Sodium-Ion Battery at Low Temperature: Challenges and Strategies
Abstract
:1. Introduction
2. Research Progress of SIBs in LT Environment
2.1. Anode
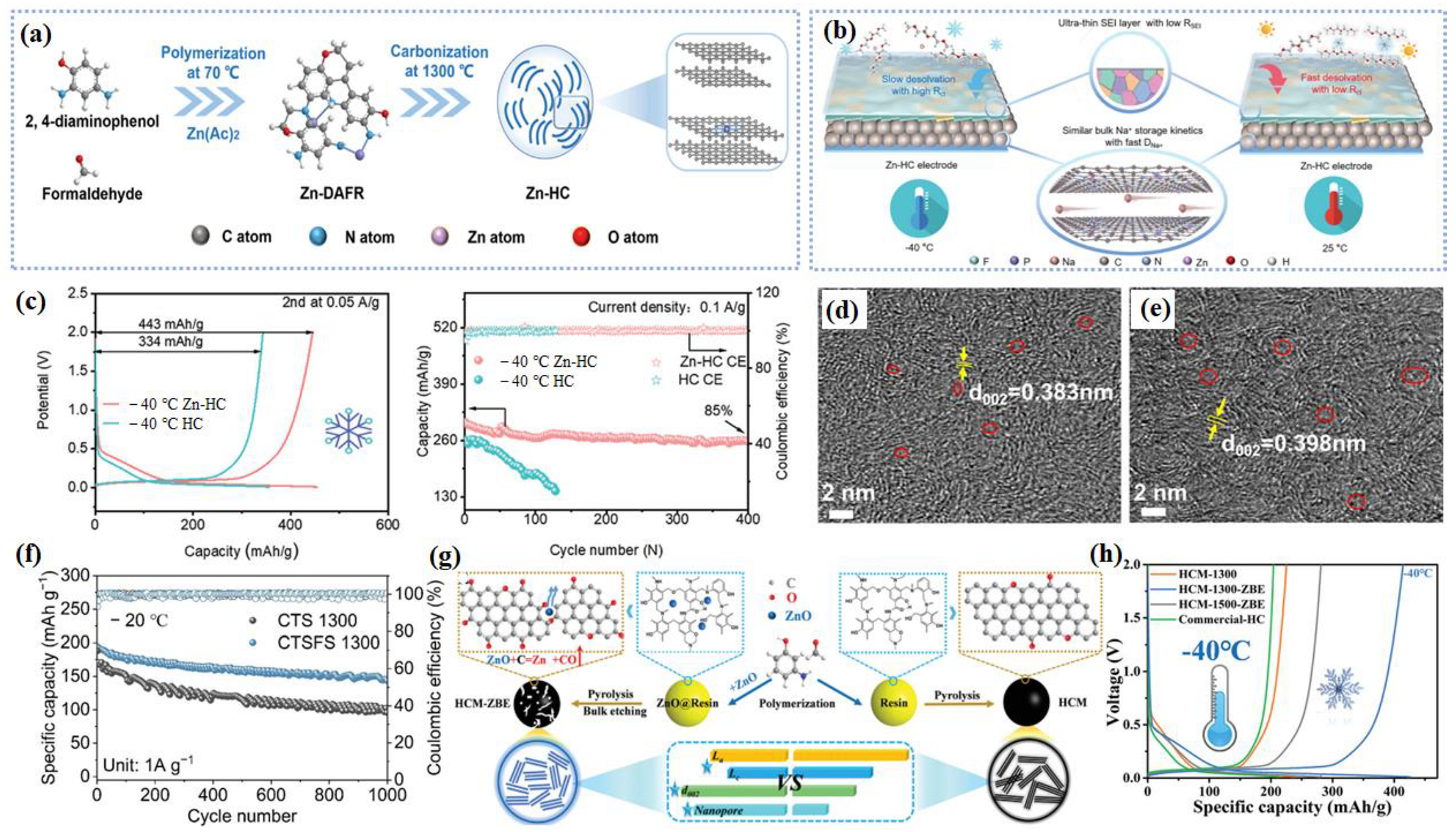
2.1.1. Carbon-Based Materials
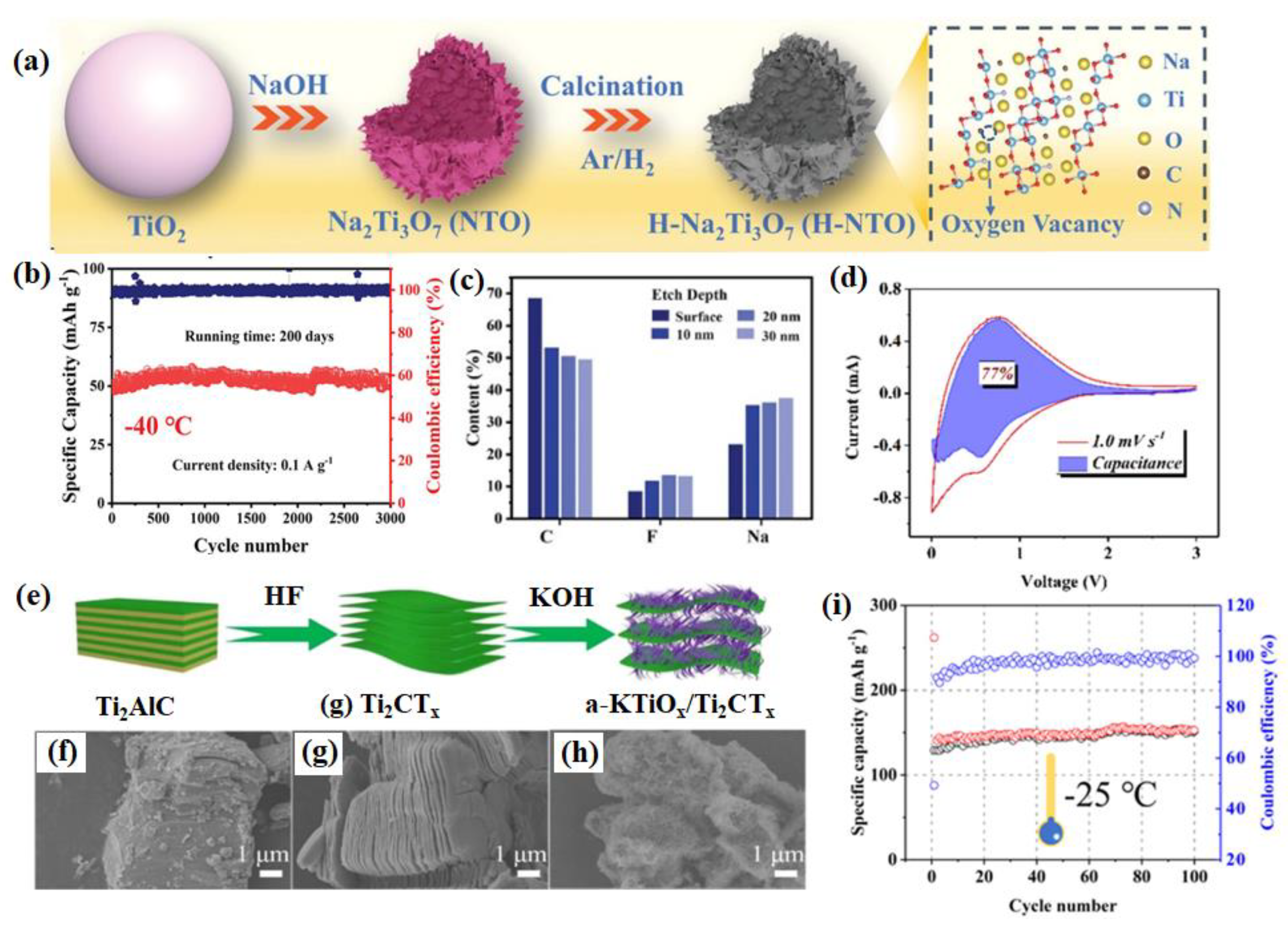
2.1.2. Titanium-Based Materials
2.1.3. Conversion and Alloy-Based Materials
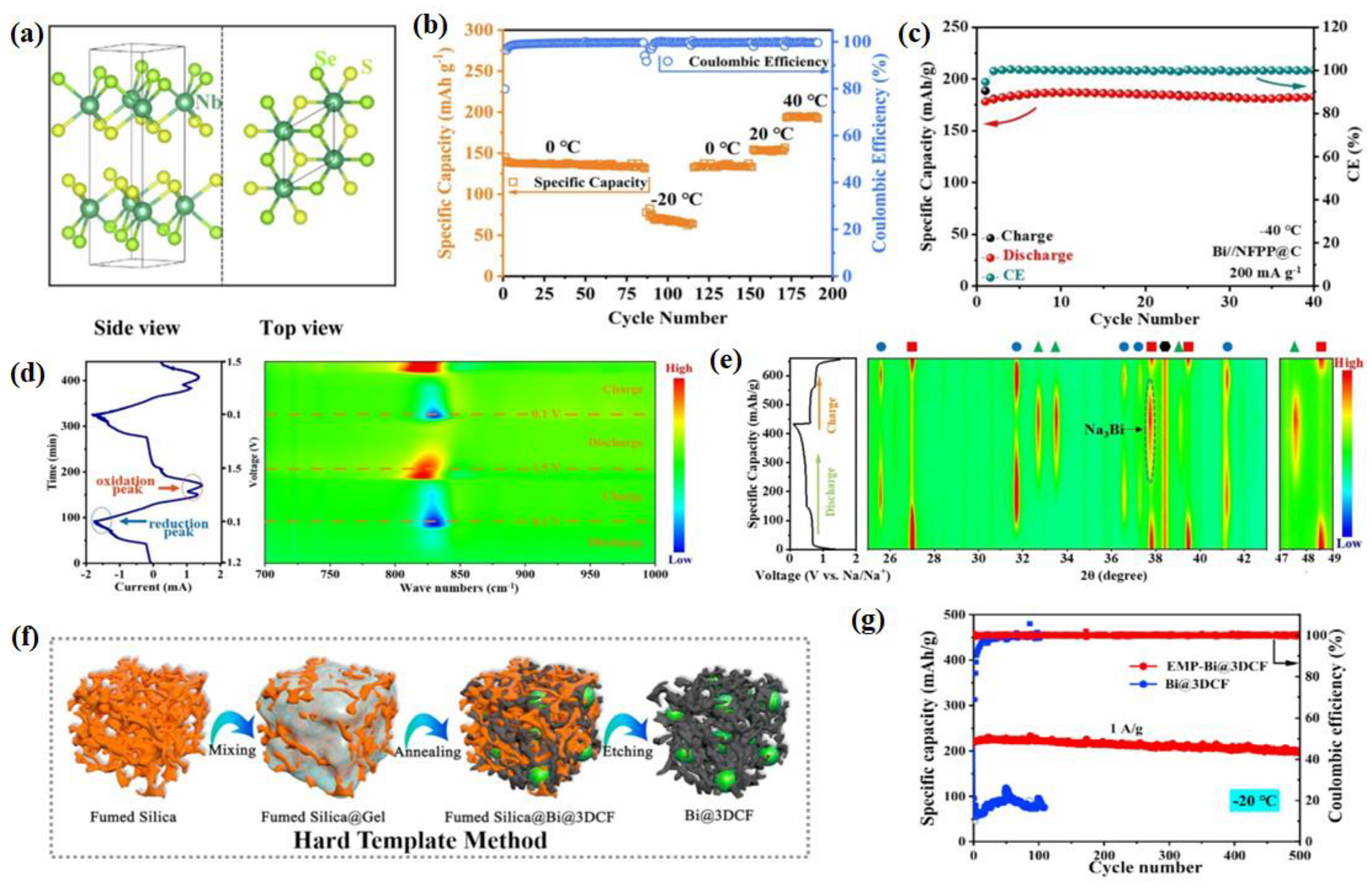
2.2. Cathode
2.2.1. Polyanionic Compounds
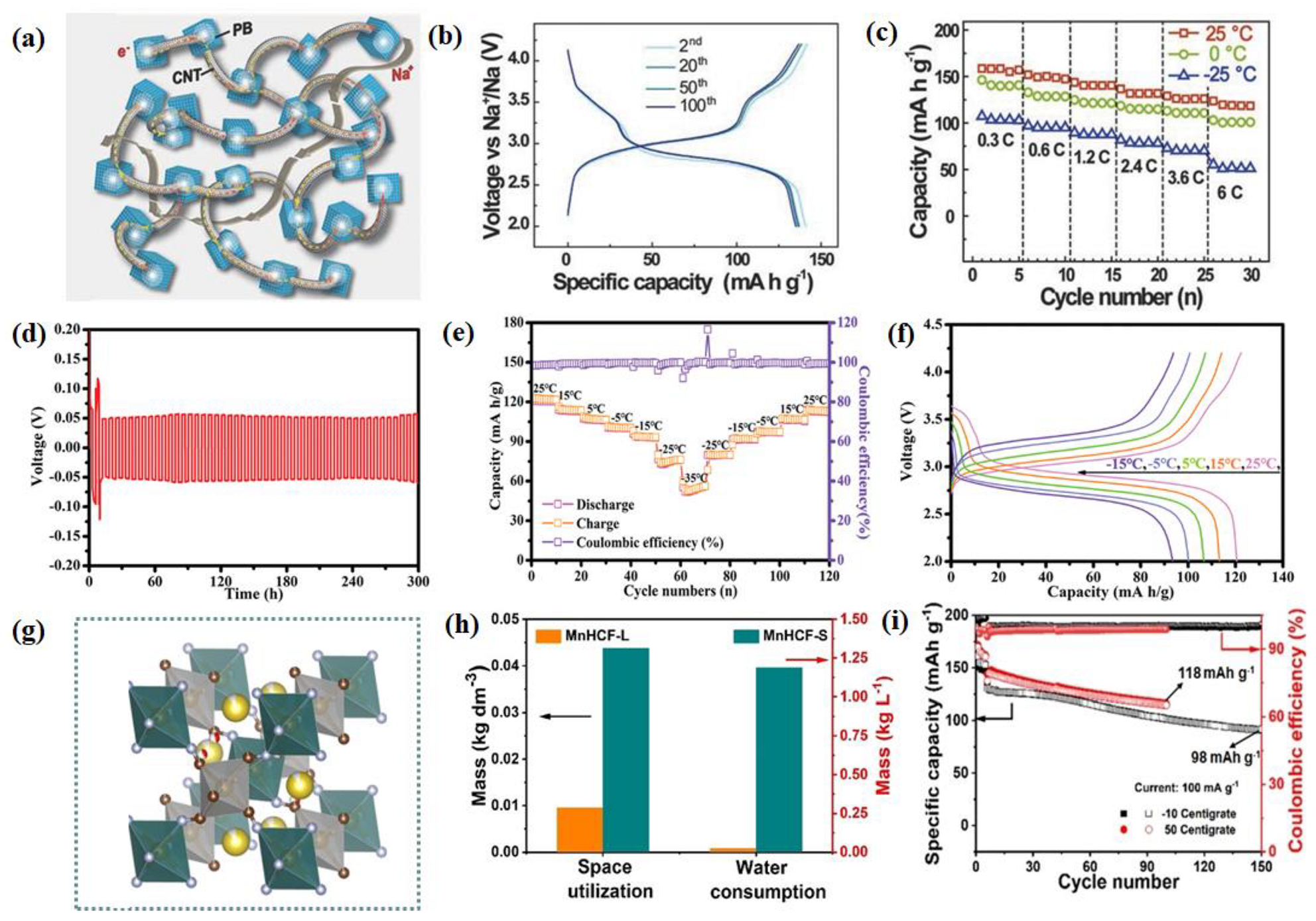
2.2.2. Prussian Blue and Prussian Blue Analogs
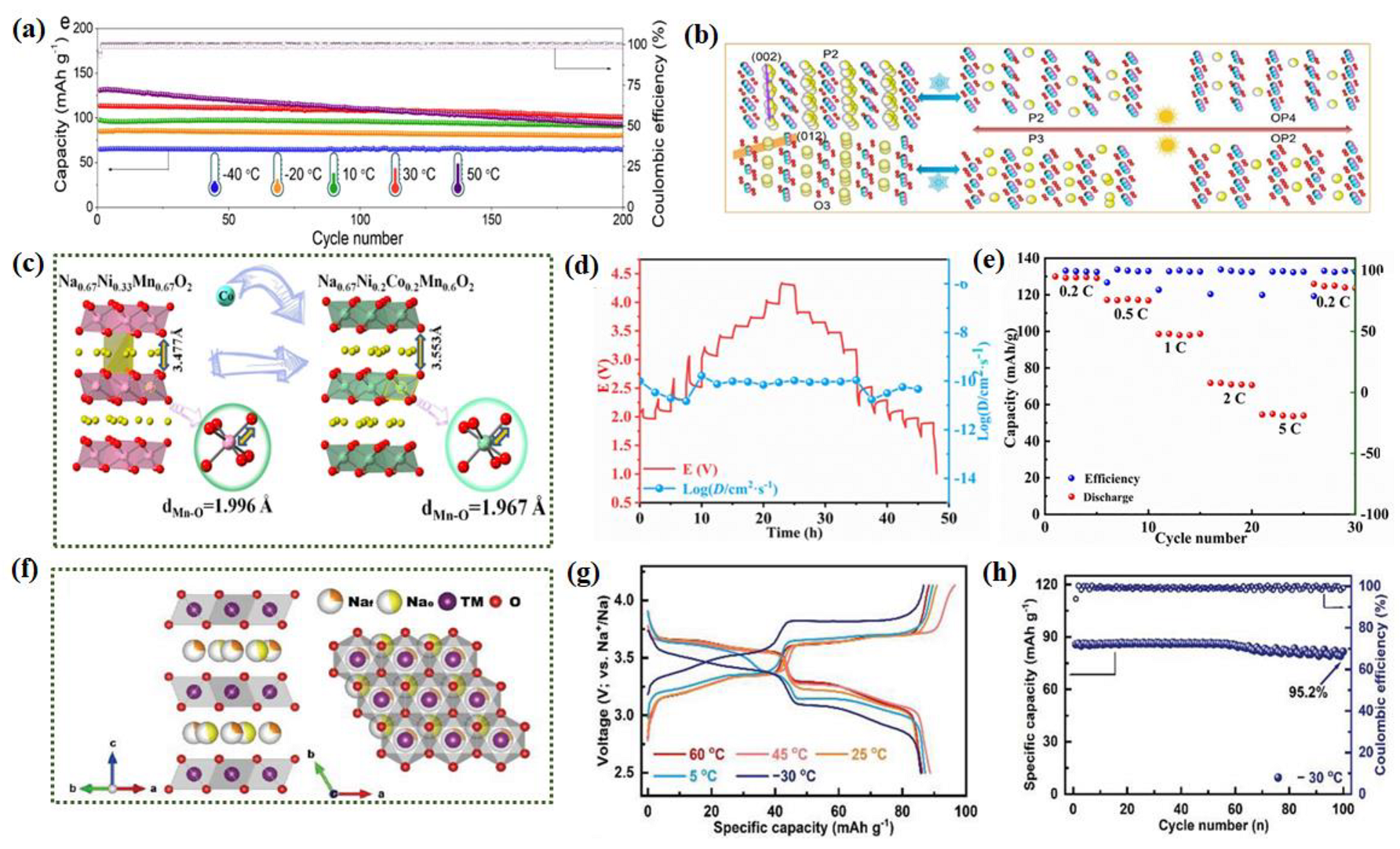
2.2.3. Layered Transition Metal Oxides
2.3. Electrolyte
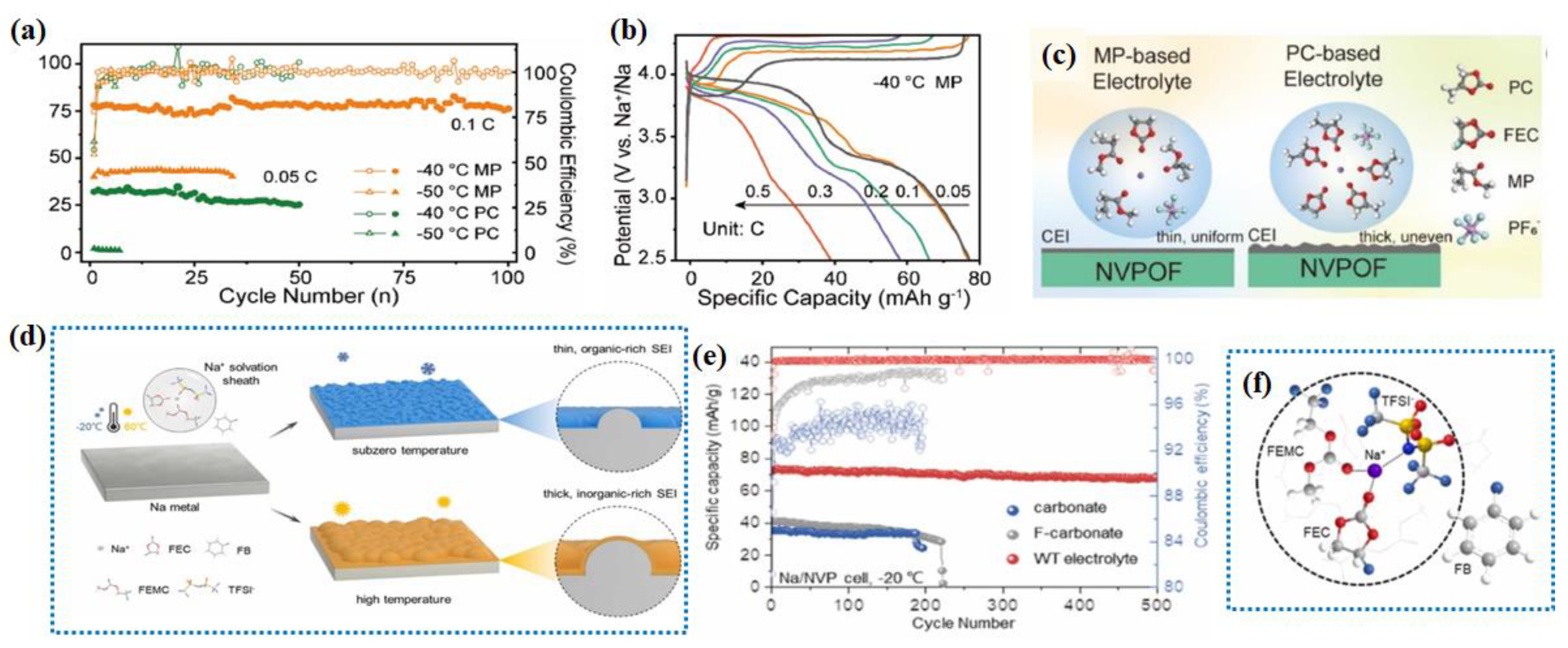
2.3.1. Ester-Based Electrolytes
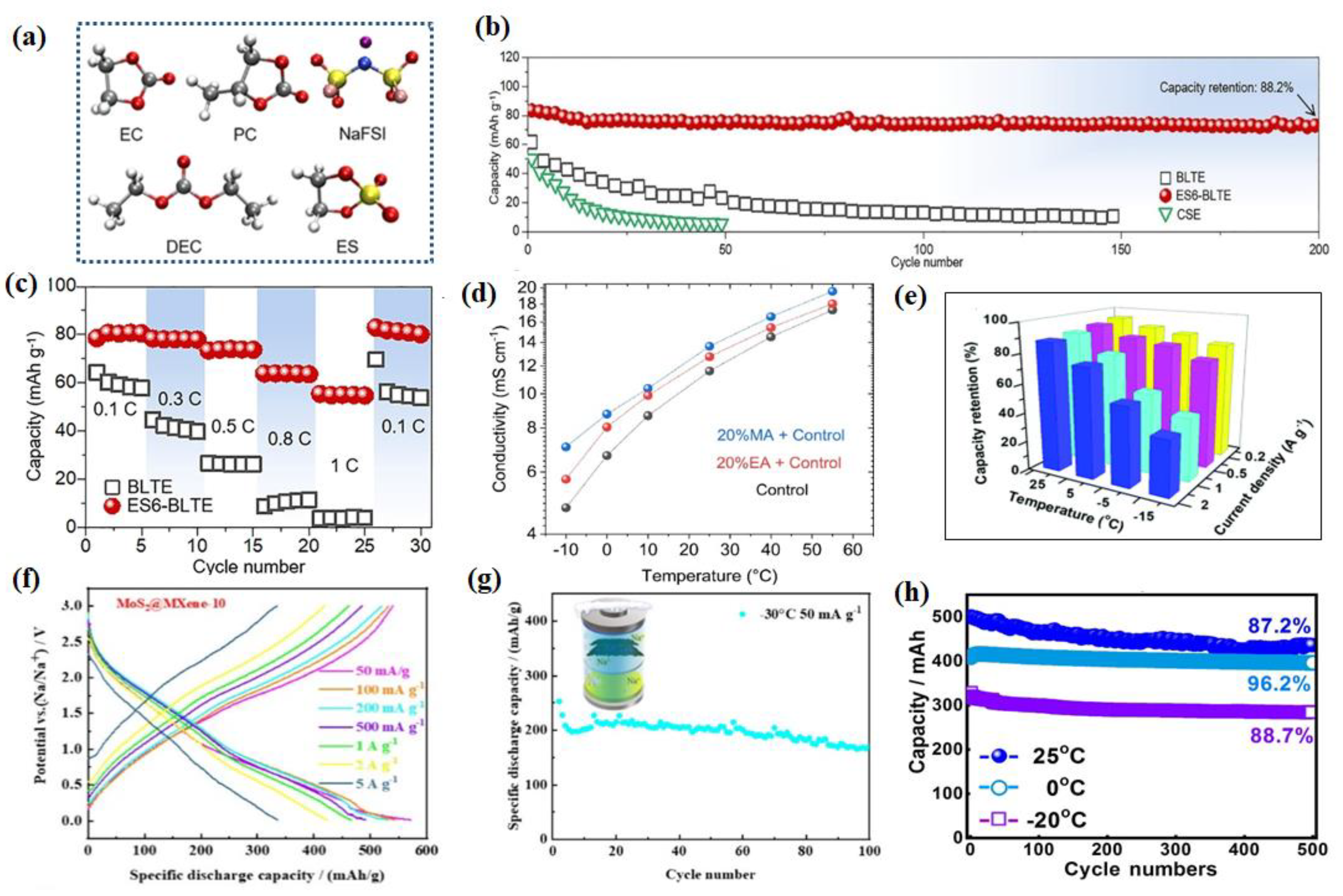
2.3.2. Ether-Based Electrolyte
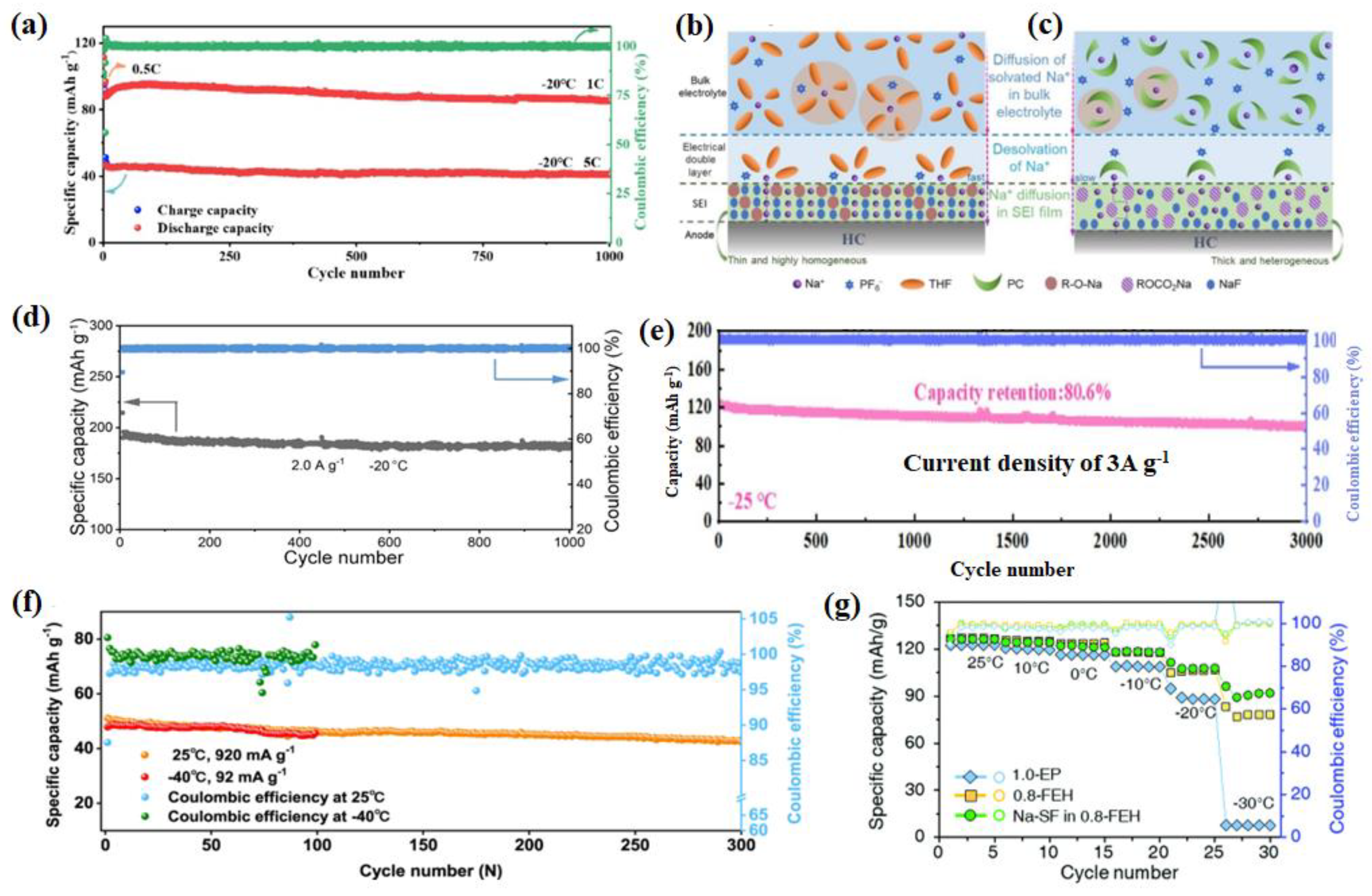
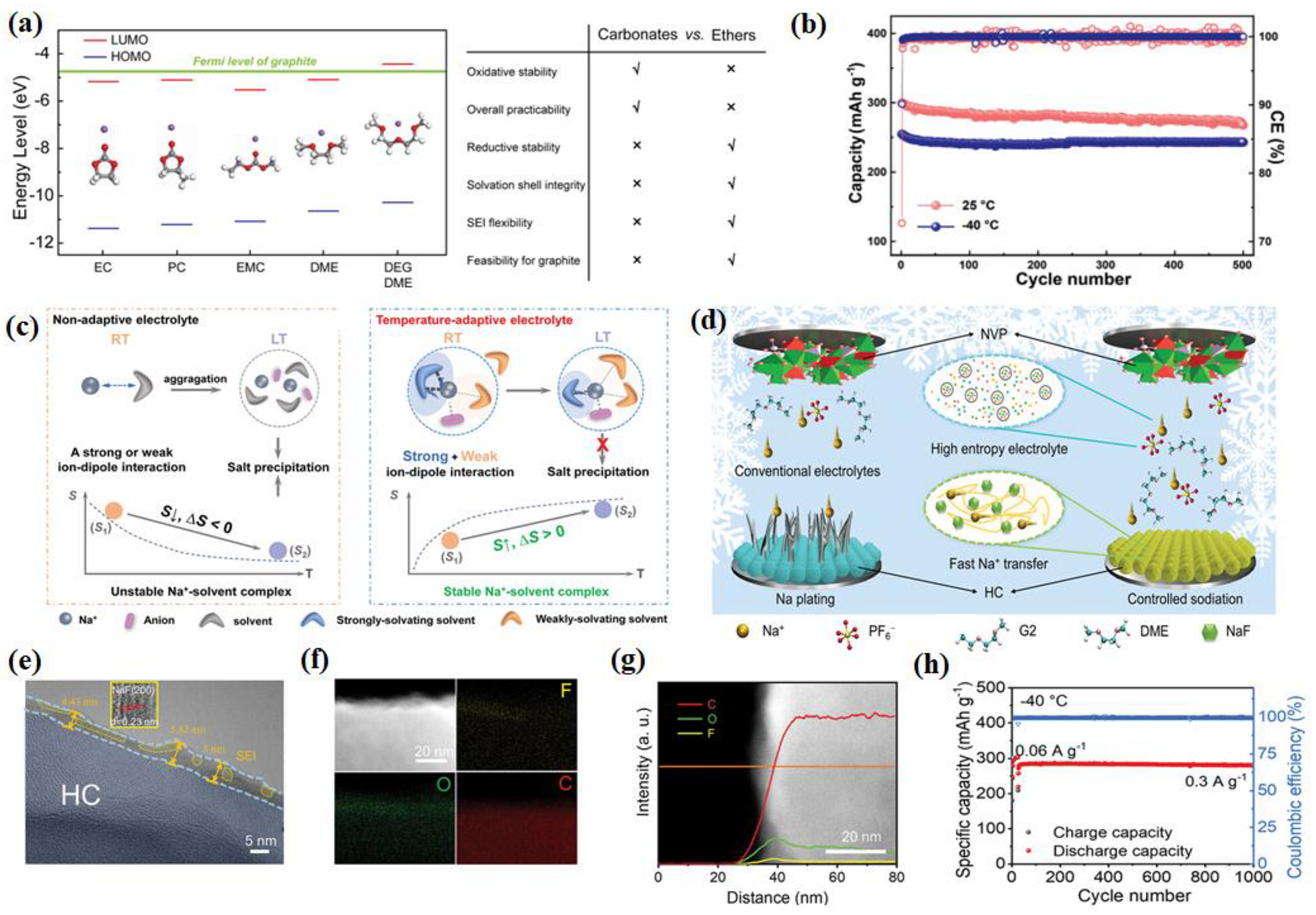
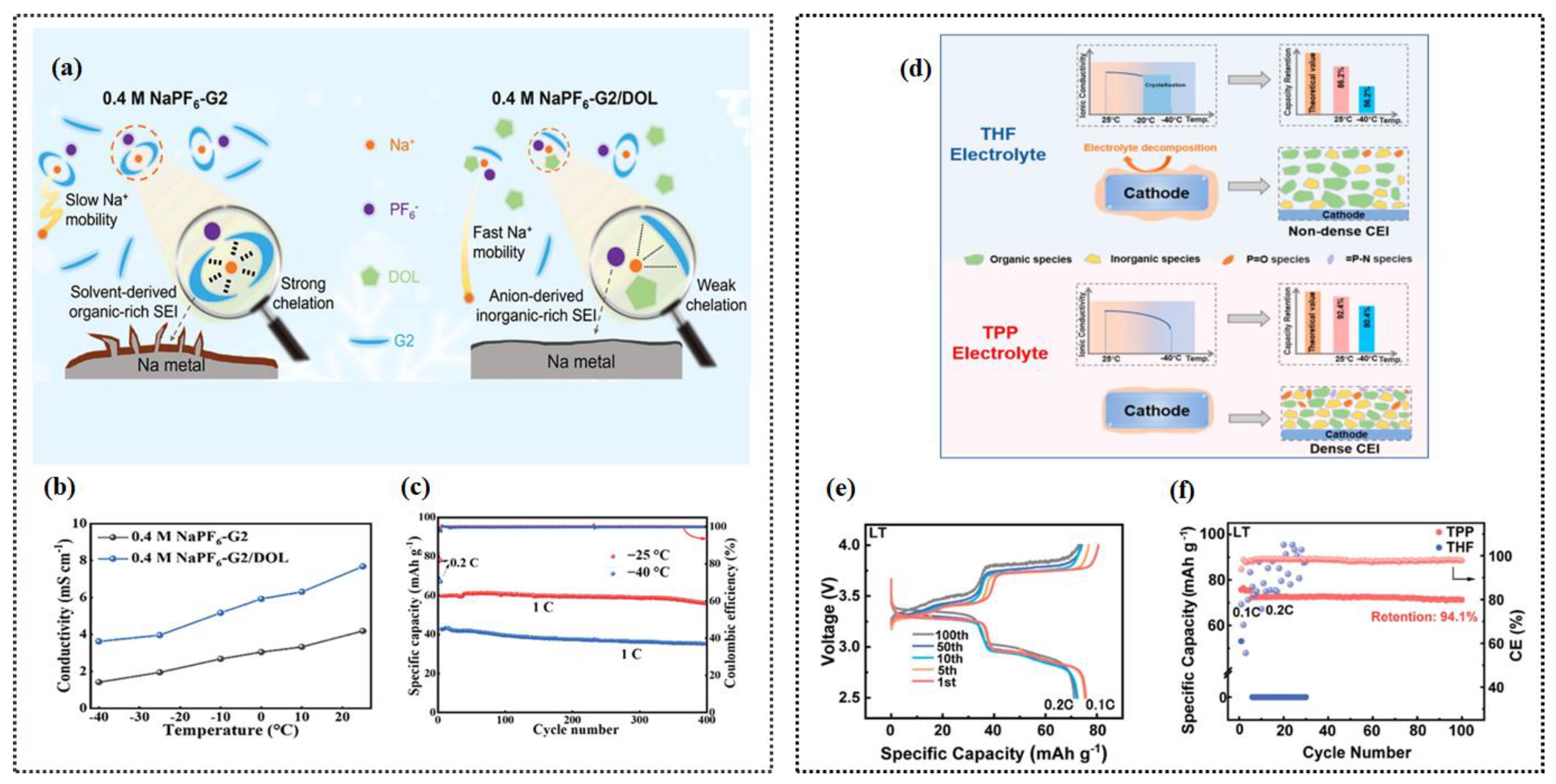
2.3.3. Other Electrolytes
3. Conclusions and Perspective
- (1)
- Innovative materials: New materials can revolutionize battery technology, especially SIBs. The advancement of batteries heavily relies on material development. For instance, materials with lots of