Enhancing the Efficacy of Active Pharmaceutical Ingredients in Medicinal Plants through Nanoformulations: A Promising Field
Abstract
:1. Introduction
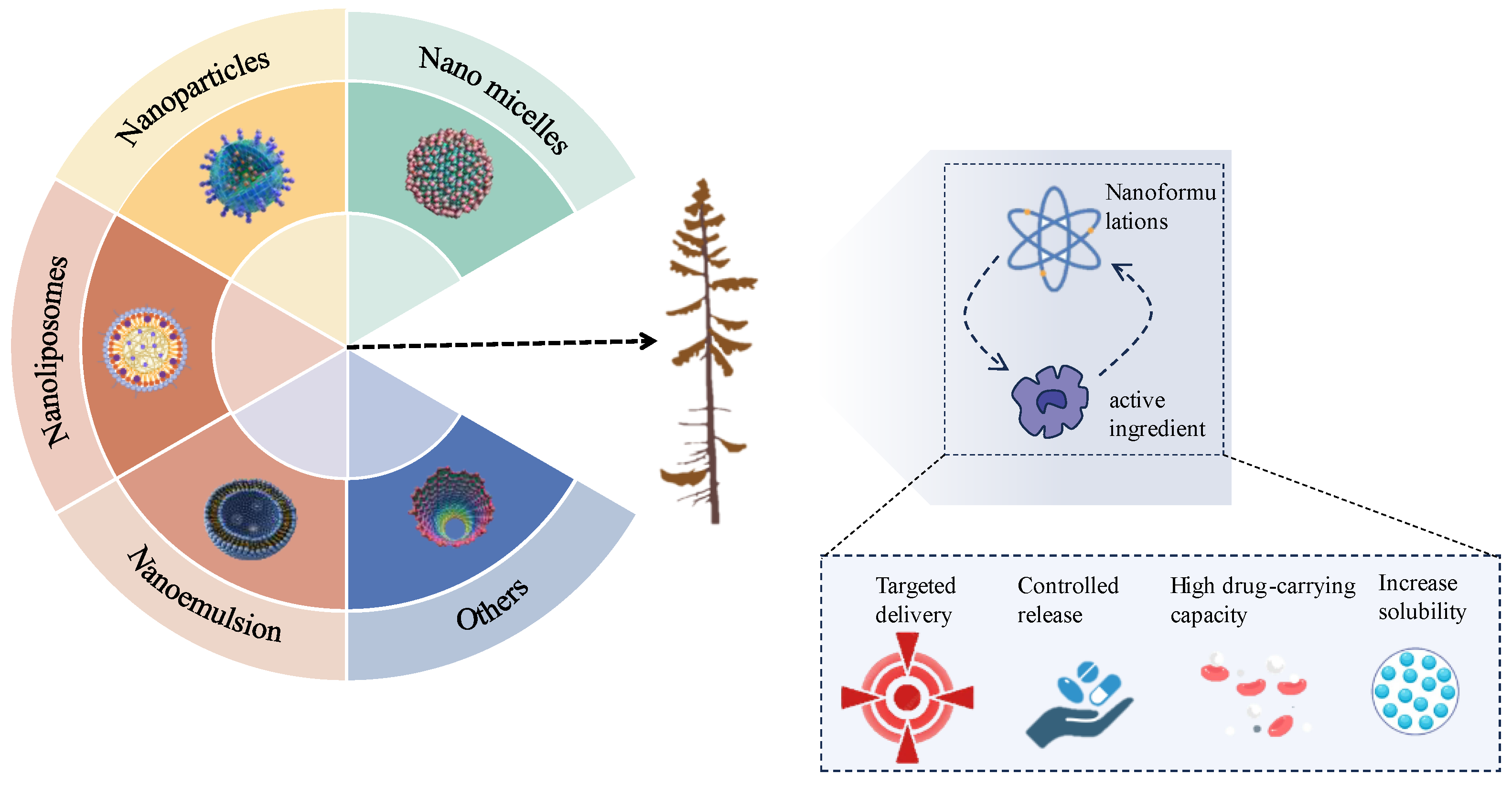
2. Nanoformulations of Medicinal Plant
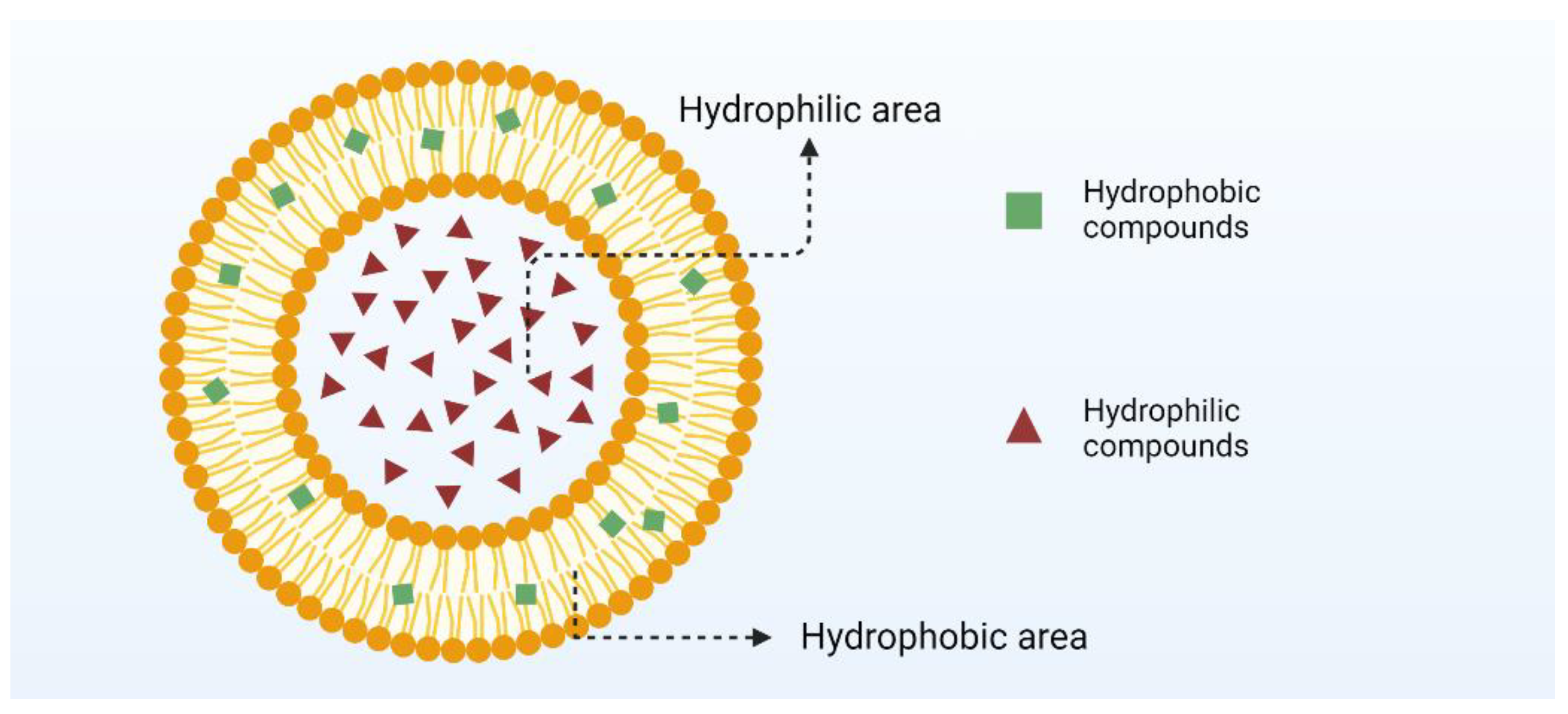
2.1. Nanoliposomes
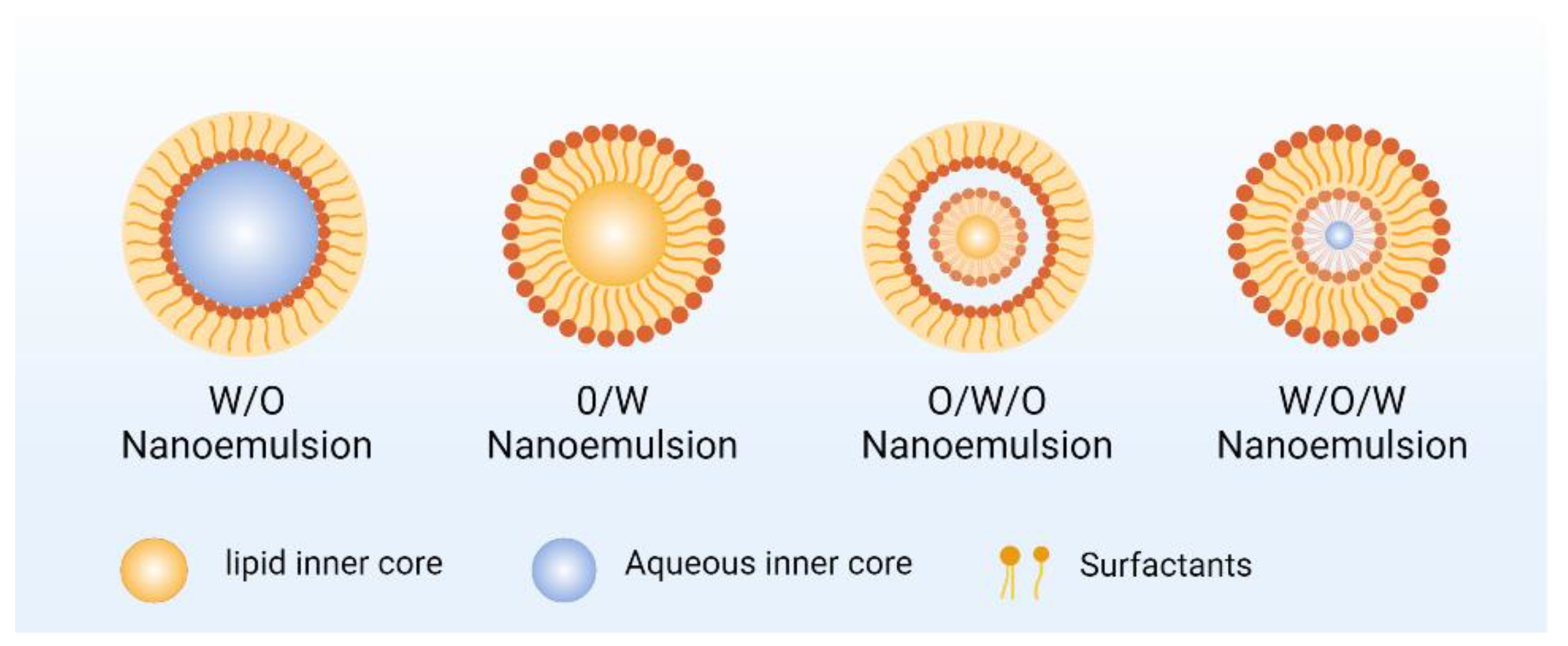
2.2. Nanoemulsion
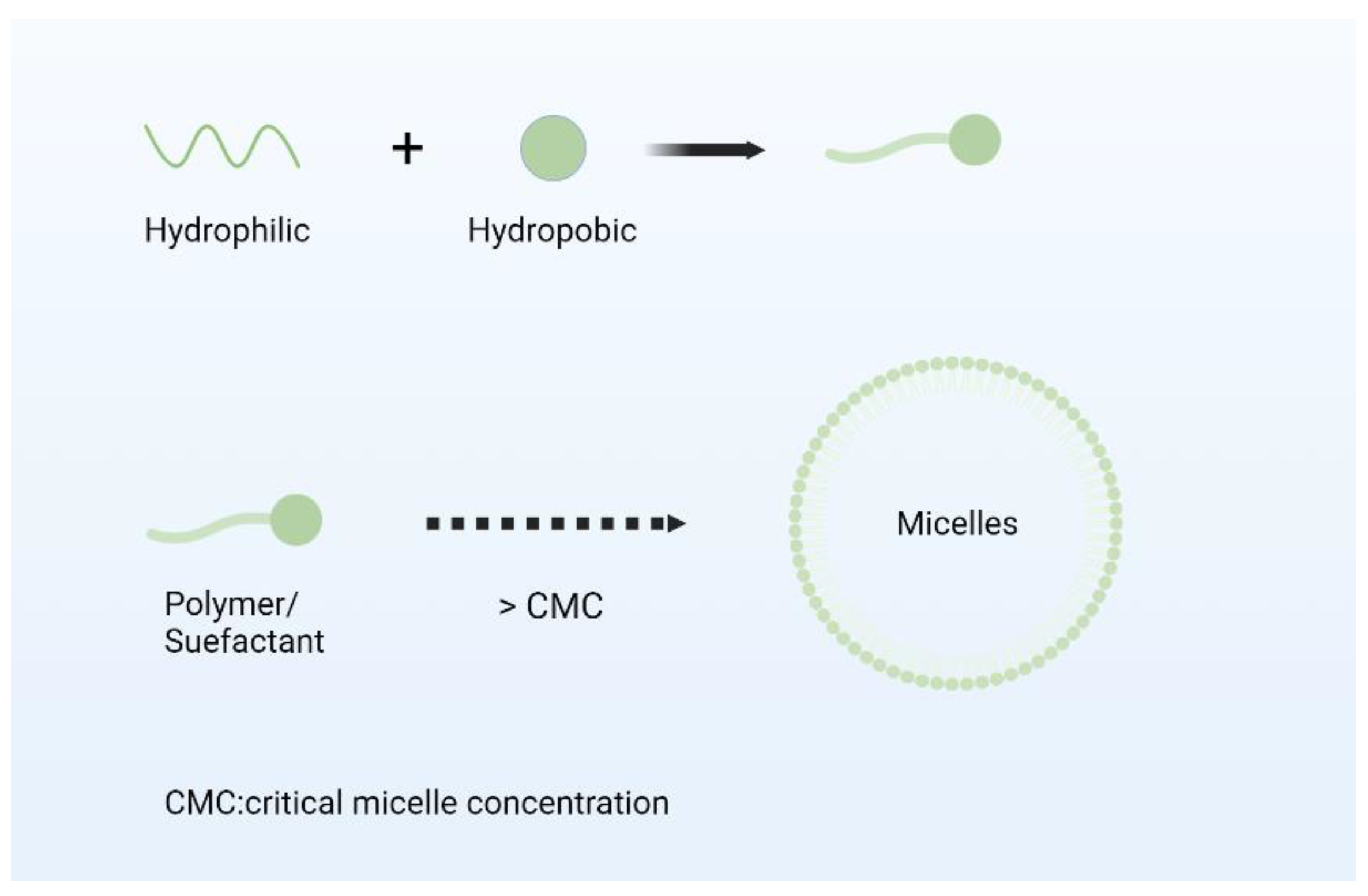
2.3. Nano Micelles
2.4. Nanoparticles
3. Advantages of Nanoformulations in Enhancing the Efficacy of Medicinal Plants
3.1. Improve Bioavailability and Drug Activity
3.2. Trigger Release
3.3. Targeted Action
4. Limitations and Potential Toxicity of Nanoformulations of Medicinal Plants
- (1)
- The particle size and distribution of nanoformulations have an important impact on their performance and biological effects. In large-scale production, it is difficult to ensure that every batch of nanoparticles has a uniform particle size and a narrow particle size distribution. Small fluctuations in the production process, such as changes in temperature, stirring speed, reaction time, etc., can lead to uneven particle size [191]. For example, in the preparation of nanoparticles by emulsion polymerization, agitation that is too fast may result in smaller particle sizes but, at the same time, increase the width of the particle size distribution. Many methods for the preparation of nanoformulations can be successfully implemented in the laboratory, but various problems are encountered when scaling up to industrial-scale production. For example, some nano-preparation methods based on microfluidic technology can accurately control the flow and reaction conditions of fluids in the laboratory to prepare high-quality nano-preparations, but in large-scale production, microfluidic equipment is expensive and complex to operate, making it difficult to achieve industrial application [192,193].
- (2)
- There is still a lot of uncertainty about the long-term toxicity and potential risks of nanomaterials. In vivo, the nanoformulations may interact with biomolecules, cells, and tissues, producing some unexpected biological effects. For example, nanoparticles may accumulate in the body, causing damage to the organs such as the liver and kidneys; or trigger an immune response, leading to adverse reactions such as allergies and inflammation [194,195]. However, due to the relatively short development and application time of nanoformulations, the long-term toxicity and potential risks of nanoformulations have not been sufficiently studied, which poses a great challenge to the safety assessment of regulatory authorities. In addition, the biocompatibility of nanoformulations is also a key issue. Some nanomaterials may interact with the immune system, blood system, etc., affecting the normal physiological functions of organisms. For example, certain nanoparticles may activate the complement system, leading to the formation of immune complexes and inflammatory responses; or bind to proteins in the blood, altering the rheological properties of the blood and increasing the risk of thrombosis [196,197].
- (3)
- The pharmacokinetic behavior of nanoformulations in vivo is very different from that of conventional drugs. Factors such as the size, shape, and surface properties of nanoparticles affect their distribution, metabolism, and excretion in the body. For example, nanoparticles with smaller particle sizes may be more likely to enter the interstitial space through the walls of blood vessels, while nanoparticles with specific ligands on the surface can enable targeted delivery to specific tissues or cells. Traditional medicines are metabolized primarily by hepatic metabolic enzymes. Nanoformulations may be taken up by immune cells such as macrophages, where different metabolic processes occur within the cell. For example, some lipid nanoformulations may be degraded by lysosomes within the cells [46]. However, these unique in vivo behaviors make traditional pharmacokinetic research methods no longer applicable, and new research methods and models need to be developed to accurately assess the pharmacokinetic properties of nanoformulations.
- (4)
- At present, the international regulatory standards for nanoformulations are not uniform, and there are differences in regulatory requirements in different countries and regions. This makes enterprises face great confusion when carrying out the research and development and declaration of nanoformulations and increases the difficulty and cost of listing products in different regions. The established (and in some cases, unwanted regulations) set by drug regulatory authorities are another challenge on the road to new nano dosage forms. Regulations from regulatory agencies such as the NMPA, EMA, and FDA are different from one another, and these regulations are always subject to change on a regular basis. As a result, such regulations can significantly impact the entire process of a clinical trial.
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Wang, K.L.; Chen, Q.; Shao, Y.Y.; Yin, S.S.; Liu, C.Y.; Liu, Y.M.; Wang, R.; Wang, T.; Qiu, Y.L.; Yu, H.Y. Anticancer activities of TCM and their active components against tumor metastasis. Biomed. Pharmacother. 2021, 133, 111044. [Google Scholar] [CrossRef] [PubMed]
- WHO. Selection and Use of Essential Medicines: Report of the WHO Expert Committee on Selection and Use of Essential Medicines, 2019 (including the 21st WHO Model List of Essential Medicines and the 7th WHO Model List of Essential Medicines for Children). In Selection and Use of Essential Medicines: Report of the WHO Expert Committee on Selection and Use of Essential Medicines; WHO: Geneva, Switzerland, 2019. [Google Scholar]
- Tripathy, V.; Basak, B.B.; Varghese, T.S.; Saha, A. Residues and contaminants in medicinal herbs—A review. Phytochem. Lett. 2015, 14, 67–78. [Google Scholar] [CrossRef]
- Theodoridis, S.; Drakou, E.G.; Hickler, T.; Thines, M.; Nogues-Bravo, D. Evaluating natural medicinal resources and their exposure to global change. Lancet Planet. Health 2023, 7, E155–E163. [Google Scholar] [CrossRef] [PubMed]
- Vaou, N.; Stavropoulou, E.; Voidarou, C.; Tsigalou, C.; Bezirtzoglou, E. Towards Advances in Medicinal Plant Antimicrobial Activity: A Review Study on Challenges and Future Perspectives. Microorganisms 2021, 9, 2041. [Google Scholar] [CrossRef]
- Fan, P.H.; Wu, L.W.; Wang, Q.; Wang, Y.; Luo, H.M.; Song, J.Y.; Yang, M.H.; Yao, H.; Chen, S.L. Physiological and molecular mechanisms of medicinal plants in response to cadmium stress: Current status and future perspective. J. Hazard. Mater. 2023, 450, 131008. [Google Scholar] [CrossRef]
- Lv, G.S.; Li, Z.H.; Zhao, Z.Y.; Liu, H.L.; Li, L.; Li, M.H. The factors affecting the development of medicinal plants from a value chain perspective. Planta 2024, 259, 108. [Google Scholar] [CrossRef]
- Shan, Z.J.; Ye, J.F.; Hao, D.C.; Xiao, P.G.; Chen, Z.D.; Lu, A.M. Distribution patterns and industry planning of commonly used traditional Chinese medicinal plants in China. Plant Divers. 2022, 44, 255–261. [Google Scholar] [CrossRef]
- Li, Z.G.; Wang, Y.X.; Xu, M.W.; Liu, H.Y.; Li, L.; Xu, D.L. Molecular mechanism overview of metabolite biosynthesis in medicinal plants. Plant Physiol. Biochem. 2023, 204, 108125. [Google Scholar] [CrossRef]
- Noor, F.; ul Qamar, M.T.; Ashfaq, U.A.; Albutti, A.; Alwashmi, A.S.S.; Aljasir, M.A. Network Pharmacology Approach for Medicinal Plants: Review and Assessment. Pharmaceuticals 2022, 15, 572. [Google Scholar] [CrossRef]
