Structural and Optical Properties of Nickel-Doped Zinc Sulfide
Abstract
:1. Introduction
2. Experimental Section
2.1. Synthesis of Zn1−xNixS Samples
2.2. Characterization of the Samples
3. Results and Discussion
3.1. X-ray Diffraction (XRD) Measurements
3.2. Fourier-Transform Infrared Spectroscopy (FT-IR) Measurements
3.3. Scanning Electron Microscope (SEM) Measurements
3.4. Nitrogen Isotherms and Pore Size Distribution
3.5. X-ray Photoelectron Spectroscopy (XPS) Assessment
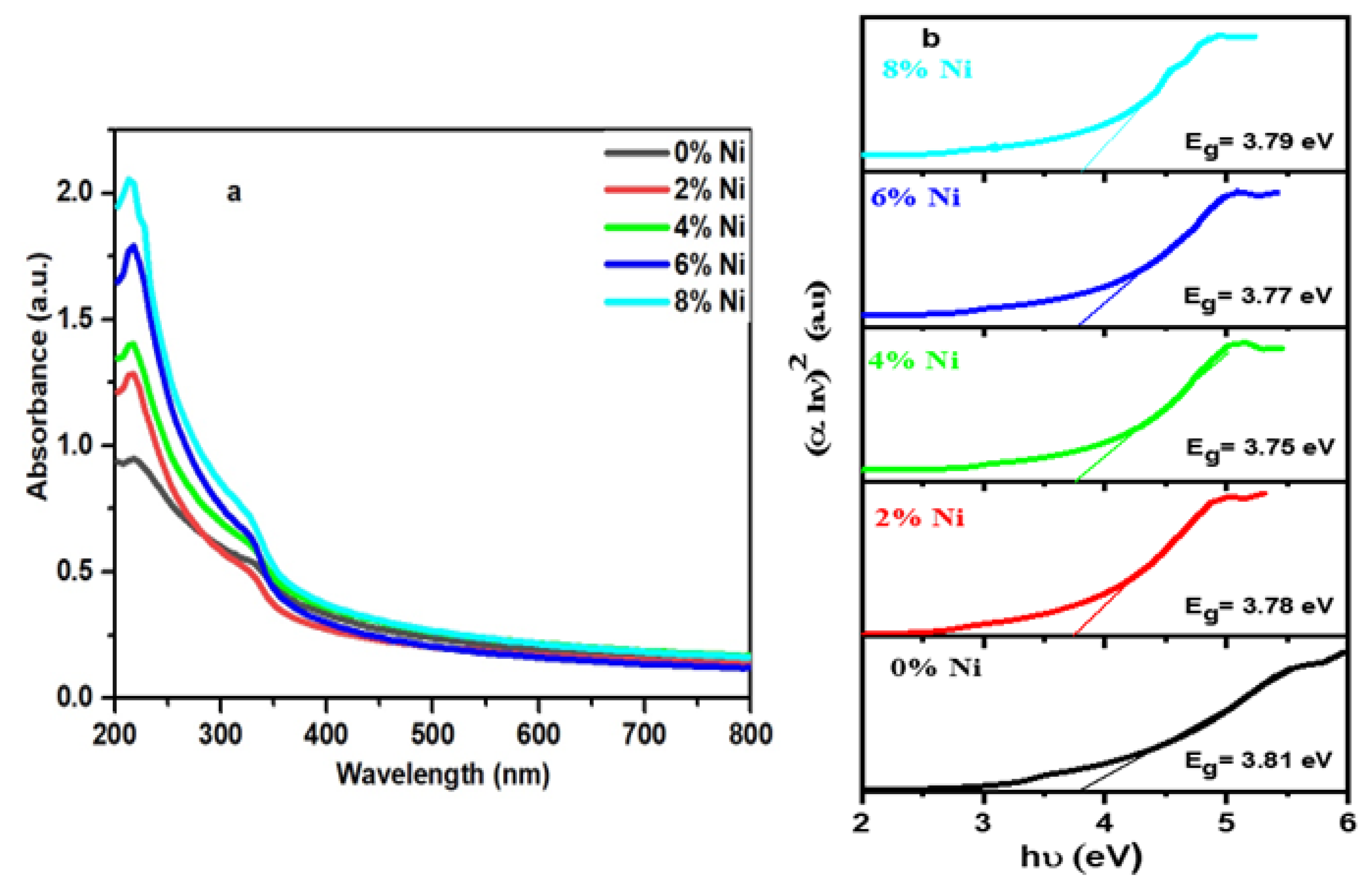
3.6. UV–Vis Absorption Measurements
3.7. Energy Transfer Mechanism
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hou, M.; Zhou, Z.; Xu, A.; Xiao, K.; Li, J.; Qin, D.; Xu, W.; Hou, L. Synthesis of Group II–VI Semiconductor Nanocrystals via Phosphine Free Method and Their Application in Solution Processed Photovoltaic Devices. Nanomaterials 2021, 11, 2071. [Google Scholar] [CrossRef] [PubMed]
- Slejko, E.A.; Lughi, V. Size Control at Maximum Yield and Growth Kinetics of Colloidal II–VI Semiconductor Nanocrystals. J. Phys. Chem. C 2018, 123, 1421–1428. [Google Scholar] [CrossRef]
- Lippens, P.E.; Lannoo, M. Optical properties of II-VI semiconductor nanocrystals. Semicond. Sci. Technol. 1991, 6, A157–A160. [Google Scholar] [CrossRef]
- Sarma, M. Structural and Optical Properties of ZnS and Cr-ZnS Thin Films Prepared by Chemical Bath Deposition Method. Am. J. Mater. Sci. Technol. 2015, 4, 58–71. [Google Scholar] [CrossRef]
