Cui, J. Encapsulated MAPbBr3 in Nickel Oxide Nanotubes and Their Electroluminescence. Nanoscale 2022, 14, 6417–6424. [Google Scholar] [CrossRef]
- Abdi-Jalebi, M.; Andaji-Garmaroudi, Z.; Cacovich, S.; Stavrakas, C.; Philippe, B.; Richter, J.M.; Alsari, M.; Booker, E.P.; Hutter, E.M.; Pearson, A.J.; et al. Maximizing and Stabilizing Luminescence from Halide Perovskites with Potassium Passivation. Nature 2018, 555, 497–501. [Google Scholar] [CrossRef]
- Xiong, S.; Hou, Z.; Zou, S.; Lu, X.; Yang, J.; Hao, T.; Zhou, Z.; Xu, J.; Zeng, Y.; Xiao, W.; et al. Direct Observation on P- to n-Type Transformation of Perovskite Surface Region during Defect Passivation Driving High Photovoltaic Efficiency. Joule 2021, 5, 467–480. [Google Scholar] [CrossRef]
- Fang, H.-H.; Yang, J.; Tao, S.; Adjokatse, S.; Kamminga, M.E.; Ye, J.; Blake, G.R.; Even, J.; Loi, M.A. Unravelling Light-Induced Degradation of Layered Perovskite Crystals and Design of Efficient Encapsulation for Improved Photostability. Adv. Funct. Mater. 2018, 28, 1800305. [Google Scholar] [CrossRef]
- Raja, S.N.; Bekenstein, Y.; Koc, M.A.; Fischer, S.; Zhang, D.; Lin, L.; Ritchie, R.O.; Yang, P.; Alivisatos, A.P. Encapsulation of Perovskite Nanocrystals into Macroscale Polymer Matrices: Enhanced Stability and Polarization. ACS Appl. Mater. Interfaces 2016, 8, 35523–35533. [Google Scholar] [CrossRef] [PubMed]
- Konidakis, I.; Karagiannaki, A.; Stratakis, E. Advanced Composite Glasses with Metallic, Perovskite, and Two-Dimensional Nanocrystals for Optoelectronic and Photonic Applications. Nanoscale 2022, 14, 2966–2989. [Google Scholar] [CrossRef] [PubMed]
- He, H.; Cui, Y.; Li, B.; Wang, B.; Jin, C.; Yu, J.; Yao, L.; Yang, Y.; Chen, B.; Qian, G. Confinement of Perovskite-QDs within a Single MOF Crystal for Significantly Enhanced Multiphoton Excited Luminescence. Adv. Mater. 2019, 31, 1806897. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.; Fan, X.; Yang, X.; Zheng, X.; Huang, W.; Shangguan, Z.; Wang, Y.; Kuo, H.; Wu, T.; Chen, Z. Remarkable Black-Phase Robustness of CsPbI3 Nanocrystals Sealed in Solid SiO2/AlOx Sub-Micron Particles. Small 2021, 17, 2103510. [Google Scholar] [CrossRef]
- Park, S.M.; Wei, M.; Xu, J.; Atapattu, H.R.; Eickemeyer, F.T.; Darabi, K.; Grater, L.; Yang, Y.; Liu, C.; Teale, S.; et al. Engineering Ligand Reactivity Enables High-Temperature Operation of Stable Perovskite Solar Cells. Science 2023, 381, 209–215. [Google Scholar] [CrossRef]
- Fan, X.; Wang, S.; Yang, X.; Zhong, C.; Chen, G.; Yu, C.; Chen, Y.; Wu, T.; Kuo, H.; Lin, Y.; et al. Brightened Bicomponent Perovskite Nanocomposite Based on Förster Resonance Energy Transfer for Micro-LED Displays. Adv. Mater. 2023, 35, 2300834. [Google Scholar] [CrossRef]