- Shedoeva, A.; Leavesley, D.; Upton, Z.; Fan, C. Wound Healing and the Use of Medicinal Plants. Evid.-Based Complement. Altern. Med. 2019, 2019, 2684108. [Google Scholar] [CrossRef]
- Tomeh, M.A.; Hadianamrei, R.; Zhao, X.B. A Review of Curcumin and Its Derivatives as Anticancer Agents. Int. J. Mol. Sci. 2019, 20, 1033. [Google Scholar] [CrossRef]
- Chen, S.L.; Yu, H.; Luo, H.M.; Wu, Q.; Li, C.F.; Steinmetz, A. Conservation and sustainable use of medicinal plants: Problems, progress, and prospects. Chin. Med. 2016, 11, 37. [Google Scholar] [CrossRef] [PubMed]
- Li, R.L.; Zhou, J.N.; Zhang, X.Y.; Wang, Y.J.; Wang, J.; Zhang, M.; He, C.W.; Zhuang, P.W.; Chen, H.X. Construction of the Gal-NH2/mulberry leaf polysaccharides-lysozyme/ luteolin nanoparticles and the amelioration effects on lipid accumulation. Int. J. Biol. Macromol. 2023, 253, 126780. [Google Scholar] [CrossRef]
- Hu, Y.B.; Li, Y.M.; Cao, Y.; Shen, Y.Z.; Zou, X.J.; Liu, J.X.; Zhao, J. Advancements in enzymatic biotransformation and bioactivities of rare ginsenosides: A review. J. Biotechnol. 2024, 392, 78–89. [Google Scholar] [CrossRef] [PubMed]
- Donthi, M.R.; Munnangi, S.R.; Krishna, K.V.; Saha, R.N.; Singhvi, G.; Dubey, S.K. Nanoemulgel: A Novel Nano Carrier as a Tool for Topical Drug Delivery. Pharmaceutics 2023, 15, 164. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.L.; Dong, X.; Yang, S.C.; Lai, X.; Liu, H.J.; Gao, Y.H.; Feng, H.Y.; Zhu, M.H.; Yuan, Y.H.; Lu, Q.; et al. Biomimetic Liposomal Nanoplatinum for Targeted Cancer Chemophototherapy. Adv. Sci. 2021, 8, 2003679. [Google Scholar] [CrossRef]
- Kong, B.; Liu, R.; Guo, J.H.; Lu, L.; Zhou, Q.; Zhao, Y.J. Tailoring micro/nano-fibers for biomedical applications. Bioact. Mater. 2023, 19, 328–347. [Google Scholar] [CrossRef]
- Reda, F.M.; El-Saadony, M.T.; Elnesr, S.S.; Alagawany, M.; Tufarelli, V. Effect of Dietary Supplementation of Biological Curcumin Nanoparticles on Growth and Carcass Traits, Antioxidant Status, Immunity and Caecal Microbiota of Japanese Quails. Animals 2020, 10, 754. [Google Scholar] [CrossRef]
- Qu, N.; Wang, C.Y.; Meng, Y.M.; Gao, Y.H. Superior Anticancer Potential of Nano-Paclitaxel Combined Bevacizumab Treatment in Ovarian Cancer. Curr. Pharm. Biotechnol. 2023, 24, 1204–1212. [Google Scholar] [CrossRef]
- Bi, F.Y.; Qin, Y.; Chen, D.; Kan, J.; Liu, J. Development of active packaging films based on chitosan and nano-encapsulated luteolin. Int. J. Biol. Macromol. 2021, 182, 545–553. [Google Scholar] [CrossRef]
- Shi, P.Z.; Cheng, Z.R.; Zhao, K.C.; Chen, Y.H.; Zhang, A.R.; Gan, W.K.; Zhang, Y.K. Active targeting schemes for nano-drug delivery systems in osteosarcoma therapeutics. J. Nanobiotechnol. 2023, 21, 103. [Google Scholar] [CrossRef] [PubMed]
- Adepu, S.; Ramakrishna, S. Controlled Drug Delivery Systems: Current Status and Future Directions. Molecules 2021, 26, 5905. [Google Scholar] [CrossRef] [PubMed]
- Bonifácio, B.V.; da Silva, P.B.; Ramos, M.A.D.; Negri, K.M.S.; Bauab, T.M.; Chorilli, M. Nanotechnology-based drug delivery systems and herbal medicines: A review. Int. J. Nanomed. 2014, 9, 1–15. [Google Scholar] [CrossRef]
- Kumar, M.; Keshwania, P.; Chopra, S.; Mahmood, S.; Bhatia, A. Therapeutic Potential of Nanocarrier-Mediated Delivery of Phytoconstituents for Wound Healing: Their Current Status and Future Perspective. Aaps Pharmscitech 2023, 24, 155. [Google Scholar] [CrossRef] [PubMed]
- Bernela, M.; Seth, M.; Kaur, N.; Sharma, S.; Pati, P.K. Harnessing the potential of nanobiotechnology in medicinal plants. Ind. Crops Prod. 2023, 194, 116266. [Google Scholar] [CrossRef]
- Churilov, G.; Ivanycheva, J.; Kiryshin, V. Influence of Nano-Crystal Metals on Texture and Biological Properties of Water Soluble Polysaccharides of Medicinal Plants. In Proceedings of the 3rd International Youth Conference on Interdisciplinary Problems of Nanotechnology, Biomedicine and Nanotoxicology (Nanobiotech), Tambov, Russia, 21–22 May 2015. [Google Scholar]
- Barman, A.; Kotal, A.; Das, M. Synthesis of metal based nano particles from Moringa Olifera and its biomedical applications: A review. Inorg. Chem. Commun. 2023, 158, 111438. [Google Scholar] [CrossRef]
- Liu, Y.L.; Yang, Y.; Zhang, Q.R.; Lu, D.H.; Li, S.Y.; Li, J.F.; Yang, G.C.; Shan, Y.P. Dynamics of delivering aptamer targeted nano-drugs into cells. J. Mater. Chem. B 2021, 9, 952–957. [Google Scholar] [CrossRef]
- Rajput, D.; Singh, M.; Sahu, P.; Jain, D.; Kashaw, S.K.; Patil, U.K. Advances in Nanogel as Drug Delivery System for Cancer Therapeutics: An Overview. Mini-Rev. Med. Chem. 2023, 23, 2053–2072. [Google Scholar] [CrossRef]
- Pretorius, D.; Serpooshan, V.; Zhang, J.Y. Nano-Medicine in the Cardiovascular System. Front. Pharmacol. 2021, 12, 640182. [Google Scholar] [CrossRef]
- Zou, J.H.; Li, M.; Liu, Z.W.; Luo, W.; Han, S.Q.; Xiao, F.; Tao, W.; Wu, Q.B.; Xie, T.; Kong, N. Unleashing the potential: Integrating nano-delivery systems with traditional Chinese medicine. Nanoscale 2024, 16, 8791–8806. [Google Scholar] [CrossRef]
- Lopus, M. Nano-ayurvedic medicine and its potential in cancer treatment. J. Integr. Med. JIM 2023, 21, 117–119. [Google Scholar] [CrossRef]
- Dai, Y.X.; Wang, Y.H.; Yang, Z. Anti-inflammatory effects of traditional chinese medicine ingredients based on nano-Strychnos liposomes for external skin application. Ferroelectrics 2021, 581, 186–199. [Google Scholar] [CrossRef]
- Shang, Q.; Liu, W.D.; Leslie, F.; Yang, J.P.; Guo, M.M.; Sun, M.J.; Zhang, G.J.; Zhang, Q.; Wang, F.H. Nano-formulated delivery of active ingredients from traditional Chinese herbal medicines for cancer immunotherapy. Acta Pharm. Sin. B 2024, 14, 1525–1541. [Google Scholar] [CrossRef] [PubMed]
- Kang, C.L.; Wang, J.W.; Li, R.T.; Gong, J.N.; Wang, K.R.; Wang, Y.X.; Wang, Z.H.; He, R.Z.; Li, F.Y. Smart Targeted Delivery Systems for Enhancing Antitumor Therapy of Active Ingredients in Traditional Chinese Medicine. Molecules 2023, 28, 5955. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.H.; Wang, Y.; Xia, M.Y.; Gao, Y.; Zhang, L.; Song, Y.N.; Zhang, C. The combination of nanotechnology and traditional Chinese medicine (TCM) inspires the modernization of TCM: Review on nanotechnology in TCM-based drug delivery systems. Drug Deliv. Transl. Res. 2022, 12, 1306–1325. [Google Scholar] [CrossRef] [PubMed]
- Faghihi, H.; Mozafari, M.R.; Bumrungpert, A.; Parsaei, H.; Taheri, S.V.; Mardani, P.; Dehkharghani, F.M.; Pudza, M.Y.; Alavi, M. Prospects and challenges of synergistic effect of fluorescent carbon dots, liposomes and nanoliposomes for theragnostic applications. Photodiagn. Photodyn. Ther. 2023, 42, 103614. [Google Scholar] [CrossRef]
- Akhavan, S.; Assadpour, E.; Katouzian, I.; Jafari, S.M. Lipid nano scale cargos for the protection and delivery of food bioactive ingredients and nutraceuticals. Trends Food Sci. Technol. 2018, 74, 132–146. [Google Scholar] [CrossRef]
- Farjadian, F.; Ghasemi, A.; Gohari, O.; Roointan, A.; Karimi, M.; Hamblin, M.R. Nanopharmaceuticals and nanomedicines currently on the market: Challenges and opportunities. Nanomedicine 2019, 14, 93–126. [Google Scholar] [CrossRef]
- Azarashkan, Z.; Farahani, S.; Abedinia, A.; Akbarmivehie, M.; Motamedzadegan, A.; Heidarbeigi, J.; Hayaloglu, A.A. Co-encapsulation of broccoli sprout extract nanoliposomes into basil seed gum: Effects on in vitro antioxidant, antibacterial and anti-Listeria activities in ricotta cheese. Int. J. Food Microbiol. 2022, 376, 109761. [Google Scholar] [CrossRef]
- Ozpolat, B.; Sood, A.K.; Lopez-Berestein, G. Liposomal siRNA nanocarriers for cancer therapy. Adv. Drug Deliv. Rev. 2014, 66, 110–116. [Google Scholar] [CrossRef]