- Fang, X.; Zhai, T.; Gautam, U.K.; Li, L.; Wu, L.; Bando, Y.; Golberg, D. ZnS nanostructures: From synthesis to applications. Prog. Mater. Sci. 2011, 56, 175–287. [Google Scholar] [CrossRef]
- Zhang, Y.; Liu, W.; Wang, R. From ZnS nanoparticles, nanobelts, to nanotetrapods: The ethylenediamine modulated anisotropic growth of ZnS nanostructures. Nanoscale 2012, 4, 2394. [Google Scholar] [CrossRef]
- Liu, Y.; Li, Y.; Kang, H.; Jin, T.; Jiao, L. Design, synthesis, and energy-related applications of metal sulfides. Mater. Horiz. 2016, 3, 402–421. [Google Scholar] [CrossRef]
- Ma, Q.; Wang, Y.; Kong, J.; Jia, H. Tunable synthesis, characterization and photocatalytic properties of various ZnS nanostructures. Ceram. Int. 2016, 42, 2854–2860. [Google Scholar] [CrossRef]
- Liu, S.; Li, S.; Sekar, K.; Li, R.; Zhu, Y.; Xing, R.; Fujishima, A. Hierarchical ZnS@ C@ MoS2 core-shell nanostructures as efficient hydrogen evolution electrocatalyst for alkaline water electrolysis. Int. J. Hydrog. Energy 2019, 44, 25310–25318. [Google Scholar] [CrossRef]
- Raffah, B.M.; Hassan, H.; Iqbal, M.W.; Al-Hadeethi, Y. Synergistic Co-MOF/ZnS Composite: Advancing Supercapattery Performance and Catalyzing Hydrogen Evolution Reaction. Energy Fuels 2024, 38, 3477–3490. [Google Scholar] [CrossRef]
- Othman, A.; Osman, M.; Ali, M.A.; Ibrahim, E. Influence of transition metals dopant type on the structural, optical, magnetic, and dielectric properties of ZnS nanoparticles prepared by ultrasonication process. Mater. Sci. Eng. B 2021, 270, 115195. [Google Scholar] [CrossRef]