active sites and good mass transfer can ensure fast reactions, high capacity, and long-lasting performance. Precisely arranging materials at a microscopic or even mesoscale using “precision chemistry” might enhance ion and electron movement in LT conditions. Ideally, materials with minimal energy barriers for ion and electron transfer could be universally applicable in such environments.
- (2)
- More suitable electrolytes: Traditional electrolytes usually use ester-based solvents and have been extensively studied in LIBs and SIBs. In contrast, emerging electrolytes based on ether solvents lack a comprehensive understanding of their fundamental properties and interactions with lithium/sodium ions and solvated structures. In-depth basic research on ether solvents, especially their relationship with sodium ions and various salts at LTs, including the effects of different solvents and salts on ion transport performance, electrochemical stability, and thermal stability, can provide valuable insights to guide the development of new LT electrolytes for SIBs.
- (3)
- Development of theoretical mechanisms: In recent decades, there have been advancements in new energy technologies, but our scientific understanding has not deepened much. While energy storage materials are progressing and new ideas are emerging, electrochemistry itself has not seen much conceptual innovation. Understanding the electrode and electrolyte interface, along with the dynamic nature of batteries, remains complex. A unified theory for electrochemistry at the system level is still lacking. Looking ahead, integrating computer technology may help establish more innovative theoretical frameworks.
- (4)
- Around safety: Solid electrolytes are promising for enhancing the safety and stability of sodium metal batteries (SMBs) by avoiding issues like leaks and flammability seen with liquid electrolytes. Future research will likely concentrate on boosting the electrical conductivity and strength of solid electrolytes to better match SMB requirements. Advanced techniques for characterization will be crucial for understanding how these cells work, especially at the nanoscale and during operation. This exploration will provide valuable insights for subsequent optimization efforts.