- Ashrafizadeh, M.; Delfi, M.; Zarrabi, A.; Bigham, A.; Sharifi, E.; Rabiee, N.; Paiva-Santos, A.C.; Kumar, A.P.; Tan, S.C.; Hushmandi, K.; et al. Stimuli-responsive liposomal nanoformulations in cancer therapy: Pre-clinical & clinical approaches. J. Control. Release 2022, 351, 50–80. [Google Scholar] [CrossRef] [PubMed]
- Shirane, M. Lipid Transfer-Dependent Endosome Maturation Mediated by Protrudin and PDZD8 in Neurons. Front. Cell Dev. Biol. 2020, 8, 615600. [Google Scholar] [CrossRef] [PubMed]
- Vanic, Z.; Barnert, S.; Süss, R.; Schubert, R. Fusogenic activity of PEGylated pH-sensitive liposomes. J. Liposome Res. 2012, 22, 148–157. [Google Scholar] [CrossRef]
- Hamer, I.; Van Beersel, G.; Arnould, T.; Jadot, M. Lipids and Lysosomes. Curr. Drug Metab. 2012, 13, 1371–1387. [Google Scholar] [CrossRef] [PubMed]
- Albright, J.M.; Sydor, M.J.; Shannahan, J.; Ferreira, C.R.; Holian, A. Imipramine Treatment Alters Sphingomyelin, Cholesterol, and Glycerophospholipid Metabolism in Isolated Macrophage Lysosomes. Biomolecules 2023, 13, 1732. [Google Scholar] [CrossRef]
- Jaishy, B.; Abel, E.D. Lipids, lysosomes, and autophagy. J. Lipid Res. 2016, 57, 1619–1635. [Google Scholar] [CrossRef]
- Kumari, A.; Raina, N.; Wahi, A.; Goh, K.W.; Sharma, P.; Nagpal, R.; Jain, A.; Ming, L.C.; Gupta, M. Wound-Healing Effects of Curcumin and Its Nanoformulations: A Comprehensive Review. Pharmaceutics 2022, 14, 2288. [Google Scholar] [CrossRef]
- Agrawal, M.; Ajazuddin; Tripathi, D.K.; Saraf, S.; Saraf, S.; Antimisiaris, S.G.; Mourtas, S.; Hammarlund-Udenaes, M.; Alexander, A. Recent advancements in liposomes targeting strategies to cross blood-brain barrier (BBB) for the treatment of Alzheimer’s disease. J. Control. Release 2017, 260, 61–77. [Google Scholar] [CrossRef]
- Homayoonfal, M.; Aminianfar, A.; Asemi, Z.; Yousefi, B. Application of Nanoparticles for Efficient Delivery of Quercetin in Cancer Cells. Curr. Med. Chem. 2024, 31, 1107–1141. [Google Scholar] [CrossRef]
- Li, Q.B.; Lv, L.A.; Liang, W.Q.; Chen, Z.B.; Deng, Q.; Sun, L.J.; Wang, Y.L.; Liu, Y. Screening, characterization and mechanism of a potential stabiliser for nisin nanoliposomes with high encapsulation efficiency. Food Chem. 2024, 457, 140185. [Google Scholar] [CrossRef]
- Huang, M.G.; Cong, L.X.; Ying, R.F.; Ahmad, M.; Hao, G.; Hayat, K.; Salamatullah, A.M. Polysaccharide-coated quercetin-loaded nanoliposomes mitigate bitterness: A comparison of carrageenan, pectin, and trehalose. Int. J. Biol. Macromol. 2024, 259, 129410. [Google Scholar] [CrossRef] [PubMed]
- Rai, V.K.; Mishra, N.; Yadav, K.S.; Yadav, N.P. Nanoemulsion as pharmaceutical carrier for dermal and transdermal drug delivery: Formulation development, stability issues, basic considerations and applications. J. Control. Release 2018, 270, 203–225. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.C.; Deng, L.A.; Yang, J.; Yang, G.W.; Fan, H.T.; Yin, Y.Q.; Luo, S.; Li, S.S.; Liu, L.Y.; Yang, M. Preparation and evaluation of a nanoemulsion containing cordycepin and its protective effect on skin. J. Dispers. Sci. Technol. 2023, 44, 1094–1102. [Google Scholar] [CrossRef]
- Deshpande, D.; Kethireddy, S.; Gattacceca, F.; Amiji, M. Comparative pharmacokinetics and tissue distribution analysis of systemically administered 17-β-estradiol and its metabolites in vivo delivered using a cationic nanoemulsion or a peptide-modified nanoemulsion system for targeting atherosclerosis. J. Control. Release 2014, 180, 117–124. [Google Scholar] [CrossRef]
- Choudhary, A.; Jain, P.; Mohapatra, S.; Mustafa, G.; Ansari, M.J.; Aldawsari, M.F.; Alalaiwe, A.S.; Mirza, M.A.; Iqbal, Z. A Novel Approach of Targeting Linezolid Nanoemulsion for the Management of Lymph Node Tuberculosis. ACS Omega 2022, 7, 15688–15694. [Google Scholar] [CrossRef]
- Yang, S.L.; Lin, H.S.; Zhang, L.; Ho, P.C.L. Formulating 10-hydroxycamptothecin into nanoemulsion with functional excipient tributyrin: An innovative strategy for targeted hepatic cancer chemotherapy. Int. J. Pharm. 2024, 654, 123945. [Google Scholar] [CrossRef]
- Gazolu-Rusanova, D.; Lesov, I.; Tcholakova, S.; Denkov, N.; Ahtchi, B. Food grade nanoemulsions preparation by rotor-stator homogenization. Food Hydrocoll. 2020, 102, 105579. [Google Scholar] [CrossRef]
- Sneha, K.; Kumar, A. Nanoemulsions: Techniques for the preparation and the recent advances in their food applications. Innov. Food Sci. Emerg. Technol. 2022, 76, 102914. [Google Scholar] [CrossRef]
- Jaiswal, M.; Dudhe, R.; Sharma, P.K. Nanoemulsion: An advanced mode of drug delivery system. 3 Biotech 2015, 5, 123–127. [Google Scholar] [CrossRef]
- Shehabeldine, A.M.; Doghish, A.S.; El-Dakroury, W.A.; Hassanin, M.M.H.; Al-Askar, A.A.; AbdElgawad, H.; Hashem, A.H. Antimicrobial, Antibiofilm, and Anticancer Activities of Syzygium aromaticum Essential Oil Nanoemulsion. Molecules 2023, 28, 5812. [Google Scholar] [CrossRef]
- Choi, S.J.; McClements, D.J. Nanoemulsions as delivery systems for lipophilic nutraceuticals: Strategies for improving their formulation, stability, functionality and bioavailability. Food Sci. Biotechnol. 2020, 29, 149–168. [Google Scholar] [CrossRef] [PubMed]
- Chu, Y.F.; Gao, C.C.; Liu, X.Y.; Zhang, N.; Xu, T.; Feng, X.; Yang, Y.L.; Shen, X.C.; Tang, X.Z. Improvement of storage quality of strawberries by pullulan coatings incorporated with cinnamon essential oil nanoemulsion. LWT-Food Sci. Technol. 2020, 122, 109054. [Google Scholar] [CrossRef]
- Liu, Y.C.; Zhao, L.T.; Chen, H.Y.; Ye, Z.M.; Guo, L.; Zhou, Z.Q. Nobiletin enhances the antifungal activity of eugenol nanoemulsion against Penicillium italicum in both in vitro and in vivo settings. Int. J. Food Microbiol. 2024, 420, 110769. [Google Scholar] [CrossRef] [PubMed]
- Cagel, M.; Tesan, F.C.; Bernabeu, E.; Salgueiro, M.J.; Zubillaga, M.B.; Moretton, M.A.; Chiappetta, D.A. Polymeric mixed micelles as nanomedicines: Achievements and perspectives. Eur. J. Pharm. Biopharm. 2017, 113, 211–228. [Google Scholar] [CrossRef] [PubMed]
- Avramovic, N.; Mandic, B.; Savic-Radojevic, A.; Simic, T. Polymeric Nanocarriers of Drug Delivery Systems in Cancer Therapy. Pharmaceutics 2020, 12, 298. [Google Scholar] [CrossRef]
- Perumal, S.; Atchudan, R.; Lee, W. A Review of Polymeric Micelles and Their Applications. Polymers 2022, 14, 2510. [Google Scholar] [CrossRef]
- Chen, D.Q.; Wang, G.H.; Song, W.G.; Zhang, Q. Novel CD44 receptor targeting multifunctional “nano-eggs” based on double pH-sensitive nanoparticles for co-delivery of curcumin and paclitaxel to cancer cells and cancer stem cells. J. Nanopart. Res. 2015, 17, 421. [Google Scholar] [CrossRef]
- Delorme, V.; Lichon, L.; Mahindad, H.; Hunger, S.; Laroui, N.; Daurat, M.; Godefroy, A.; Coudane, J.; Gary-Bobo, M.; Van den Berghe, H. Reverse poly(ε-caprolactone)-g-dextran graft copolymers. Nano-carriers for intracellular uptake of anticancer drugs. Carbohydr. Polym. 2020, 232, 115764. [Google Scholar] [CrossRef]
- Cabral, H.; Kataoka, K. Progress of drug-loaded polymeric micelles into clinical studies. J. Control. Release 2014, 190, 465–476. [Google Scholar] [CrossRef]
- Elmowafy, M.; Shalaby, K.; Elkomy, M.H.; Alsaidan, O.A.; Gomaa, H.A.M.; Abdelgawad, M.A.; Mostafa, E.M. Polymeric Nanoparticles for Delivery of Natural Bioactive Agents: Recent Advances and Challenges. Polymers 2023, 15, 1123. [Google Scholar] [CrossRef]
- Muhamad, N.; Plengsuriyakarn, T.; Na-Bangchang, K. Application of active targeting nanoparticle delivery system for chemotherapeutic drugs and traditional/herbal medicines in cancer therapy: A systematic review. Int. J. Nanomed. 2018, 13, 3921–3935. [Google Scholar] [CrossRef] [PubMed]