- Wang, C.; Li, Q.; Hu, B. Preparation and characterization of ZnS nanoparticles prepared by hydrothermal method. Int. J. Mod. Phys. B 2017, 31, 1744055. [Google Scholar] [CrossRef]
- Othman, A.A.; Osman, M.A.; Ali, M.A.; Mohamed, W.S.; Ibrahim, E.M.M. Sonochemically synthesized Ni-doped ZnS nanoparticles: Structural, optical, and photocatalytic properties. J. Mater. Sci. Mater. Electron. 2020, 31, 1752–1767. [Google Scholar] [CrossRef]
- Kaur, J.; Sharma, M.; Pandey, O.P. Photoluminescence and photocatalytic studies of metal ions (Mn and Ni) doped ZnS nanoparticles. Opt. Mater. 2015, 47, 7–17. [Google Scholar] [CrossRef]
- Poornaprakash, B.; Chalapathi, U.; Vattikuti, S.P. Compositional, morphological, structural, microstructural, optical, and magnetic properties of Fe, Co, and Ni doped ZnS nanoparticles. Appl. Phys. A 2017, 123, 1–10. [Google Scholar] [CrossRef]
- Kumar, R.S.; Veeravazhuthi, V.; Muthukumarasamy, N.; Thambidurai, M.; Shankar, D.V. Effect of nickel doping on structural and optical properties of ZnS nanoparticles. Superlattices Microstruct. 2015, 86, 552–558. [Google Scholar] [CrossRef]
- Mamiyev, Z.Q.; Balayeva, N.O. Optical and structural studies of ZnS nanoparticles synthesized via chemical in situ technique. Chem. Phys. Lett. 2016, 646, 69–74. [Google Scholar] [CrossRef]
- Talantikite-Touati, D.; Merzouk, H.; Haddad, H.; Tounsi, A. Effect of dopant concentration on structural and optical properties Mn doped ZnS films prepared by CBD method. Optik 2017, 136, 362–367. [Google Scholar] [CrossRef]