Funding
Conflicts of Interest
References
- Goodenough, J.B.; Park, K.-S. The Li-Ion Rechargeable Battery: A Perspective. J. Am. Chem. Soc. 2013, 135, 1167–1176. [Google Scholar] [CrossRef] [PubMed]
- Perveen, T.; Siddiq, M.; Shahzad, N.; Ihsan, R.; Ahmad, A.; Shahzad, M.I. Prospects in Anode Materials for Sodium Ion Batteries—A Review. Renew. Sustain. Energy Rev. 2020, 119, 109549. [Google Scholar] [CrossRef]
- Cui, X.; Wang, Y.; Wu, H.; Lin, X.; Tang, S.; Xu, P.; Liao, H.; Zheng, M.; Dong, Q. A Carbon Foam with Sodiophilic Surface for Highly Reversible, Ultra-Long Cycle Sodium Metal Anode. Adv. Sci. 2021, 8, 2003178. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.; Li, B.; Jiao, X.; Zhang, C.; Dai, X.; Song, J. Stable Cycling of Phosphorus Anode for Sodium-Ion Batteries through Chemical Bonding with Sulfurized Polyacrylonitrile. Adv. Funct. Mater. 2018, 28, 1801010. [Google Scholar] [CrossRef]
- Zheng, X.; Gu, Z.; Liu, X.; Wang, Z.; Wen, J.; Wu, X.; Luo, W.; Huang, Y. Bridging the Immiscibility of an All-Fluoride Fire Extinguishant with Highly-Fluorinated Electrolytes toward Safe Sodium Metal Batteries. Energy Environ. Sci. 2020, 13, 1788–1798. [Google Scholar] [CrossRef]
- Liu, W.; Chen, Z.; Zhang, Z.; Jiang, P.; Chen, Y.; Paek, E.; Wang, Y.; Mitlin, D. Lithium-Activated Sns–Graphene Alternating Nanolayers Enable Dendrite-Free Cycling of Thin Sodium Metal Anodes in Carbonate Electrolyte. Energy Environ. Sci. 2021, 14, 382–395. [Google Scholar] [CrossRef]
- Liu, X.; Lei, X.; Wang, Y.-G.; Ding, Y. Prevention of Na Corrosion and Dendrite Growth for Long-Life Flexible Na–Air Batteries. ACS Cent. Sci. 2021, 7, 335–344. [Google Scholar] [CrossRef]
- Nan, Z.; Deng, T.; Zhang, S.; Wang, C.; Chen, L.; Wang, C.; Fan, X. Critical Review on Low-Temperature Li-Ion/Metal Batteries. Adv. Mater. 2022, 34, 2107899. [Google Scholar]
- Xin, S.; Zhang, R.; Shi, P.; Chen, X.; Zhang, Q. How Does External Pressure Shape Li Dendrites in Li Metal Batteries? Adv. Energy Mater. 2021, 11, 2003416. [Google Scholar]
- He, Y.; Shang, W.; Tan, P. Insight into Rechargeable Batteries in Extreme Environment for Deep Space Exploration. Carbon Neutralization 2024, 3, 773–780. [Google Scholar] [CrossRef]
- Zheng, X.; Gu, Z.; Fu, J.; Wang, H.; Ye, X.; Huang, L.; Liu, X.; Wu, X.; Luo, W.; Huang, Y. Knocking Down the Kinetic Barriers Towards Fast-Charging and Low-Temperature Sodium Metal Batteries. Energy Environ. Sci. 2021, 14, 4936–4947. [Google Scholar] [CrossRef]
- Qiu, X.; Chen, Y.; Sun, Y.; Wang, Y.; Liang, Z.; Zhou, G.; Xue, Y.; Shi, L.; Jiang, J.; Kong, X.; et al. Research on Low-Temperature Sodium-Ion Batteries: Challenges, Strategies and Prospect. Energy Storage Mater. 2024, 72, 103760. [Google Scholar] [CrossRef]
- Irisarri, E.; Ponrouch, A.; Palacin, M.R. Review—Hard Carbon Negative Electrode Materials for Sodium-Ion Batteries. J. Electrochem. Soc. 2015, 162, A2476. [Google Scholar] [CrossRef]
- Ponrouch, A.; Palacín, M. On the High and Low Temperature Performances of Na-Ion Battery Materials: Hard Carbon as a Case Study. Electrochem. Commun. 2015, 54, 51–54. [Google Scholar] [CrossRef]
- Prabeer, B.; Liu, G.; Ling, C.D.; Tamaru, M.; Avdeev, M.; Chung, S.; Yamada, Y.; Yamada, A. Na2fep2o7: A Safe Cathode for Rechargeable Sodium-Ion Batteries. Chem. Mater. 2013, 25, 3480–3487. [Google Scholar]
- Zhao, J.; Mu, L.; Qi, Y.; Hu, Y.; Liu, H.; Dai, S. A Phase-Transfer Assisted Solvo-Thermal Strategy for Low-Temperature Synthesis of Na3(Vo1−Xpo4)2f1+2x Cathodes for Sodium-Ion Batteries. Chem. Commun. 2015, 51, 7160–7163. [Google Scholar] [CrossRef]
- Bai, Z.; Yao, Q.; Wang, M.; Meng, W.; Dou, S.; Liu, H.; Wang, N. Low-Temperature Sodium-Ion Batteries: Challenges and Progress. Adv. Energy Mater. 2024, 14, 2303788. [Google Scholar] [CrossRef]
- Zhu, G.; Wen, K.; Lv, W.; Zhou, X.; Liang, Y.; Yang, F.; Chen, Z.; Zou, M.; Li, J.; Zhang, Y.; et al. Materials Insights into Low-Temperature Performances of Lithium-Ion Batteries. J. Power Sources 2015, 300, 29–40. [Google Scholar] [CrossRef]
- Zhang, S.; Xu, K.; Jow, T. Charge and Discharge Characteristics of a Commercial LiCoO2-Based 18650 Li-Ion Battery. J. Power Sources 2006, 160, 1403–1409. [Google Scholar] [CrossRef]
- Ali, S.; Zhang, Y.; Yang, H.; Xu, T.; Wang, Y.; Cui, J.; Elshof, J.E.T.; Shan, C.; Xu, H.; Yuan, H. Altering the Alkaline Metal Ions in Lepidocrocite-Type Layered Titanate for Sodium-Ion Batteries. ACS Appl. Mater. Interfaces 2023, 15, 5028–5037. [Google Scholar] [CrossRef]
- Weng, J.; Zou, D.; Yuan, W.; Zhou, P.; Ding, M.; Zhou, J.; Cong, H.; Cheng, F. Bimetallic Selenide Heterostructure with Directional Built-in Electric-Field Confined in N-Doped Carbon Nanofibers for Superior Sodium Storage with Ultralong Lifespan. J. Energy Chem. 2024, 91, 407–416. [Google Scholar] [CrossRef]
- Wei, Z.; Zhang, Y.; Li, M.; Shu, C.; Deng, D. Hierarchically Interconnected Ws2/Graphene Foam for High-Performance Sodium Storage. J. Energy Storage 2024, 81, 110115. [Google Scholar] [CrossRef]
- Park, J.; Han, J.; Gim, J.; Garcia, J.; Iddir, H.; Ahmed, S.; Xu, G.-L.; Amine, K.; Johnson, C.; Jung, Y.; et al. Evidence of Zintl Intermediate Phase and Its Impacts on Li and Na Storage Performance of Pb-Based Alloying Anodes. Chem. Mater. 2023, 35, 4171–4180. [Google Scholar] [CrossRef]
- Kang, H.; Liu, Y.; Cao, K.; Zhao, Y.; Jiao, L.; Wang, Y.; Yuan, H. Update on Anode Materials for Na-Ion Batteries. J. Mater. Chem. A 2015, 3, 17899–17913. [Google Scholar] [CrossRef]
- Chen, C.-C.; Maier, J. Space Charge Storage in Composites: Thermodynamics. Phys. Chem. Chem. Phys. 2017, 19, 6379–6396. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.; Dou, S.; Yu, Y. The State and Challenges of Anode Materials Based on Conversion Reactions for Sodium Storage. Small 2018, 14, e1703671. [Google Scholar] [CrossRef]
- Li, X.; Zeng, L.; Lai, W.; Lei, Z.; Ge, M.; Fang, C.; Weng, B.; Chen, Q.; Wei, M.; Qian, Q. Bisb Alloy Anchored on Selenium Doped Carbon Nanofibers as Highly Stable Anode Materials for Sodium/Potassium-Ion Batteries. Energy Fuels 2024, 38, 16966–16975. [Google Scholar] [CrossRef]
- Iqbal, S.; Wang, L.; Kong, Z.; Zhai, Y.; Wang, F.; Jing, Z.; Sun, X.; Wang, B.; He, X.; Dou, J.; et al. 2d Se-Rich Znse/Cose2@C Heterostructured Composite as Ultrastable Anodes for Alkaline-Ion Batteries. Small 2024, 2404193. [Google Scholar] [CrossRef]
- Wang, H.; Niu, H.; Shu, K.; Sun, L.; Wang, Y.; Du, Y.; Han, Y.; Yang, C.; Kang, Y.-M. Regulating the “Core-Shell” Microstructure of Hard Carbon through Sodium Hydroxide Activation for Achieving High-Capacity Sibs Anode. J. Mater. Sci. Technol. 2025, 209, 161–170. [Google Scholar] [CrossRef]
- Zhao, B.; Suo, G.; Mu, R.; Lin, C.; Li, J.; Hou, X.; Ye, X.; Yang, Y.; Zhang, L. Constructing Hierarchical Mos2/Ws2 Heterostructures in Dual Carbon Layer for Enhanced Sodium Ions Batteries Performance. J. Colloid Interface Sci. 2024, 668, 565–574. [Google Scholar] [CrossRef]
- Lan, D.; Zhao, Y.; Liu, Y.; Zhu, N.; Cui, J. Self-Assembled Nano-Mns@N,P Dual-Doped Lignite Based Carbon as High-Performance Sodium-Ion Batteries Anode. J. Energy Storage 2024, 90, 111827. [Google Scholar] [CrossRef]
- Mehek, R.; Iqbal, N.; Javed, O.; Noor, T.; Liu, W. Improved Rate Capability and Long Cycle Life of Metal-Organic Framework Derived TiO2@V2o5 Composite as an Efficient Cathode for Sodium-Ion Batteries. J. Energy Storage 2024, 78, 109921. [Google Scholar] [CrossRef]
- Lee, M.; Kim, M.-S.; Oh, J.-M.; Park, J.K.; Paek, S.-M. Hybridization of Layered Titanium Oxides and Covalent Organic Nanosheets into Hollow Spheres for High-Performance Sodium-Ion Batteries with Boosted Electrical/Ionic Conductivity and Ultralong Cycle Life. ACS Nano 2023, 17, 3019–3036. [Google Scholar] [CrossRef] [PubMed]