- Rabouw, F.T.; den Hartog, S.A.; Senden, T.; Meijerink, A. Photonic Effects on the Förster Resonance Energy Transfer Efficiency. Nat. Commun. 2014, 5, 3610. [Google Scholar] [CrossRef]
- Zhang, B.; Lyu, G.; Kelly, E.A.; Evans, R.C. Förster Resonance Energy Transfer in Luminescent Solar Concentrators. Adv. Sci. 2022, 9, 2201160. [Google Scholar] [CrossRef]
- Mishra, L.; Behera, R.K.; Panigrahi, A.; Sarangi, M.K. Förster Resonance Energy Transfer Assisted Enhancement in Optoelectronic Properties of Metal Halide Perovskite Nanocrystals. J. Phys. Chem. Lett. 2022, 13, 4357–4364. [Google Scholar] [CrossRef]
- Song, Y.; Zhang, C.; Liu, W.; Li, X.; Long, H.; Wang, K.; Wang, B.; Lu, P. High-Efficiency Energy Transfer in Perovskite Heterostructures. Opt. Express 2018, 26, 18448. [Google Scholar] [CrossRef] [PubMed]
- Panuganti, S.; Besteiro, L.V.; Vasileiadou, E.S.; Hoffman, J.M.; Govorov, A.O.; Gray, S.K.; Kanatzidis, M.G.; Schaller, R.D. Distance Dependence of Förster Resonance Energy Transfer Rates in 2D Perovskite Quantum Wells via Control of Organic Spacer Length. J. Am. Chem. Soc. 2021, 143, 4244–4252. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Liu, G.; Liu, Q.; Nie, L.; Yao, G.; Zeng, F.; He, Y.; Xiang, W. Ultrastable Zero-Dimensional Cs4PbBr6 Perovskite Quantum Dot Glass. ACS Sustain. Chem. Eng. 2020, 8, 10646–10652. [Google Scholar] [CrossRef]
- Kim, H.; Park, J.H.; Kim, K.; Lee, D.; Song, M.H.; Park, J. Highly Emissive Blue Quantum Dots with Superior Thermal Stability via In Situ Surface Reconstruction of Mixed CsPbBr3–Cs4PbBr6 Nanocrystals. Adv. Sci. 2022, 9, 2104660. [Google Scholar] [CrossRef] [PubMed]
- Leitão, M.F.; Islim, M.S.; Yin, L.; Viola, S.; Watson, S.; Kelly, A.; Dong, Y.; Li, X.; Zeng, H.; Videv, S.; et al. Pump-Power-Dependence of a CsPbBr3-in-Cs4PbBr6 Quantum Dot Color Converter. Opt. Mater. Express 2019, 9, 3504. [Google Scholar] [CrossRef]
- Gonzalez-Rodriguez, R.; Hathaway, E.; Paulette, H.; Coffer, J.L.; Lin, Y.; Cui, J. Two-Dimensional Quantum-Confined CsPbBr3 in Silicene for LED Applications. ACS Appl. Nano Mater. 2023, 6, 4028–4033. [Google Scholar] [CrossRef]
- Saidaminov, M.I.; Almutlaq, J.; Sarmah, S.; Dursun, I.; Zhumekenov, A.A.; Begum, R.; Pan, J.; Cho, N.; Mohammed, O.F.; Bakr, O.M. Pure Cs4PbBr6: Highly Luminescent Zero-Dimensional Perovskite Solids. ACS Energy Lett. 2016, 1, 840–845. [Google Scholar] [CrossRef]
- Gonzalez-Rodriguez, R.; del Castillo, R.M.; Hathaway, E.; Lin, Y.; Coffer, J.L.; Cui, J. Silicene/Silicene Oxide Nanosheets for Broadband Photodetectors. ACS Appl. Nano Mater. 2022, 5, 4325–4335. [Google Scholar] [CrossRef]
- Zhao, H.; Sun, R.; Wang, Z.; Fu, K.; Hu, X.; Zhang, Y. Zero-Dimensional Perovskite Nanocrystals for Efficient Luminescent Solar Concentrators. Adv. Funct. Mater. 2019, 29, 1902262. [Google Scholar] [CrossRef]