- Joseph, T.M.; Mahapatra, D.K.; Esmaeili, A.; Piszczyk, L.; Hasanin, M.S.; Kattali, M.; Haponiuk, J.; Thomas, S. Nanoparticles: Taking a Unique Position in Medicine. Nanomaterials 2023, 13, 574. [Google Scholar] [CrossRef] [PubMed]
- Yazdanian, M.; Rostamzadeh, P.; Rahbar, M.; Alam, M.; Abbasi, K.; Tahmasebi, E.; Tebyaniyan, H.; Ranjbar, R.; Seifalian, A.; Yazdanian, A. The Potential Application of Green-Synthesized Metal Nanoparticles in Dentistry: A Comprehensive Review. Bioinorg. Chem. Appl. 2022, 2022, 2311910. [Google Scholar] [CrossRef] [PubMed]
- Khan, Y.; Sadia, H.; Shah, S.Z.A.; Khan, M.N.; Shah, A.A.; Ullah, N.; Ullah, M.F.; Bibi, H.; Bafakeeh, O.T.; Ben Khedher, N.; et al. Classification, Synthetic, and Characterization Approaches to Nanoparticles, and Their Applications in Various Fields of Nanotechnology: A Review. Catalysts 2022, 12, 1386. [Google Scholar] [CrossRef]
- Selmani, A.; Kovacevic, D.; Bohinc, K. Nanoparticles: From synthesis to applications and beyond. Adv. Colloid Interface Sci. 2022, 303, 102640. [Google Scholar] [CrossRef]
- Zhang, Z.B.; Ren, J.J.; Dai, W.B.; Zhang, H.; Wang, X.Q.; He, B.; Zhang, Q. Fast and Dynamic Mapping of the Protein Corona on Nanoparticle Surfaces by Photocatalytic Proximity Labeling. Adv. Mater. 2023, 35, 2206636. [Google Scholar] [CrossRef]
- Rezaei, G.; Daghighi, S.M.; Haririan, I.; Yousefi, I.; Raoufi, M.; Rezaee, F.; Dinarvand, R. Protein corona variation in nanoparticles revisited: A dynamic grouping strategy. Colloids Surf. B Biointerfaces 2019, 179, 505–516. [Google Scholar] [CrossRef]
- García-Alvarez, R.; Hadjidemetriou, M.; Sánchez-Iglesias, A.; Liz-Marzán, L.M.; Kostarelos, K. In vivo formation of protein corona on gold nanoparticles. The effect of their size and shape. Nanoscale 2018, 10, 1256–1264. [Google Scholar] [CrossRef]
- Natte, K.; Friedrich, J.F.; Wohlrab, S.; Lutzki, J.; von Klitzing, R.; Österle, W.; Orts-Gil, G. Impact of polymer shell on the formation and time evolution of nanoparticle-protein corona. Colloids Surf. B Biointerfaces 2013, 104, 213–220. [Google Scholar] [CrossRef]
- Singh, U.; Saifi, Z.; Kumar, M.; Reimers, A.; Krishnananda, S.D.; Adelung, R.; Baum, M. Role of structural specificity of ZnO particles in preserving functionality of proteins in their corona. Sci. Rep. 2021, 11, 15945. [Google Scholar] [CrossRef]
- Sun, H.A.; Wang, Y.Y.; Zhang, L.M. Tuning the Microstructure of Protein Corona by Nanoparticle Hydrophobicity: A Dissipative Particle Dynamics Study. Chem. Lett. 2023, 52, 242–245. [Google Scholar] [CrossRef]
- Dai, L.; Sun, C.X.; Li, R.R.; Mao, L.K.; Liu, F.G.; Gao, Y.X. Structural characterization, formation mechanism and stability of curcumin in zein-lecithin composite nanoparticles fabricated by antisolvent co-precipitation. Food Chem. 2017, 237, 1163–1171. [Google Scholar] [CrossRef] [PubMed]
- Luther, D.C.; Huang, R.; Jeon, T.; Zhang, X.Z.; Lee, Y.W.; Nagaraj, H.; Rotello, V.M. Delivery of drugs, proteins, and nucleic acids using inorganic nanoparticles. Adv. Drug Deliv. Rev. 2020, 156, 188–213. [Google Scholar] [CrossRef] [PubMed]
- Yetisgin, A.A.; Cetinel, S.; Zuvin, M.; Kosar, A.; Kutlu, O. Therapeutic Nanoparticles and Their Targeted Delivery Applications. Molecules 2020, 25, 2193. [Google Scholar] [CrossRef] [PubMed]
- Anselmo, A.C.; Mitragotri, S. A Review of Clinical Translation of Inorganic Nanoparticles. AAPS J. 2015, 17, 1041–1054. [Google Scholar] [CrossRef]
- McClements, D.J. Advances in edible nanoemulsions: Digestion, bioavailability, and potential toxicity. Prog. Lipid Res. 2021, 81, 101081. [Google Scholar] [CrossRef]
- Jo, M.J.; Jin, I.S.; Park, C.W.; Hwang, B.Y.; Chung, Y.B.; Kim, J.S.; Shin, D.H. Revolutionizing technologies of nanomicelles for combinatorial anticancer drug delivery. Arch. Pharm. Res. 2020, 43, 100–109. [Google Scholar] [CrossRef]
- Ma, Z.M.; Gao, X.Z.; Raza, F.; Zafar, H.; Huang, G.H.; Yang, Y.Y.; Shi, F.; Wang, D.Q.; He, X. Design of GSH-Responsive Curcumin Nanomicelles for Oesophageal Cancer Therapy. Pharmaceutics 2022, 14, 1802. [Google Scholar] [CrossRef]
- Zarrabi, A.; Abadi, M.A.A.; Khorasani, S.; Mohammadabadi, M.R.; Jamshidi, A.; Torkaman, S.; Taghavi, E.; Mozafari, M.R.; Rasti, B. Nanoliposomes and Tocosomes as Multifunctional Nanocarriers for the Encapsulation of Nutraceutical and Dietary Molecules. Molecules 2020, 25, 638. [Google Scholar] [CrossRef]
- Mozafari, M.R. Nanoliposomes: Preparation and Analysis. In Liposomes: Methods and Protocols, Volume 1: Pharmaceutical Nanocarriers; Weissig, V., Ed.; Humana Press: Totowa, NJ, USA, 2010. [Google Scholar]
- Hamadou, A.H.; Zhang, J.Y.; Chao, C.; Xu, B. Stability of rutin using pectin-chitosan dual coating nanoliposomes. LWT-Food Sci. Technol. 2022, 170, 114084. [Google Scholar] [CrossRef]
- Smruthi, M.R.; Nallamuthu, I.; Anand, T. A comparative study of optimized naringenin nanoformulations using nano-carriers (PLA/PVA and zein/pectin) for improvement of bioavailability. Food Chem. 2022, 369, 130950. [Google Scholar] [CrossRef] [PubMed]
- Ovais, M.; Mukherjee, S.; Pramanik, A.; Das, D.; Mukherjee, A.; Raza, A.; Chen, C.Y. Designing Stimuli-Responsive Upconversion Nanoparticles that Exploit the Tumor Microenvironment. Adv. Mater. 2020, 32, 2000055. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.L.; Hong, L.H.; Wu, Y.; Gu, Y.C.; Luo, J.G.; Kong, L.Y. A dual recognition-based strategy employing Ni-modified metal-organic framework for in situ screening of SIRT1 inhibitors from Chinese herbs. Talanta 2024, 274, 125975. [Google Scholar] [CrossRef] [PubMed]
- Bazzazan, S.; Moeinabadi-Bidgoli, K.; Lalami, Z.A.; Bazzazan, S.; Mehrarya, M.; Yeganeh, F.E.; Hejabi, F.; Akbarzadeh, I.; Noorbazargan, H.; Jahanbakhshi, M.; et al. Engineered UIO-66 metal-organic framework for delivery of curcumin against breast cancer cells: An in vitro evaluation. J. Drug Deliv. Sci. Technol. 2023, 79, 104009. [Google Scholar] [CrossRef]
- Xie, Y.L.; Liu, H.K.; Xie, X.L.; Li, Y.; Peng, F.; Zhao, Y.; Xu, H. Active ingredients biosynthesis and genic response of traditional Chinese medicine Ligusticum sinense cv. Chuanxiong under different cadmium level. Ind. Crops Prod. 2023, 202, 117074. [Google Scholar] [CrossRef]
- Sharma, R.; Bhardwaj, R.; Gautam, V.; Kohli, S.K.; Kaur, P.; Bali, R.S.; Saini, P.; Thukral, A.K.; Arora, S.; Vig, A.P. Microbial Siderophores in Metal Detoxification and Therapeutics: Recent Prospective and Applications. In Plant Microbiome: Stress Response; Egamberdieva, D., Ahmad, P., Eds.; Springer: Singapore, 2018. [Google Scholar]
- Lai, H.H.; Ming, P.T.; Liu, Y.X.; Wang, S.M.; Zhou, Q.; Zhai, H.Y. MWCNTs and ZnO-based Ce-MOF nanocomposites as enhanced sensing platform for electrochemical detection of carbendazim in Chinese traditional herbs samples. Microchim. Acta 2023, 190, 281. [Google Scholar] [CrossRef]
- Algahtani, M.S.; Ahmad, M.Z.; Nourein, I.H.; Albarqi, H.A.; Alyami, H.S.; Alyami, M.H.; Alqahtani, A.A.; Alasiri, A.; Algahtani, T.S.; Mohammed, A.A.; et al. Preparation and Characterization of Curcumin Nanoemulgel Utilizing Ultrasonication Technique for Wound Healing: In Vitro, Ex Vivo, and In Vivo Evaluation. Gels 2021, 7, 213. [Google Scholar] [CrossRef]
- Patra, S.; Pradhan, B.; Nayak, R.; Behera, C.; Rout, L.; Jena, M.; Efferth, T.; Bhutia, S.K. Chemotherapeutic efficacy of curcumin and resveratrol against cancer: Chemoprevention, chemoprotection, drug synergism and clinical pharmacokinetics. Semin. Cancer Biol. 2021, 73, 310–320. [Google Scholar] [CrossRef]