- Herreros-Lucas, C.; Vila-Fungueiriño, J.M.; Giménez-López, M.d.C. Electrochemically Versatile Graphite Nanoplatelets Prepared by a Straightforward, Highly Efficient, and Scalable Route. ACS Appl. Mater. Interfaces 2023, 15, 21375–21383. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Hou, B.; Guo, J.; Ning, Q.; Pang, W.; Wang, J.; Lü, C.; Wu, X. An Ultralong Lifespan and Low-Temperature Workable Sodium-Ion Full Battery for Stationary Energy Storage. Adv. Energy Mater. 2018, 8, 1703252. [Google Scholar] [CrossRef]
- Tian, Z.; Zhang, Y.; Zhu, J.; Li, Q.; Liu, T.; Antonietti, M. A Reanalysis of the Diverse Sodium Species in Carbon Anodes for Sodium Ion Batteries: A Thermodynamic View. Adv. Energy Mater. 2021, 11, 2102489. [Google Scholar] [CrossRef]
- Dou, X.; Hasa, I.; Saurel, D.; Vaalma, C.; Wu, L.; Buchholz, D.; Bresser, D.; Komaba, S.; Passerini, S. Hard Carbons for Sodium-Ion Batteries: Structure, Analysis, Sustainability, and Electrochemistry. Mater. Today 2019, 23, 87–104. [Google Scholar] [CrossRef]
- Shao, W.; Shi, H.; Jian, X.; Wu, Z.-S.; Hu, F. Hard-Carbon Anodes for Sodium-Ion Batteries: Recent Status and Challenging Perspectives. Adv. Energy Sustain. Res. 2022, 3, 2200009. [Google Scholar] [CrossRef]
- Chu, Y.; Zhang, J.; Zhang, Y.; Li, Q.; Jia, Y.; Dong, X.; Xiao, J.; Tao, Y.; Yang, Q. Reconfiguring Hard Carbons with Emerging Sodium-Ion Batteries: A Perspective. Adv. Mater. 2023, 35, 2212186. [Google Scholar] [CrossRef]
- Lu, Z.; Wang, J.; Feng, W.; Yin, X.; Feng, X.; Zhao, S.; Li, C.; Wang, R.; Huang, Q.A.; Zhao, Y. Zinc Single-Atom-Regulated Hard Carbons for High-Rate and Low-Temperature Sodium-Ion Batteries. Adv. Mater. 2023, 35, e2211461. [Google Scholar] [CrossRef]
- Yuan, M.; Yu, S.; Wang, K.; Mi, C.; Shen, L. Ultrafast Synthesis of Hard Carbon for High-Rate and Low-Temperature Sodium-Ion Storage through Flash Joule Heating. Solid State Ion. 2024, 414, 116622. [Google Scholar] [CrossRef]
- Yin, X.; Lu, Z.; Wang, J.; Feng, X.; Roy, S.; Liu, X.; Yang, Y.; Zhao, Y.; Zhang, J. Enabling Fast Na+ Transfer Kinetics in the Whole-Voltage-Region of Hard-Carbon Anodes for Ultrahigh-Rate Sodium Storage. Adv. Mater. 2022, 34, 2109282. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Zeng, M.; She, Y.; Lin, X.; Yang, D.; Qin, Y.; Rui, X. Enhanced Low-Temperature Sodium Storage Kinetics in a Nati2(Po4)3@C Nanocomposite. J. Power Sources 2020, 477, 228735. [Google Scholar] [CrossRef]
- Lin, D.; Li, K.; Wang, Q.; Lyu, L.; Li, B.; Zhou, L. Rate-Independent and Ultra-Stable Low-Temperature Sodium Storage in Pseudocapacitive TiO2 Nanowires. J. Mater. Chem. A 2019, 7, 19297–19304. [Google Scholar] [CrossRef]
- Zhong, W.; Tao, M.; Tang, W.; Gao, W.; Yang, T.; Zhang, Y.; Zhan, R.; Bao, S.-J.; Xu, M. Mxene-Derivative Pompon-Like Na2ti3o7@C Anode Material for Advanced Sodium Ion Batteries. Chem. Eng. J. 2019, 378, 122209. [Google Scholar] [CrossRef]
- Pan, J.; Wang, N.; Li, L.; Zhang, F.; Cheng, Z.; Li, Y.; Yang, J.; Qian, Y. Improved Na Storage and Coulombic Efficiency in Tip2o7@C Microflowers for Sodium Ion Batteries. Nano Res. 2021, 14, 139–147. [Google Scholar] [CrossRef]
- Meng, W.; Dang, Z.; Li, D.; Jiang, L. Long-Cycle-Life Sodium-Ion Battery Fabrication via a Unique Chemical Bonding Interface Mechanism. Adv. Mater. 2023, 35, 2301376. [Google Scholar] [CrossRef]
- Lai, Q.; Mu, J.; Liu, Z.; Zhao, L.; Gao, X.; Yang, D.; Chen, H.; Luo, W. Tunnel-Type Na2ti6o13@Carbon Nanowires as Anode Materials for Low-Temperature Sodium-Ion Batteries. Batter. Supercaps 2023, 6, e202200549. [Google Scholar] [CrossRef]
- Li, J.; Tang, S.; Li, Z.; Ding, Z.; Wang, T.; Wang, C. Cross-Linked Amorphous Potassium Titanate Nanobelts/Titanium Carbide Mxene Nanoarchitectonics for Efficient Sodium Storage at Low Temperature. J. Colloid Interface Sci. 2023, 629, 461–472. [Google Scholar] [CrossRef]
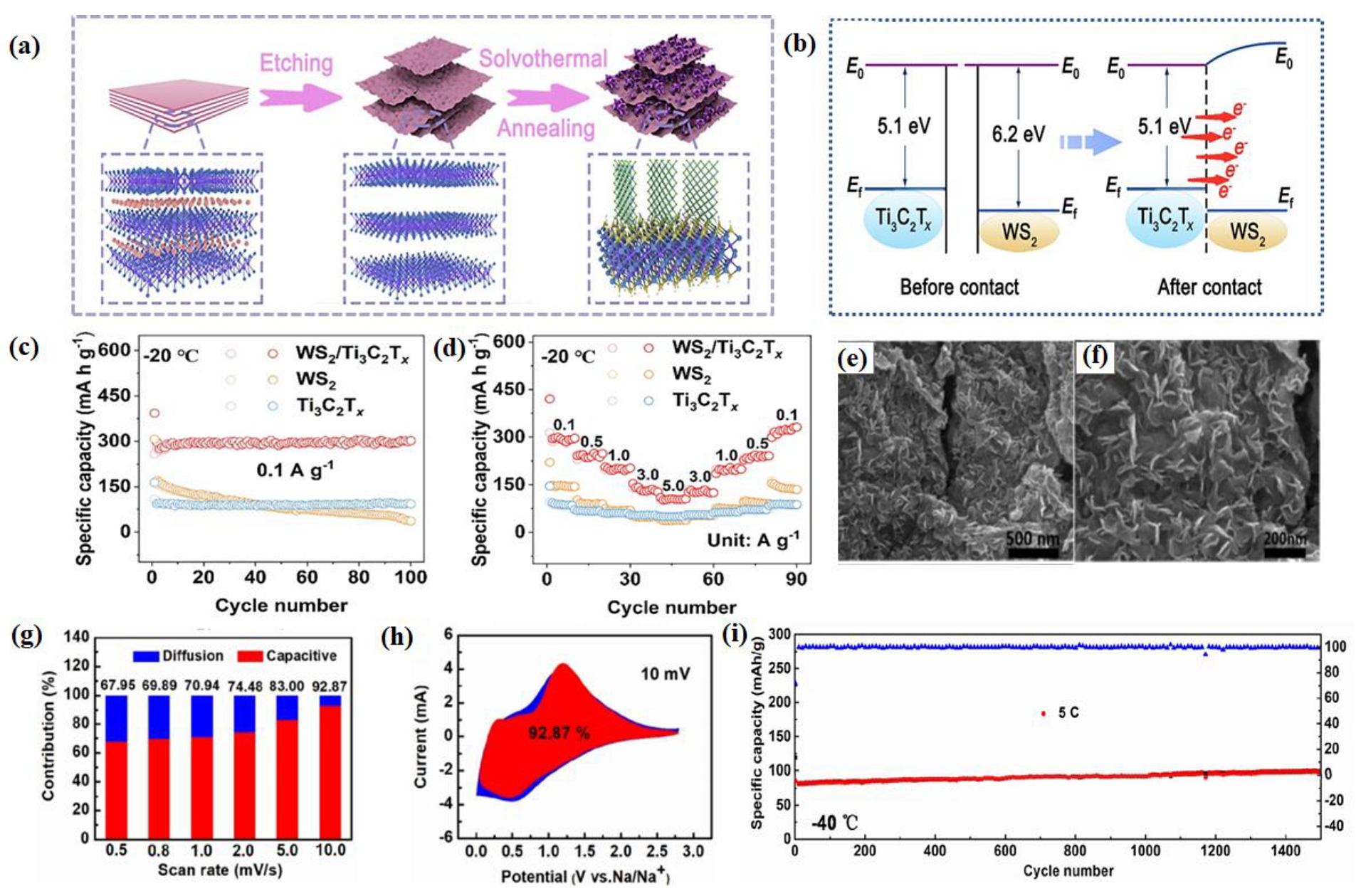
- Li, J.; Tang, S.; Hao, J.; Yuan, Q.; Wang, T.; Pan, L.; Li, J.; Yang, S.; Wang, C. Interfacial Built-in Electric Field and Crosslinking Pathways Enabling Ws2/Ti3c2t Heterojunction with Robust Sodium Storage at Low Temperature. J. Energy Chem. 2024, 89, 635–645. [Google Scholar] [CrossRef]
- Deng, D.; Cui, X.; Wu, Q.; Zheng, M.; Dong, Q. In-Situ Synthesis TiO2 Nanosheets@Rgo for Ultrafast Sodium Ion Storage at Both Room and Low Temperatures. J. Alloys Compd. 2020, 835. [Google Scholar] [CrossRef]
- Chen, C.; Wen, Y.; Hu, X.; Ji, X.; Yan, M.; Mai, L.; Hu, P.; Shan, B.; Huang, Y. Na+ Intercalation Pseudocapacitance in Graphene-Coupled Titanium Oxide Enabling Ultra-Fast Sodium Storage and Long-Term Cycling. Nat. Commun. 2015, 6, 6929. [Google Scholar] [CrossRef] [PubMed]
- Tian, Y.; Lu, J.; Tang, H.; Wang, X.; Zhang, L.; Hu, P.; Zhou, L.; Wang, Y.; Guo, Y.; Khatoon, R.; et al. An Ultra-Stable Anode Material for High/Low-Temperature Workable Super-Fast Charging Sodium-Ion Batteries. Chem. Eng. J. 2021, 422, 130054. [Google Scholar] [CrossRef]
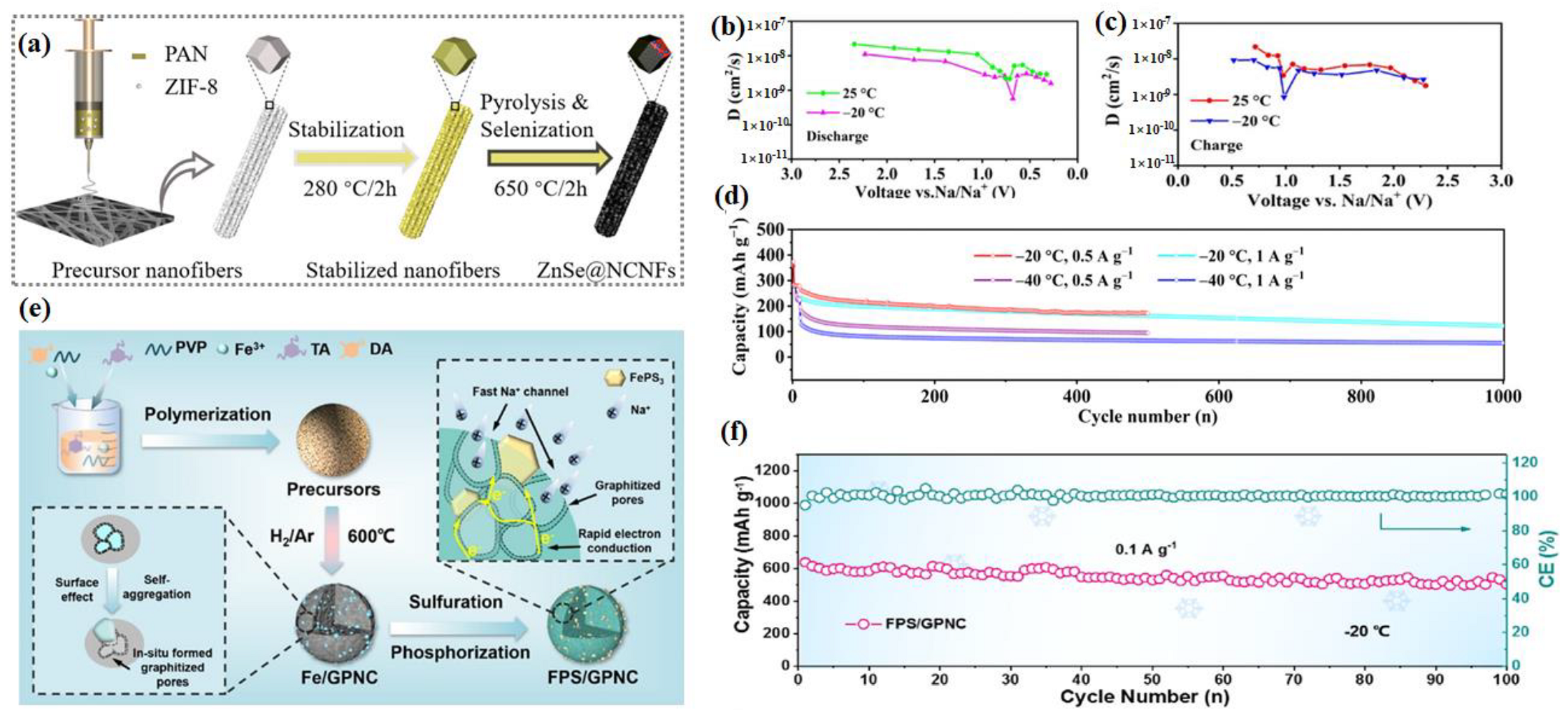
- Wang, X.; Zhao, W.; Zhang, W.; Wong, K.W.; Wu, J.; Chen, T.; Huang, S. Ultrafine Znse Encapsulated in Nitrogen-Doped Porous Carbon Nanofibers for Superior Na-Ion Batteries with a Long Lifespan and Low-Temperature Performance. ACS Sustain. Chem. Eng. 2021, 9, 11705–11713. [Google Scholar] [CrossRef]
- Huang, S.; Ye, M.; Zhang, Y.; Tang, Y.; Liu, X.; Li, C.C. Achieving Ultrahigh-Rate and Low-Temperature Sodium Storage of Feps3 Via in Situ Construction of Graphitized Porous N-Doped Carbon. ACS Appl. Mater. Interfaces 2022, 14, 42048–42056. [Google Scholar] [CrossRef]
- Mo, F.; Lian, Z.; Fu, B.; Song, Y.; Wang, P.; Fang, F.; Zhou, Y.-N.; Peng, S.; Sun, D. A Novel Composite Strategy to Build a Sub-Zero Temperature Stable Anode for Sodium-Ion Batteries. J. Mater. Chem. A 2019, 7, 9051–9058. [Google Scholar] [CrossRef]
- Hu, L.; Pan, J.; Zhao, P.; Shi, G.; Wang, B.; Huang, F. A New Method of Synthesis of Sb2se3/Rgo as a High-Rate and Low-Temperature Anode for Sodium–Ion Batteries. Mater. Adv. 2022, 3, 3554–3561. [Google Scholar] [CrossRef]
- Sun, G.; Lin, H.; Tian, R.; Wei, Z.; Wang, X.; Jin, X.; Yao, S.; Chen, G.; Shen, Z.; Du, F. Rational Design and Synthesis of Nanosheets Self-Assembled Hierarchical Flower-Ball-Like Cufes2 for Boosted Wide Temperature Sodium-Ion Batteries. Nano Res. 2023, 16, 9407–9415. [Google Scholar] [CrossRef]
- Zhang, H.; Song, J.; Li, J.; Feng, J.; Ma, Y.; Ma, L.; Liu, H.; Qin, Y.; Zhao, X.; Wang, F. Interlayer-Expanded Mos2 Nanoflowers Vertically Aligned on Mxene@Dual-Phased TiO2 as High-Performance Anode for Sodium-Ion Batteries. ACS Appl. Mater. Interfaces 2022, 14, 16300–16309. [Google Scholar] [CrossRef]
- Zhou, L.-F.; Gao, X.-W.; Du, T.; Gong, H.; Liu, L.-Y.; Luo, W.-B. Two-Dimensional Nbsse as Anode Material for Low-Temperature Sodium-Ion Batteries. Chem. Eng. J. 2022, 435, 134838. [Google Scholar] [CrossRef]
- Tian, M.; Ben, L.; Jin, Z.; Ji, H.; Yu, H.; Zhao, W.; Huang, X. Excellent Low-Temperature Electrochemical Cycling of an Anode Consisting of Si Nanoparticles Seeded in Sn Nanowires for Lithium-Ion Batteries. Electrochim. Acta 2021, 396, 139224. [Google Scholar] [CrossRef]
- Wei, Y.; He, J.; Zhang, J.; Ou, M.; Guo, Y.; Chen, J.; Zeng, C.; Xu, J.; Han, J.; Zhai, T.; et al. Seamlessly Merging the Capacity of P into Sb at Same Voltage with Maintained Superior Cycle Stability and Low-Temperature Performance for Li-Ion Batteries. Energy Environ. Mater. 2023, 6, e12336. [Google Scholar] [CrossRef]