- Husain, S.; Rahman, F.; Ali, N.; Alvi, P.A. Nickel sub-lattice effects on the optical properties of ZnO nanocrystals. J. Optoelectron. Eng. 2013, 1, 28–32. [Google Scholar]
- Siddique, M.N.; Ali, T.; Ahmed, A.; Tripathi, P. Enhanced electrical and thermal properties of pure and Ni substituted ZnO Nanoparticles. Nano-Struct. Nano-Objects 2018, 16, 156–166. [Google Scholar] [CrossRef]
- Qadri, S.B.; Skelton, E.F.; Hsu, D.; Dinsmore, A.D.; Yang, J.; Gray, H.F.; Ratna, B.R. Size-induced transition-temperature reduction in nanoparticles of ZnS. Phys. Rev. B 1999, 60, 9191. [Google Scholar] [CrossRef]
- Rema Devi, B.S.; Raveendran, R.; Vaidyan, A.V. Synthesis and characterization of Mn2+-doped ZnS nanoparticles. Pramana 2007, 68, 679–687. [Google Scholar] [CrossRef]
- Weng, Y.C.; Chou, Y.D.; Chang, C.J.; Chan, C.C.; Chen, K.Y.; Su, Y.F. Screening of ZnS-based photocatalysts by scanning electrochemical microscopy and characterization of potential photocatalysts. Electrochim. Acta 2014, 125, 354–361. [Google Scholar] [CrossRef]
- Zhu, Y.P.; Li, J.; Ma, T.Y.; Liu, Y.P.; Du, G.; Yuan, Z.Y. Sonochemistry-assisted synthesis and optical properties of mesoporous ZnS nanomaterials. J. Mater. Chem. A 2014, 2, 1093–1101. [Google Scholar] [CrossRef]
- Tauc, J. Optical Properties of Solids, Abeles, F., Ed.; North-Holland: Amsterdam, The Netherlands, 1969. [Google Scholar]