- Abdallah, M.H.; Abu Lila, A.S.; Unissa, R.; Elsewedy, H.S.; Elghamry, H.A.; Soliman, M.S. Preparation, characterization and evaluation of anti-inflammatory and anti-nociceptive effects of brucine-loaded nanoemulgel. Colloids Surf. B Biointerfaces 2021, 205, 111868. [Google Scholar] [CrossRef]
- Du, X.Q.; Hu, M.; Liu, G.N.; Qi, B.K.; Zhou, S.J.; Lu, K.Y.; Xie, F.Y.; Zhu, X.Q.; Li, Y. Development and evaluation of delivery systems for quercetin: A comparative study between coarse emulsion, nano-emulsion, high internal phase emulsion, and emulsion gel. J. Food Eng. 2022, 314, 110784. [Google Scholar] [CrossRef]
- Kandemir, K.; Tomas, M.; McClements, D.J.; Capanoglu, E. Recent advances on the improvement of quercetin bioavailability. Trends Food Sci. Technol. 2022, 119, 192–200. [Google Scholar] [CrossRef]
- Dario, M.F.; Oliveira, C.A.; Cordeiro, L.R.G.; Rosado, C.; Mariz, I.D.A.; Maçôas, E.; Santos, M.; da Piedade, M.E.M.; Baby, A.R.; Velasco, M.V.R. Stability and safety of quercetin-loaded cationic nanoemulsion: In vitro and in vivo assessments. Colloids Surf. A Physicochem. Eng. Asp. 2016, 506, 591–599. [Google Scholar] [CrossRef]
- Marques, B.M.; Machado, P.A.; Santos, A.P.; Carrett-Dias, M.; Araújo, S.G.; Alves, B.D.; de Oliveira, B.S.; da Silva, F.M.R.; Dora, L.C.; Cañedo, D.A.; et al. Anti-MDR Effects of Quercetin and its Nanoemulsion in Multidrug-Resistant Human Leukemia Cells. Anti-Cancer Agents Med. Chem. 2021, 21, 1911–1920. [Google Scholar] [CrossRef] [PubMed]
- Zou, H.Y.; Ye, H.Q.; Kamaraj, R.; Zhang, T.H.; Zhang, J.; Pavek, P. A review on pharmacological activities and synergistic effect of quercetin with small molecule agents. Phytomedicine 2021, 92, 153736. [Google Scholar] [CrossRef]
- Yi, T.Q.; Huang, J.; Chen, X.W.; Xiong, H.Y.; Kang, Y.; Wu, J. Synthesis, characterization, and formulation of poly-puerarin as a biodegradable and biosafe drug delivery platform for anti-cancer therapy. Biomater. Sci. 2019, 7, 2152–2164. [Google Scholar] [CrossRef]
- Maity, S.; Mukhopadhyay, P.; Kundu, P.P.; Chakraborti, A.S. Alginate coated chitosan core-shell nanoparticles for efficient oral delivery of naringenin in diabetic animals-An in vitro and in vivo approach. Carbohydr. Polym. 2017, 170, 124–132. [Google Scholar] [CrossRef]
- Motallebi, M.; Bhia, M.; Rajani, H.F.; Bhia, I.; Tabarraei, H.; Mohammadkhani, N.; Pereira-Silva, M.; Kasaii, M.S.; Nouri-Majd, S.; Mueller, A.L.; et al. Naringenin: A potential flavonoid phytochemical for cancer therapy. Life Sci. 2022, 305, 120752. [Google Scholar] [CrossRef]
- Moghaddam, R.H.; Samimi, Z.; Moradi, S.Z.; Little, P.J.; Xu, S.W.; Farzaei, M.H. Naringenin and naringin in cardiovascular disease prevention: A preclinical review. Eur. J. Pharmacol. 2020, 887, 173535. [Google Scholar] [CrossRef]
- Stabrauskiene, J.; Kopustinskiene, D.M.; Lazauskas, R.; Bernatoniene, J. Naringin and Naringenin: Their Mechanisms of Action and the Potential Anticancer Activities. Biomedicines 2022, 10, 1686. [Google Scholar] [CrossRef]
- Sathishkumar, P.; Li, Z.F.; Govindan, R.; Jayakumar, R.; Wang, C.Y.; Gu, F.L. Zinc oxide-quercetin nanocomposite as a smart nano-drug delivery system: Molecular-level interaction studies. Appl. Surf. Sci. 2021, 536, 147741. [Google Scholar] [CrossRef]
- Demirturk, E.; Kaplan, A.B.U.; Cetin, M.; Akillioglu, K.; Kutlu, M.D.; Kose, S.; Aksu, F. Assessment of Pharmacokinetic Parameters of Daidzein-Containing Nanosuspension and Nanoemulsion Formulations After Oral Administration to Rats. Eur. J. Drug Metab. Pharmacokinet. 2022, 47, 247–257. [Google Scholar] [CrossRef] [PubMed]
- Essa, D.; Kondiah, P.P.D.; Kumar, P.; Choonara, Y.E. Design of Chitosan-Coated, Quercetin-Loaded PLGA Nanoparticles for Enhanced PSMA-Specific Activity on LnCap Prostate Cancer Cells. Biomedicines 2023, 11, 1201. [Google Scholar] [CrossRef]
- Su, M.; Dai, Q.X.; Chen, C.; Zeng, Y.; Chu, C.C.; Liu, G. Nano-Medicine for Thrombosis: A Precise Diagnosis and Treatment Strategy. Nano-Micro Lett. 2020, 12, 96. [Google Scholar] [CrossRef]
- Wang, Q.Y.; Ou, Y.J.; Hu, G.M.; Wen, C.; Yue, S.S.; Chen, C.; Xu, L.; Xie, J.W.; Dai, H.; Xiao, H.; et al. Naringenin attenuates non-alcoholic fatty liver disease by down-regulating the NLRP3/NF-κB pathway in mice. Br. J. Pharmacol. 2020, 177, 1806–1821. [Google Scholar] [CrossRef] [PubMed]
- Kalinova, R.G.; Dimitrov, I.V.; Ivanova, D.I.; Ilieva, Y.E.; Tashev, A.N.; Zaharieva, M.M.; Angelov, G.; Najdenski, H.M. Polycarbonate-Based Copolymer Micelles as Biodegradable Carriers of Anticancer Podophyllotoxin or Juniper Extracts. J. Funct. Biomater. 2024, 15, 53. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Du, S.Y.; Zhong, W.; Liu, K.G.; Qu, L.H.; Chu, F.Y.; Yang, J.J.; Han, X. Accurate delivery of pristimerin and paclitaxel by folic acid-linked nano-micelles for enhancing chemosensitivity in cancer therapy. Nano Converg. 2022, 9, 52. [Google Scholar] [CrossRef]
- Negi, P.; Sharma, G.; Verma, C.; Garg, P.; Rathore, C.; Kulshrestha, S.; Lal, U.R.; Gupta, B.; Pathania, D. Novel thymoquinone loaded chitosan-lecithin micelles for effective wound healing: Development, characterization, and preclinical evaluation. Carbohydr. Polym. 2020, 230, 115659. [Google Scholar] [CrossRef]
- Feng, Y.; Zarei, V.; Mousavipour, N. Provision and assessment properties of nanoliposomes containing macroalgae extracts of Sargassum boveanume and Padina pavonica. LWT Food Sci. Technol. 2023, 175, 114194. [Google Scholar] [CrossRef]
- Al-Samydai, A.; Alshaer, W.; Al-Dujaili, E.A.S.; Azzam, H.; Aburjai, T. Preparation, Characterization, and Anticancer Effects of Capsaicin-Loaded Nanoliposomes. Nutrients 2021, 13, 3995. [Google Scholar] [CrossRef]
- Ancic, D.; Orsolic, N.; Odeh, D.; Tomasevic, M.; Pepic, I.; Ramic, S. Resveratrol and its nanocrystals: A promising approach for cancer therapy? Toxicol. Appl. Pharmacol. 2022, 435, 115851. [Google Scholar] [CrossRef]
- Yao, F.; Lin, L.Z.; Shi, W.; Li, C.S.; Liang, Z.J.; Huang, C.L. Inhibitory Effect of Poly(lactic-co-glycolic acid) Nanoparticles Loaded with Resveratrol and Phosphatase and Tensin Homolog Deleted on Chromosome Ten (PTEN) siRNA on Lung Cancer Cells. Sci. Adv. Mater. 2022, 14, 810–817. [Google Scholar] [CrossRef]
- Qiao, F.X.; Zhao, Y.; Zhang, W.N.; Yang, J.H. Isoliquiritigenin Nanosuspension Enhances Cytostatic Effects in A549 Lung Cancer Cells. Planta Med. 2020, 86, 538–547. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.J.; Shrestha, R.; Zhang, Y. Encapsulation of Photosensitizers and Upconversion Nanocrystals in Lipid Micelles for Photodynamic Therapy. Part. Part. Syst. Charact. 2014, 31, 228–235. [Google Scholar] [CrossRef]
- Liu, Y.; Liu, W.; Xiong, S.; Luo, J.S.; Li, Y.; Zhao, Y.Y.; Wang, Q.; Zhang, Z.X.; Chen, X.J.; Chen, T.K. Highly stabilized nanocrystals delivering Ginkgolide B in protecting against the Parkinson’s disease. Int. J. Pharm. 2020, 577, 119053. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.; Li, X.; Xu, J.; Shao, J.; Ding, M.; Shi, S. Preparation, Characterization, and Evaluation of Breviscapine Nanosuspension and Its Freeze-Dried Powder. Pharmaceutics 2022, 14, 923. [Google Scholar] [CrossRef]
- Yu, P.; He, X.M.; Baer, M.; Beirinckx, S.; Tian, T.; Moya, Y.A.T.; Zhang, X.C.; Deichmann, M.; Frey, F.P.; Bresgen, V.; et al. Plant flavones enrich rhizosphere Oxalobacteraceae to improve maize performance under nitrogen deprivation. Nat. Plants 2021, 7, 481–499. [Google Scholar] [CrossRef]
- Yan, D.W.; Tajima, H.; Cline, L.C.; Fong, R.Y.; Ottaviani, J.; Shapiro, H.Y.; Blumwald, E. Genetic modification of flavone biosynthesis in rice enhances biofilm formation of soil diazotrophic bacteria and biological nitrogen fixation. Plant Biotechnol. J. 2022, 20, 2135–2148. [Google Scholar] [CrossRef]