- Benin, B.M.; Dirin, D.N.; Morad, V.; Wörle, M.; Yakunin, S.; Rainò, G.; Nazarenko, O.; Fischer, M.; Infante, I.; Kovalenko, M.V. Highly Emissive Self-Trapped Excitons in Fully Inorganic Zero-Dimensional Tin Halides. Angew. Chem. Int. Ed. 2018, 57, 11329–11333. [Google Scholar] [CrossRef]
- Yin, J.; Zhang, Y.; Bruno, A.; Soci, C.; Bakr, O.M.; Brédas, J.-L.; Mohammed, O.F. Intrinsic Lead Ion Emissions in Zero-Dimensional Cs4PbBr6 Nanocrystals. ACS Energy Lett. 2017, 2, 2805–2811. [Google Scholar] [CrossRef]
- Almutlaq, J.; Yin, J.; Mohammed, O.F.; Bakr, O.M. The Benefit and Challenges of Zero-Dimensional Perovskites. J. Phys. Chem. Lett. 2018, 9, 4131–4138. [Google Scholar] [CrossRef] [PubMed]
- Wen, X.; Zhang, P.; Smith, T.A.; Anthony, R.J.; Kortshagen, U.R.; Yu, P.; Feng, Y.; Shrestha, S.; Coniber, G.; Huang, S. Tunability Limit of Photoluminescence in Colloidal Silicon Nanocrystals. Sci. Rep. 2015, 5, 12469. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Lin, X.; Zhou, L.; Sun, X.; Li, R.; Chen, M.; Yang, Y.; Hou, W.; Wu, L.; Cao, W.; et al. Blue Light-Emitting Diodes Based on Colloidal Quantum Dots with Reduced Surface-Bulk Coupling. Nat. Commun. 2023, 14, 284. [Google Scholar] [CrossRef]
- Li, Y.; Shao, W.; Chen, L.; Wang, J.; Nie, J.; Zhang, H.; Zhang, S.; Gao, R.; Ouyang, X.; Ouyang, X.; et al. Lead-Halide Cs4PbBr6 Single Crystals for High-Sensitivity Radiation Detection. NPG Asia Mater. 2021, 13, 40. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mondragon, A.H.; Gonzalez Rodriguez, R.; Hurley, N.; Varghese, S.; Jiang, Y.; Squires, B.; Cheng, M.; Davis, B.; Jiang, Q.; Mortazavi, M.; et al. Förster Resonance Energy Transfer and Enhanced Emission in Cs4PbBr6 Nanocrystals Encapsulated in Silicon Nano-Sheets for Perovskite Light Emitting Diode Applications. Nanomaterials 2024, 14, 1596. https://doi.org/10.3390/nano14191596
Mondragon AH, Gonzalez Rodriguez R, Hurley N, Varghese S, Jiang Y, Squires B, Cheng M, Davis B, Jiang Q, Mortazavi M, et al. Förster Resonance Energy Transfer and Enhanced Emission in Cs4PbBr6 Nanocrystals Encapsulated in Silicon Nano-Sheets for Perovskite Light Emitting Diode Applications. Nanomaterials. 2024; 14(19):1596. https://doi.org/10.3390/nano14191596
Chicago/Turabian StyleMondragon, Araceli Herrera, Roberto Gonzalez Rodriguez, Noah Hurley, Sinto Varghese, Yan Jiang, Brian Squires, Maoding Cheng, Brooke Davis, Qinglong Jiang, Mansour Mortazavi, and et al. 2024. "Förster Resonance Energy Transfer and Enhanced Emission in Cs4PbBr6 Nanocrystals Encapsulated in Silicon Nano-Sheets for Perovskite Light Emitting Diode Applications" Nanomaterials 14, no. 19: 1596. https://doi.org/10.3390/nano14191596