- Davis, E.A.; Mott, N. Conduction in non-crystalline systems, V. Conductivity, optical absorption and photoconductivity in amorphous semiconductors. Philos. Mag. 1970, 22, 903–922. [Google Scholar] [CrossRef]
- Apostolova, I.; Apostolov, A.; Wesselinowa, J. Magnetic, Phonon and Optical Properties of Transition Metal and Rare Earth Ion Doped ZnS Nanoparticles. Nanomaterials 2022, 13, 79. [Google Scholar] [CrossRef]
- Mamiyev, Z.; Balayeva, N.O. PbS nanostructures: A review of recent advances. Mater. Today Sustain. 2023, 21, 100305. [Google Scholar] [CrossRef]
- Islam, M.R.; Podder, J. Optical properties of ZnO nano fiber thin films grown by spray pyrolysis of zinc acetate precursor. Cryst. Res. Technol. J. Exp. Ind. Crystallogr. 2009, 44, 286–292. [Google Scholar] [CrossRef]
- Viezbicke, B.D.; Patel, S.; Davis, B.E.; Birnie, D.P., III. Evaluation of the Tauc method for optical absorption edge determination: ZnO thin films as a model system. Phys. Status Solidi B 2015, 252, 1700–1710. [Google Scholar] [CrossRef]
- Wu, M.; Wei, Z.; Zhao, W.; Wang, X.; Jiang, J. Optical and magnetic properties of Ni doped ZnS diluted magnetic semiconductors synthesized by hydrothermal method. J. Nanomater. 2017, 2017, 1603450. [Google Scholar] [CrossRef]
- Kim, K.J.; Park, Y.R. Optical investigation of Zn1−xFexO films grown on Al2O3 (0001) by radio-frequency sputtering. J. Appl. Phys. 2004, 96, 4150–4153. [Google Scholar] [CrossRef]