- Zhao, C.N.; Liu, X.J.; Gong, Q.; Cao, J.P.; Shen, W.X.; Yin, X.R.; Grierson, D.; Zhang, B.; Xu, C.J.; Li, X.; et al. Three AP2/ERF family members modulate flavonoid synthesis by regulating type IV chalcone isomerase in citrus. Plant Biotechnol. J. 2021, 19, 671–688. [Google Scholar] [CrossRef]
- Bangar, S.P.; Kajla, P.; Chaudhary, V.; Sharma, N.; Ozogul, F. Luteolin: A flavone with myriads of bioactivities and food applications. Food Biosci. 2023, 52, 102366. [Google Scholar] [CrossRef]
- Huang, T.; Liu, Y.A.; Zhang, C.L. Pharmacokinetics and Bioavailability Enhancement of Baicalin: A Review. Eur. J. Drug Metab. Pharmacokinet. 2019, 44, 159–168. [Google Scholar] [CrossRef]
- Kong, N.; Chen, X.Y.; Feng, J.; Duan, T.; Liu, S.P.; Sun, X.N.; Chen, P.; Pan, T.; Yan, L.L.; Jin, T.; et al. Baicalin induces ferroptosis in bladder cancer cells by downregulating FTH1. Acta Pharm. Sin. B 2021, 11, 4045–4054. [Google Scholar] [CrossRef] [PubMed]
- Fan, Z.Y.; Cai, L.L.; Wang, S.N.; Wang, J.; Chen, B.H. Baicalin Prevents Myocardial Ischemia/Reperfusion Injury Through Inhibiting ACSL4 Mediated Ferroptosis. Front. Pharmacol. 2021, 12, 628988. [Google Scholar] [CrossRef] [PubMed]
- Hu, Q.C.; Zhang, W.W.; Wu, Z.; Tian, X.; Xiang, J.B.; Li, L.X.; Li, Z.H.; Peng, X.; Wei, S.Z.; Ma, X.; et al. Baicalin and the liver-gut system: Pharmacological bases explaining its therapeutic effects. Pharmacol. Res. 2021, 165, 105444. [Google Scholar] [CrossRef] [PubMed]
- Jin, X.; Liu, M.Y.; Zhang, D.F.; Zhong, X.; Du, K.; Qian, P.; Yao, W.F.; Gao, H.; Wei, M.J. Baicalin mitigates cognitive impairment and protects neurons from microglia-mediated neuroinflammation via suppressing NLRP3 inflammasomes and TLR4/NF-κB signaling pathway. CNS Neurosci. Ther. 2019, 25, 575–590. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.; Meena, A.; Luqman, S. Baicalin mediated regulation of key signaling pathways in cancer. Pharmacol. Res. 2021, 164, 105387. [Google Scholar] [CrossRef]
- Mi, X.; Hu, M.G.; Dong, M.R.; Yang, Z.H.; Zhan, X.; Chang, X.Y.; Lu, J.; Chen, X. Folic Acid Decorated Zeolitic Imidazolate Framework (ZIF-8) Loaded with Baicalin as a Nano-Drug Delivery System for Breast Cancer Therapy. Int. J. Nanomed. 2021, 16, 8337–8352. [Google Scholar] [CrossRef]
- Wei, Y.M.; Liang, J.; Zheng, X.L.; Pi, C.; Liu, H.; Yang, H.R.; Zou, Y.G.; Ye, Y.; Zhao, L. Lung-targeting drug delivery system of baicalin-loaded nanoliposomes: Development, biodistribution in rabbits, and pharmacodynamics in nude mice bearing orthotopic human lung cancer. Int. J. Nanomed. 2017, 12, 251–261. [Google Scholar] [CrossRef]
- Xu, Q.; Zhou, A.; Wu, H.F.; Bi, Y.J. Development and in vivo evaluation of baicalin-loaded W/O nanoemulsion for lymphatic absorption. Pharm. Dev. Technol. 2019, 24, 1155–1163. [Google Scholar] [CrossRef]
- Wang, X.; Lu, J.; Cao, Y.; Liang, Y.D.; Dai, X.L.; Liu, K.; Xie, L.; Li, X.F. Does binary blend emulsifier enhance emulsifier performance? Preparation of baicalin nanoemulsions using tea saponins and glycyrrhizic acid as binary blend emulsifier. J. Drug Deliv. Sci. Technol. 2023, 84, 104398. [Google Scholar] [CrossRef]
- Memariani, Z.; Abbas, S.Q.; Ul Hassan, S.S.; Ahmadi, A.; Chabra, A. Naringin and naringenin as anticancer agents and adjuvants in cancer combination therapy: Efficacy and molecular mechanisms of action, a comprehensive narrative review. Pharmacol. Res. 2021, 171, 105264. [Google Scholar] [CrossRef]
- Fernández, J.; Silván, B.; Entrialgo-Cadierno, R.; Villar, C.J.; Capasso, R.; Uranga, J.A.; Lombó, F.; Abalo, R. Antiproliferative and palliative activity of flavonoids in colorectal cancer. Biomed. Pharmacother. 2021, 143, 112241. [Google Scholar] [CrossRef] [PubMed]
- Bhia, M.; Motallebi, M.; Abadi, B.; Zarepour, A.; Pereira-Silva, M.; Saremnejad, F.; Santos, A.C.; Zarrabi, A.; Melero, A.; Jafari, S.M.; et al. Naringenin Nano-Delivery Systems and Their Therapeutic Applications. Pharmaceutics 2021, 13, 291. [Google Scholar] [CrossRef] [PubMed]
- Slika, H.; Mansour, H.; Wehbe, N.; Nasser, S.A.; Iratni, R.; Nasrallah, G.; Shaito, A.; Ghaddar, T.; Kobeissy, F.; Eid, A.H. Therapeutic potential of flavonoids in cancer: ROS-mediated mechanisms. Biomed. Pharmacother. 2022, 146, 112442. [Google Scholar] [CrossRef] [PubMed]
- Lesjak, M.; Beara, I.; Simin, N.; Pintac, D.; Majkic, T.; Bekvalac, K.; Orcic, D.; Mimica-Dukic, N. Antioxidant and anti-inflammatory activities of quercetin and its derivatives. J. Funct. Foods 2018, 40, 68–75. [Google Scholar] [CrossRef]
- Wang, Z.X.; Ma, J.; Li, X.Y.; Wu, Y.; Shi, H.; Chen, Y.; Lu, G.; Shen, H.M.; Lu, G.D.; Zhou, J. Quercetin induces p53-independent cancer cell death through lysosome activation by the transcription factor EB and Reactive Oxygen Species-dependent ferroptosis. Br. J. Pharmacol. 2021, 178, 1133–1148. [Google Scholar] [CrossRef] [PubMed]
- Hemati, M.; Haghiralsadat, F.; Yazdian, F.; Jafari, F.; Moradi, A.; Malekpour-Dehkordi, Z. Development and characterization of a novel cationic PEGylated niosome-encapsulated forms of doxorubicin, quercetin and siRNA for the treatment of cancer by using combination therapy. Artif. Cells Nanomed. Biotechnol. 2019, 47, 1295–1311. [Google Scholar] [CrossRef]
- Karole, A.; Parvez, S.; Thakur, R.S.; Mudavath, S.L. Effervescent based nano-gas carrier enhanced the bioavailability of poorly aqueous soluble drug: A comprehensive mechanistic understanding. J. Drug Deliv. Sci. Technol. 2022, 69, 103167. [Google Scholar] [CrossRef]
- Lee, J.H.; Yeo, Y. Controlled drug release from pharmaceutical nanocarriers. Chem. Eng. Sci. 2015, 125, 75–84. [Google Scholar] [CrossRef]
- Herdiana, Y.; Wathoni, N.; Shamsuddin, S.; Muchtaridi, M. Drug release study of the chitosan-based nanoparticles. Heliyon 2022, 8, e08674. [Google Scholar] [CrossRef]
- Kashkooli, F.M.; Soltani, M.; Souri, M. Controlled anti-cancer drug release through advanced nano-drug delivery systems: Static and dynamic targeting strategies. J. Control. Release 2020, 327, 316–349. [Google Scholar] [CrossRef]
- Borandeh, S.; van Bochove, B.; Teotia, A.; Seppälä, J. Polymeric drug delivery systems by additive manufacturing. Adv. Drug Deliv. Rev. 2021, 173, 349–373. [Google Scholar] [CrossRef] [PubMed]
- Kamaly, N.; Yameen, B.; Wu, J.; Farokhzad, O.C. Degradable Controlled-Release Polymers and Polymeric Nanoparticles: Mechanisms of Controlling Drug Release. Chem. Rev. 2016, 116, 2602–2663. [Google Scholar] [CrossRef] [PubMed]
- Huh, K.M.; Kang, H.C.; Lee, Y.J.; Bae, Y.H. pH-sensitive polymers for drug delivery. Macromol. Res. 2012, 20, 224–233. [Google Scholar] [CrossRef]
- Anirudhan, T.S.; Manjusha, V.; Sekhar, V.C. A new biodegradable nano cellulose-based drug delivery system for pH-controlled delivery of curcumin. Int. J. Biol. Macromol. 2021, 183, 2044–2054. [Google Scholar] [CrossRef]
- Gong, X.; Qiu, X.; Zhao, Y.; Wang, J. Synthesis and characterization of pH responsive tea polyphenols/mesoporous zinc oxide nano-complex with antioxidant and antibacterial activity. J. Funct. Mater. 2022, 53, 08186. [Google Scholar]
- Weinstain, R.; Slanina, T.; Kand, D.; Klán, P. Visible-to-NIR-Light Activated Release: From Small Molecules to Nanomaterials. Chem. Rev. 2020, 120, 13135–13272. [Google Scholar] [CrossRef]
- Zhou, Z.X.; Vázquez-González, M.; Willner, I. Stimuli-responsive metal-organic framework nanoparticles for controlled drug delivery and medical applications. Chem. Soc. Rev. 2021, 50, 4541–4563. [Google Scholar] [CrossRef]