- Liu, X.; Zhang, T.; Shi, X.; Ma, Y.; Song, D.; Zhang, H.; Liu, X.; Wang, Y.; Zhang, L. Hierarchical Sulfide-Rich Modification Layer on Sio/C Anode for Low-Temperature Li-Ion Batteries. Adv. Sci. 2022, 9, 2104531. [Google Scholar] [CrossRef] [PubMed]
- Huang, K.-C.; Guo, J.-Z.; Li, H.-H.; Fan, H.-H.; Liu, D.-H.; Zheng, Y.-P.; Li, W.-L.; Wu, X.-L.; Zhang, J.-P. Layer-Stacked Sb@Graphene Micro/Nanocomposite with Decent Na-Storage, Full-Cell and Low-Temperature Performances. J. Alloys Compd. 2018, 731, 881–888. [Google Scholar] [CrossRef]
- Wang, B.; Yang, H.; Feng, Y.; Zeng, S.; Tan, L.; Xu, R.; Wang, L.; Hu, R.; Yu, Y. Boosting Low-Temperature Sodium/Potassium Storage Performance of Bi Via Novel Electrochemical Milling Process. Mater. Today Energy 2021, 20, 100627. [Google Scholar] [CrossRef]
- Chen, J.; Fan, X.; Ji, X.; Gao, T.; Hou, S.; Zhou, X.; Wang, L.; Wang, F.; Yang, C.; Chen, L.; et al. Intercalation of Bi Nanoparticles into Graphite Results in an Ultra-Fast and Ultra-Stable Anode Material for Sodium-Ion Batteries. Energy Environ. Sci. 2018, 11, 1218–1225. [Google Scholar] [CrossRef]
- Li, Z.; Zhang, Y.; Zhang, J.; Cao, Y.; Chen, J.; Liu, H.; Wang, Y. Sodium-Ion Battery with a Wide Operation-Temperature Range from −70 to 100 °C. Angew. Chem. Int. Ed. 2022, 61, e202116930. [Google Scholar] [CrossRef]
- Essehli, R.; Alkhateeb, A.; Mahmoud, A.; Boschini, F.; Yahia, H.B.; Amin, R.; Belharouak, I. Optimization of the Compositions of Polyanionic Sodium-Ion Battery Cathode NaFe2−XVx(PO4)(SO4)2. J. Power Sources 2020, 469, 228417. [Google Scholar] [CrossRef]
- Lv, Z.; Ling, M.; Yue, M.; Li, X.; Song, M.; Zheng, Q.; Zhang, H. Vanadium-Based Polyanionic Compounds as Cathode Materials for Sodium-Ion Batteries: Toward High-Energy and High-Power Applications. J. Energy Chem. 2021, 55, 361–390. [Google Scholar] [CrossRef]
- Du, G.; Pang, H. Recent Advancements in Prussian Blue Analogues: Preparation and Application in Batteries. Energy Storage Mater. 2021, 36, 387–408. [Google Scholar] [CrossRef]
- Zhao, X.; Fan, L.-Z.; Zhou, Z. Cationic Potential: An Effective Descriptor for Rational Design of Layered Oxides for Sodium-Ion Batteries. Green Energy Environ. 2020, 6, 455–457. [Google Scholar] [CrossRef]
- Yang, Y.; Wei, W.-F. Electrochemical Mechanism of High Na-Content P2-Type Layered Oxides for Sodium-Ion Batteries. Rare Met. 2020, 39, 332–334. [Google Scholar] [CrossRef]
- Gupta, Y.; Siwatch, P.; Karwasra, R.; Sharma, K.; Tripathi, S. Recent Progress of Layered Structured P2- and O3-Type Transition Metal Oxides as Cathode Material for Sodium-Ion Batteries. Renew. Sustain. Energy Rev. 2024, 192, 114167. [Google Scholar] [CrossRef]
- Liu, Y.; Yao, Z.; Vanaphuti, P.; Yang, X.; Mei, L.; Zhu, X.; Liu, S.; Wang, Y. Stable Fast-Charging Sodium-Ion Batteries Achieved by a Carbomethoxy-Modified Disodium Organic Material. Cell Rep. Phys. Sci. 2023, 4, 101240. [Google Scholar] [CrossRef]
- Guo, J.-Z.; Gu, Z.-Y.; Du, M.; Zhao, X.-X.; Wang, X.-T.; Wu, X.-L. Emerging Characterization Techniques for Delving Polyanion-Type Cathode Materials of Sodium-Ion Batteries. Mater. Today 2023, 66, 221–244. [Google Scholar] [CrossRef]
- Jin, T.; Li, H.; Zhu, K.; Wang, P.-F.; Liu, P.; Jiao, L. Polyanion-Type Cathode Materials for Sodium-Ion Batteries. Chem. Soc. Rev. 2020, 49, 2342–2377. [Google Scholar] [CrossRef]
- Lin, Z.; Shi, H.-Y.; Lin, L.; Yang, X.; Wu, W.; Sun, X. A High Capacity Small Molecule Quinone Cathode for Rechargeable Aqueous Zinc-Organic Batteries. Nat. Commun. 2021, 12, 4424. [Google Scholar] [CrossRef]
- Zhao, B.; Si, Y.; Guo, W.; Fu, Y. Insoluble Naphthoquinone-Derived Molecular Cathode for High-Performance Lithium Organic Battery. Adv. Funct. Mater. 2022, 32, 2112225. [Google Scholar] [CrossRef]
- Jin, S.; Allam, O.; Jang, S.S.; Lee, S.W. Covalent Organic Frameworks: Design and Applications in Electrochemical Energy Storage Devices. InfoMat 2022, 4, e12277. [Google Scholar] [CrossRef]
- Sun, T.; Xie, J.; Guo, W.; Li, D.; Zhang, Q. Covalent–Organic Frameworks: Advanced Organic Electrode Materials for Rechargeable Batteries. Adv. Energy Mater. 2020, 10, 1904199. [Google Scholar] [CrossRef]
- Zhou, Q.; Wang, L.; Li, W.; Zhao, K.; Liu, M.; Wu, Q.; Yang, Y.; He, G.; Parkin, I.P.; Shearing, P.R.; et al. Sodium Superionic Conductors (Nasicons) as Cathode Materials for Sodium-Ion Batteries. Electrochem. Energy Rev. 2021, 4, 793–823. [Google Scholar] [CrossRef]
- Fang, Y.; Xiao, L.; Ai, X.; Cao, Y.; Yang, H. Hierarchical Carbon Framework Wrapped Na3V2(PO4)3 as a Superior High-Rate and Extended Lifespan Cathode for Sodium-Ion Batteries. Adv. Mater. 2015, 27, 5895–5900. [Google Scholar] [CrossRef] [PubMed]
- Rui, X.; Sun, W.; Wu, C.; Yu, Y.; Yan, Q. An Advanced Sodium-Ion Battery Composed of Carbon Coated Na3V2(PO4)3 in a Porous Graphene Network. Adv. Mater. 2015, 27, 6670–6676. [Google Scholar] [CrossRef] [PubMed]
- Guo, J.; Wang, P.; Wu, X.; Zhang, X.; Yan, Q.; Chen, H.; Zhang, J.; Guo, Y. High-Energy/Power and Low-Temperature Cathode for Sodium-Ion Batteries: In Situ Xrd Study and Superior Full-Cell Performance. Adv. Mater. 2017, 29, 1701968. [Google Scholar] [CrossRef] [PubMed]
- Rao, X.; Wang, J.; Yang, M.-A.; Zhao, H.; Li, Z. A Superior Na3v2(Po4)3-Based Cathode Enhanced by Nb-Doping for High-Performance Sodium-Ion Battery. APL Mater. 2022, 10, 010701. [Google Scholar] [CrossRef]
- Lim, S.; Han, D.; Nam, D.; Hong, K.; Eom, J.; Ryu, W.; Kwon, H. Structural Enhancement of Na3V2(PO4)3/C Composite Cathode Materials by Pillar Ion Doping for High Power and Long Cycle Life Sodium-Ion Batteries. J. Mater. Chem. A 2014, 2, 19623–19632. [Google Scholar] [CrossRef]
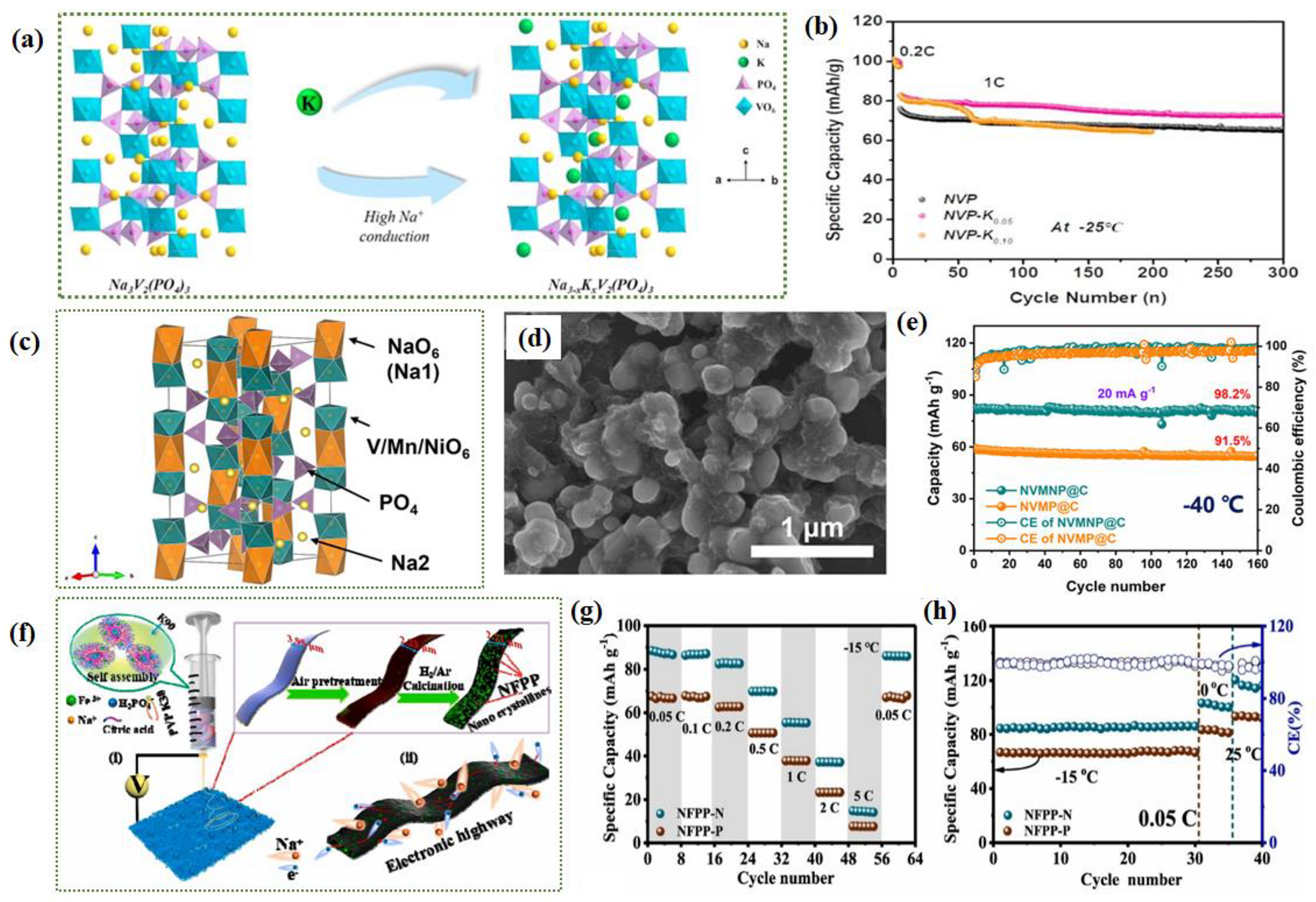
- Shen, L.; Li, Y.; Hu, C.; Huang, Z.; Wang, B.; Wang, Y.; Liu, Q.; Ye, F. A High-Rate Cathode Material Based on Potassium-Doped Na3V2(PO4)3 for High/Low-Temperature Sodium-Ion Batteries. Mater. Today Chem. 2023, 30. [Google Scholar] [CrossRef]
- Xu, S.; Zhu, W.; Yang, Y.; Yao, Y.; Ali, G.; Zhang, X.; Rui, X.; Yu, Y. Bimetal-Substituted Polyanion Cathode for Sodium-Ion Batteries: Less Vanadium and Boosted Low-Temperature Kinetics. Small Struct. 2024, 5, 2300369. [Google Scholar] [CrossRef]
- Zhang, J.; Tang, L.; Zhang, Y.; Li, X.; Xu, Q.; Liu, H.; Ma, Z.-F. Polyvinylpyrrolidone Assisted Synthesized Ultra-Small Na4Fe3(PO4)2(P2O7) Particles Embedded in 1d Carbon Nanoribbons with Enhanced Room and Low Temperature Sodium Storage Performance. J. Power Sources 2021, 498, 229907. [Google Scholar] [CrossRef]
- Li, J.; Li, Z.; Tang, S.; Wang, T.; Wang, K.; Pan, L.; Wang, C. Sodium Titanium Phosphate Nanocube Decorated on Tablet-Like Carbon for Robust Sodium Storage Performance at Low Temperature. J. Colloid Interface Sci. 2023, 629, 121–132. [Google Scholar] [CrossRef]
- Zhou, R.; Wen, X.; Tang, Z.; Wang, C.; Wang, T.; Wang, L.; Zhong, H.; Jiang, Y. Mn–Ni Foreign Ions Codoping Polyanionic Iron-Based Compounds of Na3.12Fe2.44(P2O7)2 for Long-Life and High-Rate Sodium-Ion Battery Cathode. ACS Appl. Energy Mater. 2024, 7, 1756–1765. [Google Scholar] [CrossRef]
- Chen, Y.; Chen, Y.; Zeng, G.; Zeng, G.; Zhang, B.; Zhang, B.; Chen, L.; Chen, L.; Yin, J.; Yin, J.; et al. From Li to Na: Exploratory Analysis of Fe-Based Phosphates Polyanion-Type Cathode Materials by Mn Substitution. Small 2023, 19, 2303929. [Google Scholar] [CrossRef] [PubMed]
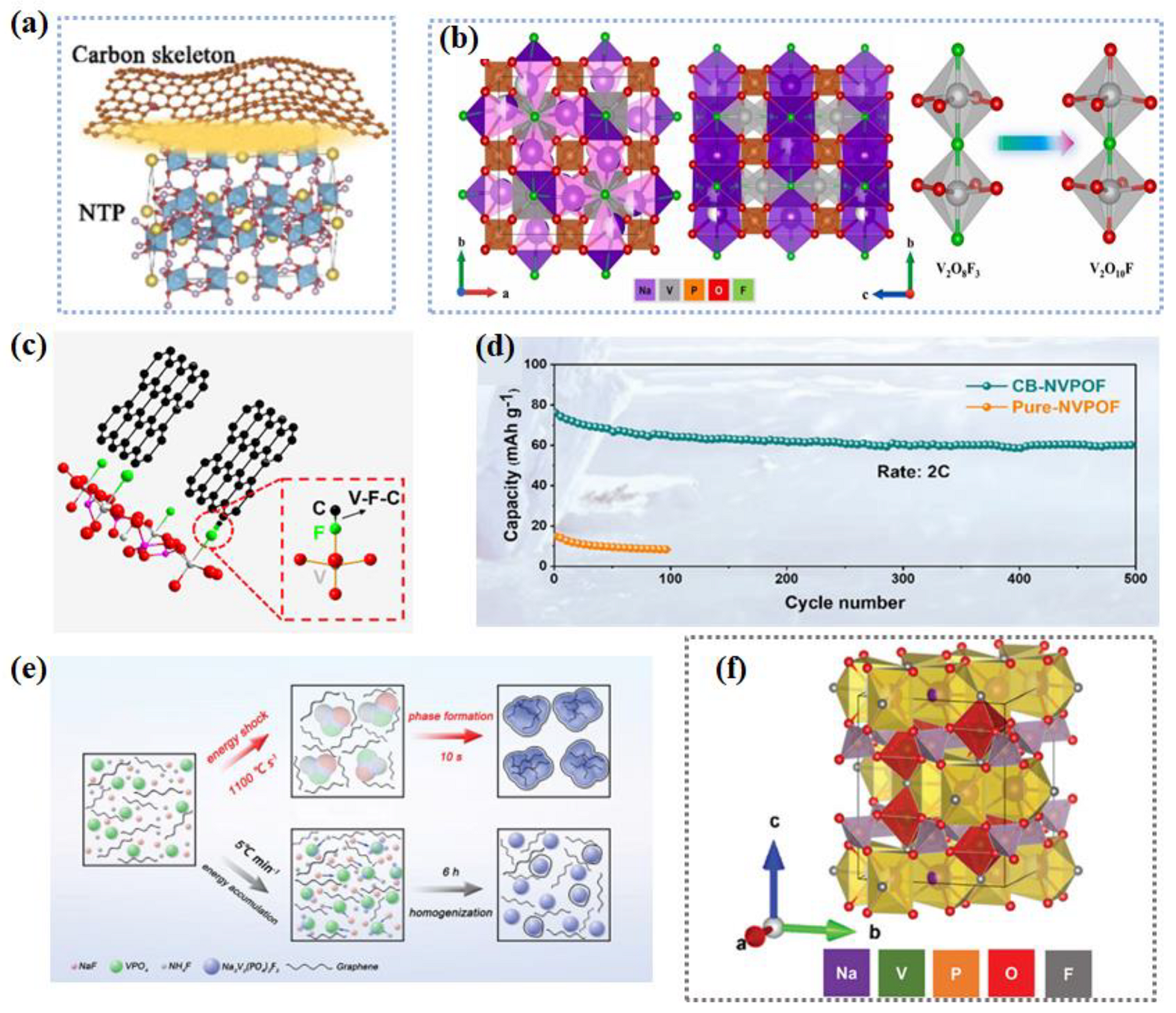
- Deng, L.; Yu, F.-D.; Xia, Y.; Jiang, Y.-S.; Sui, X.-L.; Zhao, L.; Meng, X.-H.; Que, L.-F.; Wang, Z.-B. Stabilizing Fluorine to Achieve High-Voltage and Ultra-Stable Na3V2(PO4)2F3 Cathode for Sodium Ion Batteries. Nano Energy 2021, 82, 105659. [Google Scholar] [CrossRef]