- Malek, M.F.; Mamat, M.H.; Md Sin, N.D.; Rusop, M. Effects of Sn dopant on structural and optical properties of zno thin film prepared by sol-gel route. Appl. Mech. Mater. 2015, 773, 617–621. [Google Scholar] [CrossRef]
- Manimozhi, T.; Logu, T.; Archana, J.; Navaneethan, M.; Sethuraman, K.; Ramamurthi, K. Enhanced photo-response of CdTe Thin film via Mo doping prepared using electron beam evaporation technique. J. Mater. Sci. Mater. Electron. 2020, 31, 21059–21072. [Google Scholar] [CrossRef]
- Kumaravel, R.; Bhuvaneswari, S.; Ramamurthi, K.; Krishnakumar, V. Structural, optical and electrical properties of molybdenum-doped cadmium oxide thin films prepared by spray pyrolysis method. Appl. Phys. A 2012, 109, 579–584. [Google Scholar] [CrossRef]
- Gahlawat, S.; Singh, J.; Yadav, A.K.; Ingole, P.P. Exploring Burstein—Moss type effects in nickel doped hematite dendrite nanostructures for enhanced photo-electrochemical water splitting. Phys. Chem. Chem. Phys. 2019, 21, 20463–20477. [Google Scholar] [CrossRef]
- Coutts, J.; Hintze, P.; Meier, A.; Devor, R.; Surma, J.; Maloney, P.; Bauer, B.; Shah, M.; Mazyck, D. Visible-light-responsive photocatalysis: Ag-doped TiO2 catalyst development and reactor design testing. In Proceedings of the 46th International Conference on Environmental Systems, Vienna, Austria, 10–14 July 2016. [Google Scholar]
- Khaparde, R.; Acharya, S. Effect of isovalent dopants on photodegradation ability of ZnS nanoparticles. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2016, 163, 49–57. [Google Scholar] [CrossRef]