- Qian, C.G.; Yu, J.C.; Chen, Y.L.; Hu, Q.Y.; Xiao, X.Z.; Sun, W.J.; Wang, C.; Feng, P.J.; Shen, Q.D.; Gu, Z. Light-Activated Hypoxia-Responsive Nanocarriers for Enhanced Anticancer Therapy. Adv. Mater. 2016, 28, 3313–3320. [Google Scholar] [CrossRef]
- Hu, D.; Zhang, C.; Sun, C.; Bai, H.; Xie, J.; Gu, Y.; Li, M.; Jiang, J.; Le, A.; Qiu, J.; et al. Carvacrol combined with NIR light-responsive nano-drug delivery system with specific anti-bacteria anti-inflammation, and immunomodulation for periodontitis. Nano Res. 2023, 16, 7199–7215. [Google Scholar] [CrossRef]
- Wen, X.; Liu, N.; Ren, J.; Jiao, X.; Lv, J.; Akhtar, M.H.; Qi, H.; Zhu, J.; Yu, C.; Li, Y. In situ synthesis of a functional ZIF-8 nanocomposite for synergistic photodynamic-chemotherapy and pH and NIR-stimulated drug release. New J. Chem. 2022, 46, 6966–6970. [Google Scholar] [CrossRef]
- Meerovich, I.; Nichols, M.G.; Dash, A.K. Low-intensity light-induced paclitaxel release from lipid-based nano-delivery systems. J. Drug Target. 2019, 27, 971–983. [Google Scholar] [CrossRef] [PubMed]
- Li, F.Y.; Pei, Z.R.; Chen, S.T.; Li, G.; Liu, M.Y.; Ding, L.Q.; Liu, J.B.; Qiu, F. Multifunctional nano-herb based on tumor microenvironment for enhanced tumor therapy of gambogic acid. Chin. Chem. Lett. 2024, 35, 108752. [Google Scholar] [CrossRef]
- Hafezi, M.; Rostami, M.; Hosseini, A.; Rahimi-Nasrabadi, M.; Fasihi-Ramandi, M.; Badiei, A.; Ahmadi, F. Cur-loaded ZnFe2O4@mZnO@N-GQDs biocompatible nano-carriers for smart and controlled targeted drug delivery with pH-triggered and ultrasound irradiation. J. Mol. Liq. 2021, 322, 114875. [Google Scholar] [CrossRef]
- Xu, H.Y.; Zhang, Y.Q.; Liu, Z.M.; Chen, T.; Lv, C.Y.; Tang, S.H.; Zhang, X.B.; Zhang, W.; Li, Z.Y.; Zhou, R.R.; et al. ETCM: An encyclopaedia of traditional Chinese medicine. Nucleic Acids Res. 2019, 47, D976–D982. [Google Scholar] [CrossRef]
- Kou, L.F.; Bhutia, Y.D.; Yao, Q.; He, Z.G.; Sun, J.; Ganapathy, V. Transporter-Guided Delivery of Nanoparticles to Improve Drug Permeation across Cellular Barriers and Drug Exposure to Selective Cell Types. Front. Pharmacol. 2018, 9, 27. [Google Scholar] [CrossRef]
- Shi, S.Z.; Zhong, H.B.; Zhang, Y.; Mei, Q.S. Targeted delivery of nano-radiosensitizers for tumor radiotherapy. Coord. Chem. Rev. 2024, 518, 216101. [Google Scholar] [CrossRef]
- Duan, L.; Yang, L.; Jin, J.; Yang, F.; Liu, D.; Hu, K.; Wang, Q.X.; Yue, Y.B.; Gu, N. Micro/nano-bubble-assisted ultrasound to enhance the EPR effect and potential theranostic applications. Theranostics 2020, 10, 462–483. [Google Scholar] [CrossRef]
- Cheng, X.X.; Xie, Q.R.; Sun, Y. Advances in nanomaterial-based targeted drug delivery systems. Front. Bioeng. Biotechnol. 2023, 11, 1177151. [Google Scholar] [CrossRef]
- Ren, H.W.; He, Y.W.; Liang, J.M.; Cheng, Z.K.; Zhang, M.; Zhu, Y.; Hong, C.; Qin, J.; Xu, X.C.; Wang, J.X. Role of Liposome Size, Surface Charge, and PEGylation on Rheumatoid Arthritis Targeting Therapy. ACS Appl. Mater. Interfaces 2019, 11, 20304–20315. [Google Scholar] [CrossRef]
- Meng, Q.W.; Li, J.W.; Wang, C.S.; Shan, A.S. Biological function of resveratrol and its application in animal production: A review. J. Anim. Sci. Biotechnol. 2023, 14, 25. [Google Scholar] [CrossRef]
- Tian, B.R.; Liu, J.Y. Resveratrol: A review of plant sources, synthesis, stability, modification and food application. J. Sci. Food Agric. 2020, 100, 1392–1404. [Google Scholar] [CrossRef] [PubMed]
- Vijayakumar, M.R.; Kumari, L.; Patel, K.K.; Vuddanda, P.R.; Vajanthri, K.Y.; Mahto, S.K.; Singh, S. Intravenous administration of trans-resveratrol-loaded TPGS-coated solid lipid nanoparticles for prolonged systemic circulation, passive brain targeting and improved in vitro cytotoxicity against C6 glioma cell lines. RSC Adv. 2016, 6, 50336–50348. [Google Scholar] [CrossRef]
- Fang, X.L.; Cao, J.J.; Shen, A.Z. Advances in anti-breast cancer drugs and the application of nano-drug delivery systems in breast cancer therapy. J. Drug Deliv. Sci. Technol. 2020, 57, 101662. [Google Scholar] [CrossRef]
- Wang, M.J.; Xue, W.X.; Yuan, H.H.; Wang, Z.C.; Yu, L. Nano-Drug Delivery Systems Targeting CAFs: A Promising Treatment for Pancreatic Cancer. Int. J. Nanomed. 2024, 19, 2823–2849. [Google Scholar] [CrossRef] [PubMed]
- Tu, Z.X.; Zhong, Y.L.; Hu, H.Z.; Shao, D.; Haag, R.N.; Schirner, M.; Lee, J.; Sullenger, B.; Leong, K.W. Design of therapeutic biomaterials to control inflammation. Nat. Rev. Mater. 2022, 7, 557–574. [Google Scholar] [CrossRef]
- Takahashi, T.; Utoguchi, N.; Takara, A.; Yamamoto, N.; Nakanishi, T.; Tanaka, K.; Audus, K.L.; Watanabe, Y. Carrier-mediated transport of folic acid in BeWo cell monolayers as a model of the human trophoblast. Placenta 2001, 22, 863–869. [Google Scholar] [CrossRef]
- Roger, E.; Kalscheuer, S.; Kirtane, A.; Guru, B.R.; Grill, A.E.; Whittum-Hudson, J.; Panyam, J. Folic Acid Functionalized Nanoparticles for Enhanced Oral Drug Delivery. Mol. Pharm. 2012, 9, 2103–2110. [Google Scholar] [CrossRef]
- Tong, L.X.; Chen, W.; Wu, J.; Li, H.X. Folic acid-coupled nano-paclitaxel liposome reverses drug resistance in SKOV3/TAX ovarian cancer cells. Anti-Cancer Drugs 2014, 25, 244–254. [Google Scholar] [CrossRef]
- Du, Z.J.; Mao, Y.; Zhang, P.F.; Hu, J.; Fu, J.J.; You, Q.J.; Yin, J. TPGS-Galactose-Modified Polydopamine Co-delivery Nanoparticles of Nitric Oxide Donor and Doxorubicin for Targeted Chemo-Photothermal Therapy against Drug-Resistant Hepatocellular Carcinoma. ACS Appl. Mater. Interfaces 2021, 13, 35518–35532. [Google Scholar] [CrossRef]
- Chen, Q.B.; Li, Q.; Liang, Y.Q.; Zu, M.H.; Chen, N.X.; Canup, B.S.B.; Luo, L.Y.; Wang, C.H.; Zeng, L.; Xiao, B. Natural exosome-like nanovesicles from edible tea flowers suppress metastatic breast cancer via ROS generation and microbiota modulation. Acta Pharm. Sin. B 2022, 12, 907–923. [Google Scholar] [CrossRef]
- Nie, W.D.; Wu, G.H.; Zhang, J.F.; Huang, L.L.; Ding, J.J.; Jiang, A.Q.; Zhang, Y.H.; Liu, Y.H.; Li, J.C.; Pu, K.Y.; et al. Responsive Exosome Nano-bioconjugates for Synergistic Cancer Therapy. Angew. Chem.-Int. Ed. 2020, 59, 2018–2022. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.H.; Wang, B.Y.; Wang, S.S.; Chen, J.Q.; Zhi, W.W.; Guan, Y.B.; Cai, B.R.; Zhu, Y.H.; Jia, Y.Y.; Huang, S.N.; et al. Injectable in situ intelligent thermo-responsive hydrogel with glycyrrhetinic acid-conjugated nano graphene oxide for chemo-photothermal therapy of malignant hepatocellular tumor. J. Biomater. Appl. 2022, 37, 151–165. [Google Scholar] [CrossRef] [PubMed]
- Iskusnykh, I.Y.; Zakharova, A.A.; Pathak, D. Glutathione in Brain Disorders and Aging. Molecules 2022, 27, 324. [Google Scholar] [CrossRef] [PubMed]
- Zhan, Y.; Qiu, Y.; Wang, H.; Wang, Z.; Xu, J.; Fan, G.; Xu, J.; Li, W.; Cao, Y.; Le, V.-M.; et al. Bufalin reverses multidrug resistance by regulating stemness through the CD133/nuclear factor-κB/MDR1 pathway in colorectal cancer. Cancer Sci. 2020, 111, 1619–1630. [Google Scholar] [CrossRef]
- Guo, H.C.; Liu, F.; Yang, S.G.; Xue, T. Emodin alleviates gemcitabine resistance in pancreatic cancer by inhibiting MDR1/P-glycoprotein and MRPs expression. Oncol. Lett. 2020, 20, 167. [Google Scholar] [CrossRef]
- Yang, S.D.; Zhu, W.J.; Zhu, Q.L.; Chen, W.L.; Ren, Z.X.; Li, F.; Yuan, Z.Q.; Li, J.Z.; Liu, Y.; Zhou, X.F.; et al. Binary-copolymer system base on low-density lipoprotein-coupled N-succinyl chitosan lipoic acid micelles for co-delivery MDR1 siRNA and paclitaxel, enhances antitumor effects via reducing drug. J. Biomed. Mater. Res. Part B Appl. Biomater. 2017, 105, 1114–1125. [Google Scholar] [CrossRef]