- Xu, S.; Yao, K.; Yang, D.; Chen, D.; Lin, C.; Liu, C.; Wu, H.; Zeng, J.; Liu, L.; Zheng, Y.; et al. Interfacial Engineering of Na3V2(PO4)2O2F Cathode for Low-Temperature (−40 °C) Sodium-Ion Batteries. ACS Appl. Mater. Interfaces 2023, 15, 14329–14338. [Google Scholar] [CrossRef]
- Song, Z.; Liu, Y.; Guo, Z.; Liu, Z.; Li, Z.; Zhou, J.; Liu, W.; Liu, R.; Zhang, J.; Luo, J.; et al. Ultrafast Synthesis of Large-Sized and Conductive Na3V2(PO4)2F3 Simultaneously Approaches High Tap Density, Rate and Cycling Capability. Adv. Funct. Mater. 2024, 34, 2313998. [Google Scholar] [CrossRef]
- Li, L.; Qin, Y.; Zhang, S.; Zhao, H.; Zhao, J.; Li, X.; Zhao, J.; Wu, H.; Su, Y.; Ding, S. Ion Transport through Carbon Nanotubes Enable Highly Crystalline Na3V2(PO4)2F3 Cathode for Ultra-Stable Sodium-Ion Storage. J. Power Sources 2023, 576, 233226. [Google Scholar] [CrossRef]
- Wang, S.-J.; Liang, K.; Li, J.-B.; Huang, X.-B.; Ren, Y.-R. Surfactant-Assisted Synthesis of Self-Assembled Na3V2(PO4)2F3@C Microspheres as the Cathode for Na-Ion Batteries. Vacuum 2023, 211, 111894. [Google Scholar] [CrossRef]
- Liang, K.; Wang, S.; Zhao, H.; Huang, X.; Ren, Y.; He, Z.; Mao, J.; Zheng, J. A Facile Strategy for Developing Uniform Hierarchical Na3V2(PO4)2F3@Carbonized Polyacrylonitrile Multi-Clustered Hollow Microspheres for High-Energy-Density Sodium-Ion Batteries. Chem. Eng. J. 2022, 428, 131780. [Google Scholar] [CrossRef]
- Li, Y.; Liang, X.; Zhong, G.; Wang, C.; Wu, S.; Xu, K.; Yang, C. Fiber-Shape Na3V2(PO4)2F3@N-Doped Carbon as a Cathode Material with Enhanced Cycling Stability for Na-Ion Batteries. ACS Appl. Mater. Interfaces 2020, 12, 25920–25929. [Google Scholar] [CrossRef]
- Rui, X.; Zhang, X.; Xu, S.; Tan, H.; Jiang, Y.; Gan, L.Y.; Feng, Y.; Li, C.C.; Yu, Y. A Low-Temperature Sodium-Ion Full Battery: Superb Kinetics and Cycling Stability. Adv. Funct. Mater. 2020, 31, 2009458. [Google Scholar] [CrossRef]
- Liu, Q.; Hu, Z.; Chen, M.; Zou, C.; Jin, H.; Wang, S.; Chou, S.; Liu, Y.; Dou, S. The Cathode Choice for Commercialization of Sodium-Ion Batteries: Layered Transition Metal Oxides versus Prussian Blue Analogs. Adv. Funct. Mater. 2020, 30, 1909530. [Google Scholar] [CrossRef]
- Qian, J.; Wu, C.; Cao, Y.; Ma, Z.; Huang, Y.; Ai, X.; Yang, H. Prussian Blue Cathode Materials for Sodium-Ion Batteries and Other Ion Batteries. Adv. Energy Mater. 2018, 8, 1702619. [Google Scholar] [CrossRef]
- Lim, C.; Xuan, Q.; Wang, T.; Ong, E.W.Y.; Tan, Z.-K. High-Capacity Sodium–Prussian Blue Rechargeable Battery through Chelation-Induced Nano-Porosity. Adv. Mater. Interfaces 2020, 7, 2000853. [Google Scholar] [CrossRef]
- Yan, X.; Yang, Y.; Liu, E.; Sun, L.; Wang, H.; Liao, X.-Z.; He, Y.; Ma, Z.-F. Improved Cycling Performance of Prussian Blue Cathode for Sodium Ion Batteries by Controlling Operation Voltage Range. Electrochim. Acta 2017, 225, 235–242. [Google Scholar] [CrossRef]
- Wang, X.; Wang, B.; Tang, Y.; Bin Xu, B.; Liang, C.; Yan, M.; Jiang, Y. Manganese Hexacyanoferrate Reinforced by Pedot Coating Towards High-Rate and Long-Life Sodium-Ion Battery Cathode. J. Mater. Chem. A 2020, 8, 3222–3227. [Google Scholar] [CrossRef]
- Xie, B.; Du, Y.; Ma, Y.; Liu, S.; Wang, Y.; Zuo, P.; Gao, Y.; Yin, G. Interface Reinforcement of a Prussian Blue Cathode Using a Non-Flammable Co-Solvent Cresyl Diphenyl Phosphate for a High-Safety Na-Ion Battery. ACS Sustain. Chem. Eng. 2021, 9, 5809–5817. [Google Scholar] [CrossRef]
- Feng, F.; Chen, S.; Zhao, S.; Zhang, W.; Miao, Y.; Che, H.; Liao, X.-Z.; Ma, Z.-F. Enhanced Electrochemical Performance of Mnfe@Nife Prussian Blue Analogue Benefited from the Inhibition of Mn Ions Dissolution for Sodium-Ion Batteries. Chem. Eng. J. 2021, 411, 128518. [Google Scholar] [CrossRef]
- Zhao, Y.; Peng, J.; Chen, K.; Luo, L.; Chen, H.; Zhang, H.; Chou, S.; Feng, X.; Chen, W.; Cao, R.; et al. Boosting the Sodium Storage Performance of Prussian Blue Analogues Via Effective Etching. Sci. China Chem. 2023, 66, 3154–3160. [Google Scholar] [CrossRef]
- Wang, L.; Lu, Y.; Liu, J.; Xu, M.; Cheng, J.; Zhang, D.; Goodenough, J.B. A Superior Low-Cost Cathode for a Na-Ion Battery. Angew. Chem. Int. Ed. 2013, 52, 1964–1967. [Google Scholar] [CrossRef]
- Song, J.; Wang, L.; Lu, Y.; Liu, J.; Guo, B.; Xiao, P.; Lee, J.-J.; Yang, X.-Q.; Henkelman, G.; Goodenough, J.B. Goodenough. Removal of Interstitial H2O in Hexacyanometallates for a Superior Cathode of a Sodium-Ion Battery. J. Am. Chem. Soc. 2015, 137, 2658–2664. [Google Scholar] [CrossRef]
- You, Y.; Wu, X.-L.; Yin, Y.-X.; Guo, Y.-G. High-Quality Prussian Blue Crystals as Superior Cathode Materials for Room-Temperature Sodium-Ion Batteries. Energy Environ. Sci. 2014, 7, 1643–1647. [Google Scholar] [CrossRef]
- You, Y.; Yao, H.-R.; Xin, S.; Yin, Y.-X.; Zuo, T.-T.; Yang, C.-P.; Guo, Y.-G.; Cui, Y.; Wan, L.-J.; Goodenough, J.B. Subzero-Temperature Cathode for a Sodium-Ion Battery. Adv. Mater. 2016, 28, 7243–7248. [Google Scholar] [CrossRef] [PubMed]
- Du, G.; Tao, M.; Li, J.; Yang, T.; Gao, W.; Deng, J.; Qi, Y.; Bao, S.; Xu, M. Low-Operating Temperature, High-Rate and Durable Solid-State Sodium-Ion Battery Based on Polymer Electrolyte and Prussian Blue Cathode. Adv. Energy Mater. 2019, 10, 1903351. [Google Scholar] [CrossRef]
- Peng, J.; Gao, Y.; Zhang, H.; Liu, Z.; Zhang, W.; Li, L.; Qiao, Y.; Yang, W.; Wang, J.; Dou, S.; et al. Ball Milling Solid-State Synthesis of Highly Crystalline Prussian Blue Analogue Na2−XMnFe(CN)6 Cathodes for All-Climate Sodium-Ion Batteries. Angew. Chem. Int. Ed. 2022, 61, e202205867. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.; Zhang, Z.; Cheng, S.; Han, X.; Fu, J.; Sui, M.; Yan, P. Precipitate-Stabilized Surface Enabling High-Performance Na0.67Ni0.33−XMn0.67ZnxO2 for Sodium-Ion Battery. eScience 2022, 2, 529–536. [Google Scholar] [CrossRef]
- Cheng, C.; Hu, H.; Yuan, C.; Xia, X.; Mao, J.; Dai, K.; Zhang, L. Precisely Modulating the Structural Stability and Redox Potential of Sodium Layered Cathodes through the Synergetic Effect of Co-Doping Strategy. Energy Storage Mater. 2022, 52, 10–18. [Google Scholar] [CrossRef]
- Zhou, P.; Che, Z.; Liu, J.; Zhou, J.; Wu, X.; Weng, J.; Zhao, J.; Cao, H.; Zhou, J.; Cheng, F. High-Entropy P2/O3 Biphasic Cathode Materials for Wide-Temperature Rechargeable Sodium-Ion Batteries. Energy Storage Mater. 2023, 57, 618–627. [Google Scholar] [CrossRef]
- Wang, X.; Yin, X.; Feng, X.; Li, Y.; Dong, X.; Shi, Q.; Zhao, Y.; Zhang, J. Rational Design of Na0.67Ni0.2CO0.2Mn0.6O2 Microsphere Cathode Material for Stable and Low Temperature Sodium Ion Storage. Chem. Eng. J. 2022, 428, 130990. [Google Scholar] [CrossRef]
- Liu, S.; Wan, J.; Ou, M.; Zhang, W.; Chang, M.; Cheng, F.; Xu, Y.; Sun, S.; Luo, C.; Yang, K.; et al. Regulating Na Occupation in P2-Type Layered Oxide Cathode for All-Climate Sodium-Ion Batteries. Adv. Energy Mater. 2023, 13, 2203521. [Google Scholar] [CrossRef]
- Li, Q.; Liu, G.; Cheng, H.; Sun, Q.; Zhang, J.; Ming, J. Low-Temperature Electrolyte Design for Lithium-Ion Batteries: Prospect and Challenges. Chemistry 2021, 27, 15842–15865. [Google Scholar] [CrossRef]
- Li, Y.; Wu, F.; Li, Y.; Liu, M.; Feng, X.; Bai, Y.; Wu, C. Ether-Based Electrolytes for Sodium Ion Batteries. Chem. Soc. Rev. 2022, 51, 4484–4536. [Google Scholar] [CrossRef] [PubMed]
- Tian, Z.; Zou, Y.; Liu, G.; Wang, Y.; Yin, J.; Ming, J.; Alshareef, H.N. Alshareef. Electrolyte Solvation Structure Design for Sodium Ion Batteries. Adv. Sci. 2022, 9, 2201207. [Google Scholar] [CrossRef] [PubMed]