- Reddy, D.A.; Liu, C.; Vijayalakshmi, R.P.; Reddy, B.K. Effect of Al doping on the structural, optical and photoluminescence properties of ZnS nanoparticles. J. Alloys Compd. 2014, 582, 257–264. [Google Scholar] [CrossRef]
- Amaranatha Reddy, D.; Murali, G.; Vijayalakshmi, R.P.; Reddy, B.K.; Sreedhar, B. Effect of Cr doping on the structural and optical properties of ZnS nanoparticles. Cryst. Res. Technol. 2011, 46, 731–736. [Google Scholar] [CrossRef]
- Derbali, A.; Attaf, A.; Saidi, H.; Aida, M.S.; Benamra, H.; Attaf, R.; Attaf, N.; Ezzaouia, H.; Derbali, L. Br doping effect on structural, optical and electrical properties of ZnS thin films deposited by ultrasonic spray. Mater. Sci. Eng. B 2021, 268, 115135. [Google Scholar] [CrossRef]
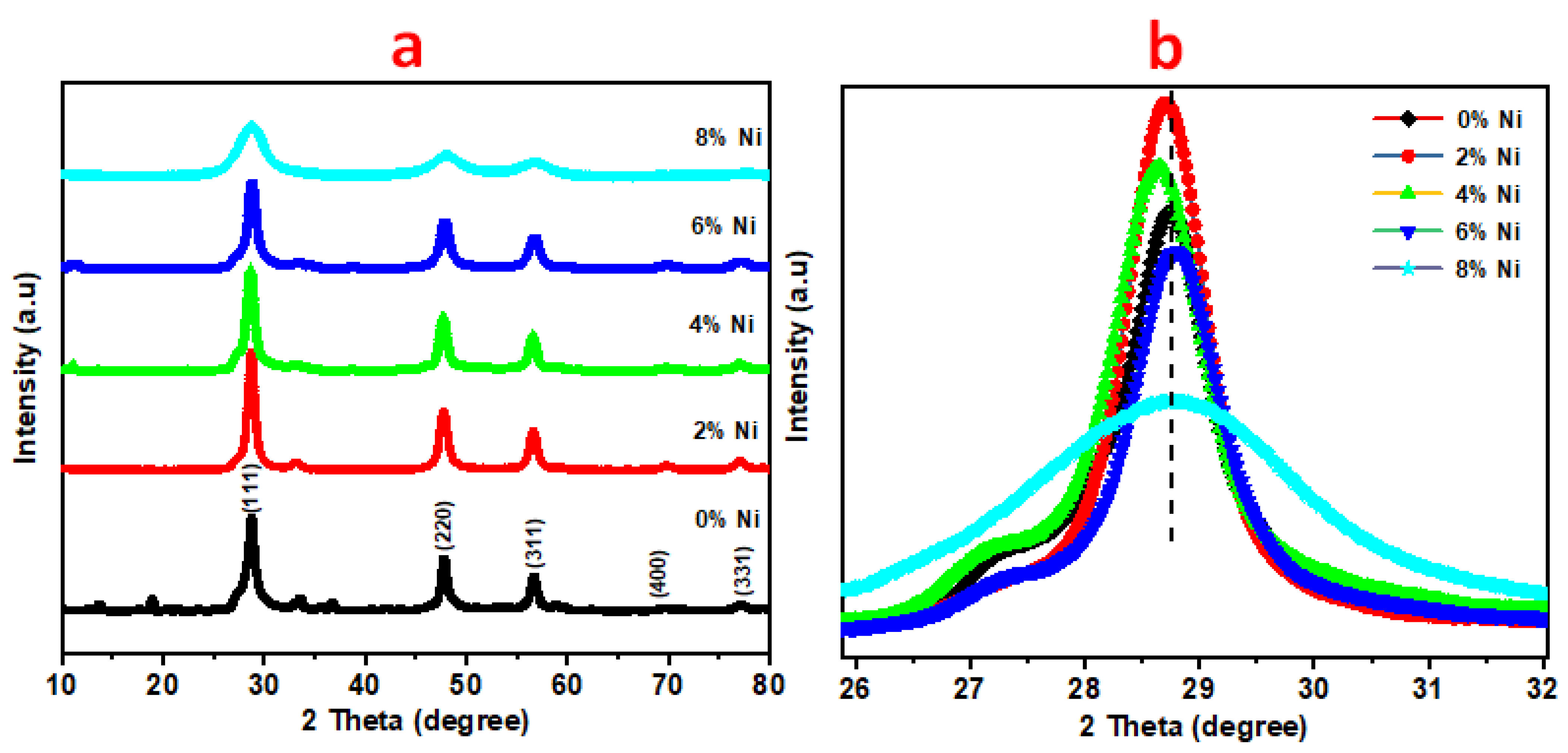

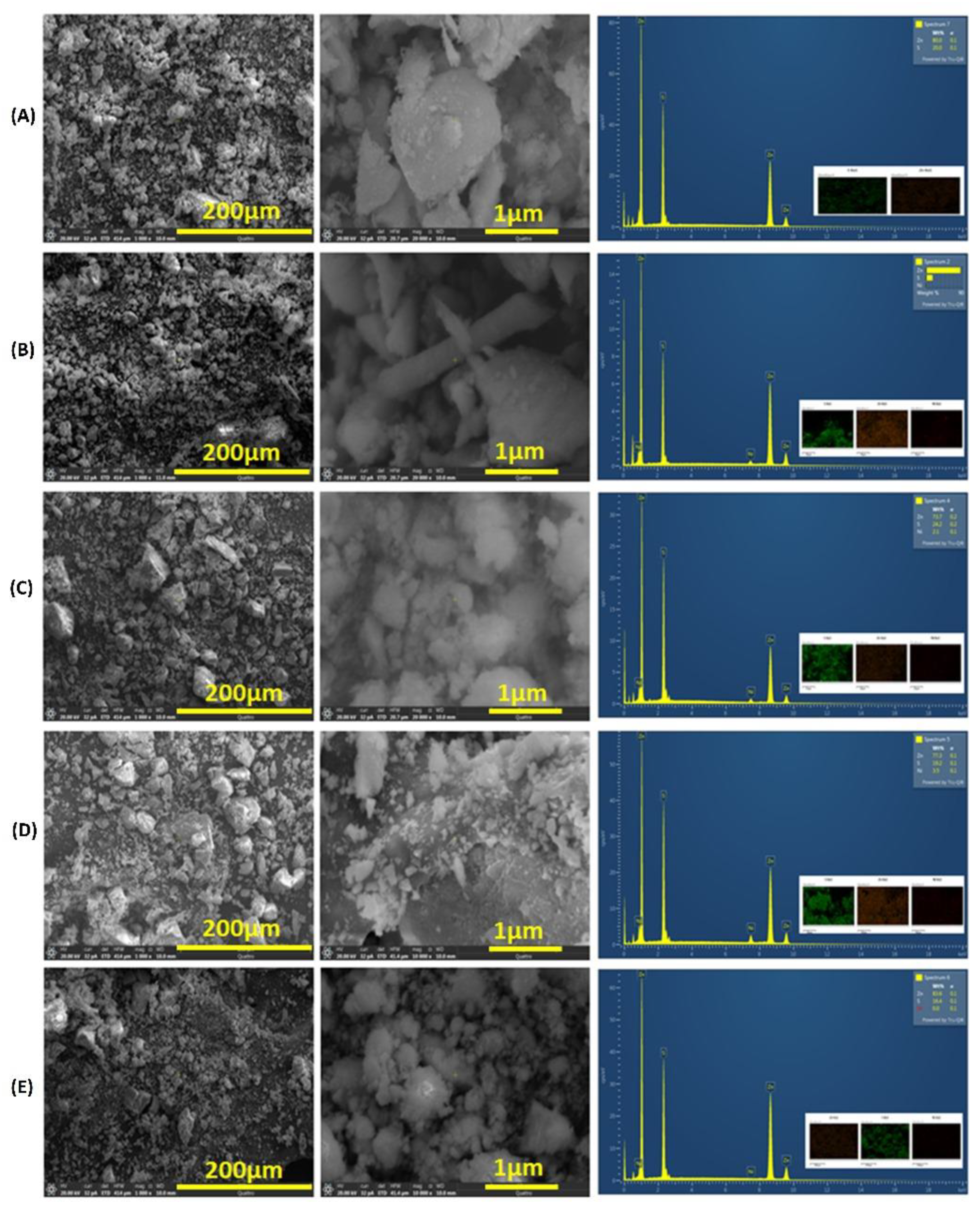
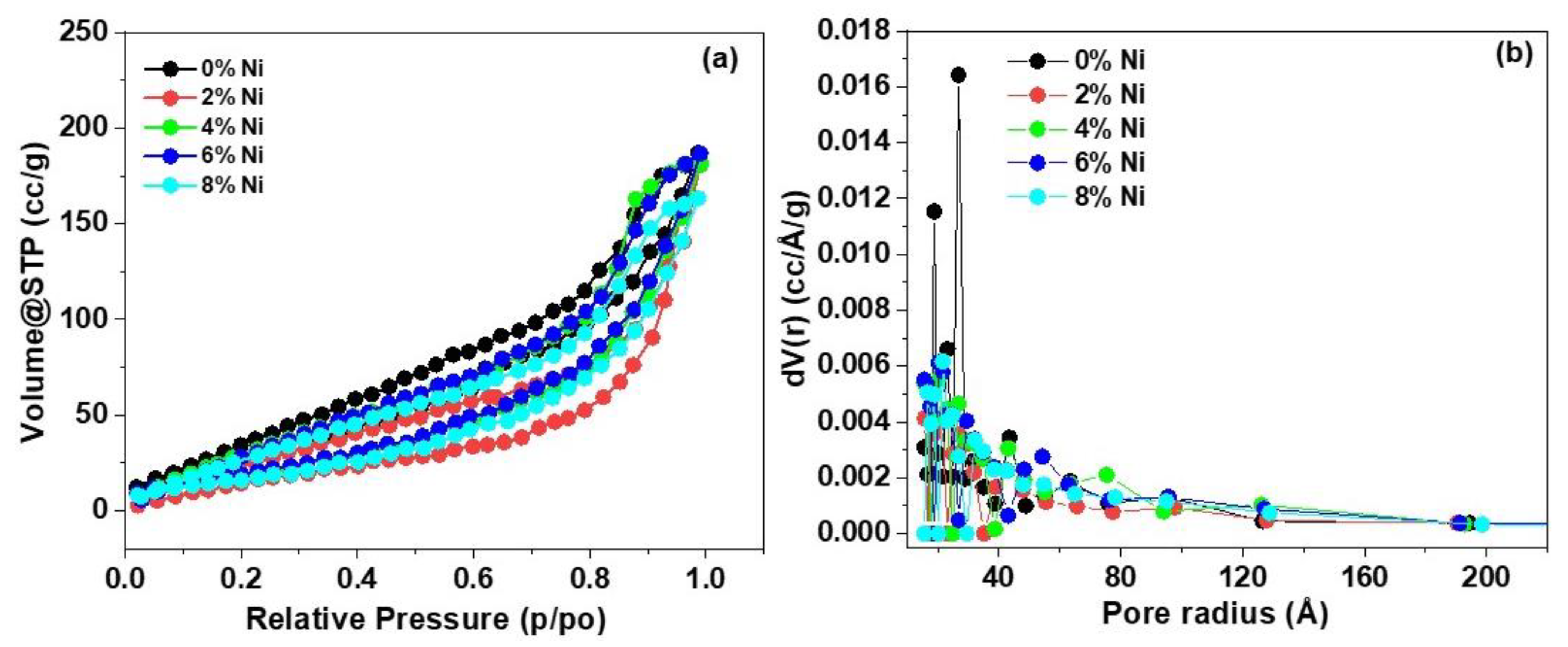
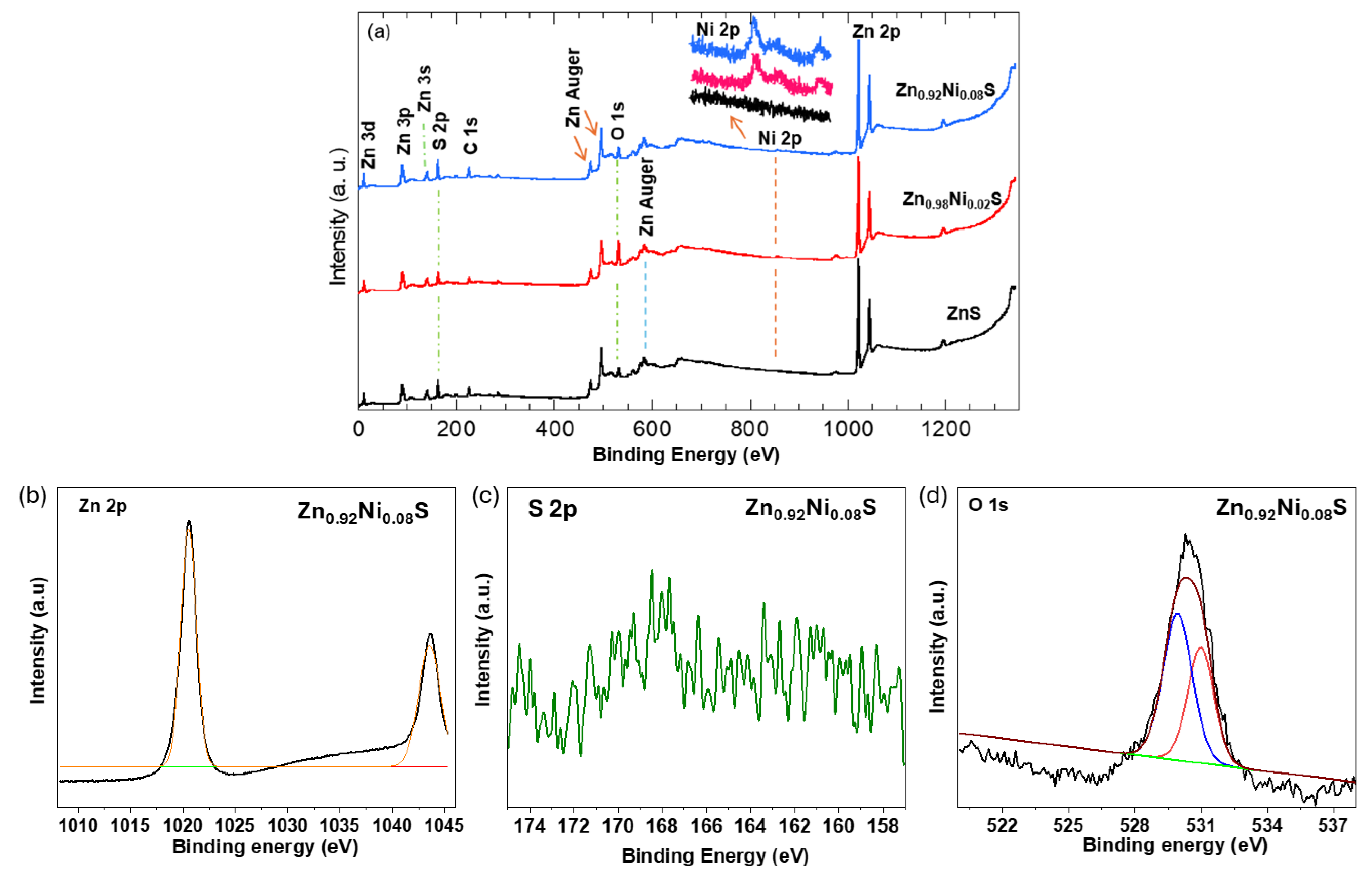
| Ni % | hkl | dhkl | Average Lattice Constant a | Average Crystal Size |
|---|---|---|---|---|
| 0 (ZnS) | 111 | 3.09257 | 5.364 | 5.27 nm |
| 220 | 1.89537 | |||
| 311 | 1.62041 | |||
| 2 | 111 | 3.08734 | 5.356 | 5.45 nm |
| 220 | 1.89301 | |||
| 311 | 1.61831 | |||
| 4 | 111 | 3.07986 | 5.358 | 5.83 nm |
| 220 | 1.89571 | |||
| 311 | 1.62147 | |||
| 6 | 111 | 3.0762 | 5.351 | 5.71 nm |
| 220 | 1.89377 | |||
| 311 | 1.61852 | |||
| 8 | 111 | 3.10657 | 5.386 | 5.60 nm |
| 220 | 1.90367 | |||
| 311 | 1.62588 |
| Materials | SBET/m2 g−1 | DBJH/Å | VBJH/cm3 g−1 |
|---|---|---|---|