- Ouyang, L.; Wang, L.S.; Schork, F.J. Synthesis and nucleation mechanism of inverse emulsion polymerization of acrylamide by RAFT polymerization: A comparative study. Polymer 2011, 52, 63–67. [Google Scholar] [CrossRef]
- Maeki, M.; Kimura, N.; Sato, Y.; Harashima, H.; Tokeshi, M. Advances in microfluidics for lipid nanoparticles and extracellular vesicles and applications in drug delivery systems. Adv. Drug Deliv. Rev. 2018, 128, 84–100. [Google Scholar] [CrossRef]
- Cooley, M.; Sarode, A.; Hoore, M.; Fedosov, D.A.; Mitragotri, S.; Sen Gupta, A. Influence of particle size and shape on their margination and wall-adhesion: Implications in drug delivery vehicle design across nano-to-micro scale. Nanoscale 2018, 10, 15350–15364. [Google Scholar] [CrossRef]
- Zhang, T.H.; Wang, L.S.; He, X.Y.; Lu, H.L.; Gao, L. Cytocompatibility of pH-sensitive, chitosan-coated Fe3O4 nanoparticles in gynecological cells. Front. Med. 2022, 9, 799145. [Google Scholar] [CrossRef]
- Yin, M.Z.; Xu, W.G.; Cui, B.C.; Dai, H.L.; Han, Y.C.; Yin, Y.X.; Li, S.P. Effects of the Interaction between Hydroxyapatite Nanoparticles and Hepatoma Cells. J. Wuhan Univ. Technol.-Mater. Sci. Ed. 2014, 29, 635–642. [Google Scholar] [CrossRef]
- Kim, J.E.; Kim, H.; An, S.S.A.; Maeng, E.H.; Kim, M.K.; Song, Y.J. In vitro cytotoxicity of SiO2 or ZnO nanoparticles with different sizes and surface charges on U373MG human glioblastoma cells. Int. J. Nanomed. 2014, 9, 235–241. [Google Scholar] [CrossRef]
- Tay, C.Y.; Cai, P.Q.; Setyawati, M.I.; Fang, W.R.; Tan, L.P.; Hong, C.H.L.; Chen, X.D.; Leong, D.T. Nanoparticles Strengthen Intracellular Tension and Retard Cellular Migration. Nano Lett. 2014, 14, 83–88. [Google Scholar] [CrossRef] [PubMed]
| Type | Comparative Advantage | Limitations | References |
|---|---|---|---|
| Nanoemulsion | Strong drug-loading capacity. The particle size is small and uniform. High bioavailability. | Potential toxicity of surfactants; the storage conditions are demanding. | [59,60,62,88] |
| Nano micelles | High loading capacity, good stability in blood, prolonged circulation time, low number of side effects, and protects internal drugs from degradation. | Stability still needs to be improved; complex behavior in vivo | [89,90] |
| Nanoliposomes | Passive targeting of drugs, highly efficient cargo delivery, reducing cargo toxicity. | The storage and transportation conditions are demanding; stability issues. | [38,91,92,93,94] |
| Upconversion nanoparticles | Unique optical properties, low toxicity and good biocompatibility, and easy surface functionalization. | The optical conversion efficiency needs to be improved. The drug load is relatively low. | [95] |
| Metal-organic framework | High specific surface area and porosity, biodegradability, structure and performance can be adjusted. | Synthesis and preparation are complex. There are limited studies on drug loading and release kinetics. | [96,97] |
| Nanometallic elemental | Low cytotoxicity, controlled size and surface, easy synthesis, high cell permeability, ability to bind many molecules on their surface, high drug-loading capacity. | Clearance problems in the body; difficult to achieve complex drug delivery patterns. | [98,99] |
| Carbon dots | Very high elastic modulus and mechanical strength, high electrical and thermal conductivity, prolonged circulating time, cell membrane permeability, high aspect ratio allowing for high drug loading. | Limited drug loading. Preparation is complex and costly. | [100] |
| Nanoformulations | Active Ingredient | Impacts | Reference |
|---|---|---|---|
| Nanoemulsion | Curcumin | Compared with curcumin dispersed in conventional hydrogel systems, the developed curcumin nanolatex exhibits thixotropic rheological behavior with a significant increase in skin permeability. | [12,49,101,102] |
| Nanoemulsion | Brucine | Brucine-loaded nanolatex exhibits superior anti-inflammatory and antinociceptive activity in reducing hind paw swelling and inhibiting acetic acid-induced abdominal writhing compared to brucine-loaded gels or brucine-loaded latex. | [54,55,60,61,63,103] |
| Nanoemulsion | Quercetin | In four delivery systems, the highest bioavailability of quercetin was observed in nanoemulsions. | [104,105,106,107,108] |
| Nanoparticles | Puerarin | Compared with free puerarin, poly-puerarin nanoparticles have the best anti-tumor effect. | [72,76,77,109] |
| Nanoparticles | Naringenin | In experimental rat models, naringenin-loaded nanoparticles were more effective than free naringenin in improving Streptozotocin-induced diabetogenic effects. | [94,110,111,112,113] |
| Nanoparticles | Quercetin | The amount of quercetin loaded on the ZnO nanoparticles reached 210 μg/mg, and the half maximal inhibitory concentration value of ZnO-quercetin nanocomposites for Michigan Cancer Foundation-7 breast cancer cells was 0.01 (0.07 μg/mL for free quercetin). | [51,105,114,115,116] |
| Nano micelles | Naringenin | Compared with pure bovine beta-casein micelles, naringenin-containing bovine beta-casein micelles had a lower critical micelle concentration and a larger aggregation number, which greatly increased the concentration of naringenin in aqueous solution. | [111,112,117,118] |
| Nano micelles | Podophyllotoxin | Compared with the other two cytotoxic agent-loaded micelles, podophyllotoxin-loaded micelles had the highest activity. | [89,119,120] |
| Nano micelles | Thymoquinone | Thymoquinone polymer micelles exhibit better wound-healing effects than natural thymoquinone and silver sulfadiazine. | [90,120,121] |
| Nanoliposomes | Sargassum boveanum | After encapsulating the extract with nanoliposomes, Sargassum boveanum retained a high proportion of phenolic compounds for antioxidant properties. | [92,93,122] |
| Nanoliposomes | Capsaicin | Compared with capsaicin, nanoliposomes encapsulating capsaicin have improved pharmacokinetic properties, enhanced anticancer activity, and selectivity. | [38,91,123] |
| Nanoliposomes | Lutin | Better protection of the vitality of rutin bioactive compounds. | [52,93,123] |
| Nanocrystals | Resveratrol | Compared to nonnanocrystalline forms, resveratrol nanocrystals exhibit better anti-tumor effects than resveratrol itself. | [102,124,125] |
| Nanocrystals | Isoliquiritigenin | Compared with the free form of isoliquiritigenin, it has higher solubility and lower toxicity to cells. | [126,127,128] |
| Nanocrystals | Breviscapine | Compared with brevisanthin microparticle formulations, brevisanthin nanocrystals can provide relatively stable drug concentrations in plasma for a long time. | [124,129] |
| Nanocrystals | Ginkgolide B | Ginkgolide B nanocrystals show higher drug plasma levels and neuronal drug distribution compared to free ginkgolide B. | [127,128] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, Y.; Tang, Y.; Li, Y.; Rui, Y.; Zhang, P. Enhancing the Efficacy of Active Pharmaceutical Ingredients in Medicinal Plants through Nanoformulations: A Promising Field. Nanomaterials 2024, 14, 1598. https://doi.org/10.3390/nano14191598
Chen Y, Tang Y, Li Y, Rui Y, Zhang P. Enhancing the Efficacy of Active Pharmaceutical Ingredients in Medicinal Plants through Nanoformulations: A Promising Field. Nanomaterials. 2024; 14(19):1598. https://doi.org/10.3390/nano14191598
Chicago/Turabian StyleChen, Yuhao, Yuying Tang, Yuanbo Li, Yukui Rui, and Peng Zhang. 2024. "Enhancing the Efficacy of Active Pharmaceutical Ingredients in Medicinal Plants through Nanoformulations: A Promising Field" Nanomaterials 14, no. 19: 1598. https://doi.org/10.3390/nano14191598