- Che, H.; Yang, X.; Yu, Y.; Pan, C.; Wang, H.; Deng, Y.; Li, L.; Ma, Z.-F. Engineering Optimization Approach of Nonaqueous Electrolyte for Sodium Ion Battery with Long Cycle Life and Safety. Green Energy Environ. 2021, 6, 212–219. [Google Scholar] [CrossRef]
- Deng, L.; Goh, K.; Yu, F.-D.; Xia, Y.; Jiang, Y.-S.; Ke, W.; Han, Y.; Que, L.-F.; Zhou, J.; Wang, Z.-B. Self-Optimizing Weak Solvation Effects Achieving Faster Low-Temperature Charge Transfer Kinetics for High-Voltage Na3V2(PO4)2F3 Cathode. Energy Storage Mater. 2022, 44, 82–92. [Google Scholar] [CrossRef]
- Xu, J.; Zhang, J.; Pollard, T.P.; Li, Q.; Tan, S.; Hou, S.; Wan, H.; Chen, F.; He, H.; Hu, E.; et al. Electrolyte Design for Li-Ion Batteries under Extreme Operating Conditions. Nature 2023, 614, 694–700. [Google Scholar] [CrossRef] [PubMed]
- Mo, Y.; Liu, G.; Yin, Y.; Tao, M.; Chen, J.; Peng, Y.; Wang, Y.; Yang, Y.; Wang, C.; Dong, X.; et al. Fluorinated Solvent Molecule Tuning Enables Fast-Charging and Low-Temperature Lithium-Ion Batteries. Adv. Energy Mater. 2023, 13, 2301285. [Google Scholar] [CrossRef]
- Liu, Y.-T.; Liang, H.-J.; Du, M.; Yang, J.-L.; Gu, Z.-Y.; Wang, X.-T.; Tang, Y.-Z.; Guo, J.-Z.; Wu, X.-L. Ester-Based Anti-Freezing Electrolyte Achieving Ultra-Low Temperature Cycling for Sodium-Ion Batteries. J. Mater. Sci. Technol. 2024, 182, 111–118. [Google Scholar] [CrossRef]
- Liu, X.; Zheng, X.; Qin, X.; Deng, Y.; Dai, Y.; Zhao, T.; Wang, Z.; Yang, H.; Luo, W. Temperature-Responsive Solid-Electrolyte-Interphase Enabling Stable Sodium Metal Batteries in a Wide Temperature Range. Nano Energy 2022, 103, 107746. [Google Scholar] [CrossRef]
- Zhong, S.; Yu, Y.; Yang, Y.; Yao, Y.; Wang, L.; He, S.; Yang, Y.; Liu, L.; Sun, W.; Feng, Y.; et al. Molecular Engineering on Solvation Structure of Carbonate Electrolyte toward Durable Sodium Metal Battery at −40 °C. Angew. Chem. Int. Ed. 2023, 62, e202301169. [Google Scholar] [CrossRef]
- Desai, P.; Abou-Rjeily, J.; Tarascon, J.-M.; Mariyappan, S. Practicality of Methyl Acetate as a Co-Solvent for Fast Charging Na-Ion Battery Electrolytes. Electrochim. Acta 2022, 416, 140217. [Google Scholar] [CrossRef]
- Cui, G.; Wang, H.; Yu, F.; Che, H.; Liao, X.; Li, L.; Yang, W.; Ma, Z. Scalable Synthesis of Na3V2(PO4)3/C with High Safety and Ultrahigh-Rate Performance for Sodium-Ion Batteries. Chin. J. Chem. Eng. 2022, 46, 280–286. [Google Scholar] [CrossRef]
- Liu, B.; Zhang, Q.; Li, L.; Zhang, L.; Jin, Z.; Wang, C.; Su, Z. Achieving Highly Electrochemically Active Maricite Nafepo4 with Ultrafine NaFePO4@C Subunits for High Rate and Low Temperature Sodium-Ion Batteries. Chem. Eng. J. 2021, 405, 126689. [Google Scholar] [CrossRef]
- Liang, H.-J.; Gu, Z.; Zhao, X.; Guo, J.; Yang, J.; Li, W.; Li, B.; Liu, Z.; Li, W.; Wu, X. Ether-Based Electrolyte Chemistry towards High-Voltage and Long-Life Na-Ion Full Batteries. Angew. Chem. Int. Ed. 2021, 60, 26837–26846. [Google Scholar] [CrossRef] [PubMed]
- Dong, R.; Zheng, L.; Bai, Y.; Ni, Q.; Li, Y.; Wu, F.; Ren, H.; Wu, C. Elucidating the Mechanism of Fast Na Storage Kinetics in Ether Electrolytes for Hard Carbon Anodes. Adv. Mater. 2021, 33, 2008810. [Google Scholar] [CrossRef]
- Yang, C.; Liu, X.; Lin, Y.; Yin, L.; Lu, J.; You, Y. Entropy-Driven Solvation toward Low-Temperature Sodium-Ion Batteries with Temperature-Adaptive Feature. Adv. Mater. 2023, 35, 2301817. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Lan, H.; Dong, S.; Zhu, Q.; Cheng, L.; Wang, H.; Wang, L.; Wang, S.; Tang, M.; Shodievich, K.M.; et al. A High-Power Rechargeable Sodium-Ion Full Battery Operating at −40 °C. Adv. Funct. Mater. 2024, 34, 2315498. [Google Scholar] [CrossRef]
- Hu, L.; Deng, J.; Lin, Y.; Liang, Q.; Ge, B.; Weng, Q.; Bai, Y.; Li, Y.; Deng, Y.; Chen, G.; et al. Restructuring Electrolyte Solvation by a Versatile Diluent toward Beyond 99.9% Coulombic Efficiency of Sodium Plating/Stripping at Ultralow Temperatures. Adv. Mater. 2024, 36, 2312161. [Google Scholar] [CrossRef]
- Yin, L.; Wang, M.; Xie, C.; Yang, C.; Han, J.; You, Y. High-Voltage Cyclic Ether-Based Electrolytes for Low-Temperature Sodium-Ion Batteries. ACS Appl. Mater. Interfaces 2023, 15, 9517–9523. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Wang, Y.; Wang, J.; Liu, Y.; Li, Y.; Cheng, L.; Ding, Y.; Dong, S.; Zhu, Q.; Tang, M.; et al. Low-Temperature and High-Rate Sodium Metal Batteries Enabled by Electrolyte Chemistry. Energy Storage Mater. 2022, 50, 47–54. [Google Scholar] [CrossRef]
- Tang, Z.; Wang, H.; Wu, P.; Zhou, S.; Huang, Y.; Zhang, R.; Sun, D.; Tang, Y.; Wang, H. Electrode–Electrolyte Interfacial Chemistry Modulation for Ultra-High Rate Sodium-Ion Batteries. Angew. Chem. Int. Ed. 2022, 61, e202200475. [Google Scholar] [CrossRef]
- Sun, M.-Y.; Yu, F.-D.; Xia, Y.; Deng, L.; Jiang, Y.-S.; Que, L.-F.; Zhao, L.; Wang, Z.-B. Trigger Na+-Solvent Co-Intercalation to Achieve High-Performance Sodium-Ion Batteries at Subzero Temperature. Chem. Eng. J. 2022, 430, 132750. [Google Scholar] [CrossRef]
- Chen, J.; Peng, Y.; Yin, Y.; Fang, Z.; Cao, Y.; Wang, Y.; Dong, X.; Xia, Y. A Desolvation-Free Sodium Dual-Ion Chemistry for High Power Density and Extremely Low Temperature. Angew. Chem. Int. Ed. 2021, 60, 23858–23862. [Google Scholar] [CrossRef] [PubMed]
- Shi, Q.; Qi, R.; Feng, X.; Wang, J.; Li, Y.; Yao, Z.; Wang, X.; Li, Q.; Lu, X.; Zhang, J.; et al. Niobium-Doped Layered Cathode Material for High-Power and Low-Temperature Sodium-Ion Batteries. Nat. Commun. 2022, 13, 3205. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Zhang, M.; Chen, Z.; Du, X.; Huang, S.; Tang, B.; Dong, T.; Wu, H.; Yu, Z.; Zhang, J.; et al. Flame-Retardant Quasi-Solid Polymer Electrolyte Enabling Sodium Metal Batteries with Highly Safe Characteristic and Superior Cycling Stability. Nano Res. 2019, 12, 2230–2237. [Google Scholar] [CrossRef]
- Lu, Y.; Alonso, J.A.; Yi, Q.; Lu, L.; Wang, Z.L.; Sun, C. A High-Performance Monolithic Solid-State Sodium Battery with Ca2+ Doped Na3Zr2Si2PO12 Electrolyte. Adv. Energy Mater. 2019, 9, 1901205. [Google Scholar] [CrossRef]
- Kim, J.-K.; Lim, Y.J.; Kim, H.; Cho, G.-B.; Kim, Y. A Hybrid Solid Electrolyte for Flexible Solid-State Sodium Batteries. Energy Environ. Sci. 2015, 8, 3589–3596. [Google Scholar] [CrossRef]
- Gao, H.; Xin, S.; Xue, L.; Goodenough, J.B. Goodenough. Stabilizing a High-Energy-Density Rechargeable Sodium Battery with a Solid Electrolyte. Chem 2018, 4, 833–844. [Google Scholar] [CrossRef]
- Zhao, C.; Liu, L.; Qi, X.; Lu, Y.; Wu, F.; Zhao, J.; Yu, Y.; Hu, Y.-S.; Chen, L. Solid-State Sodium Batteries. Adv. Energy Mater. 2018, 8, 1703012. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, Y.; Zhang, Z.; Zheng, Y.; Luo, Y.; Jiang, X.; Wang, Y.; Wang, Z.; Wu, Y.; Zhang, Y.; Liu, X.; et al. Sodium-Ion Battery at Low Temperature: Challenges and Strategies. Nanomaterials 2024, 14, 1604. https://doi.org/10.3390/nano14191604
Zhao Y, Zhang Z, Zheng Y, Luo Y, Jiang X, Wang Y, Wang Z, Wu Y, Zhang Y, Liu X, et al. Sodium-Ion Battery at Low Temperature: Challenges and Strategies. Nanomaterials. 2024; 14(19):1604. https://doi.org/10.3390/nano14191604
Chicago/Turabian StyleZhao, Yan, Zhen Zhang, Yalong Zheng, Yichao Luo, Xinyu Jiang, Yaru Wang, Zhoulu Wang, Yutong Wu, Yi Zhang, Xiang Liu, and et al. 2024. "Sodium-Ion Battery at Low Temperature: Challenges and Strategies" Nanomaterials 14, no. 19: 1604. https://doi.org/10.3390/nano14191604
APA StyleZhao, Y., Zhang, Z., Zheng, Y., Luo, Y., Jiang, X., Wang, Y., Wang, Z., Wu, Y., Zhang, Y., Liu, X., & Fang, B. (2024). Sodium-Ion Battery at Low Temperature: Challenges and Strategies. Nanomaterials, 14(19), 1604. https://doi.org/10.3390/nano14191604