| 0% Ni (ZnS) | 52.66 | 26.73 | 0.2804 |
| 2% Ni | 33.78 | 20.15 | 0.2287 |
| 4% Ni | 41.49 | 21.54 | 0.2931 |
| 6% Ni | 44.45 | 20.20 | 0.3014 |
| 8% Ni | 42.94 | 21.59 | 0.2637 |
| Materials | Preparation Method | Energy Band Gap (eV) | References |
|---|---|---|---|
| ZnS | Co-precipitation | 3.96 | [38] |
| ZnS:Mn | 4.07 | [38] | |
| ZnS:Cu | 3.92 | [38] | |
| Zn0.9Al0.1S | Co-precipitation | 4.01 | [39] |
| Zn0.92Al0.08S | 3.94 | [39] | |
| Zn0.97Cr0.03S | Co-precipitation | 4.03 | [16] |
| ZnS | Co-precipitation | 3.8 | [40] |
| Ni-doped ZnS | 3.7 | [40] | |
| ZnS:Br (2%) | Ultrasonic spraying | 3.8 | [41] |
| P25 | Standard | 3.15 | [37] |
| ZnS | Hydrothermal | 3.81 | This Work |
| Zn0.96Ni0.04S | Hydrothermal | 3.75 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alhassan, S.; Alshammari, A.H.; Alotibi, S.; Alshammari, K.; Mohamed, W.S.; Hadia, N.M.A. Structural and Optical Properties of Nickel-Doped Zinc Sulfide. Nanomaterials 2024, 14, 1599. https://doi.org/10.3390/nano14191599
Alhassan S, Alshammari AH, Alotibi S, Alshammari K, Mohamed WS, Hadia NMA. Structural and Optical Properties of Nickel-Doped Zinc Sulfide. Nanomaterials. 2024; 14(19):1599. https://doi.org/10.3390/nano14191599
Chicago/Turabian StyleAlhassan, Sultan, Alhulw H. Alshammari, Satam Alotibi, Khulaif Alshammari, W. S. Mohamed, and N. M. A. Hadia. 2024. "Structural and Optical Properties of Nickel-Doped Zinc Sulfide" Nanomaterials 14, no. 19: 1599. https://doi.org/10.3390/nano14191599





